Published online Nov 19, 2021. doi: 10.5498/wjp.v11.i11.981
Peer-review started: March 2, 2021
First decision: June 5, 2021
Revised: June 9, 2021
Accepted: August 23, 2021
Article in press: August 23, 2021
Published online: November 19, 2021
Major depressive disorder (MDD) is a disabling and highly prevalent mood disorder as well as a common cause of suicide. Chronic stress, inflammation, and intestinal dysbiosis have all been shown to play crucial roles in the patho
Core Tip: One of the main challenges in the advancement of antidepressant therapy is the establishment of safe and effective fast-acting antidepressants. Ketamine is a prototype for rapid-onset antidepressant responses. Agmatine has been shown to produce fast antidepressant-like effect by stimulating mechanistic target of rapamycin complex 1 signaling pathway, similar to ketamine. Moreover, NLR family pyrin domain containing 3 and microbiota-gut-brain axis may be novel targets for fast antidepressant responses. These targets have also been postulated to play a role in the antidepressant effect of both ketamine and agmatine.
- Citation: Valverde AP, Camargo A, Rodrigues ALS. Agmatine as a novel candidate for rapid-onset antidepressant response. World J Psychiatr 2021; 11(11): 981-996
- URL: https://www.wjgnet.com/2220-3206/full/v11/i11/981.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i11.981
Major depressive disorder (MDD) affects more than 300 million people and is a major cause of disability and suicidal deaths worldwide[1]. Despite the high prevalence of this psychiatric disorder, its neurobiological basis remains to be fully elucidated, and its treatment remains a challenge. Although the monoaminergic hypothesis of MDD has played a crucial role in its pharmacotherapy, it is now considered overly simplistic[2]. One of the limitations of this hypothesis is the fact that the drugs currently used to treat MDD exert their therapeutic effect only after 3-4 wk and many patients fail to respond to these drugs. Antidepressants also feature side effects that may include nausea, dizziness, insomnia, weight gain, sleep disturbances, and sexual dysfunction. This scenario underscores the strong demand for developing novel antidepressants with a fast antidepressant response, better efficacy, and fewer adverse effects[3,4].
In this regard, the year 2000 marked a turning point in the history of MDD pharmacotherapy, and Berman et al[5] showed for the first time that a single dose of ketamine, an N-methyl-d-aspartic acid (NMDA) receptor antagonist, produced a fast-acting antidepressant response in MDD patients. This discovery was reinforced by several subsequent studies that demonstrated that a single dose of ketamine elicited rapid and long-lasting antidepressant effects, even in treatment-resistant patients with suicidal ideation[6-8]. Although the discovery of ketamine is considered the major breakthrough in MDD pharmacotherapy and opened new perspectives to manage refractory patients at risk of suicide, ketamine has knock-on effects that limit its widespread clinical use[9]. For these reasons, ketamine has emerged as a prototype for screening novel fast-acting antidepressant agents. Agmatine, an endogenous neuromodulator, shares some common molecular mechanisms with ketamine and the ability to elicit fast antidepressant-like effects in preclinical studies[10]. Therefore, agmatine could be a novel candidate to elicit fast antidepressant responses.
Therefore, this narrative review presents evidence that agmatine has a rapid antidepressant effect and provides an overview of the possible mechanisms underlying this effect versus those already described for ketamine. The PubMed, SCOPUS, and SciSearch databases were searched for original manuscripts and contemporary reviews published in English.
A milestone for the development of drugs with a rapid antidepressant effect has emerged over the past two decades[5,6]. Berman et al[5] demonstrated for the first time that a single subanesthetic dose of ketamine elicited fast (within 4 h) and long-lasting (for up to 3 d) antidepressant effects in MDD patients. These findings were reinforced and extended by Zarate et al[6], who showed that a single dose of ketamine effectively improved the MDD symptoms in treatment-refractory patients, as evidenced by an effect observed within 110 min that was sustained for up to 7 d. Importantly, a rapid resolution of suicidal ideation after a single infusion of ketamine in patients with treatment-resistant MDD was also observed, supporting the premise that ketamine has rapid beneficial effects even in severely depressed individuals at risk of suicide[7,8]. Due to the ability of ketamine to alleviate MDD symptoms, several research groups have investigated its underlying molecular mechanisms.
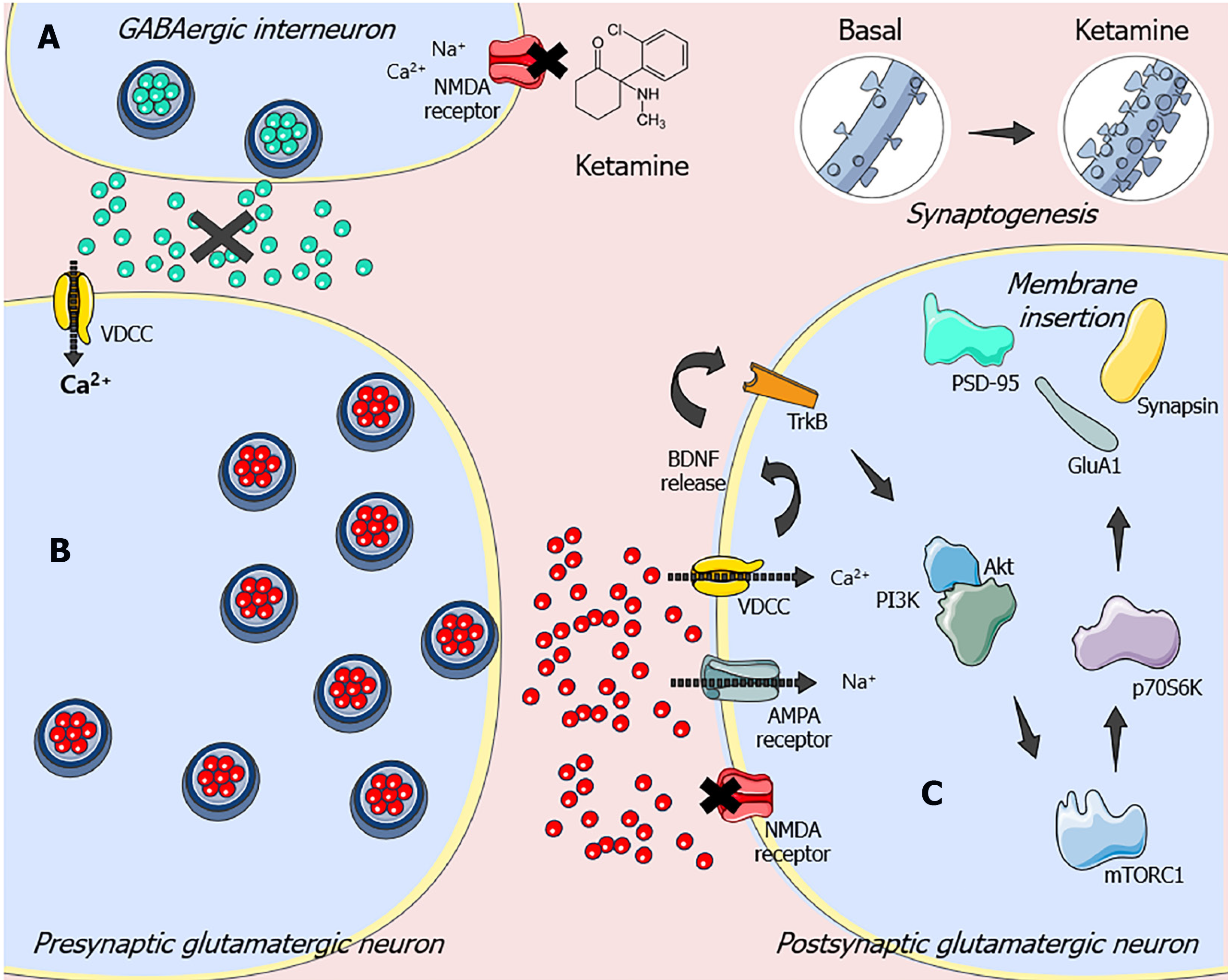
Although ketamine has been well characterized as an NMDA receptor antagonist, its molecular effects extend far beyond this level. It is worth noting that this drug has a window of therapeutic efficacy that surpasses its short half-life of a few hours[11,12]. Experimental studies have provided evidence that the antidepressant-like effect of ketamine depends on mechanistic target of rapamycin complex 1 (mTORC1) activation, a key pathway required for protein synthesis–dependent synaptic plasticity [13-15]. It has been postulated that ketamine, by antagonizing NMDA receptors in GABAergic interneurons, attenuates the inhibitory action of this system on glutamatergic tonus. This blockade causes the disinhibition of pyramidal cells, which causes a burst of glutamatergic transmission[11]. In particular, the glutamate released under these conditions preferentially stimulates alpha-amino-3-hydroxy-methyl-5-4-isoxazole propionic acid (AMPA) receptors, promoting a transient sodium influx that depolarizes the cell and activates the voltage-dependent calcium channels (VDCC). This event causes the exocytosis of synaptic vesicles containing the brain-derived neurotrophic factor (BDNF) in the synaptic cleft, as a result of calcium influx by VDCC[16].
BDNF activates tropomyosin receptor kinase B (TrkB), which stimulates the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mTORC1 signaling pathway[12]. In turn, mTORC1 controls the translation of proteins involved in new dendritic spine formation and synaptogenesis [e.g., postsynaptic density protein-95 kDa (PSD-95), glutamate AMPA receptor subunit 1 (GluA1), and synapsin] by activating the 70-kDa ribosomal protein S6 kinase (p70S6K) and inhibiting the eukaryotic initiation factor 4E-binding protein[13,14,17]. Although mTORC1-dependent synaptogenesis induced by ketamine was first demonstrated in the prefrontal cortex of rodents[13,15], it has been shown that similar events also occur in the hippocampus[18-20]. Therefore, these findings suggest that targeting the mTORC1-driven signaling pathway may produce rapid-onset and long-lasting antidepressant-like responses. The molecular mechanisms underlying ketamine’s antidepressant responses are shown in Figure 1.
Importantly, almost 20 years after the groundbreaking discovery that ketamine effectively produces rapid and sustained antidepressant effects, particularly in March 2019, the United States Food and Drug Administration approved the use of (S)-ketamine nasal spray (Spravato™) for treatment-resistant MDD. In the same year, (S)-ketamine nasal spray was approved for use in treatment-resistant depression in Europe[21]. Despite the fast and long-lasting antidepressant effects of ketamine, there is much concern about its abuse potential and serious adverse effects[9]. For this reason, ketamine is only available through a restricted distribution system, limiting its widespread clinical use. Although there are some drawbacks associated with its use, it may serve as a prototype for screening novel fast antidepressant agents. Given this scenario, the search for ketamine-like compounds has emerged as a promising therapeutic strategy. In this regard, our research group and others have shown that agmatine is also able to produce fast antidepressant responses and shares some mechanisms of action with ketamine[10].
Agmatine, a cationic amine produced from the L-arginine in a reaction catalyzed by the enzyme arginine decarboxylase, is widely distributed in human tissues, including the brain[22-24]. For almost a century, it was wrongly believed that agmatine was produced by bacteria, plants, and fish but not mammals[25]. Agmatine was “rediscovered” in 1994 during the search for an endogenous ligand for imidazoline binding sites[24]. In this study, a molecule was isolated from the mammalian brain and identified as agmatine[24]. This was the starting point for many studies that have evaluated the biological properties and possible beneficial effects of agmatine on a wide variety of diseases[26].
Soon after providing evidence of the presence of agmatine in mammalian nervous tissue, Piletz et al[27] documented its neuroprotective effects. The neuroprotective effects of agmatine reportedly involve protection mechanisms against excitotoxicity, since agmatine may block NMDA receptors and inhibit the increase in intracellular calcium concentrations in different neuronal cell cultures[28-30]. The NMDA receptor is an ion channel controlled by glutamatergic excitation, which is essential for the normal functioning of the central nervous system (CNS), including cognitive function, locomotion, and breathing[31-33]. This type of receptor is located on the membranes of neuronal and glial cells[34], and is implicated in the development and maintenance of acute and chronic diseases of the CNS, such as stroke, Parkinson’s disease, Alzhei
The first evidence of the antidepressant effects of agmatine was reported in a study that examined its impact on behavioral tests related to depression (immobility time in the tail suspension test and forced swimming test) in mice[38]. Since then, other studies have confirmed the antidepressant efficacy of agmatine in behavioral tests in rodents[39-41]. Subsequent studies implicated several molecular targets in the antidepressant effect of agmatine, namely the modulation of: (1) K+ channels[42]; (2) NO synthesis[43,44]; and (3) several neurotransmitter receptors including NMDA receptors[38,45], AMPA receptors[46,47], GABA receptors[48], serotonin receptors[49,50], and opioid system receptors[51].
In 2010, a human clinical trial showed the safety of oral agmatine[52]. In 2013, Shopsin et al[53] provided the first evidence that agmatine may effectively treat MDD, but this study included only three patients. None of these three patients treated with agmatine relapsed after the joint administration of a serotonin-depleting drug, indicating that the mechanism underlying the antidepressant action of agmatine is likely unrelated to the serotonergic system[53]. It was also reported that 8-wk treatment with the standard antidepressant bupropion normalized plasma agmatine levels[54]. In brain autopsies of chronically depressed patients, Bernstein et al[55] reported a significant increase in agmatinase immunoreactivity in hippocampal neurons, suggesting the role of the agmatinergic system in MDD pathophysiology. However, it was not possible to determine the exact reason why levels of this enzyme increased in hippocampal neurons due to the use of antidepressants.
In 2018, a gas chromatography–mass spectrometry study quantified agmatine levels in the brains of post-mortem humans who died by suicide and showed reduced agmatine levels in the suicide cortex regardless of these individuals formerly meeting the criteria for MDD versus controls[56].
Weiss et al[57] presented evidence of the activity of the agmatinergic system in habenular nuclei and investigated the actions of agmatine and agmatinase in the rat and human habenular systems. It is important to highlight that the role of habenular nuclei in mental disorders, including MDD, has already been considered[58,59]. In this study, agmatine was demonstrated responsible for the strong decrease in the spontaneous action potential of medial habenular neurons by activating type I1 imidazoline receptors. It was also reported that increased activity of the agmatinergic system in habenular nuclei may strengthen the dopaminergic activity of the midbrain. This evidence suggests dysregulation in the habenular-interpeduncular axis in patients with MDD[57].
In summary, these results present the possible role of agmatine in the neurobiology of MDD and highlight the possible benefits of agmatine as antidepressant therapy.
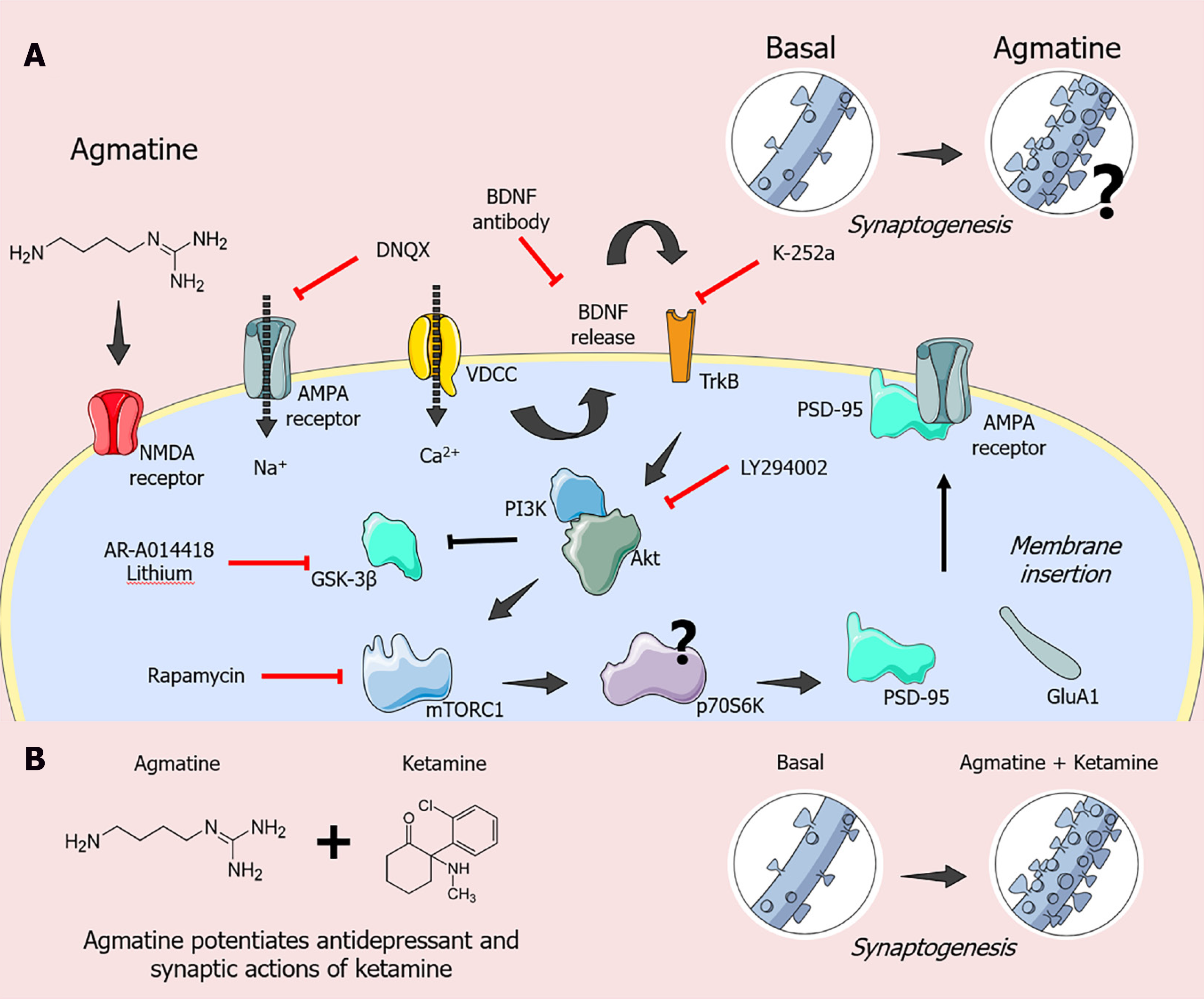
Recent evidence has also demonstrated the possible fast antidepressant-like actions of agmatine (Figure 2)[10]. In this context, Neis et al[46] reported that the antidepressant-like effect of agmatine administered orally to mice subjected to the tail suspension test is dependent on the modulation of molecular targets associated with the fast antidepressant-like effect displayed by ketamine. In particular, the antidepressant-like effect elicited by the acute administration of agmatine in the tail suspension test appears to involve inhibition of NMDA receptors since it enhanced the antidepressant potency of MK-801 (an NMDA receptor antagonist) up to 100-fold[60]. Moreover, the antidepressant-like effect of agmatine in the tail suspension test is dependent on AMPA and TrkB receptor activation since the administration of 6,7-dinitroquinoxaline-2,3-dione (DNQX; an AMPA receptor antagonist) or K-252a (a TrkB receptor antagonist) completely abolished its antidepressant-like response[46]. A single dose of agmatine also increased BDNF levels in the prefrontal cortex of mice, and its antidepressant-like effect in the tail suspension test was abrogated by the administration of anti-BDNF antibody. Of note, the antidepressant-like effect of agm
Supporting the assumption that agmatine could elicit a fast antidepressant-like effect, a study by Neis et al[61] demonstrated that a single dose of agmatine effectively reversed the depressive-like behavior induced by chronic unpredictable stress. In this study, mice were exposed to the stress protocol for 14 d and received a single oral dose of agmatine, ketamine, or fluoxetine. The results indicated that a single dose of agmatine or ketamine (after 24 h), but not fluoxetine, counteracted the depressive-like behavior induced by the stress protocol in the tail suspension test[61].
Expanding upon these findings, Neis et al[62] reinforced the ability of agmatine to rapidly reverse the depressive-like behavior induced by the 21-d administration of corticosterone, a pharmacological model of stress in mice. In the tail suspension test, a single dose of agmatine or ketamine abolished the depressive-like behavior of mice chronically exposed to corticosterone. In addition, treatment increased GluA1 immunocontent in the hippocampus of control animals[62]. Notably, a single dose of fluoxetine did not produce the same effects as ketamine or agmatine[62]. Chronic unpredictable stress and chronic corticosterone administration models are sensitive to chronic but not acute administration of conventional antidepressants, but a single dose of ketamine sufficiently produced antidepressant responses in these models[62]. Moreover, a single dose of agmatine or ketamine counteracted the depressive-like phenotype of cAMP-responsive element binding protein–regulated transcription coactivator 1 knockout mice in the forced swimming test, reinforcing the notion that agmatine could have a rapid antidepressant-like effect[63].
Subsequent studies provided novel evidence that a low-dose combination of ketamine plus agmatine produced neuroprotective, antidepressant-like, and synaptogenic effects[11,64,65]. A study using the HT-22 mouse hippocampal neuronal cell line reported that the combined use of sub-effective concentrations of ketamine and agmatine prevented the neuronal damage caused by corticosterone[64]. Of note, this effect was associated with increased phosphorylation of Akt (Ser473), p70S6K (Thr389), and PSD-95 immunocontent[65]. These data support the idea that ketamine and agmatine share common molecular targets and could work in tandem to protect neuronal cells from the harmful effects of corticosterone by activating Akt/ mTORC1/p70S6K signaling, resulting in synaptic protein expression[11,65].
Reinforcing these findings, Freitas et al[47] investigated the ability of agmatine to potentiate the effects of the antidepressant and synaptic actions of ketamine in mice. Of special interest, the combination of single subthreshold doses of ketamine and agmatine exerted antidepressant-like and pro-synaptogenic actions in a time-dependent manner. In particular, agmatine plus ketamine produced fast (1 and 24 h) and sustained (7-d) antidepressant-like effects in the tail suspension test[47]. Furthermore, this combined treatment increased p70S6K phosphorylation and GluA1 immunocontent in the prefrontal cortex 1 h after treatment. This same protocol increased the PSD-95 immunocontent, an effect that persisted for up to 7 d. The combined treatment also increased the complexity of the dendritic branches after 24 h, and this effect lasted up to 7 d. Likewise, ketamine plus agmatine treatment effectively increased the dendritic spinal density after 1 h later, a response that lasted up to 24 h[47]. These results reinforce the notion that agmatine and ketamine share common molecular targets and expand the findings regarding the ability of agmatine to enhance the antidepressant-like and synaptic actions of ketamine[47].
Taken together, these results support the hypothesis that agmatine can act as a ketamine-like compound, and further studies are crucial to investigate whether the rapid antidepressant effects of agmatine are reproducible in patients with MDD. Moreover, the use of agmatine in the clinic would be highly promising owing to its safety, even at high doses, without evident effects of toxicity[26,52].
In addition to focusing on the importance of the mTORC1-mediated signaling pathway for the antidepressant effect of agmatine, some studies investigated other signaling pathways that may play a role in its antidepressant effect. Understanding other pathways influenced by agmatine is important to its establishment as a therapeutic alternative in the clinical setting.
Regarding the factors that may influence the mechanisms associated with MDD symptoms, neuroinflammation has received much attention in recent years. Neuroinflammation reportedly plays an essential role in several neuropathologies, such as multiple sclerosis, Alzheimer’s disease, and MDD[66,67]. It was demonstrated in both humans and animals that immunological challenges may induce depressive behavior[68]. It is important to note that, since the 1990s, several studies reported a strong correlation between MDD and peripheral inflammatory markers[69,70].
In the last few years, three meta-analyses aimed to better understand the relationship between neuroinflammation and the development/maintenance of MDD. Kappelmann et al[71] published a meta-analysis in 2018 that analyzed data from four randomized controlled studies that examined the effects of pro-inflammatory cytokine inhibitors. In these studies, adalimumab and etanercept were used, and both treatments improved depressive symptoms in patients. In 2019, a randomized clinical study showed more pronounced antidepressant results in patients with higher high-sensitivity C-reactive protein levels[72]. In this study, the use of anti-inflammatory drugs improved clinical signs of depression, such as motor retardation, suicidal thoughts, and depressed mood[72]. Another meta-analysis evaluated 36 studies and assessed the effects of anti-inflammatory drugs in almost 10000 patients[73]. The findings suggested that the use of anti-inflammatory drugs sufficiently reversed depressive symptoms. The latest meta-analysis combined 26 randomized clinical trials of over 1500 patients[74]. This study also verified the improvement in depressive symptoms in patients with anti-inflammatory agent use[74].
Therefore, inflammatory pathways play an important role in the development and maintenance of depressive symptoms. Increasing evidence has shown that agmatine also acts on neuroinflammatory-related pathways that may participate in rapid-onset antidepressant responses.
The early administration of agmatine for 7 d prevented the depressive-like behavior caused by lipopolysaccharide (LPS) challenge in mice[75]. Agmatine pretreatment counteracted LPS-induced neuroinflammation by preventing increases in interleukin (IL)-1β and tumor necrosis factor (TNF)-α level in the murine brain. In addition, agmatine positively regulates BDNF levels in the hippocampus[75]. In another study, agmatine pretreatment also normalized LPS-induced sickness behavior in rats in addition to decreasing serum concentrations of IL-6 and TNF-α[76]. Zarifkar et al[77] reported that agmatine prevented LPS-induced spatial memory impairment and hippocampal caspase-3 activation in LPS-treated rats. It is also noteworthy that agmatine effectively inhibited the LPS-induced production of nitrite and decreased body temperature in rats in a dose-dependent manner[78].
Notably, Neis et al[45] showed that agmatine effectively counteracted the depressive-like behavior induced by the pro-inflammatory cytokine TNF-α in mice. In this study, the combined treatment of sub-effective doses of agmatine and fluoxetine, imipramine, bupropion, MK-801, or 7-nitroindazole resulted in a synergistic antidepressant-like effect in mice subjected to TNF-α administration[45].
Agmatine also exhibits anti-inflammatory effects in other disease models. In particular, in a type 2 diabetes mellitus (T2DM) model induced by a high-fat diet for 12 wk, anxiety- and depressive-like behaviors were associated with an increase in pro-inflammatory cytokines, such as IL-6 and TNF-α, as well as a decrease in the BDNF immunocontent in the rat hippocampus[79]. These parameters were inhibited by agmatine treatment in the last 4 wk of the protocol. In this study, agmatine levels in the hippocampus of rats subjected to the T2DM protocol were significantly lower than those in the control animals[79].
In an Alzheimer’s disease model, the administration of amyloid-β peptide (Aβ1-42) to mice caused depressive-like behavior in the forced swimming test, an effect parallel to an increase in the pro-inflammatory cytokines IL-6 and TNF-α in the hippocampus[80]. Both depressive-like behavior and pro-inflammatory markers were reversed by agmatine treatment, suggesting that the anti-inflammatory properties of agmatine may be related to its antidepressant effect. Notably, this study also detected lower concentrations of agmatine in the brains of animals injected with Aβ1-42[80]. These data point to the action of agmatine in neuroinflammatory processes, as a pharmacological strategy to decrease depressive-like behavior, including that associated with comorbid diseases, such as T2DM and Alzheimer’s disease.
It is important to note that the activation of various types of inflammasomes is a critical target in the inflammatory response. Inflammasomes are involved in the development of several neurological diseases, including MDD[81,82]. Among them, the NLR family pyrin domain containing 3 (NLRP3) inflammasome is the most closely related to MDD due to the exaggerated activation of inflammatory and immunological responses that contribute to the pathogenesis and progression of this disorder[83]. A compelling study reported that depressive-like behavior in mice subjected to LPS administration is related to NLRP3-dependent caspase-1 activation[84]. Accordingly, anxiety-like behavior reportedly occurs in rats exposed to neonatal inflammation or inflammatory stress early in life triggered by NLRP3 inflammasome activation in animals’ brains[85]. Altogether, evidence suggests that the NLRP3 inflammasome plays an essential role in the neurobiology of MDD and may be a potential target for antidepressant treatment.
In this regard, ketamine was shown to exert an antidepressant effect in the LPS-induced model via suppressing the NLRP3 inflammasome and upregulating AMPA receptors[86]. Importantly, in this study, the authors postulated that ketamine might increase AMPA receptor expression through the NLRP3 inflammasome, suggesting that NLRP3 could be a target in fast-acting antidepressant treatment[86].
The possibility that agmatine exerts antidepressant effects by modulating neuroinflammatory mechanisms has also been investigated. Sahin et al[43] investigated the effects of agmatine in a model of restraint stress–induced depressive-like behavior. The authors demonstrated that agmatine rescued anti-inflammatory cytokine IL-4 and IL-10 levels that were impaired by stress[43]. Moreover, the 6-wk treatment with agmatine counteracted the depressive-like behavior of animals exposed to chronic unpredictable stress by suppressing NLRP3 and IL-1β[43].
The exact role of the NLRP3 inflammasome–driven signaling pathway in MDD pathophysiology and antidepressant responses is still not well established. However, it has been proposed that the gut microbiota may influence activation of the NLRP3 inflammasome and neuroinflammatory processes through the microbiota-gut-brain axis.
The microbiome, a complex ecosystem in the human gut, includes bacteria, viruses, archaea, and fungi. The bacteria present in this system regulate aspects of the host’s health, mainly brain development and functioning[87,88]. The microbiome is a dynamic structure that is affected by delivery type, sex, age, nutrition, stress, and medications[89]. These interferences can compromise the balance between pathogenic and commensal bacteria[90], promoting the development of a process called dysbiosis, which can change the permeability of the intestinal wall, allowing bacteria and their products to leak into the sterile cavity and activate the immune response[91]. Immune response activation increases the levels of pro-inflammatory cytokines, which, together with other toxic metabolites, damage the blood–brain barrier and trigger neuroinflammation[92].
The immune and brain mechanisms involved in intestinal dysbiosis may include microglial activation[93]. Microglia are responsible for releasing pro-inflammatory cytokines in the brain when activated by stress, a mechanism that is altered in MDD[94,95]. On the other hand, a balanced and healthy microbiota can regulate the activation of these stress response pathways through the synthesis of hormones and neurotransmitters, minimizing the effects of such stressors[96].
Studies have shown that there is a “microbiota of MDD” due to the difference in composition between depressed patients and healthy controls. In a microbiome study of patients with MDD and irritable bowel syndrome, less bacterial diversity, an effect associated with increased levels of bacteria from the phylum Bacteroidetes, and increased colon inflammation were noted in patients compared to healthy controls[97]. In a Chinese cohort, the microbiota of patients with MDD showed higher concentration of Proteobacteria and decreased concentrations of Firmicutes[98].
Several studies have suggested the direct modulation of bacteria in the immune system. Proteo mirabilis, a proteobacterium, can activate the NLRP3 inflammasome and interleukin IL-1b production[99]. Other components of Proteobacteria, such as the LPS produced by Pseudomonas, are related to the development of MDD symptoms via activation of the NLRP3 inflammasome and pro-inflammatory immunoglobulins[93]. In patients with MDD, an increase in some Bacteroidetes species (Parabacteroidetes and Alistepes) reportedly converts tryptophan to indole, which can influence the availability of tryptophan in the body and affect serotonergic balance[100]. Other studies confirmed an increase in Alistepes bacteria in patients with MDD, chronic fatigue syndrome, irritable bowel syndrome, and stress models[101,102].
The transplantation of fecal microbiota from patients diagnosed with MDD to germ-free microbiota mice triggered anxious-, anhedonic-, and depressive-like behaviors in the animals[103,104]. This evidence suggests that the depressive phenotype may be transmitted by gut microbiota. These data show a close relationship between the composition of the gut microbiota and brain health, mainly in the pathological mechanisms involved in the development and maintenance of depressive symptoms. Furthermore, the immune system/NLRP3 inflammasome acts as an intermediary between gut dysbiosis and brain function.
Some studies have suggested that the ability of ketamine to elicit antidepressant effects may be mediated, at least in part, by modulation of the microbiota-gut-brain axis. Two studies that investigated the effects of ketamine administration in the gut microbiota of mice following the social defeat stress model reported that the treatment attenuated the alterations in Bacteroidales, Clostridiales, Ruminococcaceae, Deltaproteobacteria, and Mollicutes bacterial levels in their feces[105,106]. Moreover, ketamine prevented the increase in the Clostridium and Butyricimonas species induced by the stress model[105,106]. Other studies showed that ketamine significantly amplified the number of healthy bacteria and decreased the number of opportunistic pathogens in Wistar rats[107]. In an inflammatory model of LPS-induced depressive-like behavior, ketamine improved the diversity of the gut microbiota, positively regulating this microsystem[108]. Together, these data suggest that ketamine influences the composition of the microbiota, a response that may underlie its antidepressant-like effects.
The relationship between gut microbiota and agmatine levels has emerged and may play a role in the ability of gut microbiota to influence mental health. Agmatine is produced and released by gut bacteria of the human microbiome[109] and can be obtained from ingested food[110,111]. The composition of the intestinal microbiota influences agmatine availability in the gut lumen for absorption, and the majority of agmatine in humans is believed to be derived from bacterial sources[27]. Interestingly, agmatine may also be obtained from foodstuffs, particularly fermented foods such as alcoholic beverages (wine, beer, sake), which suggests the role of yeast in its production[109]. The filamentous fungus Aspergillus oryzae, which is widely used for the production of various Asian fermented foods, can enhance agmatine ingestion[112].
The consumption of fermented foods has beneficial effects on mental health[113]. The use of probiotics also reportedly exerts positive effects on depressive symptoms[114,115]. The possibility that agmatine is produced in the gut following the consumption of fermented foods and probiotics may account, at least in part, for its anti-inflammatory and antidepressant effects should be investigated in future studies.
Metformin, the mainstay therapy for T2DM, reportedly influences the diversity and composition of the gut microbiota[116]. This drug has recently been shown to act on Escherichia coli, elevating agmatine production and increases the longevity of Caenorhabditis elegans[117]. Metformin has been shown to produce antidepressant effects in depressed patients with diabetes mellitus[118] and proposed as an adjunctive antidepressant approach in nondiabetic patients with MDD[119]. It remains to be determined whether agmatine levels are higher in individuals taking metformin and, if so, whether it contributes to the antidepressant effect observed with metformin treatment.
Agmatine, an endogenous cationic amine, exerted antidepressant effects in several preclinical studies[26,120]. Considering that the microbiota composition and consumption of fermented foods, or even some drugs such as metformin, may influence agmatine levels in the gut[27,109-119], it remains to be established whether agmatine derived from these sources may positively impact mood and exert antidepressant effects. Therefore, modulation of the microbiota and, consequently, gut agmatine levels may represent a novel approach to mood regulation.
In addition to the fact that agmatine may be synthesized by gut microbiota, several studies have indicated that it is safe even when administered at high doses as a nutraceutical. The sulfate salt of agmatine has been used for bodybuilding[27] and the management of neuropathic pain at doses as high as 2.6 g/day[121]. The fact that agmatine also exhibits several beneficial effects for a wide spectrum of diseases[27] suggests that it is a promising therapeutic strategy for the management of MDD and several comorbid diseases and inflammatory clinical conditions such as diabetes, obesity, pain, and neurodegenerative diseases. Of particular relevance, compelling preclinical evidence has indicated that agmatine has the ability to counteract several neuroinflammatory markers induced by models of depression and shares with ketamine the ability to elicit fast antidepressant responses[46,47,61,62,75-80]. The possibility that agmatine may afford a rapid antidepressant effect would give it an advantage over conventional antidepressants that require several weeks to alleviate depressive symptoms. In preclinical studies, agmatine elicited a synergistic effect with ketamine in mice subjected to animal models of depression as well as cell culture, and pharmacological evidence has pointed to similar molecular mechanisms of these drugs[46]. These properties of agmatine clearly warrant future clinical investigation of its beneficial effects for managing depressive symptoms as a monotherapy or adjunctive treatment. Therefore, clinical studies are warranted that investigate the possibility that agmatine may be combined with low doses of ketamine to diminish the side effects and provide synergistic antidepressant effects.
Provenance and peer review: Invited article; Externally peer reviewed
Specialty type: Psychiatry
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bernstein HG S-Editor: Zhang H L-Editor: A P-Editor: Yu HG
| 1. | World Health Organization. Depression and other common mental disorders: global health estimates. World Heal Organ. 2017;1-24. [Cited in This Article: ] |
| 2. | Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1102] [Cited by in F6Publishing: 982] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 3. | Papakostas GI, Ionescu DF. Towards new mechanisms: an update on therapeutics for treatment-resistant major depressive disorder. Mol Psychiatry. 2015;20:1142-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Kaster MP, Moretti M, Cunha MP, Rodrigues ALS. Novel approaches for the management of depressive disorders. Eur J Pharmacol. 2016;771:236-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 5. | Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2441] [Cited by in F6Publishing: 2523] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 6. | Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2275] [Cited by in F6Publishing: 2404] [Article Influence: 133.6] [Reference Citation Analysis (0)] |
| 7. | Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 428] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 8. | DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA Jr. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605-1611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 440] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 9. | Gao M, Rejaei D, Liu H. Ketamine use in current clinical practice. Acta Pharmacol Sin. 2016;37:865-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 10. | Camargo A, Rodrigues ALS. Novel Targets for Fast Antidepressant Responses: Possible Role of Endogenous Neuromodulators. Chronic Stress (Thousand Oaks). 2019;3:2470547019858083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62:35-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 395] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 12. | Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 262] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 13. | Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1881] [Cited by in F6Publishing: 2062] [Article Influence: 147.3] [Reference Citation Analysis (0)] |
| 14. | Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1255] [Cited by in F6Publishing: 1372] [Article Influence: 105.5] [Reference Citation Analysis (1)] |
| 15. | Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 786] [Cited by in F6Publishing: 821] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 16. | Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014;18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 17. | Huang K, Fingar DC. Growing knowledge of the mTOR signaling network. Semin Cell Dev Biol. 2014;36:79-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 18. | Pazini FL, Rosa JM, Camargo A, Fraga DB, Moretti M, Siteneski A, Rodrigues ALS. mTORC1-dependent signaling pathway underlies the rapid effect of creatine and ketamine in the novelty-suppressed feeding test. Chem Biol Interact. 2020;332:109281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Fraga DB, Costa AP, Olescowicz G, Camargo A, Pazini FL, E Freitas A, Moretti M, S Brocardo P, Rodrigues ALS. Ascorbic acid presents rapid behavioral and hippocampal synaptic plasticity effects. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry. 2014;29:419-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 21. | Wei Y, Chang L, Hashimoto K. A historical review of antidepressant effects of ketamine and its enantiomers. Pharmacol Biochem Behav. 2020;190:172870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 22. | Raasch W, Regunathan S, Li G, Reis DJ. Agmatine, the bacterial amine, is widely distributed in mammalian tissues. Life Sci. 1995;56:2319-2330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 200] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Feng Y, Halaris AE, Piletz JE. Determination of agmatine in brain and plasma using high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl. 1997;691:277-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 1994;263:966-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 559] [Cited by in F6Publishing: 549] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 25. | Tabor CW, Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2655] [Cited by in F6Publishing: 2559] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 26. | Neis VB, Rosa PB, Olescowicz G, Rodrigues ALS. Therapeutic potential of agmatine for CNS disorders. Neurochem Int. 2017;108:318-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Piletz JE, Aricioglu F, Cheng JT, Fairbanks CA, Gilad VH, Haenisch B, Halaris A, Hong S, Lee JE, Li J, Liu P, Molderings GJ, Rodrigues ALS, Satriano J, Seong GJ, Wilcox G, Wu N, Gilad GM. Agmatine: clinical applications after 100 years in translation. Drug Discov Today. 2013;18:880-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 28. | Feng Y, Piletz JE, Leblanc MH. Agmatine suppresses nitric oxide production and attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr Res. 2002;52:606-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Zhu MY, Piletz JE, Halaris A, Regunathan S. Effect of agmatine against cell death induced by NMDA and glutamate in neurons and PC12 cells. Cell Mol Neurobiol. 2003;23:865-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Costa VV, Del Sarto JL, Rocha RF, Silva FR, Doria JG, Olmo IG, Marques RE, Queiroz-Junior CM, Foureaux G, Araújo JMS, Cramer A, Real ALCV, Ribeiro LS, Sardi SI, Ferreira AJ, Machado FS, de Oliveira AC, Teixeira AL, Nakaya HI, Souza DG, Ribeiro FM, Teixeira MM. N-Methyl-d-Aspartate (NMDA) Receptor Blockade Prevents Neuronal Death Induced by Zika Virus Infection. mBio. 2017;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Cai SX. Glycine/NMDA receptor antagonists as potential CNS therapeutic agents: ACEA-1021 and related compounds. Curr Top Med Chem. 2006;6:651-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Missale C, Fiorentini C, Busi C, Collo G, Spano PF. The NMDA/D1 receptor complex as a new target in drug development. Curr Top Med Chem. 2006;6:801-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Laube G, Bernstein HG. Agmatine: multifunctional arginine metabolite and magic bullet in clinical neuroscience? Biochem J. 2017;474:2619-2640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 34. | Brittain MK, Brustovetsky T, Sheets PL, Brittain JM, Khanna R, Cummins TR, Brustovetsky N. Delayed calcium dysregulation in neurons requires both the NMDA receptor and the reverse Na+/Ca2+ exchanger. Neurobiol Dis. 2012;46:109-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883-1885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1262] [Cited by in F6Publishing: 1326] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 36. | Kaindl AM, Degos V, Peineau S, Gouadon E, Chhor V, Loron G, Le Charpentier T, Josserand J, Ali C, Vivien D, Collingridge GL, Lombet A, Issa L, Rene F, Loeffler JP, Kavelaars A, Verney C, Mantz J, Gressens P. Activation of microglial N-methyl-D-aspartate receptors triggers inflammation and neuronal cell death in the developing and mature brain. Ann Neurol. 2012;72:536-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 37. | Barua S, Kim JY, Kim JH, Lee JE. Therapeutic Effect of Agmatine on Neurological Disease: Focus on Ion Channels and Receptors. Neurochem Res. 2019;44:735-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Zomkowski AD, Hammes L, Lin J, Calixto JB, Santos AR, Rodrigues ALS. Agmatine produces antidepressant-like effects in two models of depression in mice. Neuroreport. 2002;13:387-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 39. | Aricioglu F, Altunbas H. Is agmatine an endogenous anxiolytic/antidepressant agent? Ann N Y Acad Sci. 2003;1009:136-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Kotagale NR, Tripathi SJ, Aglawe MM, Chopde CT, Umekar MJ, Taksande BG. Evidences for the agmatine involvement in antidepressant like effect of bupropion in mouse forced swim test. Pharmacol Biochem Behav. 2013;107:42-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Moretti M, Neis VB, Matheus FC, Cunha MP, Rosa PB, Ribeiro CM, Rodrigues ALS, Prediger RD. Effects of Agmatine on Depressive-Like Behavior Induced by Intracerebroventricular Administration of 1-Methyl-4-phenylpyridinium (MPP(+)). Neurotox Res. 2015;28:222-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Budni J, Gadotti VM, Kaster MP, Santos AR, Rodrigues ALS. Role of different types of potassium channels in the antidepressant-like effect of agmatine in the mouse forced swimming test. Eur J Pharmacol. 2007;575:87-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Sahin Ozkartal C, Tuzun E, Kucukali CI, Ulusoy C, Giris M, Aricioglu F. Antidepressant-like effects of agmatine and NOS inhibitors in chronic unpredictable mild stress model of depression in rats: The involvement of NLRP inflammasomes. Brain Res. 2019;1725:146438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Ostadhadi S, Norouzi-Javidan A, Nikoui V, Zolfaghari S, Moradi A, Dehpour AR. Nitric oxide involvement in additive antidepressant-like effect of agmatine and lithium in mice forced swim test. Psychiatry Res. 2018;266:262-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Neis VB, Manosso LM, Moretti M, Freitas AE, Daufenbach J, Rodrigues ALS. Depressive-like behavior induced by tumor necrosis factor-α is abolished by agmatine administration. Behav Brain Res. 2014;261:336-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Neis VB, Moretti M, Bettio LE, Ribeiro CM, Rosa PB, Gonçalves FM, Lopes MW, Leal RB, Rodrigues ALS. Agmatine produces antidepressant-like effects by activating AMPA receptors and mTOR signaling. Eur Neuropsychopharmacol. 2016;26:959-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Freitas AE, Heinrich IA, Moura TM, Fraga DB, Costa AP, Azevedo D, Brocardo PS, Kaster MP, Leal RB, Rodrigues ALS. Agmatine potentiates antidepressant and synaptic actions of ketamine: Effects on dendritic arbors and spines architecture and Akt/S6 kinase signaling. Exp Neurol. 2020;333:113398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Neis VB, Rosado AF, Olescowicz G, Moretti M, Rosa PB, Platt N, Rodrigues ALS. The involvement of GABAergic system in the antidepressant-like effect of agmatine. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:1931-1939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 49. | Zomkowski ADE, Rosa AO, Lin J, Santos AR, Calixto JB, Rodrigues ALS. Evidence for serotonin receptor subtypes involvement in agmatine antidepressant like-effect in the mouse forced swimming test. Brain Res. 2004;1023:253-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Freitas AE, Egea J, Buendía I, Navarro E, Rada P, Cuadrado A, Rodrigues ALS, López MG. Agmatine induces Nrf2 and protects against corticosterone effects in hippocampal neuronal cell line. Mol Neurobiol. 2015;51:1504-1519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Zomkowski AD, Santos AR, Rodrigues ALS. Evidence for the involvement of the opioid system in the agmatine antidepressant-like effect in the forced swimming test. Neurosci Lett. 2005;381:279-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Keynan O, Mirovsky Y, Dekel S, Gilad VH, Gilad GM. Safety and Efficacy of Dietary Agmatine Sulfate in Lumbar Disc-associated Radiculopathy. An Open-label, Dose-escalating Study Followed by a Randomized, Double-blind, Placebo-controlled Trial. Pain Med. 2010;11:356-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Shopsin B. The clinical antidepressant effect of exogenous agmatine is not reversed by parachlorophenylalanine: a pilot study. Acta Neuropsychiatr. 2013;25:113-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Piletz JE, Halaris A, Iqbal O, Hoppensteadt D, Fareed J, Zhu H, Sinacore J, DeVane CL. Nitric oxide branch of arginine metabolism in depression: effect of venlafaxine. Int J Heal Sci. 2009;2:274-281. [Cited in This Article: ] |
| 55. | Bernstein HG, Stich C, Jäger K, Dobrowolny H, Wick M, Steiner J, Veh R, Bogerts B, Laube G. Agmatinase, an inactivator of the putative endogenous antidepressant agmatine, is strongly upregulated in hippocampal interneurons of subjects with mood disorders. Neuropharmacology. 2012;62:237-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 56. | Chen GG, Almeida D, Fiori L, Turecki G. Evidence of Reduced Agmatine Concentrations in the Cerebral Cortex of Suicides. Int J Neuropsychopharmacol. 2018;21:895-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Weiss T, Bernard R, Bernstein HG, Veh RW, Laube G. Agmatine modulates spontaneous activity in neurons of the rat medial habenular complex-a relevant mechanism in the pathophysiology and treatment of depression? Transl Psychiatry. 2018;8:201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Rolls ET. A non-reward attractor theory of depression. Neurosci Biobehav Rev. 2016;68:47-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 59. | Loonen AJM, Ivanova SA. Commentary on "A non-reward attractor theory of depression": A proposal to include the habenula connection. Neurosci Biobehav Rev. 2017;83:736-741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Neis VB, Moretti M, Manosso LM, Lopes MW, Leal RB, Rodrigues ALS. Agmatine enhances antidepressant potency of MK-801 and conventional antidepressants in mice. Pharmacol Biochem Behav. 2015;130:9-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Neis VB, Bettio LEB, Moretti M, Rosa PB, Ribeiro CM, Freitas AE, Gonçalves FM, Leal RB, Rodrigues ALS. Acute agmatine administration, similar to ketamine, reverses depressive-like behavior induced by chronic unpredictable stress in mice. Pharmacol Biochem Behav. 2016;150-151:108-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 62. | Neis VB, Bettio LB, Moretti M, Rosa PB, Olescowicz G, Fraga DB, Gonçalves FM, Freitas AE, Heinrich IA, Lopes MW, Leal RB, Rodrigues ALS. Single administration of agmatine reverses the depressive-like behavior induced by corticosterone in mice: Comparison with ketamine and fluoxetine. Pharmacol Biochem Behav. 2018;173:44-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Meylan EM, Breuillaud L, Seredenina T, Magistretti PJ, Halfon O, Luthi-Carter R, Cardinaux JR. Involvement of the agmatinergic system in the depressive-like phenotype of the Crtc1 knockout mouse model of depression. Transl Psychiatry. 2016;6:e852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 64. | Pazini FL, Cunha MP, Rosa JM, Colla AR, Lieberknecht V, Oliveira Á, Rodrigues ALS. Creatine, Similar to Ketamine, Counteracts Depressive-Like Behavior Induced by Corticosterone via PI3K/Akt/mTOR Pathway. Mol Neurobiol. 2016;53:6818-6834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 65. | Tavares MK, Dos Reis S, Platt N, Heinrich IA, Wolin IAV, Leal RB, Kaster MP, Rodrigues ALS, Freitas AE. Agmatine potentiates neuroprotective effects of subthreshold concentrations of ketamine via mTOR/S6 kinase signaling pathway. Neurochem Int. 2018;118:275-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 66. | Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14:388-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2956] [Cited by in F6Publishing: 3552] [Article Influence: 394.7] [Reference Citation Analysis (0)] |
| 67. | Troubat R, Barone P, Leman S, Desmidt T, Cressant A, Atanasova B, Brizard B, El Hage W, Surget A, Belzung C, Camus V. Neuroinflammation and depression: A review. Eur J Neurosci. 2021;53:151-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 225] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 68. | Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1963] [Cited by in F6Publishing: 2035] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 69. | Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, Desnyder R. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. 1995;34:301-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 455] [Cited by in F6Publishing: 428] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 70. | Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40:171-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 259] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 71. | Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2018;23:335-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 386] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 72. | McIntyre RS, Subramaniapillai M, Lee Y, Pan Z, Carmona NE, Shekotikhina M, Rosenblat JD, Brietzke E, Soczynska JK, Cosgrove VE, Miller S, Fischer EG, Kramer NE, Dunlap K, Suppes T, Mansur RB. Efficacy of Adjunctive Infliximab vs Placebo in the Treatment of Adults With Bipolar I/II Depression: A Randomized Clinical Trial. JAMA Psychiatry. 2019;76:783-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 73. | Köhler-Forsberg O, N Lydholm C, Hjorthøj C, Nordentoft M, Mors O, Benros ME. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139:404-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 229] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 74. | Bai S, Guo W, Feng Y, Deng H, Li G, Nie H, Guo G, Yu H, Ma Y, Wang J, Chen S, Jing J, Yang J, Tang Y, Tang Z. Efficacy and safety of anti-inflammatory agents for the treatment of major depressive disorder: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. 2020;91:21-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 75. | Gawali NB, Bulani VD, Chowdhury AA, Deshpande PS, Nagmoti DM, Juvekar AR. Agmatine ameliorates lipopolysaccharide induced depressive-like behaviour in mice by targeting the underlying inflammatory and oxido-nitrosative mediators. Pharmacol Biochem Behav. 2016;149:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 76. | Taksande BG, Chopde CT, Umekar MJ, Kotagale NR. Agmatine attenuates lipopolysaccharide induced anorexia and sickness behavior in rats. Pharmacol Biochem Behav. 2015;132:108-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 77. | Zarifkar A, Choopani S, Ghasemi R, Naghdi N, Maghsoudi AH, Maghsoudi N, Rastegar K, Moosavi M. Agmatine prevents LPS-induced spatial memory impairment and hippocampal apoptosis. Eur J Pharmacol. 2010;634:84-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 78. | Aricioglu F, Regunathan S. Agmatine attenuates stress- and lipopolysaccharide-induced fever in rats. Physiol Behav. 2005;85:370-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Kale M, Nimje N, Aglawe MM, Umekar M, Taksande B, Kotagale N. Agmatine modulates anxiety and depression-like behaviour in diabetic insulin-resistant rats. Brain Res. 2020;1747:147045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 80. | Kotagale N, Deshmukh R, Dixit M, Fating R, Umekar M, Taksande B. Agmatine ameliorates manifestation of depression-like behavior and hippocampal neuroinflammation in mouse model of Alzheimer's disease. Brain Res Bull. 2020;160:56-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 81. | Alcocer-Gómez E, Cordero MD. NLRP3 inflammasome: a new target in major depressive disorder. CNS Neurosci Ther. 2014;20:294-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 82. | Singhal G, Jaehne EJ, Corrigan F, Toben C, Baune BT. Inflammasomes in neuroinflammation and changes in brain function: a focused review. Front Neurosci. 2014;8:315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 252] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 83. | Franklin TC, Xu C, Duman RS. Depression and sterile inflammation: Essential role of danger associated molecular patterns. Brain Behav Immun. 2018;72:2-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 84. | Jeon SA, Lee E, Hwang I, Han B, Park S, Son S, Yang J, Hong S, Kim CH, Son J, Yu JW. NLRP3 Inflammasome Contributes to Lipopolysaccharide-induced Depressive-Like Behaviors via Indoleamine 2,3-dioxygenase Induction. Int J Neuropsychopharmacol. 2017;20:896-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 85. | Lei Y, Chen CJ, Yan XX, Li Z, Deng XH. Early-life lipopolysaccharide exposure potentiates forebrain expression of NLRP3 inflammasome proteins and anxiety-like behavior in adolescent rats. Brain Res. 2017;1671:43-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 86. | Li JM, Liu LL, Su WJ, Wang B, Zhang T, Zhang Y, Jiang CL. Ketamine may exert antidepressant effects via suppressing NLRP3 inflammasome to upregulate AMPA receptors. Neuropharmacology. 2019;146:149-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 87. | Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047-3052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1983] [Cited by in F6Publishing: 2060] [Article Influence: 158.5] [Reference Citation Analysis (0)] |
| 88. | Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451-1463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2084] [Cited by in F6Publishing: 2085] [Article Influence: 189.5] [Reference Citation Analysis (0)] |
| 89. | Macedo D, Filho AJMC, Soares de Sousa CN, Quevedo J, Barichello T, Júnior HVN, Lucena DF. Antidepressants, antimicrobials or both? J Affect Disord. 2017;208:22-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 90. | Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav Immun. 2010;24:9-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 283] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 91. | Kiliaan AJ, Saunders PR, Bijlsma PB, Berin MC, Taminiau JA, Groot JA, Perdue MH. Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am J Physiol. 1998;275:G1037-G1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Roy Sarkar S, Banerjee S. Gut microbiota in neurodegenerative disorders. J Neuroimmunol. 2019;328:98-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 93. | Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett. 2008;29:117-124. [PubMed] [Cited in This Article: ] |
| 94. | Walker FR, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets. 2013;14:1262-1276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 213] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 95. | Réus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, Kapczinski F, Quevedo J. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 424] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 96. | Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1288-G1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 382] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 97. | Liu Y, Zhang L, Wang X, Wang Z, Zhang J, Jiang R, Wang K, Liu Z, Xia Z, Xu Z, Nie Y, Lv X, Wu X, Zhu H, Duan L. Similar Fecal Microbiota Signatures in Patients With Diarrhea-Predominant Irritable Bowel Syndrome and Patients With Depression. Clin Gastroenterol Hepatol 2016; 14: 1602-1611. e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 98. | Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1201] [Cited by in F6Publishing: 1339] [Article Influence: 148.8] [Reference Citation Analysis (0)] |
| 99. | Seo SU, Kamada N, Muñoz-Planillo R, Kim YG, Kim D, Koizumi Y, Hasegawa M, Himpsl SD, Browne HP, Lawley TD, Mobley HL, Inohara N, Núñez G. Distinct Commensals Induce Interleukin-1β via NLRP3 Inflammasome in Inflammatory Monocytes to Promote Intestinal Inflammation in Response to Injury. Immunity. 2015;42:744-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 234] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 100. | Song Y, Könönen E, Rautio M, Liu C, Bryk A, Eerola E, Finegold SM. Alistipes onderdonkii sp. nov. and Alistipes shahii sp. nov., of human origin. Int J Syst Evol Microbiol. 2006;56:1985-1990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 101. | Saulnier DM, Riehle K, Mistretta TA, Diaz MA, Mandal D, Raza S, Weidler EM, Qin X, Coarfa C, Milosavljevic A, Petrosino JF, Highlander S, Gibbs R, Lynch SV, Shulman RJ, Versalovic J. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. 2011;141:1782-1791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 483] [Cited by in F6Publishing: 472] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 102. | Frémont M, Coomans D, Massart S, De Meirleir K. High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe. 2013;22:50-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 103. | Kelly JR, Borre Y, O' Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 849] [Cited by in F6Publishing: 923] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 104. | Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psychiatry. 2016;21:786-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1042] [Cited by in F6Publishing: 1151] [Article Influence: 143.9] [Reference Citation Analysis (0)] |
| 105. | Qu Y, Yang C, Ren Q, Ma M, Dong C, Hashimoto K. Comparison of (R)-ketamine and lanicemine on depression-like phenotype and abnormal composition of gut microbiota in a social defeat stress model. Sci Rep. 2017;7:15725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 106. | Yang C, Qu Y, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K. Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl Psychiatry. 2017;7:1294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 107. | Getachew B, Aubee JI, Schottenfeld RS, Csoka AB, Thompson KM, Tizabi Y. Ketamine interactions with gut-microbiota in rats: relevance to its antidepressant and anti-inflammatory properties. BMC Microbiol. 2018;18:222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 108. | Huang N, Hua D, Zhan G, Li S, Zhu B, Jiang R, Yang L, Bi J, Xu H, Hashimoto K, Luo A, Yang C. Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression. Pharmacol Biochem Behav. 2019;176:93-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 109. | Galgano F, Caruso M, Condelli N, Favati F. Focused review: agmatine in fermented foods. Front Microbiol. 2012;3:199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 110. | Wang W, Snooks HD, Sang S. The Chemistry and Health Benefits of Dietary Phenolamides. J Agric Food Chem. 2020;68:6248-6267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 111. | Li P, Wu G. Composition of amino acids and related nitrogenous nutrients in feedstuffs for animal diets. Amino Acids. 2020;52:523-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 112. | Akasaka N, Fujiwara S. The therapeutic and nutraceutical potential of agmatine, and its enhanced production using Aspergillus oryzae. Amino Acids. 2020;52:181-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 113. | Selhub EM, Logan AC, Bested AC. Fermented foods, microbiota, and mental health: ancient practice meets nutritional psychiatry. J Physiol Anthropol. 2014;33:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 114. | Wallace CJK, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry. 2017;16:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 115. | Kim CS, Shin DM. Probiotic food consumption is associated with lower severity and prevalence of depression: A nationwide cross-sectional study. Nutrition. 2019;63-64:169-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 116. | Lee H, Ko G. Effect of metformin on metabolic improvement and gut microbiota. Appl Environ Microbiol. 2014;80:5935-5943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 260] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 117. | MacNeil LT, Schertzer JD, Steinberg GR. Bacteria transmit metformin-associated lifespan extension. Nat Rev Endocrinol. 2020;16:9-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 118. | Guo M, Mi J, Jiang QM, Xu JM, Tang YY, Tian G, Wang B. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin Exp Pharmacol Physiol. 2014;41:650-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 119. | Abdallah MS, Mosalam EM, Zidan AA, Elattar KS, Zaki SA, Ramadan AN, Ebeid AM. The Antidiabetic Metformin as an Adjunct to Antidepressants in Patients with Major Depressive Disorder: A Proof-of-Concept, Randomized, Double-Blind, Placebo-Controlled Trial. Neurotherapeutics. 2020;17:1897-1906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 120. | Freitas AE, Neis VB, Rodrigues ALS. Agmatine, a potential novel therapeutic strategy for depression. Eur Neuropsychopharmacol. 2016;26:1885-1899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 121. | Rosenberg ML, Tohidi V, Sherwood K, Gayen S, Medel R, Gilad GM. Evidence for Dietary Agmatine Sulfate Effectiveness in Neuropathies Associated with Painful Small Fiber Neuropathy. A Pilot Open-Label Consecutive Case Series Study. Nutrients. 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 122. | Abdallah CG, Adams TG, Kelmendi B, Esterlis I, Sanacora G, Krystal JH. Ketamine’s Mechanism of Action: A Path to Rapid-Acting Antidepressants. Depress Anxiety. 2016;33:689-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |