Published online Nov 19, 2021. doi: 10.5498/wjp.v11.i11.1116
Peer-review started: July 8, 2021
First decision: July 28, 2021
Revised: August 5, 2021
Accepted: September 22, 2021
Article in press: September 22, 2021
Published online: November 19, 2021
Subgrouping patients with major depressive disorder is a promising solution for the issue of heterogeneity. However, the link between available subtypes and distinct pathological mechanisms is weak and yields disappointing results in clinical application.
To develop a novel approach for classification of patients with time-dependent prescription patterns at first onset in real-world settings.
Drug-naive patients experiencing their first major depressive episode (n = 105) participated in this study. Psychotropic agents prescribed in the first 24 mo following disease onset were recorded monthly and categorized as antidepressants, augmentation agents, and hypnosedatives. Monthly cumulative doses of agents in each category were converted into relevant equivalents. Four parameters were used to summarize the time-dependent prescription patterns for each psychotropic load: Stability, amount, frequency, and the time trend of monthly prescriptions. A K-means cluster analysis was used to derive subgroups of participants based on these input parameters of psychotropic agents across 24 mo. Clinical validity of the resulting data-driven clusters was compared using relevant severity indicators.
Four distinct clusters were derived from K-means analysis, which matches experts’ consent: "Short-term antidepressants use", "long-term antidepressants use", "long-term antidepressants and sedatives use", and "long-term antidepressants, sedatives, and augmentation use". At the first 2 years of disease course, the four clusters differed on the number of antidepressants used at adequate dosage and duration, frequency of outpatient service use, and number of psychiatric admissions. After the first 2 years following disease onset, depression severity was differed in the four subgroups.
Our findings suggested a new approach to optimize the subgrouping of patients with major depressive disorder, which may assist future etiological and treatment response studies.
Core Tip: This study evaluated the time-dependent prescription patterns in drug-naive patients experiencing their first major depressive episode with data collected over the first 2 years after disease onset. The K-means clustering analysis was performed, along with the evaluation of four input parameters to generate data-based subgroups. Four feature-based clusters were identified, differentiated by the time-dependent prescription profiles and burden of the disease. Our novel parameters successfully captured the reciprocal interaction between physicians' prescriptions and disease status in a real-world setting. This study presents a novel clustering strategy that can be used to generate prescription-based subtypes.
- Citation: Chen HC, Hsu HH, Lu ML, Huang MC, Chen CH, Wu TH, Mao WC, Hsiao CK, Kuo PH. Subgrouping time-dependent prescribing patterns of first-onset major depressive episodes by psychotropics dissection. World J Psychiatr 2021; 11(11): 1116-1128
- URL: https://www.wjgnet.com/2220-3206/full/v11/i11/1116.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i11.1116
Major depressive disorder (MDD) is a common and challenging mental illness[1]. Even though research on the biological substrates of MDD is increasing, the heterogeneity of MDD substantially compromises the applicability of these study findings[2]. Thus, subgrouping MDD into homogeneous clusters, which could be used to investigate specific neurobiological and pathological mechanisms, is a promising solution for this.
A number of clinical features were previously used to subgroup MDD[3]. Most subtyping schemes (such as melancholic, psychotic, and atypical features) are based on differences in patterns of clinically observed characteristics. However, the link between these subtypes and distinct pathological mechanisms is weak and yields disappointing results in clinical application[3,4]. Even using data-driven latent class analyses, there is inadequate evidence to support the existence of symptom-based subtypes[5]. The heterogeneity in MDD is derived from multiple aspects of the disease, including symptom presentation, clinical features, family history, comorbi
In this study, we developed a novel approach by clustering patients with similar biologically relevant potentials. Our approach was based on three conceptual aspects. First, parameters that were correlated with biological mechanisms were preferred to theoretical or symptom-based constructs. Second, non-descriptive parameters were considered superior to linguistic-defined symptoms. Third, creating parameters that enrich the available information helped sub-classify patients into data-driven clusters[6]. While practicing physicians commonly initiate treatment according to clinical guidelines, the ensuing adjustments to the regimen are essentially based on the temporal change of clinical response. The medication class and dose of prescribed psychotropics evolve naturally and reflect the underlying disease entity, as well as the patient’s preference and treatment response at a given moment. The time-dependent change in regimen is dynamically determined by considering factors such as symptom profiles, clinical features, disease stage, treatment response, effectiveness and side effects of medications, and level of functional recovery[7]. As a result, we adopted a time-dependent prescription pattern in the early disease stage as the preferred indicator for MDD subgrouping.
In summary, we aimed to develop a novel approach to tackle the heterogeneity in drug-naive patients experiencing their first episode of MDD, using detailed prescription patterns of major psychotropic classes during the early disease course. Specifically, we developed new parameters to capture time-dependent information on prescription patterns in the first two years after the initial diagnosis. Using this information, we performed a K-means clustering analysis for data-driven subgrouping to yield empirical subtypes of MDD.
This study used the cohort established by the Research Collaborating Group for New Insight, Strategy and Evaluation–Treatment-Resistant Depression Program (RECOGNISE-TRD program). Between October 2010 and April 2016, remitted individuals with a DSM-IV-TR diagnosis of MDD and between 18 and 65 years old were recruited to participate in this study[8]. Participants were eligible if they had more than 2 years of complete medical records from their first episode to the date of recruitment. The duration of the MDD course beyond the 2 years after onset to the date of recruitment differed between participants. The medical records should document all of the participants' psychiatric and medical history throughout the disease course, including prescriptions, use of outpatient and inpatient services, and information on their comorbidities. If participants discontinued their treatments within 2 years of disease onset because of full remission, they were contacted to ensure the absence of any depressive episode before recruitment. Participants were excluded if they had incomplete medical records, were comorbid with organic brain syndromes, dementia, substance abuse, psychotic disorders, and schizoaffective disorders or if their diagnosis had been revised to bipolar affective disorder. A total of 105 participants were eligible. This study was approved by the research ethics committees of National Taiwan University Hospital, Taipei Municipal Wanfang Hospital, and Taipei City Hospital, Songde Branch. Written informed consent was obtained from each participant. All methods were performed in accordance with the relevant guidelines and regulations.
Three major classes of psychotropic agents are used to treat MDD, including antidepressants and adjunctive agents (augmentation agents and hypnosedatives). Following disease onset, the drug class, dosage, date of administration, and duration of prescription were recorded in 4-wk intervals. Since the natural course of a major depressive episode is usually between 6-13 mo[9], the present study evaluated the prescription pattern of psychotropics using monthly data collected over the first 2 years after disease onset. Observations collected over this period provided adequate time for patients to experience all treatment regimens suggested by the treatment guidelines.
In this study, pharmacological dissection was used to categorize the psychotropic agents, including antidepressants, augmentation agents and hypnosedatives[10]. Antidepressants used in this study include selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, tricyclic antidepressants, mirtazapine, bupropion, moclobemide, and agomelatine. Augmentation agents included anticonvulsants, antipsychotics, lithium, thyroxin, and methylphenidate. All benzodiazepine receptor agonists (BzRA) were categorized as hypnosedatives. Cumulative doses of each psychotropic medication class were converted into equivalents and designated as antidepressant load (ADL), augmentation load (AUGL), and sedative load (SL). These three equivalent-based psychotropic loads have been used in the literature[10]. For each participant, the monthly cumulative equivalents for 3 classes of psychotropic loads were calculated in 4-wk intervals, starting with the initiation of treatment.
Doses of antidepressants and augmentation agents were transformed into equivalents using the Anatomical Therapeutic Chemical (ATC) system[11]. In analyzing hypnosedative use, equivalent doses of all BzRA were calculated, including benzodiazepines (BZDs) and the Z-drugs (zaleplon, zolpidem, zopiclone, and eszopiclone). As the defined daily dose in the ATC system for hypnosedatives do not adequately represent the clinical usage of BZDs as anxiolytics or hypnotics[12], the BZDs equivalents suggested by the Ashton manual were adopted in the present study[13]. Since conversion ratios for a number of BZD drugs commonly used in Taiwan are not provided in the original Ashton manual (including brotizolam, midazolam, oxazolam, and fludiazepam), study investigators defined the equivalents for these agents by consensus. The full list of BZD equivalents is provided in Supple
In this study, anxiety disorders and physical comorbidities were incorporated as covariates in the multivariable models evaluating the differences in several clinical severity indicators between empirical clusters. The presence of an anxiety disorder was defined as more than three consecutive instances that matched the DSM-IV-TR diagnostic criteria during the first two years following MDD onset. Additionally, physical comorbidities were evaluated as the number of medical diseases identifiable using the Charlson comorbidity index[14].
Several domains of clinical severity were used to examine the validity of data-driven clusters. Firstly, the total number of prescribed antidepressants and the number of prescribed antidepressants with adequate dosage and adequate duration (ADAD) were calculated within the first two years after disease onset. The adequate dosage used was as per the dose suggested by the Ministry of Health and Welfare, Taiwan, for treating MDD[15]. An adequate duration was defined as at least two weeks of treatment[16,17]. Secondly, the cumulative frequency of patients' utilization of psychiatric facilities, including outpatient services and psychiatric admissions, were documented for within 2 years of disease onset and from 2 years after disease onset to the date of recruitment, respectively. Additionally, the severity of depression was scored on the recruitment date using the Chinese version of the Beck Depression Inventory-II (BDI-II): Level 0 for scores 0–16 (euthymic); 1 for 17–22 (mild); 2 for 23–30 (moderate); and 3 for 31–63 (severe)[18].
Data-driven clustering analyses: A K-means clustering analysis was used for empirical subtyping. To optimize the data analyses, biological treatment history was longitudinally recorded for each participant in 4-wk intervals, yielding 24 sets of data points. Four parameters were used to capture the time-dependent prescription pattern for each class of psychotropic agents. In each participant, 24 data points for psychotropic load were calculated to determine the stability of prescribed dosage, frequency of high-dosage prescriptions, frequency of prescriptions, and the time trend of prescribed dosage. The stability of the prescribed dosage was a reflection of dosage fluctuation over the 2 year period. It was evaluated by estimating the coefficient of variance within the 24 points of data for each psychotropic load. Secondly, a count of high dosage prescriptions was obtained as an index of the need for high levels of specific interventions to achieve symptomatic control. The median of each class of psychotropic load was calculated for all participants using dichotomized cutoffs. The 24 data points for each class of psychotropic load in individual participants were compared with the cutoff. The total number of points in which the psychotropic loads were equal or greater than the median was recorded. Higher counts corresponded to a greater frequency of high-dosage prescription. Thirdly, the frequency of the prescription denoted the count of the presence of psychotropic load in the 24 data points. It represents the frequency of utilization of outpatient services and indicates the intensity of psychiatric care. Finally, the trend over time of the prescribed dosage evaluated the increments or decrements in psychotropic dosage. The correlation coefficient between each psychotropic load and time was determined for each participant to quantify the time trend of the prescribed dosage. A total of 12 parameters were generated for each participant. A K-means clustering analysis was performed using all collected parameters. The number of cluster K was an input parameter, and it was set at a priori between 3 and 5. The final class of subgroups was determined based on clinical relevance and implications. Additionally, we noted that if the scales of the input parameters are notably different, the contribution of these parameters may be uneven. Because the scales of ‘the frequency of prescription’ and ‘the level of dosage’ are greater than the other variables, we conducted sensitivity analysis to use these two parameters only, and perform the clustering analysis again. We then compared the differences of resultant clusters for evaluating the robustness of the current findings.
Between-cluster comparisons: After data-driven clustering analyses were completed using the K-means method, several clinical severity indicators were used to examine between-cluster differences. In these analyses, a Chi-square and analysis of variance (ANOVA) were used as univariate analyses. For multivariable regression analyses (excluding analyses of the severity of depression), all clinical severity data were taken into account, and Poisson regression analyses were conducted because of count data. General linear models were constructed in consideration of the between-cluster differences in depression severity at the end of the follow-up period. Data were analyzed using the SPSS version 17.0 (SPSS Inc., Chicago, IL, United States) statistical package. The statistical significance level was set at a P value of < 0.05.
A total of 105 individuals with first-onset MDD participated in the study. The average age of participants was 51.5 ± 13.2 years old, their age at MDD onset was 44.5 ± 13.6 years old, 75.5% were female, and 20.8% had concurrent anxiety disorders (Table 1).
| Total | Simple pharmacy, | Simple pharmacy, | Complex pharmacy, | Complex pharmacy, | P value for univariate omnibus test | |
| Demographic and general clinical data | ||||||
| Age (yr) | 51.5 (13.2) | 42.6 (14.4) | 50.6 (15.2) | 54.9 (9.1) | 56.5 (10.3) | 0.02 |
| Age of onset (yr) | 44.5 (13.6) | 36.3 (14.4) | 43.2 (15.3) | 48.6 (10.0) | 49.6 (11.0) | 0.01 |
| Female, n (%) | 80 (75.5) | 15 (78.9) | 24 (72.7) | 27 (79.4) | 13 (68.4) | 0.79 |
| Comorbid anxiety disorder, n (%) | 22 (20.8) | 6 (31.6) | 9 (27.3) | 4 (11.8) | 3 (15.8) | 0.25 |
| Number of comorbid physical diseases | 0.7 (1.3) | 0.5 (1.2) | 0.7 (1.4) | 1.1 (1.5) | 0.6 (1.2) | 0.39 |
| Follow-up duration after 2-yr of disease onset (yr) | 3.7 (3.5) | 3.2 (3.2) | 3.4 (3.1) | 3.7 (3.2) | 3.8 (3.9) | 0.91 |
| Psychotropic loads | ||||||
| Antidepressant load (defined daily dose/month) | 34.5 (18.4) | 13.3 (7.2) | 32.1 (13.1) | 42.8 (17.3) | 44.8 (17.7) | < 0.001 |
| Sedative load (equivalent dose/month) | 57.3 (56.8) | 8.1 (10.8) | 18.5 (20.9) | 91.9 (45.4) | 112.0 (56.7) | < 0.001 |
| Augmentation load (defined daily dose/month) | 1.2 (2.7) | 0.1 (0.5) | 0.3 (0.6) | 0.3 (1.0) | 5.3 (4.0) | < 0.001 |
| Clinical feature within 2-yr of disease onset | ||||||
| Number of antidepressants use | 1.7 (0.9) | 1.3 (0.6) | 1.6 (0.8) | 1.9 (1.1) | 1.9 (0.9) | 0.07 |
| Number of antidepressants use with adequate dosage and duration | 1.5 (0.9) | 1.1 (0.5) | 1.3 (0.8) | 1.7 (1.2) | 1.8 (0.8) | 0.04 |
| Visits of psychiatric outpatient service | 17.3 (10.7) | 10.6 (5.8) | 15.5 (5.1) | 20.2 (10.6) | 21.8 (17.1) | 0.002 |
| Frequencies of psychiatric admission | 0.2 (0.5) | 0.1 (0.3) | 0.2 (0.4) | 0.1 (0.3) | 0.7 (0.8) | < 0.001 |
| Clinical feature beyond 2-yr of disease onset | ||||||
| Visits of psychiatric outpatient service | 31.1 (66.7) | 9.7 (10.3) | 20.2 (24.7) | 41.4 (91.6) | 42.6 (78.4) | 0.22 |
| Frequencies of psychiatric admission | 0.8 (0.5) | 0.0 (0.0) | 0.1 (0.4) | 0.1 (0.7) | 0.0 (0.0) | 0.71 |
| Severity of depression in the recruitment1 | 1.5 (0.9) | 0.6 (1.0) | 0.9 (1.2) | 1.2 (1.3) | 1.7 (1.2) | 0.02 |
According to the results of the K-means clustering analyses, 4 feature-based clusters were identified. Based on the differences between regimens, psychotropic load, and prescription duration, the clusters were designated as short-term antidepressant use (SA, n = 19), long-term antidepressant use (LA, n = 33), long-term antidepressants and sedatives use (LAS, n = 34), and long-term antidepressants, sedatives, and augmentation use (LASA, n = 19). The parameters used for the K-means clustering analyses are detailed in Supplementary Table 2. Additionally, SA and LA clusters were denoted as the simple pharmacy group, due to the relatively simple pattern of psychotropic agent use. In contrast, the LAS and the LASA clusters comprised the complex pharmacy group. Table 1 also summarizes the univariate comparisons of various demographic characteristics and clinical indicators between the 4 clusters.
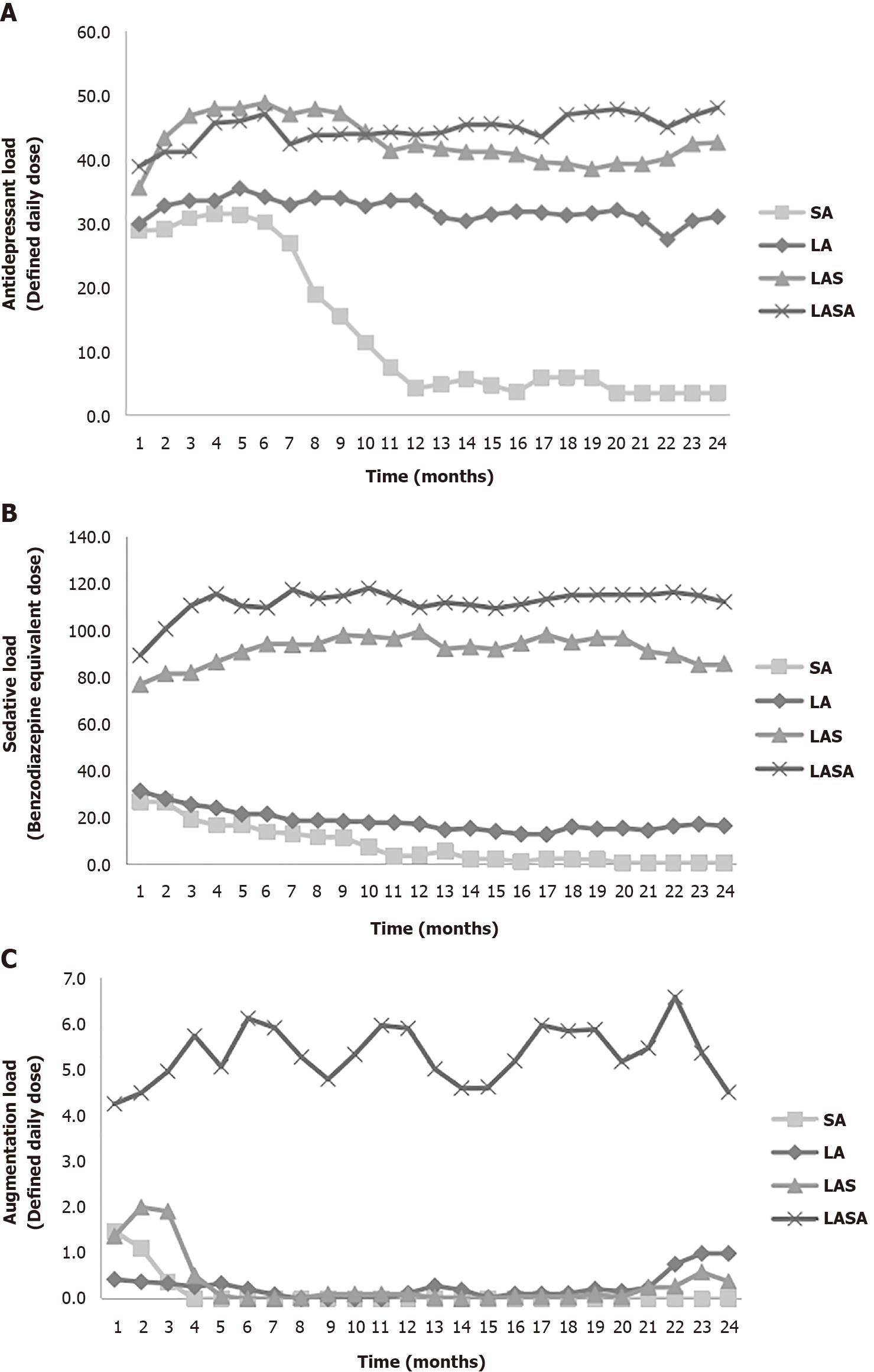
Figure 1 depicts the change over time in the monthly equivalents in the 4 clusters based on three classes of psychotropic loads. The lowest average ADL for the first 2 years following disease onset in the SA reflects the lower initial dosage and shorter exposure period of these participants (Figure 1A). Figure 1A also demonstrates that ADL in the LA cluster is consistent over time and similar to the complex pharmacy group, while the dosage of antidepressants was lower in the LA cluster. This finding is consistent with a comparison of the averaged ADL values between clusters. Additionally, Figure 1B presents the time-course of SL in the 4 clusters. Complex pharmacy group clusters exhibited steady levels and the higher equivalent of hypnosedatives, which discriminates them from the simple pharmacy group. Figure 1C illustrates the unique properties of LASA in the prescription pattern of augmentation agents. The three clusters (excluding LASA) exhibited less monthly AUGL until 4 mo after MDD onset.
Table 2 summarizes the differences in various clinical severity indicators between clusters, after controlling for the confounding effects. In the first 2 years following MDD onset, no significant difference was observed between the 4 clusters in the numbers of antidepressants used. However, the number of ADAD in the SA was significantly lower than in LAS (P = 0.04) and LASA (P = 0.03). Although the average ADL of LA is significantly lower than the complex pharmacy group (Table 1), the number of used antidepressants and ADAD did not significantly differ between the LA and the complex pharmacy group clusters. Analysis of the use of outpatient services within the first 2 years of disease course, showed a significantly lower number of visits in the clusters of the simple pharmacy group compared with those in the complex pharmacy group. Furthermore, LA exhibited greater visits to the outpatient services than SA (P < 0.001), but no significant difference was observed between the LAS and the LASA. The LASA cluster exhibited a higher frequency of psychiatric admission in the first 2 years after MDD onset, compared with the other 3 clusters. However, SA, LA, and LAS did not differ in terms of admission frequency. The simple pharmacy group exhibited a lower frequency of visits to outpatient services after 2 years from disease onset than the complex pharmacy group. However, no significant difference was observed between LAS and LASA. Finally, the clusters in the complex pharmacy group exhibited more severe depressive symptoms than the clusters of the simple pharmacy group. However, no within-group differences were found in terms of depression severity in both the simple and complex pharmacy groups (Table 2).
| Simple pharmacy, short-term antidepressants use (SA, n = 19) | Simple pharmacy, long-term antidepressants use (LA, n = 33) | Complex pharmacy, long-term antidepressants and sedatives use (LAS, n = 34) | Complex pharmacy, long-term antidepressants, sedatives and augmentation use (LASA, n = 19) | P value for pos-hoc comparison using multivariable regression test | |
| IRR (95%CI) | IRR (95%CI) | IRR (95%CI) | IRR (95%CI) | ||
| Clinical feature within 2-yr of disease onset1 | |||||
| Number of antidepressants use | 0.57 (0.32-0.82) | 0.74 (0.52-0.97) | 0.94 (0.66-1.23) | 0.96 (0.61-1.31) | |
| Number of antidepressants use with adequate dosage and duration | 0.50 (0.26-0.73) | 0.64 (0.44-0.85) | 0.87 (0.59-1.15) | 0.95 (0.60-1.31) | SA < LAS (P = 0.04); SA < LASA (P = 0.03) |
| Visits of psychiatric outpatient service | 4.76 (4.04-5.47) | 7.46 (6.75-8.17) | 10.34 (9.37-11.30) | 11.37 (10.15-12.58) | SA < LA (P < 0.001); SA < LAS (P < 0.001); SA < LASA (P < 0.001); LA < LAS (P < 0.001); LA < LASA (P < 0.001) |
| Frequencies of psychiatric admission | 0.03 (-0.02-0.08) | 0.05 (-0.002-0.10) | 0.03 (-0.01-0.08) | 0.24 (0.04-0.44) | SA < LASA (P = 0.01); LA < LASA (P = 0.004); LAS < LASA (P = 0.001) |
| Clinical feature beyond 2-yr of disease onset | |||||
| Visits of psychiatric outpatient service2 | 3.03 (2.58-3.49) | 5.81 (5.34-6.30) | 10.21 (9.49-10.93) | 10.86 (9.98-11.74) | SA < LAS (P < 0.001); SA < LA (P < 0.001); SA < LASA (P < 0.001); LA < LAS (P < 0.001); LA < LASA (P < 0.001) |
| Frequencies of psychiatric admission3 | - | - | - | - | |
| EMM (95%CI) | EMM (95%CI) | EMM (95%CI) | EMM (95%CI) | ||
| Severity of depression in the recruitment4 | 0.58 (0.23-0.93) | 0.90 (0.54-1.25) | 1.66 (1.10-2.22) | 2.20 (1.37-3.30) | SA < LAS (P = 0.002); SA < LASA (P < 0.001); LA < LAS (P = 0.02); LA < LASA (P = 0.001) |
To evaluate the robustness of the clustering results, we re-analyzed the data with various parameter combinations. Our sensitivity analysis revealed similar clustering results. By adopting only 2 parameters, the frequency of high-dosage prescriptions, and the frequency of prescriptions, the K-means clustering analysis still yielded the same 4 clusters as were generated by the original 4 parameters.
The patients were empirically classified into 4 clusters according to the K-means clustering analysis that dissected the time-dependent prescription pattern over the first 2 years following the onset of the first episode of MDD. This analysis was facilitated by novel parameters that successfully capture the reciprocal interactions between physicians' prescriptions and disease status in a real-world setting. The 4 identified clusters exhibited distinct patterns in terms of the dosage of psychotropic agents, duration of prescription, and the combinations of psychotropic agents used. Additionally, the 4 clusters also exhibited differences in severity of clinical indicators. To the best of our knowledge, this is the first attempt to classify MDD by pharmacological dissection considering time-dependent prescription patterns using a data-driven clustering analysis.
While no between-cluster differences were observed in the number of antidepressants used in this study, the SA exhibited lower number of ADAD used, compared to the clusters comprising the complex pharmacy group. In the clinical setting, the number of antidepressants used reflects the expense involved in the use of sequential trials to select appropriate antidepressants. In contrast, the number of ADAD is a reflection of inadequate treatment response after a series of interventions[19]. Therefore, data-driven clusters in the present study suggest a different level of treatment difficulty, beyond the number of trials used to identify a tolerable antidepressant regimen. Furthermore, the use of psychiatric facility (an index of clinical disease burden) showed differences between clusters. The complex pharmacy group exhibited higher number of visits to outpatient services compared to the simple pharmacy group within the first 2 years following MDD onset and beyond. The use of outpatient services reflects the need for re-evaluation or stabilization of symptoms, and serves as an indicator of long-term treatment stability. Taken together, our findings suggest that the patients in the complex pharmacy group require more intensive care to stabilize their symptoms. Similarly, the level of BDI-II-defined depression on the recruitment day corresponded to the pattern of residual symptoms over time in the complex pharmacy group. Finally, psychiatric admissions are often indicated in life-threatening conditions or situations where the treatment response remains inadequate after a series of guideline-based interventions. LASA, in comparison with other clusters with low AUGL, uniquely exhibited a highest frequency of psychiatric admissions. These findings support the clinical relevance of the prescription-based subtyping strategy.
According to the established pharmacological mechanisms of each class of psychotropic agent, the four prescription-based clusters correspond to distinct biological mechanisms. In the simple pharmacy group, SA and LA were associated with similar initial doses of antidepressants, but differed in the duration of prescription. Consistent with the clinical practice, the episodes in the SA cluster exhibited the expected response to antidepressants during the acute and maintenance phases. In contrast, LA cluster of patients needed a longer period of maintenance therapy due to vulnerability to relapse or recurrence, despite the apparent adequate antidepressant treatment. This finding suggests that the traditional monoaminergic antidepressants can correct the neurochemical deficits of MDD in clusters comprising the simple pharmacy group.
Compared with the relatively short-term and low-dose exposure to hypnosedatives in the simple pharmacy group, clusters in the complex pharmacy group were characterized by longer duration and high doses of hypnosedative use. Interestingly, the rate of comorbidity of anxiety disorders did not differ significantly between the 4 clusters. Although antidepressants were suggested as the primary therapy for anxiety[20], monoaminergic antidepressants alone seem insufficient for patients in the complex pharmacy group. Contrary to the recommendation of short-term adjunct use of hypnosedatives in the treatment guidelines[21], depending on study populations, 7.6 to 60% patients with MDD initiated antidepressant treatment with concurrent hypnosedatives, while 12% to 48% of patients received long-term combined treatment[22-28]. Indeed, the monoaminergic deficiency hypothesis was regarded as insufficient to explain the heterogeneity of MDD[29]. Additionally, monoaminergic antidepressants and benzodiazepines may alleviate the anxiety symptoms through different mechanisms[29,30]. Therefore, clusters within the complex pharmacy group may have specific pathogenic mechanisms that cannot be resolved by monoaminergic antidepressants alone, such as deficits in the gamma-aminobutyric acid-related system[29]. This finding suggests that, instead of subgrouping by comorbidity of anxiety disorders, classifying individuals by hypnosedative use pattern over 2 years may be more informative and facilitate the identification of common neurochemical deficits among these patients. Moreover, in addition to long-term hypnosedative use, LASA is characterized by long-term concomitant use of augmentation agents. Because various medications with different pharmacological mechanisms were included as augmentation agents in this study, the heterogeneity of neurochemical deficits in the LASA cluster may be more complex than originally believed.
Clusters comprising the complex pharmacy group exhibited a higher initial SL that persisted over the first 2 year after MDD onset. This is consistent with the previously published findings showing that depressed patients who used a higher daily dosage of sedatives in the initial treatment phase tend to be long-term users[28]. Additionally, the association between sedative use and clinical severity was previously found to be independent of the use of antidepressants and augmentation agents[10]. The complex pharmacy group in this study exhibited high dosage and long duration of hypnosedatives use, which correlated with worse indicators of disease severity. However, when AUGL is taken into account, participants with long-term use of hypnosedatives could be further partitioned into LAS and LASA, which differ in aspects of clinical severity (Table 2). In this study, the time-dependent pattern of ADL separated the SA, LA, and clusters comprising the complex pharmacy. When SL and AUGL are taken into consideration, a difference was detected between clusters, enhancing the clinical implications of these data-driven clusters. Therefore, simultaneous inclusion of the 3 classes of psychotropic loads has distinct and complementary function in the clustering analysis.
There are a number of limitations to this study. First, the strict inclusion criteria resulted in a sample size which is not large enough to ensure that all statistical models are sufficiently powered. However, under the current sample size, most of the analyses have successfully demonstrated between-cluster differences in terms of clinical severity indicators. This observation indirectly supports a significant extent of between-cluster differences between the resulting clusters. Additionally, people in Taiwan have high accessibility and availability to medical resources. Since eligible participants had to have at least 2 years of complete treatment history in a teaching hospital, they usually exhibited superb adherence, which may influence the selection of participants. Therefore, study findings may not be generalized to all patient groups. Second, medications compliance is unknown in our participants. Therefore, the presented psychotropic loads may not reflect the actual dose taken by the participants. Moreover, we did not know if the patients concurrently received pharmacological or non-pharmacological treatment from other hospitals, which could introduce misclassification bias. Third, baseline severity of depression was not evaluated in this study. However, findings of a previous study suggest that the initial severity of MDD is not correlated with treatment response[31]. Absence of this information is unlikely to change the clustering results. Fourth, the latency from the disease onset to the commencement of treatment is not recorded in this study. Since the delay in treatment makes it more difficulty to treat the major depressive episode, this may introduce bias in inference of homogeneity in pathogenic mechanisms between clusters. Finally, the resultant clusters in this study may merely reflect therapists' prescription preferences. According to the examination of clinical validity, we believe that these empirically-derived clusters, at least in part, reflect the underlying pathological mechanisms.
With the trend of increased data sharing of medical registries, prescription patterns are well established in most data banks. While claimed based datasets do not have detailed information on symptoms or dynamic disease status in a real-world setting, these kinds of data would have large sample size and large amounts of data regarding prescription patterns. By using such kinds of databases, researchers not only can investigate the heterogeneity of MDD but also explore the complexity feature with other comorbid health conditions, and can easily expand to study other mental disorders. Additionally, machine learning approaches, such as K-means clustering analyses can be used to optimize the subgrouping information. In the future, this technique can be enhanced by reducing the required number of input parameters and duration of prescription records. The biological validity, generalizability, and predictive value of this new clustering approach warrant further investigation.
Major depressive disorder (MDD) is a common and challenging mental illness. Even though research on the biological substrates of MDD is increasing, the heterogeneity of MDD substantially compromises the applicability of these study findings. Thus, subgrouping MDD into homogeneous clusters, which could be used to investigate specific neurobiological and pathological mechanisms, is a promising solution.
The heterogeneity in MDD is derived from multiple aspects of the disease. There is inadequate evidence to support the existence of symptom-based subtypes. Thus, we intended to develop a novel approach by clustering patients with similar biologically relevant potentials.
We aimed to develop a novel approach to tackle the heterogeneity in drug-naive patients experiencing their first episode of MDD, using detailed prescription patterns of major psychotropic classes during the early disease course.
Psychotropic agents prescribed in the first 24 mo following disease onset were recorded monthly and categorized as antidepressants, augmentation agents, and hypnosedatives. Four parameters were used to summarize the time-dependent prescription patterns for each psychotropic agent. A K-means cluster analysis was used to derive subgroups of participants based on these four parameters of psychotropic agents across 24 mo.
The patients were empirically classified into 4 clusters according to the K-means clustering analysis that dissected the time-dependent prescription pattern over the first 2 years following the onset of the first episode of MDD. The four identified clusters exhibited distinct patterns in terms of the dosage of psychotropic agents, duration of prescription, and the combinations of psychotropic agents used. Additionally, the four clusters also exhibited differences in severity of clinical indicators.
Our novel parameters successfully captured the reciprocal interaction between physicians' prescriptions and disease status in a real-world setting. This study presents a novel clustering strategy that can be used to generate prescription-based subtypes.
Machine learning approaches, such as K-means clustering analyses can be used to optimize the subgrouping information. The biological validity, generalizability, and predictive value of this new clustering approach warrant further investigation.
The authors would like to thank the Dr. Chin-Hao Chang for the assistance of statistical analysis; Dr. Chih-Kang Hsu, Dr. Hsien-hsueh Shih, Dr. Yen-Chin Wang, Dr. Hai-Ti Lin, Dr. Tzu-Yu Liu, Dr. Meng-Shiuan Shie and research assistants Miss I-Chen Huang and Miss Chieh-Chun Hu for their help with data collection.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Psychiatry
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yao XF, Yu R S-Editor: Fan JR L-Editor: A P-Editor: Guo X
| 1. | Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, de Girolamo G, de Graaf R, Demyttenaere K, Hu C, Iwata N, Karam AN, Kaur J, Kostyuchenko S, Lépine JP, Levinson D, Matschinger H, Mora ME, Browne MO, Posada-Villa J, Viana MC, Williams DR, Kessler RC. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9:90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1172] [Cited by in F6Publishing: 1210] [Article Influence: 93.1] [Reference Citation Analysis (0)] |
| 2. | McIntosh AM, Sullivan PF, Lewis CM. Uncovering the Genetic Architecture of Major Depression. Neuron. 2019;102:91-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 3. | Rush AJ. The varied clinical presentations of major depressive disorder. J Clin Psychiatry. 2007;68 Suppl 8:4-10. [PubMed] [Cited in This Article: ] |
| 4. | Uher R, Muthén B, Souery D, Mors O, Jaracz J, Placentino A, Petrovic A, Zobel A, Henigsberg N, Rietschel M, Aitchison KJ, Farmer A, McGuffin P. Trajectories of change in depression severity during treatment with antidepressants. Psychol Med. 2010;40:1367-1377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | de Vos S, Wardenaar KJ, Bos EH, Wit EC, de Jonge P. Decomposing the heterogeneity of depression at the person-, symptom-, and time-level: latent variable models versus multimode principal component analysis. BMC Med Res Methodol. 2015;15:88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | van Loo HM, de Jonge P, Romeijn JW, Kessler RC, Schoevers RA. Data-driven subtypes of major depressive disorder: a systematic review. BMC Med. 2012;10:156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 7. | Saltiel PF, Silvershein DI. Major depressive disorder: mechanism-based prescribing for personalized medicine. Neuropsychiatr Dis Treat. 2015;11:875-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. Washington, DC: American Psychiatric Association, 2000. [Cited in This Article: ] |
| 9. | Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock's Synopsis of Psychiatry: Behavioral Sciences/clinical Psychiatry. Philadelphia, United States: Lippincott Williams & Wilkins, 2014. [Cited in This Article: ] |
| 10. | Wang YC, Lin HT, Lu ML, Huang MC, Chen CH, Wu TH, Wang S, Mao WC, Kuo PH, Chen HC. The Association Between the Sedative Loads and Clinical Severity Indicators in the First-Onset Major Depressive Disorder. Front Psychiatry. 2019;10:129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | World Health Organization. WHO Collaborating Centre for Drug Statistic Methodology Oslo, Norway: WHO; 2015. [cited 10 May 2021]. Available from: http://www.whocc.no/filearchive/publications/2015_guidelines.pdf. [Cited in This Article: ] |
| 12. | Islam MM, Conigrave KM, Day CA, Nguyen Y, Haber PS. Twenty-year trends in benzodiazepine dispensing in the Australian population. Intern Med J. 2014;44:57-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Ashton H. The Benzodiazepine: what they do in the body 2002. [cited 10 May 2021]. Available from: http://www.benzo.org.uk/manual/bzcha01.htm. [Cited in This Article: ] |
| 14. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32099] [Cited by in F6Publishing: 34912] [Article Influence: 943.6] [Reference Citation Analysis (0)] |
| 15. | Taiwan Food and Drug Administration. Drug permit license Web site. 2015. [cited 10 May 2021]. Available from: http://www.fda.gov.tw/MLMS/H0001.aspx.. [Cited in This Article: ] |
| 16. | Bauer M, Pfennig A, Severus E, Whybrow PC, Angst J, Möller HJ; World Federation of Societies of Biological Psychiatry. Task Force on Unipolar Depressive Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14:334-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 397] [Cited by in F6Publishing: 373] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 17. | Liu TY, Kuo PH, Lu ML, Huang MC, Chen CH, Wu TH, Wang S, Mao WC, Chen HC. Quantifying the level of difficulty to treat major depressive disorder with antidepressants: Treatment Resistance to Antidepressants Evaluation Scale. PLoS One. 2020;15:e0227614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Lu ML, Che HH, Chang SW, Shen WW. Reliability and Validity of the Chinese Version of the Beck Depression Inventory-II. Taiwanese J Psychiatry. 2002;16:301-310. [Cited in This Article: ] |
| 19. | Montgomery SA. Selectivity of antidepressants and resistant depression. In: Amsterdam JD, editor. Advances in Neuropsychiatry and Psychopharmacology. 2. New York, United States: Raven Press, 1991: 93-104. [Cited in This Article: ] |
| 20. | Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci. 2017;19:93-107. [PubMed] [Cited in This Article: ] |
| 21. | National Collaborating Centre for Mental Health. Depression: the treatment and management of depression in adults (updated edition). NICE guideline[CG90] Web site. London2010. [cited 10 May 2021]. Available from: https://www.nice.org.uk/guidance/cg90/evidence. [Cited in This Article: ] |
| 22. | Furukawa TA, Kitamura T, Takahashi K. Treatment received by depressed patients in Japan and its determinants: naturalistic observation from a multi-center collaborative follow-up study. J Affect Disord. 2000;60:173-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Demyttenaere K, Bonnewyn A, Bruffaerts R, De Girolamo G, Gasquet I, Kovess V, Haro JM, Alonso J. Clinical factors influencing the prescription of antidepressants and benzodiazepines: results from the European study of the epidemiology of mental disorders (ESEMeD). J Affect Disord. 2008;110:84-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Sawada N, Uchida H, Suzuki T, Watanabe K, Kikuchi T, Handa T, Kashima H. Persistence and compliance to antidepressant treatment in patients with depression: a chart review. BMC Psychiatry. 2009;9:38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Pfeiffer PN, Ganoczy D, Zivin K, Valenstein M. Benzodiazepines and adequacy of initial antidepressant treatment for depression. J Clin Psychopharmacol. 2011;31:360-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Wu CS, Shau WY, Chan HY, Lai MS. Persistence of antidepressant treatment for depressive disorder in Taiwan. Gen Hosp Psychiatry. 2013;35:279-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Subramaniam M, He VY, Vaingankar JA, Abdin E, Chong SA. Prevalence of and factors related to the use of antidepressants and benzodiazepines: results from the Singapore Mental Health Study. BMC Psychiatry. 2013;13:231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Bushnell GA, Stürmer T, Gaynes BN, Pate V, Miller M. Simultaneous Antidepressant and Benzodiazepine New Use and Subsequent Long-term Benzodiazepine Use in Adults With Depression, United States, 2001-2014. JAMA Psychiatry. 2017;74:747-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 531] [Cited by in F6Publishing: 555] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 30. | Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 389] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 31. | Gibbons RD, Hur K, Brown CH, Davis JM, Mann JJ. Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69:572-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |