Published online Oct 19, 2021. doi: 10.5498/wjp.v11.i10.821
Peer-review started: February 26, 2021
First decision: May 5, 2021
Revised: May 13, 2021
Accepted: August 18, 2021
Article in press: August 18, 2021
Published online: October 19, 2021
In December 2019, a novel coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was initially reported in Wuhan, China. Previous epidemics including SARS and middle east respiratory syndrome raises concern that COVID-19 infection may pose a significant threat to the mental health of affected individuals. Studies and reviews have shown the acute psychiatric manifestations in COVID-19 patients, although long term psychiatric sequelae are predicted, there are only few review studies about the long term psychiatry outcome in COVID-19 survivors. Clinically significant post-traumatic stress disorder, anxiety, and/or depression among COVID-19 survivors during 14-90 d were observed following the diagnosis. Risk of anxiety or depression were higher in patients with more severe illness at 6 mo follow-up, early convalescence, and at 1 mo follow-up. Diagnosis of COVID-19 Led to more first diagnoses and relapses of psychiatric illness during the first 14-90 d after COVID-19 diagnosis. The possible underlying mechanisms of psychiatric sequelae in COVID-19 infection are neurotropism, immune response to SARS-CoV-2, hypothalamo-pituitary-adrenal axis hyperactivity, disrupted neuronal circuits in several brain regions, increased stress levels, neuroinflammation, and neuronal death. This study will review the psychiatric sequelae in previous coronavirus pandemics, current studies, risk factors, and thorough explanation on patho
Core Tip: Studies and reviews have shown the acute psychiatric manifestations in coronavirus disease 2019 (COVID-19) patients, and although long term psychiatric sequelae are predicted, there are only few review studies about the long-term psychiatry outcome in COVID-19 survivors. Clinically significant post-traumatic stress disorder, anxiety, and/or depression among COVID-19 survivors during 14-90 d following the diagnosis. Risk of anxiety or depression were higher in patients with more severe illness at 6 mo follow-up, early convalescence, and at 1 mo follow-up. Diagnosis of COVID-19 Led to more first diagnoses and relapses of psychiatric illness during the first 14-90 d after COVID-19 diagnosis.
- Citation: Putri C, Arisa J, Hananto JE, Hariyanto TI, Kurniawan A. Psychiatric sequelae in COVID-19 survivors: A narrative review. World J Psychiatr 2021; 11(10): 821-829
- URL: https://www.wjgnet.com/2220-3206/full/v11/i10/821.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i10.821
In December 2019, a novel coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was initially reported in Wuhan, China. This disease has caused a national outbreak of severe pneumonia in China and spread worldwide rapidly. On January 30th, 2020, the World Health Organization (WHO) declared the outbreak to be a Public Health Emergency of International Concern[1]. As of February 5th, 2021, there have been 103989900 confirmed cases of COVID-19, including 2260259 deaths, globally[2]. Previous published meta-analysis studies have identified several comorbidities[3-7], home medications[8,9], and laboratory values[10,11] which are associated with severe outcomes and the risk of dying from COVID-19.
Based on previous epidemics experiences, including SARS and middle east respiratory syndrome (MERS), it is recognized that COVID-19 infection may pose a significant threat to the mental health of affected individuals[12]. Studies have reported psychiatric symptoms in SARS survivors, including post-traumatic stress disorder (PTSD), depression, panic disorder, and obsessive-compulsive disorder at 1 to 50 mo follow up[13-15].
Previously published systematic review and meta-analysis studies have shown that the prevalence of psychological consequences of those inflicted or suspected of COVID-19, health care workers, and the general population is 26% (95%CI: 21-32). Pooled prevalence for symptoms of PTSD was 33% (0–86), anxiety 28% (21-36), stress 27% (14-43), and depression 22% (13-33)[16]. Although psychiatric sequelae are predicted, there are only a few studies about the long-term psychiatry outcome in COVID-19 survivors.
The pandemic itself is a significant psychological stressor in addition to its enormous impact on social and economic sectors worldwide. Isolation and small social networks during quarantine period limit access to external supports[17].
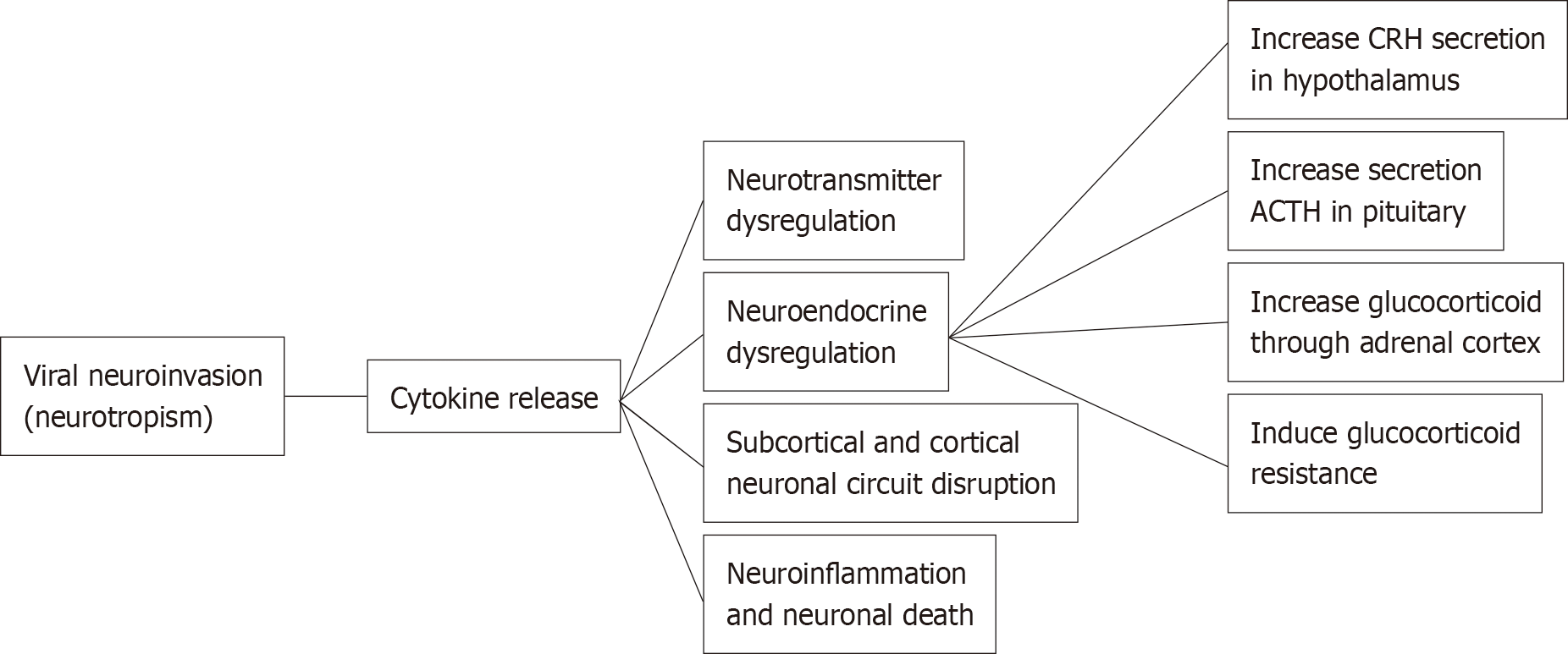
Beside the pandemic-associated psychological distress, there are several arguments that may explain the association between COVID-19 and psychological symptoms as its sequelae. Hereby, we review the current evidence regarding the characteristics of psychiatric sequelae from COVID-19 and the mechanism of how COVID-19 infection affects the communication between endocrine, immune, and central nervous systems, resulting in psychiatric sequelae in COVID-19 survivors (Figure 1).
Past pandemics have demonstrated that diverse types of neuropsychiatric symptoms, such as encephalopathy, mood changes, psychosis, neuromuscular dysfunction, or demyelinating processes, may accompany acute viral infection, or may follow infection by weeks, months, or longer in recovered patients[18].
A meta-analysis study conducted by Rogers et al[19] showed that the prevalence of PTSD in the post-illness stage among patients admitted to hospital for SARS or MERS was 32.2% (95%CI 23.7–42.0; 121 of 402 cases from 4 studies), while the prevalence of depression was 14.9% (12.1–18.2; 77 of 517 cases from 5 studies), and that of anxiety disorders was 14.8% (11.1–19.4; 42 of 284 cases from 3 studies). 446 (76.9%; 95%CI 68.1–84.6) of 580 patients from 6 studies had returned to work at a mean follow-up time of 35.3 mo (SD 40.1).
Survivors of SARS-CoV-1 were clinically diagnosed with PTSD (54.5%), depression (39%), pain disorder (36.4%), panic disorder (32.5%), and obsessive compulsive disorder (15.6%) at 31 to 50 mo post-infection, a dramatic increase from their pre-infection prevalence of any psychiatric diagnoses which is only 3%[15]. Fatigue, myalgia, depression and poor sleep were seen in a cohort of 22 patients and a post-SARS syndrome, similar to fibromyalgia or post viral chronic fatigue syndrome, was suggested, possibly as a result of the psychological trauma or neurological involve
One study showed that at 12 mo post-MERS 27% of survivors had depression and 42% had PTSD, which improved at 18 mo but was still a problem in 17% and 27% of survivors respectively[23]. At 4-6 mo after release from isolation, anxiety symptoms were observed in 3.0% (95%CI: 2.2%-3.9%). Feelings of anger were present in 6.4% (95%CI: 5.2%-7.6%)[24].
Severance et al[25] also found the increased prevalence of antibodies against 4 HCoV strains in patients with a recent psychotic episode compared to non-psychiatric controls, suggesting a possible relationship between CoV infections and psychosis, which may also occur in SARS-CoV-2. Seropositivity for coronaviruses associated with suicide and psychosis persisting 1 year after SARS[26].
Recent ambi-directional cohort study of 1733 of COVID-19 survivors in Wuhan found that risk of anxiety or depression were higher in patients with more severe illness at 6 mo follow-up. Patients showed an odds ratio OR 0.88 (0.66–1.17; P = 0.37) for scale 4 (requiring supplemental oxygen) vs scale 3 (not requiring supplemental oxygen) and OR 1.77 (1.05–2.97; P < 0.05) for scale 5–6 (requiring HFNC, NIV, or IMV) vs scale 3 for anxiety or depression. Sleep difficulties (26%, 437 of 1655) were one of the most common symptoms reported[27].
Current studies reported clinically significant PTSD, anxiety, and/or depression among COVID-19 survivors during 14-90 d were observed following the diagnosis[28-32], early convalescence[33], and at 1 mo follow-up[34]. PTSD was the most common condition reported, with female gender, past traumatic events, protracted symptoms, stigmatization, and a negative view on the COVID-19 pandemic as the predictors of symptoms severity (P < 0.05)[28]. Older survivors experienced less severe PTSD and anxiety symptoms than younger ones (P = 0.04 and P = 0.045, respectively). Older age had a significant inverse association with the severity of emotional symptoms of depression (P < 0.001)[33].
One retrospective case control cohort studies of 62354 COVID-19 cases in the USA found that diagnosis of COVID-19 Led to more first diagnoses and relapses of psychiatric illness during the first 14-90 d after COVID-19 diagnosis compared control health events (HRs between 1.58 and 2.24, all P < 0.0001). At 90 d, the estimated probability of having newly diagnosed psychiatric illness after COVID-19 diagnosis was 5.8% (95%CI: 5.2-6.4) compared with 2.5%-3.4% of patients in the comparison cohorts. The most frequent psychiatric diagnosis was anxiety disorder (HRs 1.59–2.62, all P < 0.0001). The probability of a first diagnosis of mood and psychotic disorder was 2% (95%CI: 1.7-2.4) and 0.1% (95%CI: 0.08-0.2), respectively. The rate of first or relapsed psychotic disorder diagnosis after COVID-19 diagnosis was 0.9% (95%CI: 0.8-1.1). The probability of a first diagnosis of insomnia was 1.9% (95%CI: 1.6-2.2; HRs 1.85-3.29, all P < 0.0001). The probability of being diagnosed with dementia was increased after a diagnosis of COVID-19 among patients older than 65 years the risk was 1.6% (95%CI 1.2-2.1; HRs 1.89-3.18). COVID-19 patients admitted to hospital have higher risk of psychiatric sequelae than patients not requiring admission (HR 1.40, 95%CI: 1.06-1.85; P = 0.019)[30].
Psychiatric outcomes of COVID-19 patients are affected by several biological factors (e.g., obese, older age, pregnancy) and external psychosocial stressors (e.g., social isolation, financial stress). Associated with systemic inflammation and impaired immunity, obesity not only can increase vulnerability for COVID-19 infection, but also constitutes an important risk factor for the development or worsening of psychiatric disorders[32].
Aging is related to cytokine imbalances, which are high levels of pro-inflammatory cytokines, low levels of anti-inflammatory cytokines and decrease in T-cell-mediated function[33]. These changes in elderly may be associated with higher susceptibility to viral diseases and neuropsychiatric disturbances, such as cognitive impairments[34].
Female COVID-19 survivors are at higher risk for developing psychiatric symptoms[27,30,31]. Maternal immune activation in early stages of fetus development is another important risk factor for developing neuropsychiatric disturbances, such as autism spectrum disorder[35]. The presence of family members or close relatives infected was significantly related to anxiety and depression (P < 0.001)[30]. Patients with a positive previous psychiatric diagnosis showed a significant increase in psychiatric symptoms measures[29,31].
Recent studies among COVID-19 patients found greater occurrence of depressive and anxiety disorders in people who are in quarantine, front-line workers or among family members of affected patients[36]. This finding suggested psychological stressors, such as social isolation, psychological impact of a severe and potentially fatal illness, concerns about infecting others, and stigma can lead to psychological consequences[31,36]. Loneliness has been associated with several psychiatric disorders, such as depression, anxiety, and suicide behavior[37]. In addition, it has been shown that lonely people present several immune dysregulations, such as upregulated expression of pro-inflammatory cytokine genes[38]. Studies with animal models have provided important clues on the neurobiological and the behavioral consequences of social isolation. In rodents, the stress of social isolation leads to changes in several neurotransmitter systems (e.g., dopaminergic, adrenergic, serotonergic, gabaergic, glutamatergic, nitrergic, and opioid systems). The social isolation stress can also lead to hyperactivity of the hypothalamo-pituitary-adrenal (HPA) axis through an increase in corticosterone production and release in rodents[39].
Financial problems may enhance the impact of social isolation on mental health during quarantine[40]. Studies showed that a worse socioeconomic status is directly related to higher systemic levels of inflammatory markers such as interleukin (IL)-6 and C-reactive protein[41].
The underlying mechanisms of psychiatric sequelae in COVID-19 infection are still unknown and in need further investigation. However, the relationship between COVID-19 severity and psychiatric outcome, albeit modest, might represent a dose-response relationship, suggesting that the association might be mediated by biological factors directly related to the virus (e.g., immune system, viral load)[29] (Figure 1 and Table 1).
Coronaviruses, including SARS-CoV-2 also invade the central nervous system. There is evidence of SARS-CoV-2 neurotropism based on clinical, pathological, and molecular studies. SARS-CoV-2 invades epithelial cells by binding to ACE2 on the cell membrane which is also expressed in the brain, both in neurons and glia. There are several routes for viral neuroinvasion, including trans-synaptic transfer across infected neurons in splanchnic nerves, entry via the olfactory nerve, infection of vascular endothelium, leukocyte migration across the blood-brain barrier (BBB), and/or a conjunctival route[42].
COVID-19 infection triggers a local immune response, recruiting macrophages and monocytes that release cytokines and induce T and B cell responses. In most people, this adaptive immune response is capable of resolving the infection. However, in some individuals, a dysfunctional immune response occurs which may cause severe lung damage and multiple organ failure through catalyzing enzymes such as proteases and toxic free radicals. These processes may damage immune residue in brain neural circuits among COVID-19 patients[43].
High levels of IL-1β, IL-6, interferon-γ, CXCL10, and CCL2 were observed in COVID-19 patients, suggesting an activation of T-helper-1 cell function. Moreover, unlike in SARS and MERS, elevated levels of T-helper-2 cell-secreted cytokines (such as IL-4 and IL-10) were found in COVID-19 patients[44]. Persistent with these findings, emerging evidence found that COVID-19 infection can induce a cytokine release syndrome as a part of host’s innate immune, commonly observed in cytopathic virus infections[45]. Soluble cytokines that reach the brain have significant effects on multiple neurotransmitters, including dopamine, serotonin, norepinephrine and glutamate through impact on their synthesis, release, and reuptake[46]. Changes in the metabolism of neurotransmitters contributed to the pathophysiology of various psychiatric disorders, such as depression, anxiety, and PTSD[47,48]. Pro-inflammatory cytokines increase oxidative stress which damages cellular membranes and reduce the expression of excitatory amino acid transporters that are necessary to end glutamatergic signaling that results in elevated glutamate levels[42]. Hence, the immune activation hypothesis which has been postulated for many psychiatric disorders may be a relevant mechanism for mental health issues in COVID-19 survivors[18] (Figure 1).
Since communication occurs between the endocrine, immune, and central nervous system, the activation of inflammatory responses may affect neuroendocrine processes, and vice versa. During COVID-19 infection, pro-inflammatory cytokines are released by immune cells present in the periphery (e.g., macrophages, T and NK cells) and/or in the brain (microglia). High levels cytokines can affect neuroendocrine axis and activate the HPA axis at three different levels: increasing the secretion of the corticotropin-releasing hormone in the hypothalamus, the secretion of adrenocorticotropic hormone in the pituitary, and release of glucocorticoids (e.g., cortisol) through the adrenal cortex[49]. Inflammatory cytokines and their signaling pathways including MAPK, NF-kappa B, signal transducers and activators of transcription and cyclooxygenase have been found to inhibit glucocorticoid receptor (GR) function by acting on its translocation or on GR-mediated gene transcription, thus inducing glucocorticoid resistance that results in dysfunction in the negative feedback between the HPA axis and the immune system.
HPA axis hyperactivity is one of the characteristic features of major depression (MD). Some studies have suggested that glucocorticoids also contribute to the hippocampal atrophy found in patients with MD[50]. Rates of insomnia diagnosis were also markedly elevated, in agreement with predictions that circadian disturbances will follow COVID-19 infection. The HPA axis plays important roles in modulating sleep. Therefore, dysregulation of the HPA axis at any level can disrupt sleep[51].
Findings from neuroimaging studies indicate that inflammatory cytokines impact the function of subcortical and cortical neuronal circuits in the brain, especially the basal ganglia and dorsal anterior cingulate cortex, leading to significant changes in motor activity and motivation as well as anxiety, arousal and alarm. Chronic activation of this innate behavioral and immune response may contribute to the development of depression and anxiety disorders in vulnerable individuals. Other brain regions including amygdala, hippocampus, insula, dorsolateral prefrontal cortex and subgenual anterior cingulate cortex are also involved[46].
Increased stress levels and worries during pandemic are also the major contributing factors to clinical insomnia[52]. Worry provokes cognitive arousal and may therefore disturb sleep cycle. Reduced physical fatigue and exposure to the sun, as well as increased use of electronic devices may also affect sleep homeostasis. A study showed increased prevalence of insomnia in women compared with men, suggesting that they are more prone to develop stress-related disorders such as post-traumatic stress disorder and anxiety disorders[53]. Sleep disturbances are involved in PTSD development and maintenance[54].
SARS-CoV-2 infection triggers a massive release of inflammation signals leading to BBB dysfunction, injury to astrocytes, activation of microglia and astrocytes resulting in neuroinflammation and neuronal death. Immune response and excessive inflammation in COVID-19 may also speed up the progression of brain inflammatory neurodegeneration. An early report found that one in three individuals with COVID-19 had dysexecutive syndrome at the time of hospital discharge. SARS-CoV-2 can infect endothelial cells leading to further damage of the vasculatures. The resulting hypoperfusion may disrupt energy substrates needed for maintaining neuronal networks thereby accelerating cognitive decline. Damage to limbic and cortical regions could cause retrograde and anterograde amnesia[42].
Studies above identified COVID-19 survivors at high risk for psychological problems. Good social support and a number of simple attitudes or activities should be done to prevent psychiatric sequelae in COVID-19 survivors, such as strengthen bonds with other people through social media, think and talk positively, sleep properly, balance diet, regular daily routine, relaxation exercise and other healthy lifestyle measures[49,55]. Music therapy can also be a relevant and simple strategy to improve mental health. A meta-analysis study showed music can modulate cytokine levels (including reducing IL-6 levels), as well as neuroendocrine-immune responses triggered by physical stress caused by viral infection[56]. In addition, music interferes positively in the immune system when subjected to acute stress, regulating the function of IL-6 and the HPA axis[57]. On the other hand, substance use, eating too much fast food, excessive online activity, excessive watching television, and trusting fake news should be avoided[58].
We suggested that all COVID-19 survivors should be screened for stress disorder, anxiety, and depression regularly to identify those with psychological distress for timely intervention; particularly those with the positive predictive factors (female, prior psychiatric diagnosis, presence of infected family members). Previous studies found that psychiatric sequelae occurred months after the acute infection, therefore the need for sustained follow-up beyond documenting acute stress levels, is important and urgent. Strategies aiming at minimizing mental problems during not only the acute phase of infection but also recovery phase must be designed. Since poorer mental health can be associated with shorter life expectancy and higher economic burden, political and health authorities should be aware of the mental health of COVID-19 survivors[59-61].
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country/Territory of origin: Indonesia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang MK S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang LYT
| 1. | World Health Organization. WHO director-general's statement on IHR emergency committee on novel coronavirus (2019-nCoV), 2020. [Cited in This Article: ] |
| 2. | World Health Organization. Coronavirus disease (COVID-19): Situation Dashboard. (2020). Available from: https://who.sprinklr.com/. [Cited in This Article: ] |
| 3. | Hariyanto TI, Kurniawan A. Anemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Transfus Apher Sci. 2020;59:102926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Hariyanto TI, Kurniawan A. Obstructive sleep apnea (OSA) and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis. Sleep Med. 2021;82:47-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Putri C, Hariyanto TI, Hananto JE, Christian K, Situmeang RFV, Kurniawan A. Parkinson's disease may worsen outcomes from coronavirus disease 2019 (COVID-19) pneumonia in hospitalized patients: A systematic review, meta-analysis, and meta-regression. Parkinsonism Relat Disord. 2021;87:155-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 6. | Hariyanto TI, Rosalind J, Christian K, Kurniawan A. Human immunodeficiency virus and mortality from coronavirus disease 2019: A systematic review and meta-analysis. South Afr J HIV Med. 2021;22:1220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Hariyanto TI, Putri C, Situmeang RFV, Kurniawan A. Dementia is Associated with Severe Coronavirus Disease 2019 (COVID-19) Infection. Am J Med Sci. 2021;361:394-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Hariyanto TI, Prasetya IB, Kurniawan A. Proton pump inhibitor use is associated with increased risk of severity and mortality from coronavirus disease 2019 (COVID-19) infection. Dig Liver Dis. 2020;52:1410-1412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Hariyanto TI, Kurniawan A. Dipeptidyl peptidase 4 (DPP4) inhibitor and outcome from coronavirus disease 2019 (COVID-19) in diabetic patients: a systematic review, meta-analysis, and meta-regression. J Diabetes Metab Disord. 2021;1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Soraya GV, Ulhaq ZS. Crucial laboratory parameters in COVID-19 diagnosis and prognosis: An updated meta-analysis. Med Clin (Engl Ed). 2020;155:143-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Hariyanto TI, Japar KV, Kwenandar F, Damay V, Siregar JI, Lugito NPH, Tjiang MM, Kurniawan A. Inflammatory and hematologic markers as predictors of severe outcomes in COVID-19 infection: A systematic review and meta-analysis. Am J Emerg Med. 2021;41:110-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 12. | Shuja KH, Aqeel M, Jaffar A, Ahmed A. COVID-19 Pandemic and Impending Global Mental Health Implications. Psychiatr Danub. 2020;32:32-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 13. | Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1219] [Cited by in F6Publishing: 1175] [Article Influence: 293.8] [Reference Citation Analysis (0)] |
| 14. | Cheng SK, Wong CW, Tsang J, Wong KC. Psychological distress and negative appraisals in survivors of severe acute respiratory syndrome (SARS). Psychol Med. 2004;34:1187-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 197] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 15. | Lam MH, Wing YK, Yu MW, Leung CM, Ma RC, Kong AP, So WY, Fong SY, Lam SP. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169:2142-2147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 449] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 16. | Arora T, Grey I, Östlundh L, Lam KBH, Omar OM, Arnone D. The prevalence of psychological consequences of COVID-19: A systematic review and meta-analysis of observational studies. J Health Psychol. 2020;1359105320966639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 17. | Diaz A, Baweja R, Bonatakis JK. Global health disparities in vulnerable populations of psychiatric patients during the COVID-19 pandemic. World J Psychiatry. 2021;11:94-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 26] [Cited by in F6Publishing: 24] [Article Influence: 8.0] [Reference Citation Analysis (2)] |
| 18. | Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Brain Behav Immun. 2020;87:34-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 665] [Cited by in F6Publishing: 578] [Article Influence: 144.5] [Reference Citation Analysis (0)] |
| 19. | Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, Zandi MS, Lewis G, David AS. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1673] [Cited by in F6Publishing: 1384] [Article Influence: 346.0] [Reference Citation Analysis (0)] |
| 20. | Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011;11:37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 433] [Cited by in F6Publishing: 373] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 21. | Gardner PJ, Moallef P. Psychological impact on SARS survivors: Critical review of the English language literature. Can Psychol. 2015;56:123-135. [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 22. | Wing YK, Leung CM. Mental health impact of severe acute respiratory syndrome: a prospective study. Hong Kong Med J. 2012;18 Suppl 3:24-27. [PubMed] [Cited in This Article: ] |
| 23. | Lee SH, Shin HS, Park HY, Kim JL, Lee JJ, Lee H, Won SD, Han W. Depression as a Mediator of Chronic Fatigue and Post-Traumatic Stress Symptoms in Middle East Respiratory Syndrome Survivors. Psychiatry Investig. 2019;16:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 24. | Jeong H, Yim HW, Song YJ, Ki M, Min JA, Cho J, Chae JH. Mental health status of people isolated due to Middle East Respiratory Syndrome. Epidemiol Health. 2016;38:e2016048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 623] [Cited by in F6Publishing: 599] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 25. | Severance EG, Dickerson FB, Viscidi RP, Bossis I, Stallings CR, Origoni AE, Sullens A, Yolken RH. Coronavirus immunoreactivity in individuals with a recent onset of psychotic symptoms. Schizophr Bull. 2011;37:101-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 26. | Okusaga O, Yolken RH, Langenberg P, Lapidus M, Arling TA, Dickerson FB, Scrandis DA, Severance E, Cabassa JA, Balis T, Postolache TT. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130:220-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Zhong J, Wang C, Wang J, Zhang D, Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2783] [Cited by in F6Publishing: 2580] [Article Influence: 860.0] [Reference Citation Analysis (1)] |
| 28. | Poyraz BÇ, Poyraz CA, Olgun Y, Gürel Ö, Alkan S, Özdemir YE, Balkan İİ, Karaali R. Psychiatric morbidity and protracted symptoms after COVID-19. Psychiatry Res. 2021;295:113604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 29. | Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8:130-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 736] [Cited by in F6Publishing: 805] [Article Influence: 268.3] [Reference Citation Analysis (0)] |
| 30. | Cai X, Hu X, Ekumi IO, Wang J, An Y, Li Z, Yuan B. Psychological Distress and Its Correlates Among COVID-19 Survivors During Early Convalescence Across Age Groups. Am J Geriatr Psychiatry. 2020;28:1030-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 31. | Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, Melloni EMT, Furlan R, Ciceri F, Rovere-Querini P; COVID-19 BioB Outpatient Clinic Study group, Benedetti F. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 967] [Cited by in F6Publishing: 832] [Article Influence: 208.0] [Reference Citation Analysis (0)] |
| 32. | Maffetone PB, Laursen PB. The Perfect Storm: Coronavirus (Covid-19) Pandemic Meets Overfat Pandemic. Front Public Health. 2020;8:135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 33. | Marsman D, Belsky DW, Gregori D, Johnson MA, Low Dog T, Meydani S, Pigat S, Sadana R, Shao A, Griffiths JC. Healthy ageing: the natural consequences of good nutrition-a conference report. Eur J Nutr. 2018;57:15-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Au A, Feher A, McPhee L, Jessa A, Oh S, Einstein G. Estrogens, inflammation and cognition. Front Neuroendocrinol. 2016;40:87-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 35. | Bilbo SD, Block CL, Bolton JL, Hanamsagar R, Tran PK. Beyond infection - Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp Neurol. 2018;299:241-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 187] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 36. | Qiu J, Shen B, Zhao M, Wang Z, Xie B, Xu Y. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: implications and policy recommendations. Gen Psychiatr. 2020;33:e100213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1855] [Cited by in F6Publishing: 2084] [Article Influence: 521.0] [Reference Citation Analysis (0)] |
| 37. | Beutel ME, Klein EM, Brähler E, Reiner I, Jünger C, Michal M, Wiltink J, Wild PS, Münzel T, Lackner KJ, Tibubos AN. Loneliness in the general population: prevalence, determinants and relations to mental health. BMC Psychiatry. 2017;17:97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 524] [Cited by in F6Publishing: 507] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 38. | Cole SW, Capitanio JP, Chun K, Arevalo JM, Ma J, Cacioppo JT. Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proc Natl Acad Sci U S A. 2015;112:15142-15147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 160] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 39. | Zlatković J, Todorović N, Bošković M, Pajović SB, Demajo M, Filipović D. Different susceptibility of prefrontal cortex and hippocampus to oxidative stress following chronic social isolation stress. Mol Cell Biochem. 2014;393:43-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 40. | Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, Rubin GJ. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9682] [Cited by in F6Publishing: 7558] [Article Influence: 1889.5] [Reference Citation Analysis (1)] |
| 41. | Muscatell KA, Brosso SN, Humphreys KL. Socioeconomic status and inflammation: a meta-analysis. Mol Psychiatry. 2020;25:2189-2199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 42. | de Erausquin GA, Snyder H, Carrillo M, Hosseini AA, Brugha TS, Seshadri S; CNS SARS-CoV-2 Consortium. The chronic neuropsychiatric sequelae of COVID-19: The need for a prospective study of viral impact on brain functioning. Alzheimers Dement. 2021;17:1056-1065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 104] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 43. | Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2925] [Cited by in F6Publishing: 2694] [Article Influence: 673.5] [Reference Citation Analysis (0)] |
| 44. | Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80:607-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1858] [Cited by in F6Publishing: 1823] [Article Influence: 455.8] [Reference Citation Analysis (0)] |
| 45. | Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, Bucci E, Piacentini M, Ippolito G, Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451-1454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 920] [Cited by in F6Publishing: 960] [Article Influence: 240.0] [Reference Citation Analysis (0)] |
| 46. | Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30:297-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 487] [Cited by in F6Publishing: 495] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 47. | Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17:524-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 529] [Cited by in F6Publishing: 606] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 48. | Bandelow B, Baldwin D, Abelli M, Bolea-Alamanac B, Bourin M, Chamberlain SR, Cinosi E, Davies S, Domschke K, Fineberg N, Grünblatt E, Jarema M, Kim YK, Maron E, Masdrakis V, Mikova O, Nutt D, Pallanti S, Pini S, Ströhle A, Thibaut F, Vaghi MM, Won E, Wedekind D, Wichniak A, Woolley J, Zwanzger P, Riederer P. Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18:162-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 49. | Raony Í, de Figueiredo CS, Pandolfo P, Giestal-de-Araujo E, Oliveira-Silva Bomfim P, Savino W. Psycho-Neuroendocrine-Immune Interactions in COVID-19: Potential Impacts on Mental Health. Front Immunol. 2020;11:1170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 50. | Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:722-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 352] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 51. | Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106-3114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 556] [Cited by in F6Publishing: 564] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 52. | Voitsidis P, Gliatas I, Bairachtari V, Papadopoulou K, Papageorgiou G, Parlapani E, Syngelakis M, Holeva V, Diakogiannis I. Insomnia during the COVID-19 pandemic in a Greek population. Psychiatry Res. 2020;289:113076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 256] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 53. | Li SH, Graham BM. Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? Lancet Psychiatry. 2017;4:73-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 292] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 54. | Giannakopoulos G, Kolaitis G. Sleep problems in children and adolescents following traumatic life events. World J Psychiatry. 2021;11:27-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 55. | Moradi Y, Mollazadeh F, Karimi P, Hosseingholipour K, Baghaei R. Psychological disturbances of survivors throughout COVID-19 crisis: a qualitative study. BMC Psychiatry. 2020;20:594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 56. | Fancourt D, Ockelford A, Belai A. The psychoneuroimmunological effects of music: a systematic review and a new model. Brain Behav Immun. 2014;36:15-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 57. | Koelsch S, Boehlig A, Hohenadel M, Nitsche I, Bauer K, Sack U. The impact of acute stress on hormones and cytokines, and how their recovery is affected by music-evoked positive mood. Sci Rep. 2016;6:23008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 58. | Kar SK, Arafat SY, Kabir R, Sharma P, Saxena SK. Coping with mental health challenges during COVID-19. In: Saxena SK, editor. Coronavirus Disease 2019 (COVID-19). Singapore: Springer, 2020: 199-213. [Cited in This Article: ] |
| 59. | Druss BG, Zhao L, Von Esenwein S, Morrato EH, Marcus SC. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49:599-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 334] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 60. | Nordentoft M, Wahlbeck K, Hällgren J, Westman J, Osby U, Alinaghizadeh H, Gissler M, Laursen TM. Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS One. 2013;8:e55176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 381] [Cited by in F6Publishing: 418] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 61. | Doran CM, Kinchin I. A review of the economic impact of mental illness. Aust Health Rev. 2019;43:43-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |