Published online Mar 19, 2021. doi: 10.5498/wjp.v11.i3.73
Peer-review started: December 1, 2020
First decision: December 12, 2020
Revised: December 23, 2020
Accepted: January 28, 2021
Article in press: January 28, 2021
Published online: March 19, 2021
Illness anxiety disorder (IAD) is a common, distressing, and debilitating condition with the key feature being a persistent conviction of the possibility of having one or more serious or progressive physical disorders. Because eye movements are guided by visual-spatial attention, eye-tracking technology is a comparatively direct, continuous measure of attention direction and speed when stimuli are oriented. Researchers have tried to identify selective visual attention biases by tracking eye movements within dot-probe paradigms because dot-probe paradigm can distinguish these attentional biases more clearly.
To examine the association between IAD and biased processing of illness-related information.
A case-control study design was used to record eye movements of individuals with IAD and healthy controls while participants viewed a set of pictures from four categories (illness-related, socially threatening, positive, and neutral images). Biases in initial orienting were assessed from the location of the initial shift in gaze, and biases in the maintenance of attention were assessed from the duration of gaze that was initially fixated on the picture per image category.
The eye movement of the participants in the IAD group was characterized by an avoidance bias in initial orienting to illness-related pictures. There was no evidence of individuals with IAD spending significantly more time viewing illness-related images compared with other images. Patients with IAD had an attention bias at the early stage and overall attentional avoidance. In addition, this study found that patients with significant anxiety symptoms showed attention bias in the late stages of attention processing.
Illness-related information processing biases appear to be a robust feature of IAD and may have an important role in explaining the etiology and maintenance of the disorder.
Core Tip: This is the first study which has examined patients with illness anxiety disorder (IAD) having an attention bias that is mainly manifested as attentional avoidance at the early stage and overall attentional maintenance when presented with illness-related stimuli. They also have demonstrated vigilance of attention at the early attention stage and overall attentional maintenance when presented with disease and positive/neutral stimuli. In addition, this study found that patients with suspected disorders with significant anxiety symptoms show attention bias in the late stage of attention processing and struggle to dismiss the stimulus, showing delayed detachment. This study suggests that patients with IAD have attention bias and this may have provided a new way of identification of IAD symptoms using an eye-tracking evaluation method.
- Citation: Zhang YB, Wang PC, Ma Y, Yang XY, Meng FQ, Broadley SA, Sun J, Li ZJ. Using eye movements in the dot-probe paradigm to investigate attention bias in illness anxiety disorder. World J Psychiatr 2021; 11(3): 73-86
- URL: https://www.wjgnet.com/2220-3206/full/v11/i3/73.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i3.73
Illness anxiety disorder (IAD) is a common, distressing, and debilitating condition, with the key feature being a persistent conviction of the possibility of having one or more serious or progressive physical disorders[1]. IAD was formerly named hypochondriasis in DSM-4. Currently, the reported prevalence is approximately 1%[2-5]. Patients manifest persistent somatic complaints or a persistent preoccupation with their physical appearance. They repeatedly seek assurance from medical advice for physical symptoms that they think they have, and frequently have doubt or fear about negative medical test results and doctors’ explanations[6]. This type of preoccupational thought persists despite appropriate medical evaluation and medical reassurance. The course of IAD is usually chronic and fluctuating, associated with costly and unnecessary medical reexaminations and the overuse of medical resources[5].
According to cognitive-behavioral models of IAD[1], IAD patients seem to hold faulty health beliefs. The dysfunctional assumptions about their bodily symptoms and illness may be triggered by critical incidents. It has been argued that the dysfunctional beliefs about illness and misinterpretation of bodily symptoms appear to be specific and important for patients with IAD and to be a highly specific characteristic of IAD[7,8]. They tend to show a more restrictive concept of good health than do anxiety patients and healthy people[8]. They are hypervigilant to bodily symptoms or tend to amplify benign physical sensations[9-11].
It has been proposed that these psychological processes may underpin the symptoms of IAD. A cognitive-behavior therapy (CBT) for IAD that is derived from W and S’s model has been effective. CBT patients had lower levels of health-related anxiety and less impairment of social roles, but IAD somatic symptoms were not improved significantly by CBT[11,12]. Other researchers have observed a positive correlation between the feature-positive effect (FPE) and illness anxiety concerns[13]. The FPE is the bias of overweighing positive and underweighing negative information. The FPE may explain the interesting fact of the IAD’s sensitivity to illness cues and insensitivity to medical reassurance or negative medical results, but it cannot fully explain the initial preoccupation with bodily complaints and health.
IAD is characterized by patients focusing their attention on one or two organs or systems of their body. They attend to information that confirms their worry and conviction, but simultaneously ignore and under-weigh negative results of medical investigations and evidence of good health. Thus, they display a type of information processing bias, and cognitive processes are considered relevant to the etiology and maintenance of IAD[14].
Attention bias refers to the individuals who have different attention distribution to the corresponding threat or related stimulus, relative to the neutral stimulus[15]. Researchers studying attention bias found patients with mental illness often allocate their attention towards concern-related or mood-congruent material. A key feature of attention biases is anxiety increasing patients’ attention to danger and threat-related stimuli[16]. Patients have attention bias in favor of stimuli referring to their specific disorder, and interpretational bias leads them to allocate their attention to these stimuli as more relevant. Thus, their bias is specific to the meaning of stimuli relevant to the disorder. In people with anxiety disorders, anxiety stimuli are likely to receive attention bias when they are danger or from threat-related stimuli, but in people with IAD, the content and disorder specificity of the stimuli is unclear. More recently, some non-illness-related attentional stimuli have been suggested to maintain IAD concerns; in other words, the attention bias may not be limited to illness-related stimuli but also include non-illness-related stimuli[17,18]. In sum, empirical evidence regarding measuring attention bias in IAD is scarce and equivocal.
To date, a prominent means to evaluate visual attention is the emotional stroop task. Researchers have regarded increased color-naming latencies of emotional or concern-related words as an indicator of selective attention bias, but unfortunately the emotional stroop task cannot differentiate the two possible mechanisms a facilitated engagement or a delayed disengagement[19,20]. So the precise course of such biases is obscured by the mixed nature of the test process. Alternative experimental paradigms regarding attention bias, such as the dot-probe investigation and the Posner cued-target paradigm, can distinguish these two possible biases more clearly[21,22]. Because eye movements (EMs) are guided by visual-spatial attention, eye-tracking technology is a comparatively direct, continuous measure of attention direction and speed when stimuli are oriented. ‘Persistent’, the dot-probe experiment, which uses EM tracking, has been undertaken primarily to examine attentional processing among anxiety disorders and in pain-related research. Researchers have tried to identify selective visual attention biases by tracking EMs within dot-probe paradigms with success[23,24]. These studies investigated individuals with high social anxiety, generalized anxiety disorder, and depressive disorder, using EMs and a modified probe detection task to pictures of faces. They found that anxiety individuals initially directed their gaze toward neutral faces more often, but were quicker to look at emotional faces and looked at emotional faces for less time. Therefore, anxiety individuals were consistent with a vigilant avoidant pattern of bias, compared with other people.
Using the dot-probe paradigm, our primary aim in the current study was to explore the specificity of attention bias in IAD. We hypothesized that patients with IAD, compared with healthy controls, show more attention bias for illness-related information when negative pictures, positive pictures, and neutral pictures are presented simultaneously. In addition, we hypothesized that patients display orienting attention biases, reflected by fixating first and more rapidly on illness stimuli than other alternatives. We analyzed differences in EM indices reflecting subsequent bias in attention maintenance, including duration of first fixation, average fixation and total gaze/first fixation, and overall gaze duration.
A case-control study design was used for the study, with the sample consisting of an IAD patient group and a healthy control (HC) group. Participants were recruited via e-mail and newspaper advertisement from Beijing An’Ding Hospital. The patient group fulfilled the criteria for IAD (DSM-5 and formerly named hypochondriasis in DSM-4) according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR)[25]. Patients were screened with the Structured Clinical Interview for DSM-IV-TR Axis I Disorder (SCID-I) and Structured Clinical Interview For DSM-IV-TR Axis II Disorder (SCID-II)[26] to affirm the initial axis I disorder diagnosis and axis II personality disorders. Interviews were led by independent, professionally trained clinical psychologists. The HC group was matched to the patient group in age, sex, ethnicity, and education. People who were unable to use their hands, or those with a serious head injury or psychiatric illness, were excluded from the study. All participants had normal or correct-to-normal vision and color vision and no neurological disease, psychiatric illness, or medication use for any psychiatric disorder. Their age ranged from 18 to 60 years, and their education level was 9 years or more.
Ethical approval for this research was obtained from the Beijing An’Ding Hospital, Capital Medical University Research Ethics Committee [Ethics number: (2014) Research (No. 38), Funding number: 20131107110012]. All participants were fully informed about the study research and provided with a general overview of the dot-probe task. All participants provided written informed consent before they agreed to participate in the research in compliance with the regulations of the institution and the guidelines of the Declaration of Helsinki. They were free to terminate the experiment at any time they wished.
The stimuli used in the dot-probe task consisted of 128 pictures; we collected these pictures through Baidu image search, a search engine in China, which were grouped into four main categories: Illness-related, social threat, positive, and neutral pictures. Illness-related pictures included pictures about patients in disease/hospitals/ doctors/doctors threating or checking patients and pictures about oral medicine. Social threat pictures included pictures about earthquake/quarrel/flood/ wars/fires/explosion/traffic/fighting/bridge collapse/plane crash/chasing by animals. Positive pictures included mountain/flowers/children/bird/women/ wedding/congratulation/dancing. Neutral pictures included pictures about furniture/meeting/beautiful scenery/shopping/roads/clothes. These pictures have clear meaning, clear content, and try to highlight the stimulus itself; at the same time, these pictures cover as wide range of meanings as possible. The color of the images was unified with Photoshop CS 6.0 about brightness, saturation and contrast. Illness-related and social threat pictures were included to determine whether IAD patients were associated with a specific threat-related attention bias or any threatening material. Positive pictures were included to examine whether a general emotional response accounted for any effects found. These images were randomly presented to participants for 2 s each. Each picture size had mean height of 4.6 cm and mean width of 6.5 cm.
Sixty healthy volunteers were recruited to classify the pictures into our four predefined categories in our pilot study. These volunteers had normal or correct-to-normal vision and color vision. They were not on any medication and had no diagnosed medical or mental health condition. Among 400 pictures, 272 (68%) pictures were excluded as they were not allocated to the correct picture category by at least 80% of the raters. The final number of selected pictures for trials was 128.
Stimulus pairs were presented on a DELL 19 inch TN screen, with 1980 × 1024 pixel resolution and a 60 Hz refresh rate.
EM data were recorded via an sr research eyelink 1000 device (sr research, Canada; https://www.sr-research.com/). The experiment was programmed and run with sr research experiment builder. The eye-tracker sampling rate was 1000 Hz, with a spatial accuracy greater than 0.5° and a resolution of 0.01° in the pupil-tracking mode. An infrared motion system tracked em/head motion. A forehead and chin rest kept viewing distance constant and minimized head movements. Participants sat 60 cm from the monitor screen, resulting in a 29 cm horizontal, 22 cm vertical visual field. Prior to the task, a standardized 5-point calibration procedure was performed.
EM data were recorded during each trial, starting shortly before and terminating immediately after picture-pair presentation (Figure 1).
Selective attention bias was assessed in a visual dot-probe paradigm. With traditional dot-probe tasks, stimulus pairs are presented in separate spatial locations (e.g., left vs right side of computer screen) and replaced by a probe in the location previously occupied by one stimulus. For this study, the main focus was on EMs during stimulus presentation of different image types. Before the formal study, a brief training (four trials) was undertaken to increase familiarity with task requirements; training procedures were identical to those used in the formal study with the exception that different pictures were used for the present study. Participants were told that in each trial of the forthcoming spatial perception task, a triangle would appear in the right or left of the computer screen for 500 milliseconds (ms), followed by picture pairs to which they should carefully attend. Each trial of the task began with a central fixation cross (+) shown for 500 ms and then replaced by an image pair that appeared for 2000 ms. After 500 ms, the next trial began. The formal task included 224 pairs of cards presented in four blocks with 56 trials per block. The four blocks consisted of combinations of the four categories of picture meanings, including (1) Disease–neutral (e.g., oral surgery vs rail), as shown in Figure 2; (2) Disease–positive (e.g., infusion vs scenery), as shown in Figure 2; and (3) Disease–threat (e.g., operating room vs traffic), as shown in Figure 2. Besides, there were four categories included as filling items in order to reduce the monotony of the test materials, including disease-disease, threat-threat, neutral-neutral, and positive-positive. Each pair of images was defined as a trial and each pair of pictures was presented randomly among the four blocks. Participants were given a 2-min break after the presentations of 56 pairs of pictures. On average, the task took 40 min to complete.
After providing informed consent, participants were escorted to the eye-tracking experimental area. They were seated in a height-adjustable chair with their chin in a vertically adjusted chin rest. The chin rest was used to reduce participant head movements, ensuring that participants’ eyes were level with the middle of the monitor on which the stimuli were presented, and to maintain participant eye distance from the monitor at 60 cm. They were instructed to free view the images at all times during each trial. Further, participants were asked to look at the fixation point before each trial to standardize the starting location of their gaze. On completion of the dot-probe task, participants were asked to fill in several questionnaires, including their self-reported measures of demographics. In addition, the Beck Anxiety Inventory (BAI)[27], Beck Depression Inventory[28], Illness Attitudes Scale[29], and Whiteley Index[30] were used to assess participants’ clinical symptoms.
EM data was recorded using the Eyelink1000 tracking system (Canadian sr-research) and was processed by data retrieval. First, the trials affected by invalid calibrations and uncollected EM data were removed; as each pair had two stimulus-related trials, there are total 448 trials per participant, the average invalid trials in 30 IAD participants were 13.767 (3.07%), and the average invalid trials in HC participants were 13.500 (3.01%). Then, we used data viewer software to divide the positions of the two pictures in the same picture matrix into two interest areas and selected valid EM data, including the corresponding position of the first viewpoint, the first-fixation duration, the overall gaze durations, and the overall fixation frequencies in different interest areas. Then, they were exported to Excel (Microsoft) in text format and were collated and converted in Excel to obtain the following attention bias scores: The first-fixation direction bias score = number of views (the first view of a certain type of stimulus) / effective trials; First-fixation duration bias score: The average viewing time (the first view of a certain type of stimulus); Overall gaze duration bias score = total time gazing at a certain type of stimulus/total time gazing at all stimuli in the trial; Overall fixation frequency bias score = the total number of times (viewing a certain type of stimulus)/the total number of times of viewing all stimuli in the trial. The general demographic data and scores of each clinical scale were measured using the international business machines statistical package for the social sciences studies V21.0 software package. Metrological data were represented by mean ± SD. All four EM indicators were statistically analyzed using independent sample t-test. The differences between IAD group and HC group were compared on different types of images. The mean value of the group was compared with that of independent samples test, and P < 0.05 indicated that the difference was statistically significant.
Our study mainly examined four types of EM indicators: (1) First-fixation direction bias (%): The percentage of the number of first percentile points that an individual directs to the area of interest in which a certain stimulus is located as a percentage of the total number of active trials. It mainly examines the individual's initial attentional alert feature; (2) First-fixation duration bias (ms): In the disease–neutral, disease–threat and disease–positive image pairing, the difference reflects the early attention to the stimulation information in the area of interest; (3) Overall gaze duration bias (ms): During the entire experimental process, the total focus time of an individual on the area of interest of a certain type of stimulus accounts for the percentage of the total time spent staring at two pictures in the trial; and (4) Overall fixation frequency bias (%): Throughout the experiment, the total number of times an individual looks at the area of interest of a certain type of stimulus as a percentage of the total number of times a person looks at two pictures in the trial.
Table 1 depicts demographic characteristics and clinical symptoms of the IAD patient group and HC group.
| Item | Patient group: IAD group, n = 30 | Control group: HC group, n = 30 | t/χ2 | P value |
| Age in yr | 30.03 ± 8.73 | 30.70 ± 8.61 | −0.298 | 0.767 |
| Sex, male/female | 19/11 | 14/16 | 1.684 | 0.194 |
| Educational background | ||||
| Junior high school | 5 | 5 | ||
| Senior vocational/high school | 4 | 4 | ||
| Junior college/university | 20 | 20 | ||
| Master’s degree or higher education | 1 | 1 | ||
| IAS | 47.53 ± 16.44 | 27.43 ± 13.07 | 5.240c | < 0.001 |
| WI | 7.60 ± 3.83 | 3.13 ± 1.98 | 5.677c | < 0.001 |
| BAI | 38.17 ± 12.09 | 24.57 ± 4.33 | 5.799c | < 0.001 |
| BDI | 18.10 ± 9.65 | 6.13 ± 6.71 | 5.577c | < 0.001 |
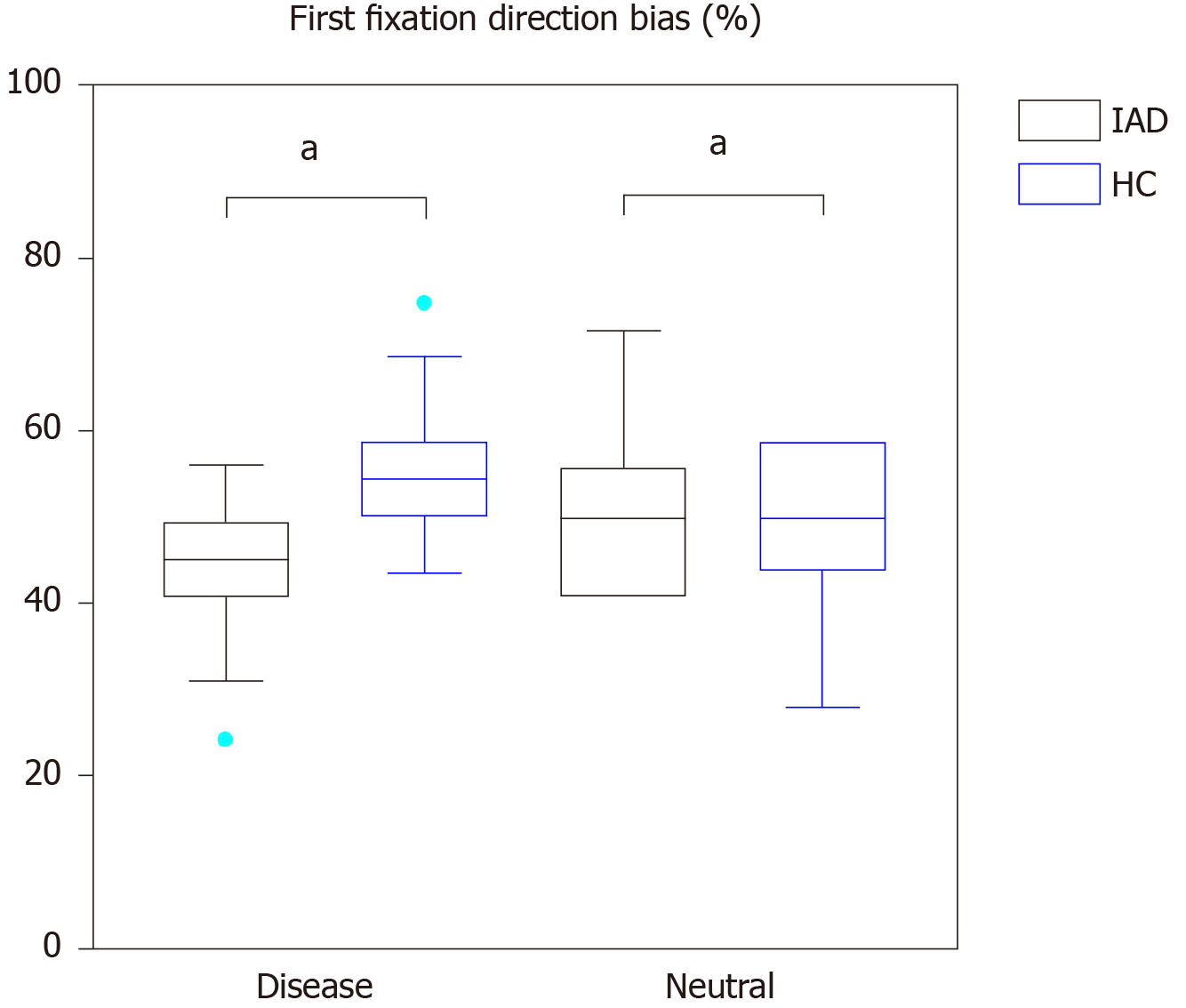
Table 2 reports attention bias-related EM data, including first-fixation direction bias (%), first-fixation duration bias (ms), overall gaze duration bias (ms), and overall fixation frequency bias (%) in the IAD group and HC group (Figure 3).
| Item | Image classification | IAD group, n = 30 | HC group, n = 30 | t | P value |
| First-fixation direction bias as % | |||||
| Disease–threat | Disease | 49.90 ± 9.93 | 49.94 ± 8.96 | 0.341 | 0.734 |
| Threat | 50.10 ± 9.93 | 50.94 ± 8.96 | -0.341 | 0.734 | |
| Disease–positive | Disease | 34.90 ± 12.23 | 32.92 ± 9.77 | 0.693 | 0.491 |
| Positive | 65.10 ± 12.23 | 67.08 ± 9.77 | -0.693 | 0.491 | |
| Disease–neutral | Disease | 45.00 ± 7.81 | 50.21 ± 8.53 | -2.467a | 0.017 |
| Neutral | 55.00 ± 7.81 | 49.79 ± 8.53 | 2.467a | 0.017 | |
| First-fixation duration bias in ms | |||||
| Disease–threat | Disease | 281.20 ± 61.47 | 246.40 ± 30.99 | 2.769b | 0.008 |
| Threat | 296.86 ± 58.13 | 266.37 ± 40.17 | 2.363a | 0.022 | |
| Disease–positive | Disease | 272.16 ± 61.83 | 246.20 ± 35.4 | 1.996 | 0.051 |
| Positive | 259.41 ± 49.95 | 238.71 ± 48.83 | 1.623 | 0.110 | |
| Disease–neutral | Disease | 280.48 ± 55.15 | 252.81 ± 45.07 | 2.128a | 0.038 |
| Neutral | 288.28 ± 59.08 | 264.67 ± 47.27 | 1.709 | 0.093 | |
| Overall gaze duration bias in ms | |||||
| Disease–threat | Disease | 44.90 ± 4.13 | 44.51 ± 3.86 | 0.388 | 0.700 |
| Threat | 55.09 ± 4.13 | 55.49 ± 3.85 | -0.388 | 0.700 | |
| Disease–positive | Disease | 46.45 ± 5.3 | 44.35 ± 6.21 | 1.407 | 0.165 |
| Positive | 53.55 ± 5.3 | 55.65 ± 6.21 | -1.407 | 0.165 | |
| Disease–neutral | Disease | 47.18 ± 4.13 | 46.97 ± 4.50 | 0.185 | 0.854 |
| Neutral | 52.82 ± 4.12 | 53.03 ± 4.50 | -0.185 | 0.854 | |
| Overall fixation frequency bias as % | |||||
| Disease–threat | Disease | 45.63 ± 3.24 | 45.59 ± 3.83 | 0.051 | 0.960 |
| Threat | 54.37 ± 3.24 | 54.41 ± 3.83 | -0.051 | 0.960 | |
| Disease–positive | Disease | 46.93 ± 4.43 | 44.67 ± 5.06 | 1.841 | 0.071 |
| Positive | 53.07 ± 4.43 | 55.33 ± 5.06 | -1.841 | 0.071 | |
| Disease–neutral | Disease | 48.44 ± 3.78 | 48.76 ± 3.50 | -0.341 | 0.735 |
| Neutral | 51.56 ± 3.78 | 51.24 ± 3.50 | 0.341 | 0.735 | |
When the participants were presented with the disease–neutral image pairs independent sample t-test showed there were significant differences in the first-fixation direction when presented with disease-related and neutral images in the HC group. When presented with disease-related images, the first-fixation direction of the IAD group was lower than that of the HC group (t = -2.467, P = 0.017); while presented with neutral images, it was higher than that of HC group (t = 2.467, P = 0.017).
When the participants were presented with the disease–neutral image pairs, independent sample t-test found that the first-fixation duration of the IAD group (280.476 ± 55.154) for the disease picture was significantly greater than that of the HC group (252.807 ± 45.075) (t = 2.128, P = 0.038). There was no difference in the neutral picture (IAD group: 288.280 ± 59.083, HC group: 264.668 ± 47.270; t = 1.709, P = 0.093).
When the participants were presented with the disease–social threat image pairs, independent sample t-test found that the first-fixation duration of the IAD group (281.20 ± 61.472) for the disease picture was significantly greater than that of the HC group (246.40 ± 30.991) (t = 2.769, P = 0.008). There was also difference in the social threat picture (IAD group: 296.86 ± 58.129, HC group: 266.37 ± 40.172; t = 2.363, P = 0.022).
When the participants were presented with disease-positive image pairs, independent sample t-test showed there were no significant differences in the first-fixation duration bias when presented with disease-related and positive-related images between the IAD group and HC group.
When the participants were presented with the disease–social threat, disease-positive, and disease-neutral pairs, independent sample t-test showed there were no significant differences between the IAD group and HC group.
When the participants were presented with the disease–positive image pairs, independent sample t-test results showed the overall fixation frequency of the two groups toward the illness-related and positive images were different. Independent sample t-test showed the overall fixation frequency bias toward the disease images in the IAD group was higher than in the HC group (t = 1.841, P = 0.071), and fixation frequency bias toward the positive images was lower than in the HC group (t = -1.841, P = 0.071).
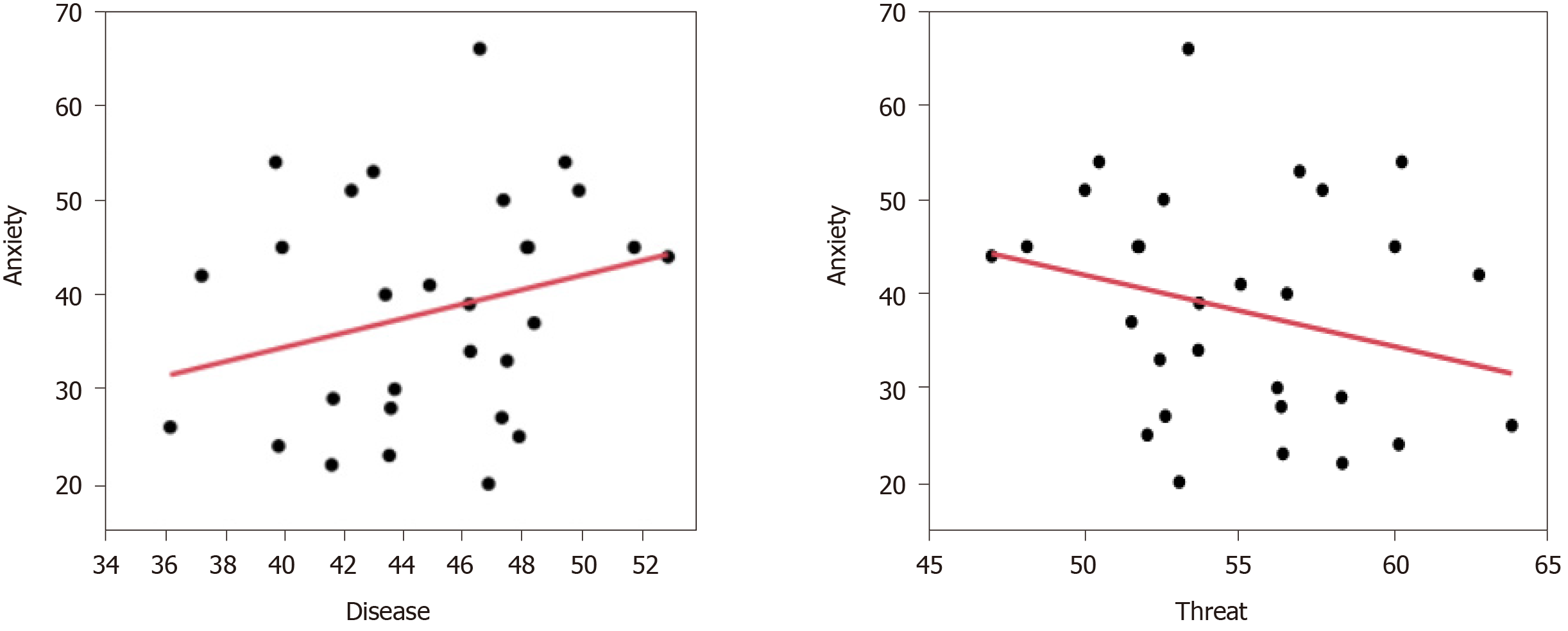
Regression analysis was used to investigate the correlations between the characteristics of the clinical symptoms of the IAD group and their EMs with various image pairs. The results showed that when the participants were presented with the disease–threat image pairs, their overall gaze duration bias toward illness-related images and BAI were found to have a positive correlation with disease-related images (β = +0.156, P = 0.045) and negative correlation with threat-related images (β = -0.156, P = 0.045) (Table 3 and Figure 4).
| Adjusted R-square | Regression coefficient (95%CI) | P value | |
| Disease image | 0.055 | 0.156 (0.004 to 0.308) | 0.045 |
| Threat image | 0.055 | -0.156 (-0.308 to -0.004) | 0.045 |
With the changes of diagnostic criteria, discussion about IAD is increasing these years. This study discovered that in the early and late stages of attention, the IAD and HC groups were different in their information processing for illness-related images and had corresponding attention mechanisms at different stages of attention.
In the early stage of attention, when presented with different pairs of socially threatening, positive, illness-related, and neutral images, participants in the IAD group did not manifest an attention bias toward illness-related images, and no vigilance attention bias was observed. However, the first-fixation direction of IAD participants on illness-related images was shorter than that of the HC group, indicating that patients with IAD had an early-stage attentional avoidance bias on illness-related stimuli. Once the early visual attention of these patients was captured by symptom-related or socially threatening stimuli, they were less likely to disengage their attention to such stimuli, thus exhibiting delayed engagement bias. At the late stage of attention, participants with IAD presented a noticeable avoidance of illness-related images, which was manifested as a less overall fixation frequency on such pictures and more fixation frequency on positive, neutral and threat-related images. Therefore, the attentional pattern of patients with IAD was shown as an attentional delayed disengagement bias toward illness-related and socially threatening images, and an overall attentional avoidance bias to illness-related images.
The hypervigilance–avoidance hypothesis suggests that individuals tend to show instant vigilance, followed by swift avoidance, when presented with threatening stimuli to prevent further processing of threatening information. This attention mechanism allows individuals to detect more potentially threatening information during the early stage of attention. The attentional avoidance mechanism then hinders such information from being objectively evaluated, leading to a cognitive bias toward the threatening stimuli and creating a selective attention, judgment, and storage effect. For this reason, this study speculated that individuals with IAD have normal attentional processing mechanisms for stimuli related to neutral, positive and threat emotions. However, their longer attention engagement toward illness and threat-related information in the early attention stage and their overall avoidance mechanism in the late stage of attention may explain why they suffer from long-term processing failure of disease-related stimuli, and hence have psychological disorders.
Current research suggests that such an attentional maintenance bias in the early attention stage may be due to the activation of disease-related schema within patients with IAD[31]. Attention schema theory suggests that if the threat stimulus is consistent with the schematic contents of an individual, then such stimulus is more likely to be processed. In addition, the activation of schemata leads to the allocation of more attentional resources to the threat stimulus at the late stage of strategic processing, manifesting as a greater frequency of selective attention. According to Salkovskis’s cognitive-behavioral model of IAD, patients with IAD tend to overvalue illness-related information, giving it greater value and significance than reality, thereby inducing a cognitive bias such as selective attention. It is possible that illness-related information is more likely to activate the illness-related interpretation of the patients, leading to a longer processing period of such stimuli and the demonstration of a longer attention maintenance toward illness-related stimuli in the early maintenance stage.
At the late stage of attention, participants with IAD presented a noticeable avoidance of illness-related images, which was manifested as a shorter overall fixation duration on such pictures and a less overall fixation frequency. Therefore, the attentional pattern of patients with IAD was shown as an attentional avoidance bias toward illness-related images at the whole attention stage, but a delayed disengagement bias once attention captured by illness or threat information. The results demonstrate the attentional patterns of the participants when presented with illness-related, positive, and neutral stimuli. The findings revealed that patients with IAD showed different attentional patterns when presented with illness-related stimuli and neutral stimuli. Specifically, patients showed an attentional maintenance bias in the early stage of attention and overall attentional avoidance toward illness symptom-related stimuli; however, they presented high levels of attention in the early attention stage and held their overall attentional maintenance when presented with normal stimuli (neutral information).
To further investigate the relationship between attention bias toward illness-related stimuli and the severity of symptoms among patients with IAD, this study utilized regression analysis. The results showed that the severity of anxiety symptoms had an impact on attention bias. In patients with mood disorder, there is a weakening of the ability to pay attention to the automatic processing of emotional information, resulting in defects and biases in the processing of emotional information[32]. Beck[33] believes that abnormal processing of emotional information in patients with mood disorders may be the main factor in the etiology of the disease and the maintenance of symptoms. Patients are sensitive to specific negative emotional information and alter their cognitive processing process, which may lead to symptom aggravation and maintenance. In many theories of clinical anxiety[19,34]. These attention biases have been regarded as initiating and maintaining clinical anxiety.
In 2009, Olatunji et al[35] proposed that the internal cognitive process indicates that IAD disorder may be related to anxiety disorder. The results also found that the more severe the anxiety symptoms, the more likely the disease stimulus attracts or occupies attention resources than other stimuli. Anxiety and IAD patients allocate excessive attention to disease information and struggle to dismiss the stimulus, showing delayed disengagement and attention bias; however, this feature is not obvious in their initial attentional stage.
This study applied EM tracking technology to investigate attention biases of patients with IAD. Compared with past measurement methods based on participants’ reactions, EM technology can effectively, directly, and continuously investigate the processing activities of attention of patients with IAD toward stimuli with threatening emotions, thereby differentiating the attention bias components more effectively. However, the method remains at the overt behavioral level and cannot analyze physiological parameters such as pupil changes and saccade during attentional processing. In addition, the method does not reveal the neurobiological mechanisms at play during attentional processing; hence, it is impossible to further explain the abnormality of brain function of patients with IAD. Future research is suggested to employ event-related potential technology (which has high time resolution) and functional magnetic resonance imaging technology (which has high spatial resolution) alongside eye-tracking technology to further investigate the characteristics of the attention bias and its mechanisms in patients with IAD. Furthermore, future study will use a machine learning classification method for the study to identify the IAD participants and estimate the accuracy of the eye-tracking method in identifying attention bias in patients with illness anxiety disorder.
Patients with IAD have an attention bias that is mainly manifested as an overall attentional avoidance when presented illness-related stimuli with disease, positive, and neutral stimuli, as well as avoidance of attention at the early attention stage; when presented with neutral stimuli, however, IAD performance showed higher delayed disengagement bias to illness and disease-related stimulus than in HCs. In addition, this study found that patients with suspected disorders with significant anxiety symptoms show attention bias in the late stage of attention processing, and struggle to dismiss the stimulus, showing delayed detachment. This study suggests that patients with IAD have attention bias and this may have provided a new way of identification of IAD symptoms using the eye-tracking evaluation method.
Illness anxiety disorder (IAD) is a common, distressing, and debilitating condition with the key feature being a persistent conviction of the possibility of having one or more serious or progressive physical disorders. Because eye movements (EMs) are guided by visual-spatial attention, eye-tracking technology is a comparatively direct, continuous measure of attention direction and speed when stimuli are oriented. Researchers have tried to identify selective visual attention biases by tracking EMs within dot-probe paradigms because the dot-probe paradigm can distinguish these attentional biases more clearly.
There are numerous studies that have investigated individuals with high social anxiety, generalized anxiety disorder, and depressive disorder, using EMs and a modified probe detection task to pictures of faces. However, no studies have provided an in-depth analysis of illness anxiety disorder using EMs.
Using the dot-probe paradigm, our primary aim in the current study was to explore the specificity of attention bias in IAD. In addition, we aimed to examine whether patients will display orienting attention biases, reflected by fixating first and more rapidly on illness stimuli than other alternatives.
A case-control study design was used for the study, with the sample consisting of an IAD patient group and a healthy control (HC) group. Participants were recruited via e-mail and newspaper advertisement from Beijing An’Ding Hospital. The patient group fulfilled the criteria for IAD (DSM-5 and formerly named hypochondriasis in DSM-4) according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). The stimuli used in the dot-probe task consisted of 128 pictures including 35 pictures from China internet sources, and 93 pictures selected from the International Affective Pictures System, which were grouped into four main categories: Illness-related, social threat, positive, and neutral pictures. These pictures were used to assess attentional bias in IAD patients. All four EM indicators were statistically analyzed using independent sample t-test. The differences between IAD group and HC group were compared on different types of images. The mean value of the group was compared with that of independent samples test, and P < 0.05 indicated that the difference was statistically significant.
When presented with disease-related images, the first-fixation direction of the IAD group was lower than that of the HC group (t = -2.467, P = 0.017); while presented with neutral images, it was higher than that of HC group (t = 2.467, P = 0.017). When the participants were presented with the disease–neutral image pairs, independent sample t-test found that the first-fixation duration of the IAD group (280.476 ± 55.154) for the disease picture was significantly greater than that of the HC group (252.807 ± 45.075) (t = 2.128, P = 0.038). When the participants were presented with the disease–social threat image pairs, independent sample t-test found that the first-fixation duration of the IAD group (281.20 ± 61.472) for the disease picture was significantly greater than that of the HC group (246.40 ± 30.991; t = 2.769, P = 0.008). There was also a difference in the social threat picture (IAD group: 296.86 ± 58.129, HC group: 266.37 ± 40.172; t = 2.363, P = 0.022). Independent sample t-test showed the overall fixation frequency bias toward the disease images in the IAD group was higher than in the HC group (t = 1.841, P = 0.071), and fixation frequency bias toward the positive images was lower than in the HC group (t = -1.841, P = 0.071).
Patients with IAD have an attention bias that is mainly manifested as an overall attentional avoidance when presented illness-related stimuli with disease, positive, and neutral stimuli, as well as avoidance of attention at the early attention stage when presented with neutral stimuli; however, IAD performance showed higher delayed disengagement bias to illness and disease-related stimulus than did the HCs. In addition, this study found that patients with suspected disorders with significant anxiety symptoms show attention bias in the late stage of attention processing, and struggle to dismiss the stimulus, showing delayed detachment.
This study suggests that patients with IAD have attention bias and this may have provided a new way of identifying IAD symptoms using the eye-tracking evaluation method.
STROBE Statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Llanes-Jurado J S-Editor: Zhang L L-Editor: Filipodia P-Editor: Li JH
| 1. | Bound F. Hypochondria. Lancet. 2006;367:105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Looper KJ, Kirmayer LJ. Hypochondriacal concerns in a community population. Psychol Med. 2001;31:577-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Martin A, Jacobi F. Features of hypochondriasis and illness worry in the general population in Germany. Psychosom Med. 2006;68:770-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Sunderland M, Newby JM, Andrews G. Health anxiety in Australia: prevalence, comorbidity, disability and service use. Br J Psychiatry. 2013;202:56-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Grandes G, Montoya I, Arietaleanizbeaskoa MS, Arce V, Sanchez A; MAS Group. The burden of mental disorders in primary care. Eur Psychiatry. 2011;26:428-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Barsky AJ, Wyshak G. Hypochondriasis and somatosensory amplification. Br J Psychiatry. 1990;157:404-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 227] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Marcus DK, Church SE. Are dysfunctional beliefs about illness unique to hypochondriasis? J Psychosom Res. 2003;54:543-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Weck F, Neng JM, Richtberg S, Stangier U. Dysfunctional beliefs about symptoms and illness in patients with hypochondriasis. Psychosomatics. 2012;53:148-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Barsky AJ, Ahern DK, Bailey ED, Saintfort R, Liu EB, Peekna HM. Hypochondriacal patients' appraisal of health and physical risks. Am J Psychiatry. 2001;158:783-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Fergus TA, Valentiner DP. Reexamining the domain of hypochondriasis: comparing the Illness Attitudes Scale to other approaches. J Anxiety Disord. 2009;23:760-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Barsky AJ, Ahern DK, Bauer MR, Nolido N, Orav EJ. A randomized trial of treatments for high-utilizing somatizing patients. J Gen Intern Med. 2013;28:1396-1404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Liu J, Gill NS, Teodorczuk A, Li ZJ, Sun J. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: A meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Rassin E, Muris P, Franken I, van Straten M. The feature-positive effect and hypochondriacal concerns. Behav Res Ther. 2008;46:263-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Creed F, Barsky A. A systematic review of the epidemiology of somatisation disorder and hypochondriasis. J Psychosom Res. 2004;56:391-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 246] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 15. | Mogg K, Bradley BP, Hyare H, Lee S. Selective attention to food-related stimuli in hunger: are attentional biases specific to emotional and psychopathological states, or are they also found in normal drive states? Behav Res Ther. 1998;36:227-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 193] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Vazquez C, Blanco I, Sanchez A, McNally RJ. Attentional bias modification in depression through gaze contingencies and regulatory control using a new eye-tracking intervention paradigm: study protocol for a placebo-controlled trial. BMC Psychiatry. 2016;16:439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Schwenzer M, Mathiak K. Hypochondriacal attitudes may reflect a general cognitive bias that is not limited to illness-related thoughts. Psychol Health. 2011;26:965-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Schwenzer M, Mathiak K. Hypochondriacal attitudes comprise heterogeneous non-illness-related cognitions. BMC Psychiatry. 2012;12:173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133:1-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2300] [Cited by in F6Publishing: 2270] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 20. | Koster EH, Crombez G, Verschuere B, De Houwer J. Selective attention to threat in the dot probe paradigm: differentiating vigilance and difficulty to disengage. Behav Res Ther. 2004;42:1183-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 433] [Cited by in F6Publishing: 454] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 21. | Avila MT, Hong E, Thaker GK. Evidence of predictive smooth pursuit eye movement deficits in schizophrenia using a newly developed cued target paradigm. Schizophrenia Res. 2003;60:265-265. [DOI] [Cited in This Article: ] |
| 22. | Asmundson GJ, Carleton RN, Ekong J. Dot-probe evaluation of selective attentional processing of pain cues in patients with chronic headaches. Pain. 2005;114:250-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Garner M, Mogg K, Bradley BP. Orienting and maintenance of gaze to facial expressions in social anxiety. J Abnorm Psychol. 2006;115:760-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 229] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 24. | Mogg K, Millar N, Bradley BP. Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. J Abnorm Psychol. 2000;109:695-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Samuel BG. Diagnostic and Statistical Manual of Mental Disorders, 4th ed (DSM-IV). American Psychiatric Association. [DOI] [Cited in This Article: ] |
| 26. | American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-IV-TR. Washington DC: American Psychiatric Association, 2000. [Cited in This Article: ] |
| 27. | Fydrich T, Dowdall D, Chambless DL. Reliability and validity of the beck anxiety inventory. J Anxiety Disord. 1992;6:55-61. [DOI] [Cited in This Article: ] [Cited by in Crossref: 454] [Cited by in F6Publishing: 455] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 28. | Jackson-Koku G. Beck Depression Inventory. Occup Med (Lond). 2016;66:174-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 29. | Sirri L, Grandi S, Fava GA. The Illness Attitude Scales. A clinimetric index for assessing hypochondriacal fears and beliefs. Psychother Psychosom. 2008;77:337-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Lee S, Ng KL, Ma YL, Tsang A, Kwok KP. A general population study of the Chinese Whiteley-7 index in Hong Kong. J Psychosom Res. 2011;71:387-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Bailey R, Wells A. Metacognitive beliefs moderate the relationship between catastrophic misinterpretation and health anxiety. J Anxiety Disord. 2015;34:8-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Bradley BP, Mogg K, White J, Groom C, de Bono J. Attentional bias for emotional faces in generalized anxiety disorder. Br J Clin Psychol. 1999;38:267-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 288] [Cited by in F6Publishing: 278] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 33. |
Aaron TB.
Cognitive therapy and the emotional disorders |
| 34. | Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: the role of awareness. Br J Clin Psychol. 1995;34:17-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 400] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 35. | Olatunji BO, Deacon BJ, Abramowitz JS. Is hypochondriasis an anxiety disorder? Br J Psychiatry. 2009;194:481-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |