Published online Jun 22, 2017. doi: 10.5498/wjp.v7.i2.128
Peer-review started: December 19, 2016
First decision: April 18, 2017
Revised: April 27, 2017
Accepted: May 12, 2017
Article in press: May 15, 2017
Published online: June 22, 2017
Increasing evidence shows that cognitive impairment and brain abnormalities can appear early in the first episodes of schizophrenia, but it is currently debated how brain changes can correlate with clinical presentation of schizophrenic patients. Of note, this report describes the case of a young schizophrenic male presenting parietal magnetic resonance/positron emission tomography abnormalities and cognitive impairment, documented by specific neuropsychological tests. In our knowledge only few studies have investigated if neuropsychological abnormalities could be concomitant with both structural and functional neuroimaging. This case shows that impairment in specific cognitive domains is associated with structural/functional brain abnormalities in the corresponding brain areas (frontal and parietal lobes), supporting the hypothesis of disconnectivity, involving a failure to integrate anatomical and functional pathways. Future research would define the role of cognitive impairment and neurodegeneration in psychiatric nosography and, in particular, their role in the early phases of illness and long-term outcome of schizophrenic patients.
Core tip: Schizophrenia is associated with impairment in executive function, verbal memory, verbal fluency and attention. Neuropsychological tests are associated with structural and functional brain alterations. This case report is an example of the potential correlation between clinical symptoms (e.g., cognitive impairment) and brain changes. These data may help in the prediction of possible outcome of schizophrenia patients.
- Citation: Grassi S, Orsenigo G, Serati M, Caletti E, Altamura AC, Buoli M. Cognitive correlates of neuroimaging abnormalities in the onset of schizophrenia: A case report. World J Psychiatr 2017; 7(2): 128-132
- URL: https://www.wjgnet.com/2220-3206/full/v7/i2/128.htm
- DOI: https://dx.doi.org/10.5498/wjp.v7.i2.128
A number of data would indicate schizophrenia as a progressive neurodegenerative disorder[1] whose outcome is influenced by many biological and clinical factors[2]. Of note, recent literature shows that neuropsychological deficits at onset may predict the clinical course of illness[3] being often associated with frontal and parietal lobe dysfunctions[4-6]. Moreover, a recent trial found that brain abnormalities of schizophrenic patients change according to age at onset. In particular, early onset patients show parietal abnormalities, while adult onset patients exhibit frontal and temporal ones[7].
To our knowledge there are few studies[8-10] associating cognitive frontal and parietal deficits with structural [magnetic resonance (MR)] and functional neuroimaging [positron emission tomography (PET)] and the anatomical and functional relationships underlying this deficit remain to be elucidated. Dysconnectivity, a failure in functional integration, is considered a key mechanism in the pathophysiology of cognitive impairments (in particular working memory performance) in individuals with schizophrenia[11].
The present paper deals with a recent diagnosed schizophrenic patient showing frontal and parietal lobe MR/PET abnormalities clinically associated with deficits in the corresponding cognitive domains.
The patient was a 19-year-old man admitted in our department. The patient showed nor psychiatric comorbidity with an Axis I disorder neither personality disorders. A neurological exam, performed by a neurologist, was negative. Diagnosis of undifferentiated schizophrenia and exclusion of comorbid conditions were assessed through the administration of semi-structured interviews based on DSM-IV criteria (SCID I and II). Patient had family history for psychiatric disorders: The father was an alcohol abuser, one schizophrenic uncle (father’s brother) committed suicide and the grandmother in mother line was affected by bipolar disorder. At the admission in our ward the patient was drug-naïve and showed persecutory delusion, auditory hallucinations, thought/behavioural disorganization and a duration of untreated psychosis of 9 mo[12]. Baseline score at Positive and Negative Syndrome Scale[13] was 84, while baseline score at Brief Psychiatric Rating Scale was 55[14]. In the first days of admission patient underwent to neuropsychological tests, cerebral MR and cerebral PET.
A neuropsychological battery was designed to encompass the areas believed to be affected by Schizophrenia[15]. Results and standard scores are summarized in Table 1. Patient’s neurocognitive performances provided evidence for impairment in the following domains: Executive function (Cognitive Estimation, Verbal fluency, Trail Making Test), verbal memory, verbal ability (Boston Naming Test, phonemic Verbal Fluency) and attention (Visual Search, Trail Making Test). In addition, the patient failed in two Wechsler Adult Intelligence Scale[16] subscales: Verbal Comprehension Index and Perceptual Organization Index.
| Test | Patient score | Normal value | Result | Z-score |
| Mini-mental state examination | 27.19 | 24-29.19 | Normal | 0.45 |
| Executive functions: Tower of London | 25 | 20-36 | Normal | -0.75 |
| Frontal assessment battery | 15.98 | 13.5-17.3 | Normal | -0.95 |
| Cognitive estimation task | 19.97 | 0-18 | Failed | 2.43 |
| Bizarreness | 6 | 0-4 | Failed | 4 |
| Problem solving: Raven’s progressive matrices | 29.05 | 18.6-33.05 | Normal | 0.89 |
| Assessment of cognitive impairment in memory | ||||
| Verbal memory and learning | ||||
| Digit Span | 5.75 | 3.75-8.75 | Normal | -0.4 |
| Verbal Learning | 10.50 | 6.50-21.50 | Normal | -0.93 |
| Recall of prose: Immediate and after 10 min | 3.50 | 8.00-27.50 | Failed | -2.92 |
| Spatial short-term memory (Corsi test) | 4.50 | 3.50-8.50 | Normal | -1.20 |
| Attention and speed information processing | ||||
| Trail making test | ||||
| Part A | 33 | < 93 s | Normal | |
| Part B, dual task | 161 | < 282 s | Normal | |
| Part B-A | 128 | < 186 s | Borderline score | -1.36 |
| Visual search | 34.25 | 31-51.25 | Borderline score | |
| Verbal fluency | ||||
| Phonemic | 23 | 17-59 | Borderline score | -1.43 |
| Categories | 32 | 25-58 | Normal | -1.15 |
| Language | ||||
| Boston naming test | 31 | 43-60 | Failed | -4.82 |
| Token test | 32 | 29-36 | Normal | -0.29 |
| Wechsler adult intelligence scale-revised | General IQ = 75 (verbal IQ = 81; performance IQ = 74) | 80-120 | Borderline score | -2.50 |
| VCI = 5.5; POI = 6.25 |
MR was performed using a circular polarized head coil and included Turbo Spin-Echo T1-weighted sequences, T2-weighted sequences and FLAIR. Imaging in three planes was performed using 5-mm slice thickness. MR revealed normal-sized ventricles, normal-sized subarachnoidal spaces, no abnormalities in gray matter, but bilaterally soft hyper-intensities in superior parietal lobe[4] periventricular white matter.
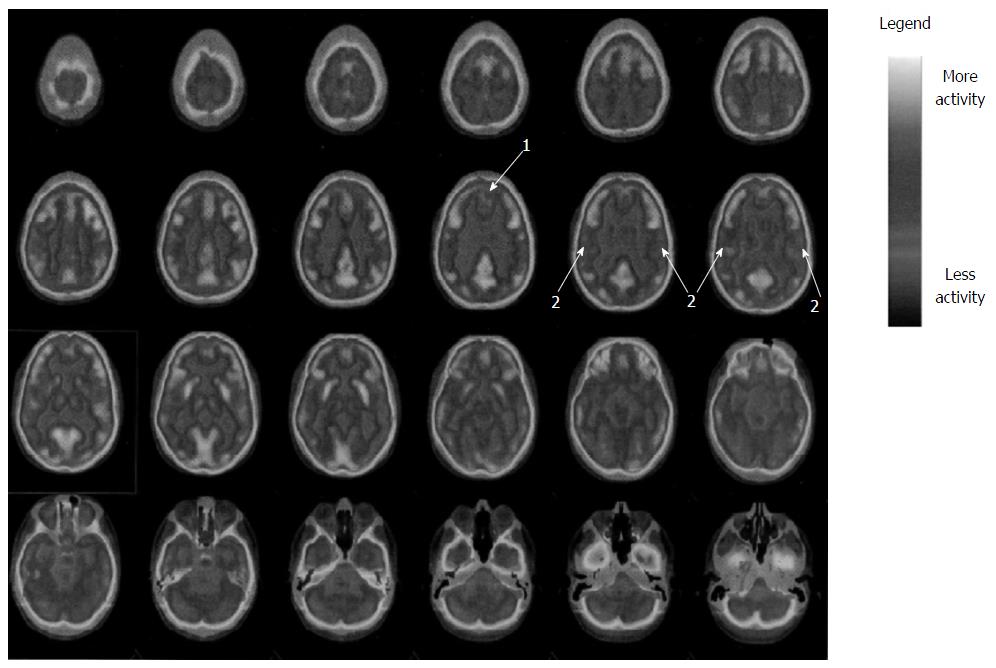
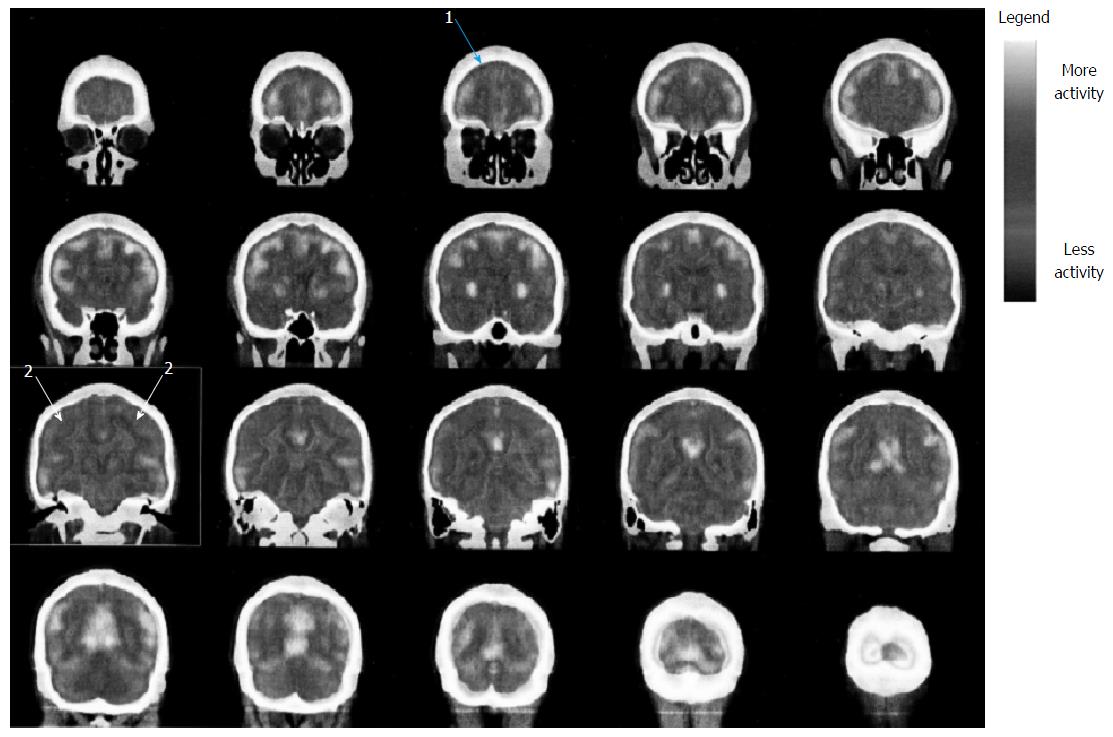
Fluorodeoxyglucose (FDG) was injected in condition of rest and fasting and after 30 min three-dimensional scan was performed. The images were compared to a cohort of normal ones. Fluoro-D-Glucose PET (Figures 1 and 2) showed glucose frontal and parietal lobes hypo-metabolism bilaterally. No further abnormalities in FDG distribution were observed.
MR and PET were performed by neuroradiologists collaborating within our department.
Of note, neuropsychological results are consistent with outlined MR abnormalities and PET images (fronto-parietal abnormalities)[17].
The present case report confirms data from literature of early cognitive deficits in the course of schizophrenia[18,19] and neuroimaging parietal abnormalities in early onset schizophrenic patients[7,20,21]. In addition, the correspondence between cognitive deficits and morphological/functional brain alterations[22] contributes to clarify the influence of brain changes in schizophrenia clinical presentation as well as to support the hypothesis of schizophrenia as a neurodegenerative disorder[23,24]. Recent trials found that brain abnormalities are more severe in patients with a longer duration of illness[25-27], novel antipsychotics are promising molecules for their efficacy in stopping the neurodegenerative process[28,29]. In this context cognitive and neuroimaging follow-up of our case can be useful to discriminate if neurodegenerative process of schizophrenia progresses in the course of illness or it is specific of early stages[24,30,31]. Finally, it would be important in the future to define the role of neuroimaging abnormalities in influencing outcome. MR and PET could be useful tools to make diagnosis and to predict long-term course of schizophrenic illness.
A 19-year-old male patient with severe schizophrenia presentation.
Patient was hospitalized because of prominent persecutory delusion, auditory hallucinations, aggressiveness and thought/behavioural disorganization.
Bipolar disorder, substance use disorder.
Routine blood tests were resulted within normal limits.
At magnetic resonance imaging bilaterally soft hyper-intensities in superior parietal lobe periventricular white matter were detected, while positron emission tomography showed glucose parietal lobes hypo-metabolism bilaterally.
Schizophrenia, acute episode.
Ziprasidone 80 mg × 2 and Gabapentin 300 mg × 3.
Severe cognitive impairment as showed by neuropsychological tests.
Dysconnectivity means abnormal functional integration among brain regions resulting in impaired modulation of neurotransmitters.
It is important to perform imaging evaluation and neuropsychological tests to better define long-term outcome of schizophrenia patients.
This case report is novel and well designed.
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Belli H, Kravos M S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | van Haren NE, Schnack HG, Cahn W, van den Heuvel MP, Lepage C, Collins L, Evans AC, Hulshoff Pol HE, Kahn RS. Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry. 2011;68:871-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 272] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 2. | Altamura AC, Bobo WV, Meltzer HY. Factors affecting outcome in schizophrenia and their relevance for psychopharmacological treatment. Int Clin Psychopharmacol. 2007;22:249-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Wölwer W, Brinkmeyer J, Riesbeck M, Freimüller L, Klimke A, Wagner M, Möller HJ, Klingberg S, Gaebel W. Neuropsychological impairments predict the clinical course in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2008;258 Suppl 5:28-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res. 2006;148:75-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 228] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 5. | Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry. 2009;166:863-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Schmidt A, Smieskova R, Simon A, Allen P, Fusar-Poli P, McGuire PK, Bendfeldt K, Aston J, Lang UE, Walter M. Abnormal effective connectivity and psychopathological symptoms in the psychosis high-risk state. J Psychiatry Neurosci. 2014;39:239-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Kyriakopoulos M, Perez-Iglesias R, Woolley JB, Kanaan RA, Vyas NS, Barker GJ, Frangou S, McGuire PK. Effect of age at onset of schizophrenia on white matter abnormalities. Br J Psychiatry. 2009;195:346-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Deserno L, Sterzer P, Wüstenberg T, Heinz A, Schlagenhauf F. Reduced prefrontal-parietal effective connectivity and working memory deficits in schizophrenia. J Neurosci. 2012;32:12-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 9. | Pujol N, Penadés R, Rametti G, Catalán R, Vidal-Piñeiro D, Palacios E, Bargallo N, Bernardo M, Junqué C. Inferior frontal and insular cortical thinning is related to dysfunctional brain activation/deactivation during working memory task in schizophrenic patients. Psychiatry Res. 2013;214:94-101. [PubMed] [Cited in This Article: ] |
| 10. | He Z, Deng W, Li M, Chen Z, Jiang L, Wang Q, Huang C, Collier DA, Gong Q, Ma X. Aberrant intrinsic brain activity and cognitive deficit in first-episode treatment-naive patients with schizophrenia. Psychol Med. 2013;43:769-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 813] [Cited by in F6Publishing: 812] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 12. | Altamura AC, Serati M, Buoli M. Is duration of illness really influencing outcome in major psychoses? Nord J Psychiatry. 2015;69:403-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl. 1989;155:59-67. [PubMed] [Cited in This Article: ] |
| 14. | Flemenbaum A, Zimmermann RL. Inter- and intra-rater reliability of the Brief Psychiatric Rating Scale. Psychol Rep. 1973;32:783-792. [PubMed] [Cited in This Article: ] |
| 15. | Stefanopoulou E, Manoharan A, Landau S, Geddes JR, Goodwin G, Frangou S. Cognitive functioning in patients with affective disorders and schizophrenia: a meta-analysis. Int Rev Psychiatry. 2009;21:336-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Kay SR. Schizophrenic WAIS pattern by diagnostic subtypes. Percept Mot Skills. 1979;48:1241-1242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Leeson VC, Barnes TR, Harrison M, Matheson E, Harrison I, Mutsatsa SH, Ron MA, Joyce EM. The relationship between IQ, memory, executive function, and processing speed in recent-onset psychosis: 1-year stability and clinical outcome. Schizophr Bull. 2010;36:400-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Fitzgerald D, Lucas S, Redoblado MA, Winter V, Brennan J, Anderson J, Harris A. Cognitive functioning in young people with first episode psychosis: relationship to diagnosis and clinical characteristics. Aust N Z J Psychiatry. 2004;38:501-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Fatouros-Bergman H, Cervenka S, Flyckt L, Edman G, Farde L. Meta-analysis of cognitive performance in drug-naïve patients with schizophrenia. Schizophr Res. 2014;158:156-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 20. | Bartholomeusz CF, Cropley VL, Wannan C, Di Biase M, McGorry PD, Pantelis C. Structural neuroimaging across early-stage psychosis: Aberrations in neurobiological trajectories and implications for the staging model. Aust N Z J Psychiatry. 2017;51:455-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Yildiz M, Borgwardt SJ, Berger GE. Parietal lobes in schizophrenia: do they matter? Schizophr Res Treatment. 2011;2011:581686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Minatogawa-Chang TM, Schaufelberger MS, Ayres AM, Duran FL, Gutt EK, Murray RM, Rushe TM, McGuire PK, Menezes PR, Scazufca M. Cognitive performance is related to cortical grey matter volumes in early stages of schizophrenia: a population-based study of first-episode psychosis. Schizophr Res. 2009;113:200-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Rund BR. Is schizophrenia a neurodegenerative disorder? Nord J Psychiatry. 2009;63:196-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70:88-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 364] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 25. | Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34:354-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 253] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Torres US, Duran FL, Schaufelberger MS, Crippa JA, Louzã MR, Sallet PC, Kanegusuku CY, Elkis H, Gattaz WF, Bassitt DP. Patterns of regional gray matter loss at different stages of schizophrenia: A multisite, cross-sectional VBM study in first-episode and chronic illness. Neuroimage Clin. 2016;12:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 27. | Buoli M, Caldiroli A, Panza G, Altamura AC. Prominent clinical dimension, duration of illness and treatment response in schizophrenia: a naturalistic study. Psychiatry Investig. 2012;9:354-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Mandl RC, Collins DL, Evans AC, Kahn RS. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32:2057-2066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 29. | Ozcelik-Eroglu E, Ertugrul A, Oguz KK, Has AC, Karahan S, Yazici MK. Effect of clozapine on white matter integrity in patients with schizophrenia: a diffusion tensor imaging study. Psychiatry Res. 2014;223:226-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 30. | Zanello A, Curtis L, Badan Bâ M, Merlo MC. Working memory impairments in first-episode psychosis and chronic schizophrenia. Psychiatry Res. 2009;165:10-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Braw Y, Bloch Y, Mendelovich S, Ratzoni G, Gal G, Harari H, Tripto A, Levkovitz Y. Cognition in young schizophrenia outpatients: comparison of first-episode with multiepisode patients. Schizophr Bull. 2008;34:544-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |