Peer-review started: April 28, 2016
First decision: May 13, 2016
Revised: May 21, 2016
Accepted: May 27, 2016
Article in press: June 2, 2016
Published online: June 9, 2016
Ganglionic long-term potentiation (gLTP) is an activity-dependent, enduring enhancement of ganglionic transmission. This phenomenon may be induced in autonomic ganglia of an organism under certain conditions where repetitive impulses surge from the central nervous system (CNS) to the periphery. Chronic stress, repetitive epileptic seizure or chronic use of CNS stimulants could induce gLTP, which would result in a long lasting heightening of sympathetic tone to the cardiovascular system causing hypertension and disturbed cardiac rhythm that may lead to sudden cardiac death. These conditions are briefly reviewed in this article.
Core tip: Heightened activity of the central nervous system (CNS) caused by epilepsy, chronic stress and CNS stimulants could provide strong preganglionic stimulation of autonomic ganglia, which may trigger expression of ganglionic long-term potentiation (gLTP). Expression of gLTP can result in cardiovascular dysfunction that may lead to morbidity and even mortality.
- Citation: Alkadhi KA. Long-term potentiation in autonomic ganglia: Potential role in cardiovascular disorders. World J Pharmacol 2016; 5(2): 51-58
- URL: https://www.wjgnet.com/2220-3192/full/v5/i2/51.htm
- DOI: https://dx.doi.org/10.5497/wjp.v5.i2.51
Before Bliss and Lomo[1] coined the term “long-term potentiation (LTP)” in the hippocampus to describe activity-dependent long-lasting potentiation, similar activity-induced enhancement of synaptic transmission was described in the mammalian sympathetic ganglia[2,3]. However, it was nearly two decades before ganglionic LTP (gLTP) was characterized in mammalian and amphibian sympathetic ganglia[4-13] as well as avian parasympathetic ciliary ganglion[14] (Table 1). Later, my laboratory identified serotonin as the neurotransmitter necessary for induction and maintenance of gLTP in the rat superior cervical ganglion[15].
| Animal species | Specific ganglia | Ref. |
| Rat | Superior cervical ganglion | Brown and McAfee[4] |
| Briggs and McAfee[6] | ||
| Alkadhi et al[15] | ||
| Alzoubi et al[25] | ||
| Alkadhi et al[28] | ||
| Alkadhi et al[29] | ||
| Alkadhi and Alzoubi[36] Alzoubi et al[30] | ||
| Cat | Superior cervical, lumbar and stellate ganglia | Alonso-deFlorida et al[7] |
| Bachoo and Polosa[8] | ||
| Guinea pig | Superior cervical ganglion | Weinreich et al[9] |
| Chick | Parasympathetic ciliary ganglion | Scott and Bennett[14] |
| Bullfrog | Sympathetic ganglia | Koyano et al[11] |
| Kumamoto and Kuba[13] | ||
| Minota et al[10] |
The expression of gLTP is due to a series of events resulting from both the postsynaptic and presynaptic regions, and including activation of enzymes, modulators and second messengers. Whereas LTP of the central nervous system (CNS) is regarded as a cellular mechanism of memory; the function of gLTP is uncertain. It is clear that hyperactivity of the CNS, as in the case of chronic stress or recurrent epileptic seizures, may provide the high frequency stimulation (HFS) necessary to induce the expression of LTP in autonomic ganglia, which may cause deleterious alterations in the cardiovascular system function.
gLTP is induced by repetitive HFS (20 Hz) of preganglionic nerve. Upon cessation of HFS of the preganglionic nerve of rat superior cervical ganglion, test stimuli (0.017 Hz) evoke initial highly potentiated ganglionic responses (compound action potentials), lasting up to 4 min, called post-tetanic potentiation[15-17]. This is followed by steady lesser-potentiated action potentials lasting up to 3 h, indicating an increase in synaptic strength[15,18-20].
Published work from this laboratory determined that initiation of gLTP entails both HFS of the preganglionic nerve and stimulation of 5-HT3 receptors by serotonin originating from certain cells within the superior cervical ganglion of rat[15]. Activation of 5-HT3 receptors is necessary for both initiation and expression of gLTP[15]. Extracellular recording revealed that, in ganglia that have expressed gLTP, serotonin 5-HT3 receptor agonists and blockers, in concentrations comparable to pharmacological doses in clinical settings, have profound effects on the magnitude of gLTP[15] (Table 2), even though the same agents produced no significant effect on basal transmission in ganglia from control rat[15]. Thus, we have established gLTP as the first serotonin-dependent LTP ever reported in a mammalian species[21,22].
| Serotonergic drugs | Mode of action | Compound AP |
| Serotonin (10-20 μmol/L) | Agonist | Increased |
| Fluoxetine (10 μmol/L) | SSRI | Increased |
| m-CPBG (1 μmol/L) | Receptor agonist | Increased |
| Tropisetron (5 μmol/L) | Receptor antagonist | Reduced |
| Ondansetron (5 μmol/L) | Receptor antagonist | Reduced |
| MDL 72222 (0.5 μmol/L) | Receptor antagonist | Reduced |
| Reserpine pretreatment (3 mg/kg) | 5-HT3 depletion | No gLTP |
| m-CPBG (1 μmol/L) + reserpine | Receptor agonist | Increased |
The 5-HT3 receptor is a ligand-gated receptor-channel complex, and a member of the superfamily that also includes the nACh receptor[23]. It is known that activation of the presynaptic 5-HT3 receptor causes upsurges in calcium concentration inside rat brain nerve terminals[24]. The role of 5-HT3 receptor in the induction and maintenance of gLTP is presently unclear. Perhaps the activation of 5-HT3 channel-receptor complex at the nerve terminals in ganglia causes localized entry of calcium ions increasing its intracellular concentration to a level adequate for activation of downstream signaling molecules, including protein kinase C (PKC), calmodulin and calcium-calmodulin kinase II (CaMKII), which are essential for expressing gLTP[25].
gLTP can be induced by HFS (20 Hz for 20 s) of the preganglionic sympathetic nerve. This frequency is within the maximum range of in vivo firing frequency of preganglionic neurons[26]. The response may be measured in vitro by intracellular or extracellular recording techniques[6]. Furthermore, gLTP has been evoked and recorded in situ from ganglia of anesthetized animals[7,8,27].
The LTP of the hippocampal CA1 region and gLTP are similar in various aspects. For example, both are saturable in that when fully expressed, another HFS will not cause additional augmentation of synaptic transmission[28]. Experiments in rat sympathetic ganglia suggest similar molecular mechanisms for the expression of gLTP and hippocampal LTP[29]. Both require a ligand-gated ion channel; here is where hippocampal LTP and gLTP differ: Whereas area CA1 hippocampal LTP requires activation of glutamate NMDA receptor, gLTP requires activation of serotonin 5-HT3 receptor. Similar to NMDA receptor, 5-HT3 receptor is very permeable to Ca2+, which is exceedingly important for launching the molecular cascades responsible for expression of LTP. Strong evidence from this laboratory reveals the involvement of a variety of signaling molecules (e.g., CaMKII, PKC, calmodulin, calcineurin, etc.) in the expression of both hippocampal LTP and gLTP[30,31].
The involvement of endogenous serotonin is indicated by absence of HFS-induced gLTP in ganglia of animals treated with reserpine (3 mg/kg) to remove serotonin. However, when these ganglia were treated with serotonin or m-CPBG (a 5-HT3-receptor agonist), HFS invariably induced expression of gLTP (Table 2)[15].
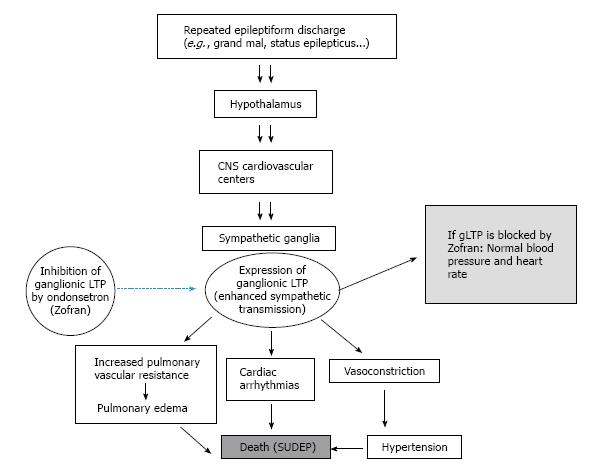
An expected outcome from in vivo manifestation of gLTP in ganglia is a long-lasting enhancement of sympathetic tone that outflows to the cardiovascular system. Work from this laboratory has established the consequences of in vivo induction of gLTP in sympathetic ganglia on blood pressure[29,32,33]. We hypothesize that CNS repetitive activity causes similar outflow to preganglionic nerves, which together with endogenous serotonin may trigger expression of gLTP of sympathetic ganglia. Expression of gLTP produces prolonged and steady increase of sympathetic tone to the cardiovascular resulting in hypertension and disturbed cardiac rhythm (Figure 1).
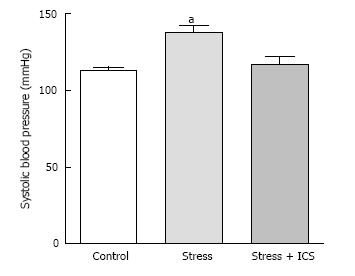
We hypothesized that chronic psychosocial stress can induce in vivo expression of gLTP in sympathetic ganglia, which results in a constant rise in sympathetic tone thus contributing to or initiating a rise of blood pressure. We tested this hypothesis in four animal models of hypertension; aged rats, spontaneously hypertensive rat (SHR), obese Zucker rat and the psychosocial stress model[28,33-35]. We investigated the existence of gLTP in ganglia from these models. For example, in psychosocially stressed hypertensive rats, treatment with tropisetron (ICS; a 5-HT3 receptor antagonist) resulted in normalizing blood pressure of these rats (Figure 2; ref. [29]). Parallel outcomes were obtained in SHR and obese Zucker rat[29,32]. This strongly indicated that the hypertension seen in these animals was, at least partly, due to expression of gLTP.
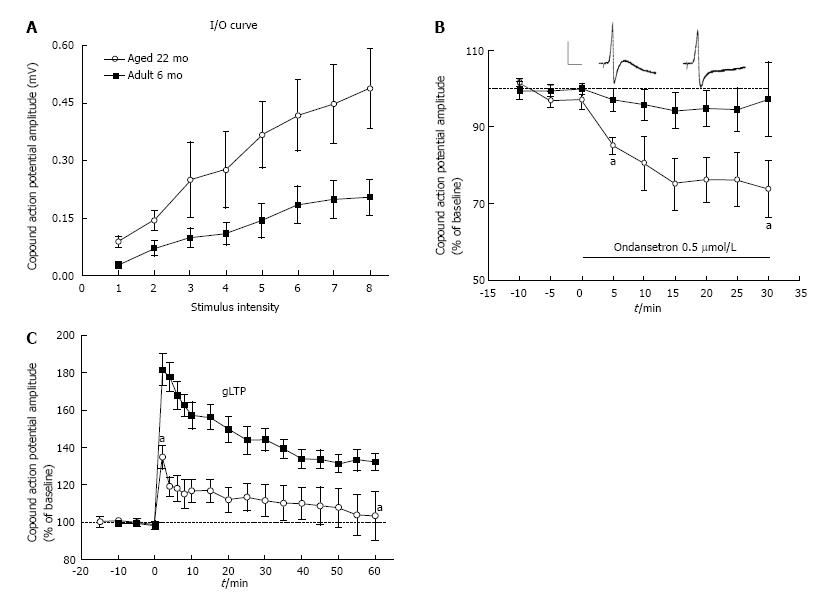
To further ascertain the existence of gLTP in ganglia isolated from these animal models we showed that “basal” transmission in these ganglia was markedly potentiated (Figure 3A) and that this potentiation was blocked when ganglia were treated with 5-HT3 receptor antagonists[29,32,33,35] (Figure 3B). In another series of experiments, we hypothesized that in vitro HFS will not induce gLTP in ganglia isolated from hypertensive old rats if, in fact, gLTP has been expressed already in these ganglia in vivo. Whereas HFS produced strong gLTP in ganglia isolated from normotensive adult rats, no gLTP was seen in ganglia from old rats[28,35] (Figure 3C). It is worthy to note that in these series, to ascertain the specificity of 5-HT3 receptor we used three different selective antagonists; bemesetron, tropisetron and ondansetron (Zofran), all were equally effective in blocking gLTP[28,33-35].
Any procedure that can induce continuous intense flow of impulses from the brain to autonomic ganglia could cause a sustained increase of sympathetic tone to the cardiovascular system, may lead to or contribute to disorders of the system[28-30,32,33,36] (Figure 1). The expression of gLTP in autonomic ganglia that may cause hypertension and cardiac arrhythmias can be a serious risk factor for morbidity and mortality. Evidence that associates expression of gLTP with hypertension has been determined for chronic psychosocial stress[28-30,32,33,36]. Other possible inducers of gLTP are discussed in the following sections.
This serious type of stress results from experiencing harsh distressing occurrences for example witnessing injuries or death, exposure to natural disasters, or experiencing a life-threatening accident. Posttraumatic stress disorder (PTSD) is an incapacitating and potentially chronic disorder characterized by substantial illness. Although similar in some features to chronic stress, PTSD has distinctive pathology[37]. In the first few years following the traumatic event, some PTSD patients may recover, but up to 40% remain chronically symptomatic for years[38]. The major brain areas implicated in the manifestation of PTSD are the prefrontal cortex, amygdala, and hippocampus[39]. During a traumatic event the amygdala sends intensifying impulses to various areas of the brain including prefrontal cortex, hypothalamus, hippocampus and brain stem nuclei. During the course of PTSD, intensified brain activity has been described. For example, PTSD patients showed augmented spontaneous activity in the amygdala and frontal cortex[40]. Moreover, PTSD is linked to increased sympathetic activity represented by elevated blood pressure increased heart rate, and/or increased adrenergic transmitter release[41]. This increase in sympathetic activity could be due to expression of gLTP in autonomic ganglia. However, whether gLTP is present in ganglia during the progression of PTSD and whether cardiovascular disorders are due to gLTP in autonomic ganglia remain to be explored in animal models of PTSD.
The excessive and abnormal cortical brain activity in epilepsy is transmitted through the brain stem to the rest of the body and can usually cause various types of seizures. Strong stimulation of the sympathetic nervous system often accompany seizures and can cause hypertension, dispersed injury of myocytes, and increased predisposition to ventricular arrhythmias[42,43]. Several areas in the brain are involved in the cardiovascular effect of epileptic seizures such as the hypothalamus and medulla oblongata, particularly nuclei of the nucleus tractus solitarius, and area postrema, which are closely engaged in regulation of cardiovascular function[44-46]. Therefore, enhanced activity of these areas is communicated to autonomic ganglia and may provide the required repetitive activity that triggers the expression of gLTP in these ganglia. The expression of gLTP results in long-term enhancement of sympathetic tone to the cardiovascular system, causing hypertension and neurogenic cardiac arrhythmias, which can be major risk factors for sudden unexpected death in epilepsy, a dangerous clinical difficulty for certain epileptic patients, especially those with chronic, inadequately controlled seizures.
Epileptiform brain activity was recorded in the brains of young rats treated with nicotine[47]. In humans, chronic use of tobacco products is known to cause enhanced cholinergic activity in the brain[48-50]. Nicotine can also augment peripheral sympathetic activity through activation of postganglionic nicotinic acetylcholine receptors[51,52]. Moreover, nicotine can release epinephrine from the adrenal medulla into the blood[53,54]. Thus, nicotine causes stimulation of the cardiovascular system that increases heart rate and causes hypertension by action on both peripheral and central sites[53]. Hence, since epileptic patients are more likely to be chronic tobacco users[55], such chronic use of nicotine may result in the expression of gLTP in ganglia or enhancement of the impacts of already expressed gLTP in epileptic tobacco users, thus intensify the risk for cardiovascular dysfunction that may cause sudden death[56].
Caffeine, a competitive inhibitor of adenosine receptors, is the most extensively used CNS stimulant because it is consumed in a variety of hot and cold drinks, as well as many prescription and over-the-counter medications. Neuroimaging studies reports show that by acting on brain cortex, caffeine enhances attention and mental arousal[57-59]. However, there is no convincing evidence that the usual doses of caffeine increase the risk of heart attack, sudden cardiac death, or disruption of cardiac rhythm. Nonetheless, a new caffeine source are the so called “energy drinks”, which contain uncommonly hefty doses of caffeine. Consumption of such energy drinks may lead to platelet and endothelial dysfunction, which can cause myocardial infarction and other cardiovascular disorders in healthy young adults[60-62] (for review see ref. [63]). Through stimulation of the CNS, heavy frequent intake of caffeine-containing drinks may trigger gLTP in sympathetic ganglia, which could be responsible for the reported cardiovascular disturbances. The danger may be even greater when such consumption of large doses of caffeine is coupled with heavy use of tobacco products.
The amphetamines work mainly by modifying the catecholamine system in the pleasure center of the brain[64]. They increase levels of major catecholamines such as dopamine and norepinephrine in a dose-dependent manner[65-67]. A case-control study has linked the use of one of the most commonly used CNS stimulant, methylphenidate (Ritalin), to sudden death in children and teenagers[68].
Cocaine inhibits the monoamine reuptake mechanism in central and peripheral sympathetic nerve terminals in humans[69-71] with end effects similar to those seen with amphetamines. This however, may not be the sole CNS effect of cocaine inasmuch as other reuptake inhibitors do not have cocaine-like effects. The abuse of cocaine is correlated with cardiovascular dysfunction including hypertension, ventricular dysrhythmia, acute myocardial infarction, and left ventricular hypertrophy. Therefore, the chronic use of CNS stimulants such as amphetamine and cocaine may trigger expression of gLTP, which may lead to morbidity and/or sudden death.
Abnormal strong brain activity as in epileptic seizures cause intense activation of ganglionic neurons, which can induce gLTP in sympathetic ganglia leading to long-term heightened sympathetic tone to the cardiovascular system with the ensuing rise in blood pressure and disturbed heart rhythm. Abnormal CNS activity can result from severe brain injuries, ongoing psychological stress, epilepsy, and regular abuse of CNS stimulating substances. Even though these disorders can cause disturbances of the function of the cardiovascular system, their possible link to gLTP has not been studied, except in chronic psychosocial stress. Therefore, it is necessary to determine such links in order to develop therapeutic plans to avoid serious consequences such as sudden cardiac death.
Manuscript source: Invited manuscript
P- Reviewer: Takahashi H, Unger M S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
| 1. | Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4427] [Cited by in F6Publishing: 4145] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 2. | Volle RL. Enhancement of neuromuscular responses to acetylcholine and succinylcholine following repetitive stimulation of the motor nerve. Arch Int Pharmacodyn Ther. 1966;160:284-293. [PubMed] [Cited in This Article: ] |
| 3. | Dunant Y, Dolivo M. Plasticity of synaptic functions in the exised sympathetic ganglion of the rat. Brain Res. 1968;10:271-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 35] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Brown TH, McAfee DA. Long-term synaptic potentiation in the superior cervical ganglion. Science. 1982;215:1411-1413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 92] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Briggs CA, McAfee DA, McCaman RE. Long-term potentiation of synaptic acetylcholine release in the superior cervical ganglion of the rat. J Physiol. 1985;363:181-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Briggs CA, McAfee DA. Long-term potentiation at nicotinic synapses in the rat superior cervical ganglion. J Physiol. 1988;404:129-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 50] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Alonso-deFlorida F, Morales MA, Minzoni AA. Modulated long-term potentiation in the cat superior cervical ganglion in vivo. Brain Res. 1991;544:203-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Bachoo M, Polosa C. Preganglionic axons from the third thoracic spinal segment fail to induce long-term potentiation in the superior cervical ganglion of the cat. Can J Physiol Pharmacol. 1992;70 Suppl:S27-S31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Weinreich D, Undem BJ, Taylor G, Barry MF. Antigen-induced long-term potentiation of nicotinic synaptic transmission in the superior cervical ganglion of the guinea pig. J Neurophysiol. 1995;73:2004-2016. [PubMed] [Cited in This Article: ] |
| 10. | Minota S, Kumamoto E, Kitakoga O, Kuba K. Long-term potentiation induced by a sustained rise in the intraterminal Ca2+ in bull-frog sympathetic ganglia. J Physiol. 1991;435:421-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Koyano K, Kuba K, Minota S. Long-term potentiation of transmitter release induced by repetitive presynaptic activities in bull-frog sympathetic ganglia. J Physiol. 1985;359:219-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 56] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Kumamoto E, Kuba K. Sustained rise in ACh sensitivity of a sympathetic ganglion cell induced by postsynaptic electrical activities. Nature. 1983;305:145-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Kumamoto E, Kuba K. Mechanism of long-term potentiation of transmitter release induced by adrenaline in bullfrog sympathetic ganglia. J Gen Physiol. 1986;87:775-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Scott TR, Bennett MR. The effect of ions and second messengers on long-term potentiation of chemical transmission in avian ciliary ganglia. Br J Pharmacol. 1993;110:461-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Alkadhi KA, Salgado-Commissariat D, Hogan YH, Akpaudo SB. Induction and maintenance of ganglionic long-term potentiation require activation of 5-hydroxytryptamine (5-HT3) receptors. J Physiol. 1996;496:479-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Martin AR, Pilar G. Presynaptic and post-synaptic events during post-tetanic potentiation and facilitation in the avian ciliary ganglion. J Physiol. 1964;175:17-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Magleby KL, Zengel JE. A quantitative description of tetanic and post-tetanic potentiation of transmitter release at the frog neuromuscular junction. J Physiol. 1975;245:183-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 89] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Bennett MR. Nitric oxide release and long term potentiation at synapses in autonomic ganglia. Gen Pharmacol. 1994;25:1541-1551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Brain KL, Bennett MR. Calcium in the nerve terminals of chick ciliary ganglia during facilitation, augmentation and potentiation. J Physiol. 1995;489:637-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Lin YQ, Bennett MR. Nitric oxide modulation of quantal secretion in chick ciliary ganglia. J Physiol. 1994;481:385-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Madison DV, Schuman EM. LTP, post or pre? A look at the evidence for the locus of long-term potentiation. New Biol. 1991;3:549-557. [PubMed] [Cited in This Article: ] |
| 22. | Johnston D, Williams S, Jaffe D, Gray R. NMDA-receptor-independent long-term potentiation. Annu Rev Physiol. 1992;54:489-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 243] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Derkach V, Surprenant A, North RA. 5-HT3 receptors are membrane ion channels. Nature. 1989;339:706-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 478] [Cited by in F6Publishing: 499] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Nichols RA, Mollard P. Direct observation of serotonin 5-HT3 receptor-induced increases in calcium levels in individual brain nerve terminals. J Neurochem. 1996;67:581-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Alzoubi KH, Bedawi AS, Aleisa AM, Alkadhi KA. Hypothyroidism impairs long-term potentiation in sympathetic ganglia: electrophysiologic and molecular studies. J Neurosci Res. 2004;78:393-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Coote JH, Westbury DR. Intracellular recordings from sympathetic preganglionic neurones. Neurosci Lett. 1979;15:171-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Bachoo M, Heppner T, Fiekers J, Polosa C. A role for protein kinase C in long term potentiation of nicotinic transmission in the superior cervical ganglion of the rat. Brain Res. 1992;585:299-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Alkadhi KA, Alzoubi KH, Aleisa AM, Tanner FL, Nimer AS. Psychosocial stress-induced hypertension results from in vivo expression of long-term potentiation in rat sympathetic ganglia. Neurobiol Dis. 2005;20:849-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Alkadhi KA, Alzoubi KH, Aleisa AM. Plasticity of synaptic transmission in autonomic ganglia. Prog Neurobiol. 2005;75:83-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Alzoubi KH, Aleisa AM, Alkadhi KA. Expression of gLTP in sympathetic ganglia of obese Zucker rats in vivo: molecular evidence. J Mol Neurosci. 2008;35:297-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Gerges NZ, Alzoubi KH, Alkadhi KA. Role of phosphorylated CaMKII and calcineurin in the differential effect of hypothyroidism on LTP of CA1 and dentate gyrus. Hippocampus. 2005;15:480-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Gerges NZ, Aleisa AM, Alhaider AA, Alkadhi KA. Reduction of elevated arterial blood pressure in obese Zucker rats by inhibition of ganglionic long-term potentiation. Neuropharmacology. 2002;43:1070-1076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Alkadhi KA, Otoom SA, Tanner FL, Sockwell D, Hogan YH. Inhibition of ganglionic long-term potentiation decreases blood pressure in spontaneously hypertensive rats. Exp Biol Med (Maywood). 2001;226:1024-1030. [PubMed] [Cited in This Article: ] |
| 34. | Gerges NZ, Aleisa AM, Schwarz LA, Alkadhi KA. Chronic psychosocial stress decreases calcineurin in the dentate gyrus: a possible mechanism for preservation of early ltp. Neuroscience. 2003;117:869-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Alzoubi KH, Aleisa AM, Alkadhi KA. In vivo expression of ganglionic long-term potentiation in superior cervical ganglia from hypertensive aged rats. Neurobiol Aging. 2010;31:805-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Alkadhi K, Alzoubi K. Role of long-term potentiation of sympathetic ganglia (gLTP) in hypertension. Clin Exp Hypertens. 2007;29:267-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Vieweg WV, Julius DA, Fernandez A, Beatty-Brooks M, Hettema JM, Pandurangi AK. Posttraumatic stress disorder: clinical features, pathophysiology, and treatment. Am J Med. 2006;119:383-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6527] [Cited by in F6Publishing: 5617] [Article Influence: 193.7] [Reference Citation Analysis (0)] |
| 39. | Brunello N, Davidson JR, Deahl M, Kessler RC, Mendlewicz J, Racagni G, Shalev AY, Zohar J. Posttraumatic stress disorder: diagnosis and epidemiology, comorbidity and social consequences, biology and treatment. Neuropsychobiology. 2001;43:150-162. [PubMed] [Cited in This Article: ] |
| 40. | Yan X, Brown AD, Lazar M, Cressman VL, Henn-Haase C, Neylan TC, Shalev A, Wolkowitz OM, Hamilton SP, Yehuda R. Spontaneous brain activity in combat related PTSD. Neurosci Lett. 2013;547:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Morris MC, Rao U. Psychobiology of PTSD in the acute aftermath of trauma: Integrating research on coping, HPA function and sympathetic nervous system activity. Asian J Psychiatr. 2013;6:3-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 42. | Ishiguro Y, Morgan JP. Effect of endogenous catecholamine on myocardial stunning in a simulated ischemia model. Fundam Clin Pharmacol. 2001;15:111-116. [PubMed] [Cited in This Article: ] |
| 43. | Shimizu M, Kagawa A, Takano T, Masai H, Miwa Y. Neurogenic stunned myocardium associated with status epileptics and postictal catecholamine surge. Intern Med. 2008;47:269-273. [PubMed] [Cited in This Article: ] |
| 44. | Colice GL. Neurogenic pulmonary edema. Clin Chest Med. 1985;6:473-489. [PubMed] [Cited in This Article: ] |
| 45. | Stauffer AZ, Dodd-o J, Lathers CM. The relationship of the lock-step phenomenon and precipitous changes in mean arterial blood pressure. Electroencephalogr Clin Neurophysiol. 1989;72:340-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Davison DL, Terek M, Chawla LS. Neurogenic pulmonary edema. Crit Care. 2012;16:212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 47. | Hralová M, Marešová D, Riljak V. Effect of the single-dose of nicotine-administration on the brain bioelectrical activity and on behaviour in immature 12-day-old rats. Prague Med Rep. 2010;111:182-190. [PubMed] [Cited in This Article: ] |
| 48. | Rose JE, Behm FM, Salley AN, Bates JE, Coleman RE, Hawk TC, Turkington TG. Regional brain activity correlates of nicotine dependence. Neuropsychopharmacology. 2007;32:2441-2452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Gloria R, Angelos L, Schaefer HS, Davis JM, Majeskie M, Richmond BS, Curtin JJ, Davidson RJ, Baker TB. An fMRI investigation of the impact of withdrawal on regional brain activity during nicotine anticipation. Psychophysiology. 2009;46:681-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Beaver JD, Long CJ, Cole DM, Durcan MJ, Bannon LC, Mishra RG, Matthews PM. The effects of nicotine replacement on cognitive brain activity during smoking withdrawal studied with simultaneous fMRI/EEG. Neuropsychopharmacology. 2011;36:1792-1800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 51. | Haass M, Kübler W. Nicotine and sympathetic neurotransmission. Cardiovasc Drugs Ther. 1997;10:657-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 185] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 52. | Adamopoulos D, van de Borne P, Argacha JF. New insights into the sympathetic, endothelial and coronary effects of nicotine. Clin Exp Pharmacol Physiol. 2008;35:458-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | Hill P, Wynder EL. Smoking and cardiovascular disease. Effect of nicotine on the serum epinephrine and corticoids. Am Heart J. 1974;87:491-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Grunberg NE, Popp KA, Bowen DJ, Nespor SM, Winders SE, Eury SE. Effects of chronic nicotine administration on insulin, glucose, epinephrine, and norepinephrine. Life Sci. 1988;42:161-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Ferguson PL, Chiprich J, Smith G, Dong B, Wannamaker BB, Kobau R, Thurman DJ, Selassie AW. Prevalence of self-reported epilepsy, health care access, and health behaviors among adults in South Carolina. Epilepsy Behav. 2008;13:529-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Rong L, Frontera AT, Benbadis SR. Tobacco smoking, epilepsy, and seizures. Epilepsy Behav. 2014;31:210-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 57. | Koppelstaetter F, Poeppel TD, Siedentopf CM, Ischebeck A, Kolbitsch C, Mottaghy FM, Felber SR, Jaschke WR, Krause BJ. Caffeine and cognition in functional magnetic resonance imaging. J Alzheimers Dis. 2010;20 Suppl 1:S71-S84. [PubMed] [Cited in This Article: ] |
| 58. | Koppelstaetter F, Poeppel TD, Siedentopf CM, Ischebeck A, Verius M, Haala I, Mottaghy FM, Rhomberg P, Golaszewski S, Gotwald T. Does caffeine modulate verbal working memory processes? An fMRI study. Neuroimage. 2008;39:492-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 59. | Park CA, Kang CK, Son YD, Choi EJ, Kim SH, Oh ST, Kim YB, Park CW, Cho ZH. The effects of caffeine ingestion on cortical areas: functional imaging study. Magn Reson Imaging. 2014;32:366-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Worthley MI, Prabhu A, De Sciscio P, Schultz C, Sanders P, Willoughby SR. Detrimental effects of energy drink consumption on platelet and endothelial function. Am J Med. 2010;123:184-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 61. | Thyagarajan B, Alagusundaramoorthy SS, Agrawal A. Atrial Fibrillation Due to Over The Counter Stimulant Drugs in A Young Adult. J Clin Diagn Res. 2015;9:OD05-OD07. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Ibrahim NK, Iftikhar R. Energy drinks: Getting wings but at what health cost? Pak J Med Sci. 2014;30:1415-1419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Lippi G, Cervellin G, Sanchis-Gomar F. Energy Drinks and Myocardial Ischemia: A Review of Case Reports. Cardiovasc Toxicol. 2016;16:207-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | Bidwell LC, McClernon FJ, Kollins SH. Cognitive enhancers for the treatment of ADHD. Pharmacol Biochem Behav. 2011;99:262-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 65. | Miller GM. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J Neurochem. 2011;116:164-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 66. | Mikkelsen E, Lake CR, Brown GL, Ziegler MG, Ebert MH. The hyperactive child syndrome: peripheral sympathetic nervous system function and the effect of d-amphetamine. Psychiatry Res. 1981;4:157-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 67. | Eiden LE, Weihe E. VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann N Y Acad Sci. 2011;1216:86-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 68. | Gould MS, Walsh BT, Munfakh JL, Kleinman M, Duan N, Olfson M, Greenhill L, Cooper T. Sudden death and use of stimulant medications in youths. Am J Psychiatry. 2009;166:992-1001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 69. | Melon PG, Boyd CJ, McVey S, Mangner TJ, Wieland DM, Schwaiger M. Effects of active chronic cocaine use on cardiac sympathetic neuronal function assessed by carbon-11-hydroxyephedrine. J Nucl Med. 1997;38:451-456. [PubMed] [Cited in This Article: ] |
| 70. | Vongpatanasin W, Mansour Y, Chavoshan B, Arbique D, Victor RG. Cocaine stimulates the human cardiovascular system via a central mechanism of action. Circulation. 1999;100:497-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 71. | Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med. 2001;345:351-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 556] [Cited by in F6Publishing: 452] [Article Influence: 19.7] [Reference Citation Analysis (0)] |