Published online Mar 9, 2015. doi: 10.5497/wjp.v4.i1.144
Peer-review started: July 15, 2014
First decision: August 28, 2014
Revised: January 27, 2015
Accepted: February 9, 2015
Article in press: February 11, 2015
Published online: March 9, 2015
Protein to protein interactions leading to homo/heteromerization of receptor is well documented in literature. These interactions leading to dimeric/oligomers formation of receptors are known to modulate their function, particularly in case of G-protein coupled receptors. The opioid receptor heteromers having changed pharmacological properties than the constituent protomers provides preferences for novel drug targets that could lead to potential analgesic activity devoid of tolerance and physical dependence. Heterodimerization of opioid receptors appears to generate novel binding properties with improved specificity and lack of side effects. Further the molecules which can interact simultaneously to both the protomers of the heteromer, or to both the binding sites (orthosteric and allosteric) of a receptor protein could be potential therapeutic molecules. This review highlights the recent advancements in exploring the plausible role of heteromerization of opioid receptors in induction of tolerance free antinociception.
Core tip: Endogenous opioid peptides are known for their analgesic effects. However their analgesic effect is downplayed by the side-effect of tolerance development. To maintain homeostasis to their effect, other endogenous anti-opioid peptides works parallel to it. The present work highlights the role of anti-opiates in development of tolerance to opiate drugs.
- Citation: Mudgal A, Pasha S. Role of opioid receptor heterodimerization in pain modulation and tolerance development. World J Pharmacol 2015; 4(1): 144-159
- URL: https://www.wjgnet.com/2220-3192/full/v4/i1/144.htm
- DOI: https://dx.doi.org/10.5497/wjp.v4.i1.144
Opioid system comprising endogenous opioid peptides and receptors is mainly expressed in the central nervous system[1]. A prominent role is played by the opioid system in modulation of nociception, neuroendocrine physiology, autonomic functions and reward processing[2]. Opioid receptors are a part of the G-protein coupled receptors (GPCRs) family. There exist three types of homologous opioid GPCRs viz. mu opioid receptor (MOR), delta opioid receptor (DOR) and Kappa opioid receptor (KOR)[3]. Extensive studies have been done to enunciate their implications in pain control, mood disorders and drug abuse[4,5]. GPCRs are seven transmembrane spanning proteins, which represent powerful targets to modulate both, the physiological and pathological states as they are involved in many biological processes. Regulation of GPCR activity is through various mechanisms viz. phosphorylation, endocytosis, desensitization, etc. is substantially evident. Among these, the interactions leading to heteromerization in which one protein receptor interacts with other protein receptor particularly modulates the GPCR function[6]. Decades of research over opioid pharmacology has discovered the complexity underlying the physiology of opioid system[7]. Various homotypic and heterotypic interactions have been revealed among the three types of opioid receptors (μ, KOR and DOR), when they are expressed in heterologous cells[8-10], which were considered as single units functionally. As a result the ligand binding and signaling properties get altered for these receptors. However occurrences of such interactions in live cells and ex vivo have not been reported until now and even no clue was there whether they were physiologically relevant. Protein complexes give rise to functional interactions within intracellular partners, resulting from shared association or downstream effectors competition. However, these interactions are highly debated whether they occur at neuronal level through signaling pathways or at molecular level exhibiting physical association of receptors by direct contact and with existing tools it is very difficult to claim any specific answer for them.
Recent reports shed some light over the functional interactions across receptors, by analysing the opioid drug effects for MOR and DOR receptors in vivo[11]. Well known example is of DORs to have implication in opioid tolerance development to morphine[12]. To further explore on this growing notion a “two-state dimer receptor model” has been recently proposed to understand and interpret heteromer operation by binding to lead molecules[13]. A receptor is a cellular macromolecular assembly which specifically transduces chemical signals inside and between cells. Whereas a receptor heterodimer is composed of two units and its biochemical properties may be different from its individual protomers[14]. This could be either due to some sort of intermolecular interaction or allosteric interaction which leads to the changes in binding properties of other protomer upon ligand binding to the first protomer. Thus generating novel pharmacological and signalling properties[15] which when targeted by specific ligands leads to improved efficacy with reduced undesirable effects[16]. Such specific ligands could be dualsteric compounds and may interact simultaneously to both the protomers or to the orthosteric site of one and then allosterically modulating the other protomer. In case of the allosteric interactions receptor subtype-selectivity is achieved and it may also modulate the efficacy as well as intracellular signaling pathway activation. The occurrence of opioid receptor heteromers uncovers a new side of novel drug targets which could be capable of combating a variety of diseases with potentially fewer side effects.
Since all the aspects of receptor physiology, pharmacology, trafficking, signaling, ligand affinities, etc. are affected due to the heteromer formation, it offers a very useful handle to obtain reliable macroscopic dissociation constant (KD) values from binding data for biphasic kinetics. A new parameter, degree of cooperativity (Dc) could quantitatively define the intramolecular communication within the dimer. This new parameter has enabled vision of the occurrence of receptor heterodimers unfolding the new functional and pharmacological perspectives for GPCRs[17].
Initially the opioid receptors heteromerization was studied in artificial cell systems but now the focus has been shifted to its in vivo relevance. Many compounds have been identified that could selectively target the opioid heteromers of DOR with KOR and MOR influencing the opioid analgesic effect and modulating its ethanol consumption side effect. In some cases the differences in receptor trafficking properties have been attributed to the specific physiological response produced by the heteromers in comparison to their homomeric counterparts. For opioid receptor heteromers the easier detection of pharmacological profile modification has been achieved which has enabled the consideration of making opioid drugs like morphine more effective while restricting its side effects[18]. One of the examples is of DOR ligands which have shown potentiation of morphine’s efficiency. Although, the molecular mechanism underlying such observations is still not clear, they have been attributed to the modulation of receptor function due to physical association between them. This hypothesis has been supported by the existence of mu-delta receptors complexes in live cells and the enhancement of their binding and signaling activity by antagonist occupancy of receptors. Thereby suggesting that heterodimeric association of opioid receptors could be used as a model to develop novel drug compounds for pain modulation[19].
An important aspect of opioid pharmacology is the establishment of the side effect, tolerance and dependence[20]. Tolerance may be defined as a phenomenon in which an increased amount of drug is required to produce the same level of drug effect after repeated use of the drug. Development of tolerance involves complex biochemical procedures at the cellular as well as sub-cellular level. Though many mechanisms have been proposed for the same but due to conflicting reports, no mechanism is yet universally accepted. Several lines of evidence propose that development of opioid tolerance may have multiple causes involving complex physiological adaptations, but majorly includes molecular level receptor trafficking.
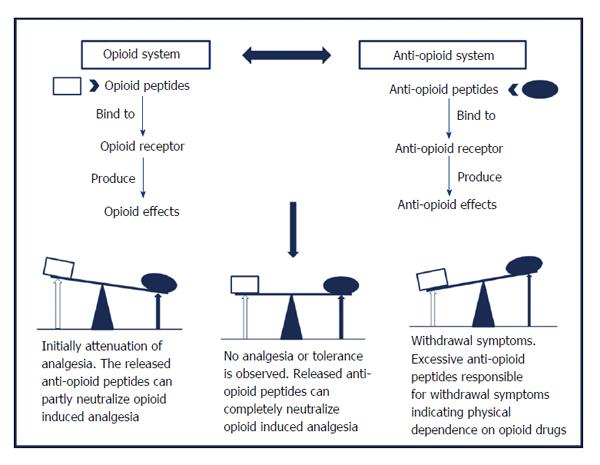
The classical approach attributes opioid tolerance development and physical dependence in the body to the changes that occur at the receptor level viz. desensitization, down-regulation or internalization of opioid receptors. But it could not completely explain all the aspects of tolerance and physical dependence which gave rise to an alternative model, the “anti-opioid model”[21], shown in Figure 1. This model postulates the existence of an anti-opioid system within the body which works parallel to the endogenous opioid system, to neutralize the antinociceptive opioid effects upon chronic treatment. Certain endogenous neuropeptides such as Phe-Met-Arg-Phe-NH2 (FMRFa), Phe-Leu-Phe-Gln-Pro-Gln-Phe-NH2 (NPFF), Tyr-Pro-Leu-Gly-NH2 (Tyr-MIF-1) and orphanin FQ/nociceptin, exhibits anti-opioid effects. These peptides mediate their biological effects through a different set of receptors, distinct from opioid receptors[22] which are also G protein-coupled receptors[23,24]. Together these peptides and their receptors are known to have role in the development of tolerance and physical dependence upon chronic administration of opioids.
Taking for example, morphine chronic treatment should increase the release of anti-opioid peptides which thereby will lead to attenuation of the morphine antinociceptive effects. Further opioid treatment will cause more release of anti-opioid which will induce tolerance to the opioid effects. The residual excess of anti-opioid left after termination of opioid administration, is partially responsible for the withdrawal syndrome. This model suggest the participation of NPFF in modulation of opioid effects and therefore present them as important targets for novel pharmacological agents which can intensify the opioid antinociceptive effects by modulating endogenous opioid function. In view of this point, the agonists of the anti-opioid system would block the opioid effect whereas their antagonists are expected to potentiate it. Such hypothesis broadens the vision to describe the role of NPFF system in the control of opioid function mainly in supraspinal regions. In particular, NPFF exhibiting anti-opioid effects emerges as a modulator of the opioid effects along with having significant role in the development of opioid tolerance and physical dependence.
This story has another side to be viewed, where experimental evidences have shown that NPFF exhibits opioid-like effects and it also potentiates in both the spinal as well as supraspinal regions the analgesic effect of morphine. Earlier NPFF has been placed in the anti-opioid category inspite of demonstration of both pro- and anti-opioid effects because the underlying mechanisms were not clear. However, recent studies are revealing the complexities of NPFF effects on opioid analgesic activity and thereby limiting the use of anti-opioid term to describe the physiological role of NPFF[25].
Opioid GPCRs appear to form homo/heteromers having altered ligand binding and activation of G-protein[26,27]. The opioid receptor heteromerization was believed to occur intracellularly, where the two receptors may have physical association, thereby operating as homo/heteromers with distinct signaling and trafficking properties as compared to their monomeric counterparts[11]. The concept of homo/heteromers prevails from the reports on heteromerization of many GPCRs including MOR and DOR[8,9]. The first example of role of heteromerization in pharmacological diversity was observed in studies on DOR and KOR receptors[28]. These studies exhibited the ligand binding, functional and trafficking properties of DOR/KOR complex that are distinct from those of each individual receptor[8]. In vivo operation of these mechanisms indeed is still unresolved in GPCR research[11]. Until now, there is very little evidence supporting the in vivo co-expression of MOR/DOR receptors. Few examples have been reported in dorsal root ganglia[29], spinal cord[19], rostroventral medulla[11] and in a limited number of brain areas which are tested by specific antibodies raised particularly against these heteromers[30]. Various studies viz. coimmunoprecipitation and bioluminescence resonance energy transfer studies suggest physical association between MOR and DOR receptors upon co-expression[19,31], although their distinct localization and activation have been recently reported in studies on mice[32]. It is revealed from one of the study that chronic administration of morphine upregulates the MOR/DOR dimers[30] and during this the activation of the DOR subunit in the dimer results in degradation of MOR and cellular response[33].
A study supporting MOR/DOR heteromer formation demonstrating oligomerization of MOR receptor dimers into heteromer complex formation with DOR receptor following prolonged morphine treatment, and the influence on these interactions of DOR receptors. The changes in association were measured by förster resonance energy transfer (FRET), the mobility of receptors by fluorescence correlation spectroscopy and the degree of oligomerization by number analysis. Moreover the structural interpretation was done via computational modelling. With morphine there was little effect on the diffusion properties and interaction with G proteins of MOR. On the other hand, presence of DOR increases the oligomerization along with association of MOR receptors with G-proteins upon morphine treatment, indicating correlation of functional properties of MOR with its oligomerization[34].
Amongst the GPCR heteromers studied so far, opioid receptors were the first to be exhaustively studied via functional (changes in pharmacology), biochemical (immunoprecipitation, cross linking) and biophysical (FRET, BRET) methods. The affinity for heteromer formation is same for all the opioid receptors[35]. For example DOR can heteromerize with either MOR[36] or/and KOR[37]. Taking a deep insight into the heteromers formed could reveal the presence of opioid receptor subtypes that have been pharmacologically defined, but not ascribed as splice variants. This could be exemplified by delta opioid receptor subtype 1 (DOR1) [preferred (D-Pen2,D-Pen5)enkephalin (DPDPE) and 7-Benzylidenenaltrexone (BNTX)] and DOR2 (preferred deltorphin II and naltriben), as DOR1 forms DOR/KOR heteromer and DOR2 forms DOR/MOR heteromer. Conversely in other study more pronounced affinity was observed for DOR/MOR heteromer by DOR1 (BNTX) ligand[10]. Supporting the previous observations, some behavioural effects viz. ethanol consumption, of DOR1 ligands are affected by disruption of MOR, partially confirming involvement of DOR1 in DOR/MOR heteromer[38]. Similarly exists the subtypes of MOR and KOR receptors. MOR antagonist naloxonazine inhibits MOR1 only out of the two MOR1 and MOR2. In case of KOR three subtypes have been pharmacologically identified out of which KOR1 binds arylacetamides, KOR2 does not, while KOR3[39] is insensitive to KOR (U50,488) ligand. Interestingly the subtypes being classified for various opioid receptors may be same, e.g., MOR2 may be DOR2[40], whereas DOR1 and KOR2 may be the same DOR/KOR heteromer[41].
Another example includes the co-immunoprecipitation of MOR1 and DOR1 in the central nervous system tissue[19] which have been selectively recognized by the antibodies for DOR/MOR heteromer in vitro and in vivo as well[42]. Furthermore, only a single ligand, 6’-guanidinonaltrindole (6’-GNTI), has been identified that selectively activates a heteromer in vitro and produces a biological effect in vivo[43]. Although there is ongoing debate over the in vivo role of DOR/MOR or DOR/KOR heteromers, some evidences do exist for their existence in vivo.
Taking together the synergism between DOR and KOR receptor agonists and the reports on co-localization of opioid receptors from spinal region, DOR receptor agonist DPDPE was proposed to interact with DOR recognition site in an allosteric DOR/KOR heteromer. This heteromer is said to be allosteric because this model considers that upon norBNI binding to KOR recognition site induces conformational changes in the other protomer of the heteromer, DOR which results in antagonism of DPDPE antinociception. Another support for this observation came from a study on porcine ileum where DOR and KOR receptors were found co-localized and norBNI significantly antagonized the DOR selective agonists[44]. Thus reinforcing the existence of DOR1 receptor as DOR/KOR heteromer in the mouse spinal cord and that the two subunits are allosterically coupled[45].
In another study the DOR agonist deltorphin-II has been reported as functional agonist of the MOR/DOR heteromer, which not only induced desensitization but also inhibited the adenylyl cyclase through a pertussis toxin-insensitive G-protein. Stimulation of the heteromer MOR/DOR lead to the activation of Gαz, which was demonstrated by the incorporation of GTPγ35S, whereas, individual activation of separate MOR and DOR receptors activated Gαi preferably. This specific behaviour has been attributed to the shared involvement of both receptors distal carboxyl tails, so that truncation of distal carboxyl tail of one of the receptor modified the selective ligand-binding pocket of the other. The significance of the role of distal carboxyl tails in the receptor interaction could be viewed from the reduction in their co-immunoprecipitation upon truncation of the carboxyl tails of both the receptors. Thereby suggesting the occurrence of interaction upon co-expression only, indicating the generation of MOR/DOR heteromer by a co-translational mechanism[46].
One of the studies further provides new understandings of the MOR/DOR receptor heteromer trafficking via clathrin and dynamin endocytic machinery. The insight of this mechanism was that MOR/DOR receptor heteromers internalization by DOR agonists needed the modification of MOR agonist binding to MOR/DOR heteromer caused by the DOR agonists which occupied both the receptors binding pockets and remained intact in a morphine tolerance model[47]. Another study suggested the existence of DOR/KOR heteromers in the sensory neurons of rat and the modulation of DOR agonist responses via KOR antagonists through the allosteric interaction of the protomers[48].
Opioid receptors are known for modulation of all levels of brain function including autonomic, sensory, emotional and cognitive processing. A very recent study has provided a proof-of-principle brain atlas using the MOR/DOR interacting model for the co-expression of GPCRs in vivo[11]. And now it has become an established fact that MOR and DOR receptors have functional interactions in vivo but still the underlying mechanism is still unresolved. To gain an insight of the existence of MOR/DOR heteromer in brain a double knock-in, redMOR/greenDOR (red protein mcherry tagged and green fluorescent protein tagged) mice model was generated. The data has been reported for the mapping of both receptors throughout the nervous system and is accessible online offering subcellular level visualization of opioid receptor atlas with associated MOR/DOR. Further where co-immunoprecipitation revealed heteromerization of receptors, high-order processing of forebrain suggested system-level interactions between MOR and DOR receptors as they were detected in separate neurons. On the contrary, subcortical networks highlighted co-localization which is crucial for eating, perception, sexual behaviours and response to aversive stimuli[11].
Although in literature all the three possible receptor heterodimer formations have been reported for the three known opioid receptors viz. KOR/MOR, MOR/DOR and KORDOR, the most importantly known and proven till date has been the heteromer MOR/DOR. Apart from this there is another interesting possibility of opioid receptors forming heteromers with other receptors outside their family having distinct pharmacological properties, different affinities for ligands as well as different signaling and receptor trafficking.
Astonishingly recent reports have identified and proposed the presence of some sort of association within the cell membrane between opioid and anti-opioid NPFF receptors. A study performed on rats revealed strong and long-lasting antinociceptive effects induced by NPFF when administered intrathecally along with potentiation of the morphine-induced analgesia[49]. This model proposed NPFF as a functional DOR/opioid autoreceptor antagonist causing an increase in the release of endogenous opioid peptides in spinal cord[50].
Another in vitro study done on SH-SY5Y cells showed a physical interaction between NPFF2 and MOR receptors, explaining the anti-opioid activity of NPFF2 receptors. By promoting a heteromeric association with MOR receptors, NPFF agonists cause changes in the diffusion properties of MOR receptors, as if they are moving MOR receptors away from their signaling associates. As a consequence, the response to opioids is reduced. The modulation of the delivery and trafficking of DOR/opioid receptors at the cell surface is assumed to be a means for regulating MOR receptor function, hence opioid analgesia and tolerance[51]. Likewise, the molecular mechanism described here for NPFF, comparable to the diffusion-trap system of the synapse, could represent another way to modulate the opioid response.
NPFF2 receptors exert a nonreciprocal antagonism on opioid receptors. Although, many other peptides including the opioid peptides, have been known to modulate the opioid receptors activity but it is only NPFF which exerts the antiopioid activity. To study this, a model SH2-D9 has been provided which enables the characterization of the molecular mechanisms involved in NPFF and opioid receptors interaction[52].
It is clearly evident at various, behavioural and receptor levels that NPFF and opioid systems interact. Though the most extensively studied physiological function is nociception, others like reward, locomotion, feeding, and intestinal motility are also affected. The two reciprocate each other as endogenous opioids are implied in analgesia upon spinal injection of NPFF and upon chronic opioid treatment, the endogenous NPFF peptides results in analgesic tolerance/hyperalgesia. These pharmacological modifications could be explained by the cellular anti-opioid effect of NPFF which has been attributed to the heteromerization mediated direct cross-talk between the two types of receptors studied in model cell lines. The validation of this hypothesis on endogenous receptors in neurons is a great challenge which will unveil some new perspectives for pain modulation and tolerance development[53]. This review focuses on the latest developments in the field of opioid receptor heteromerization and role in tolerance development.
Previously opioid receptors were considered as monomers and the selection of ligands binding to these receptors was based on observed pharmacological parameters. However, recent evidence from converging methodologies suggests that opioid receptors are expressed as homo/heterodimers. Opioid receptors homo/heteromerization is assumed to play a role in the activation of receptors and their internalization. This review focuses on advancements in the field of opioid receptor heteromerization and its impact on their pharmacological behaviour, and is summarised in Table 1. Since the theory behind heteromerization is still unknown, various models have been devised to identify the neurotransmitters binding to opioid receptors and their activation mediated by the allosteric interactions taking place between the protomers. Although there is a lack of a model predicting heteromerization signalling, functional data supports their occurrence as well as effects produced by them in response to a single neurotransmitter[54].
| Year of study | Receptors involved | Mode of study | Possible mechanismfor interaction | Changes inbinding properties | Heteromerspecific ligands | Changes in G-proteinactivation and coupling | Possible therapeutic implications | Ref. |
| 2000 | MOR/DOR | In vivo | Direct interaction (heterodimers are preferred) | Decrease in affinity for selective agonists | Pain relief | George et al[9] | ||
| 2000 | MOR/DOR | In vivo | Direct interaction (heterodimers are preferred) | Allosteric effect of DOR ligands on MOR binding | Pain relief | Gomes et al[10] | ||
| 2004 | MOR/DOR | In vivo | Direct interaction (heterodimers are preferred) | Decrease in G protein coupling | Pain relief | Gomes et al[19] | ||
| 2000 | MOR/DOR | In vivo | Direct interaction (heterodimers are preferred) | Allosteric effect of DOR ligands on MOR coupling; signaling through a β-arrestin2-mediated pathway | Pain relief | Rozenfeld et al[6] | ||
| 2007 | MOR/DOR | Shift in coupling from Gαi to Gαz | Pain relief | Hasbi et al[84] | ||||
| 2005 | MOR/DOR | COS-7 and CHO-K1 cells | Direct interaction (heterodimers are preferred) | Pain relief, tolerance development | Fan et al[46] | |||
| 2003 | KOR/DOR | Mouse spinal cord | Direct interaction (heterodimers are preferred) | Pain relief | Portoghese et al[45] | |||
| 2001 | KOR/DOR | Porcine ileum | Direct interaction (heterodimers are preferred) | Pain relief | Poonyachoti et al[44] | |||
| 2010 | MOR/DOR | HEK-293 cells | Direct interaction (heterodimers are preferred) | Pain relief | Yekkirala et al[57] | |||
| 2005 | MOR/DOR | In vivo | Direct interaction (heterodimers are preferred) | Specific heteromer activation by the agonist 6-GNTI | Pain relief | Waldhoer et al[43] | ||
| 2014 | DOR/KOR | In vivo (rat trigeminal ganglia) | Allosteric interactions | Thermal allodynia | Erbs et al[11] |
It has been very recently known that heterodimerization of opioid receptors modulates their pharmacology. The variation in the opioid receptor number, their distribution and the post-translational modifications may have great impact on the various adaptive changes following the acute (e.g., desensitization) and chronic (e.g., tolerance and down-regulation) opioid administration. Although each protomer of the heteromer is distinct, the heteromer too on the whole represents a distinct entity. Therefore depending on the heteromer different affinities and efficacies are expected for compounds. Thus the existence of opioid receptors heteromers open up a new field for the identification of compounds with improved specificity and reduced undesirable effects as they generate novel pharmacological and signaling properties.
Several laboratories have indeed started using this interesting therapeutic approach to design such compounds which may act on two receptors of the heteromer. In doing so, there arise two possibilities for the development of “dual” compounds. In first the dual compounds have moderate affinity for the two receptors whereas in second case the “dimeric” compounds would activate simultaneously both the receptors of the heterodimer. Out of these the second would serve to be an excellent tool for detection of heteromerization in natural tissues. Many such heteromer specific compounds do exist which have been identified serendipitously. These rationally designed dualsteric GPCR agonists have allowed the simultaneous exploitation of favourable characteristics of orthosteric and allosteric receptors and prove to be a promising new approach for the achievement of fine-tuned GPCR modulation[55]. Following this approach it is expected that more rational (perhaps modelling based) approaches will emerge in the future.
The dualsteric ligands would enable both in vivo heteromer localization as well as their dynamics. Moreover, tissue-selective expression of opioid receptors would also enable prevention of the opioid induced side-effects viz. constipation and respiratory depression. In addition, the alteration of heteromers expression during morphine tolerance development could represent unexplored and selective targets for the pain modulation and reversing the development of tolerance and physical dependence[56].
Out of the ligands identified so far that are selective for the opioid receptor types, some may exhibit selectivity towards the heteromer in a differential manner in comparison to the individual receptors. In this context, various studies have been initiated to evaluate the classical MOR and DOR ligands selectivity towards MOR/DOR heteromer and also some MOR/DOR heteromer selective compounds have been identified and synthesized.
Given below are some examples of ligands targeting opioid receptor heteromers having analgesic effects in vivo and their possible role in side-effects, e.g., tolerance.
Morphine has long been known as one of the choice analgesic used to treat chronic pain, but its usage is restricted due to the development of tolerance and physical dependence. To overcome this shortcoming, various strategies have been considered to enhance the potency of morphine while limiting its abuse. The most important out of them is using a combination of drugs, to increase the effectiveness of morphine.
Measurement of intracellular calcium release via chimeric G proteins or GTPγS binding has enabled the examination of the signaling properties of both the classical and clinically used MOR agonists (DAMGO, morphine, fentanyl and methadone) which is done in the cells stably expressing homo/heteromeric opioid receptors[57]. The potencies of these agonists increased to almost 7-12 folds higher in MOR/DOR heteromers than in MOR homomers and showing no significant results in DOR homomers. Whereas the DOR selective antagonist, naltrindole, antagonized the morphine, fentanyl and methadone mediated signaling exclusively in MOR/DOR heteromers expressing cells and also the antinociceptive effects of these drugs in monkeys[58]. Thus, suggesting MOR/DOR heteromers as prime targets for exhibiting antinociception and in tolerance development of these drugs.
Studies have shown that selective ligands activate distinct signaling pathways in cells expressing MOR/DOR heteromers in comparison to the cells expressing MOR homomers. As for example, DAMGO activates Gαi/o-mediated signaling in MOR alone expressing cells while β-arrestin mediated signaling is activated in MOR/DOR heteromers expressing cells[59]. Since the β-arrestin mediated signaling is known for involvement in tolerance development, the MOR/DOR heteromers are suggested to play a role in the same[60].
Various in vivo studies have revealed that modulation of morphine-mediated antinociception involves MOR/DOR heteromers like morphine antinociception is enhanced by endogenous DOR agonist, Leu-enkephalin, by synthetic analog of enkephalin, FK33824 or by DOR opioid antagonist, TIPPΨ[19].
Further studies have promoted the DOR involvement in morphine tolerance development. Like in one study DOR receptor antagonist, naltrindole have been shown to block the morphine tolerance development[61] and in other study the DOR knockout mice did not developed the antinociceptive tolerance to morphine[62,63].
Recent reports have shown increased levels of MOR/DOR heteromers in brain and spinal regions following the morphine chronic treatment, using heteromer selective antibodies[30]. Another study using (transactivator of transcription) peptide (GRKKRRQRRRPQ) (TAT) peptide which targets the transmembrane 1 of MOR, preventing its heteromerization, demonstrated prevention of morphine tolerance development upon pretreatment with TAT[33]. Thus clearly indicating the crucial role of MOR/DOR heteromers in morphine induced antinociceptive tolerance.
Further studies have shown that MOR subunits interactions or interactions with other G-proteins are not disturbed by the morphine treatment rather it destabilizes the MOR/DOR heteromers. This could be seen in a recent study which shows that activation of the DOR protomer in MOR/DOR heteromer causes degradation of MOR protomer rather than recycling and thus decreasing its cellular response[33]. This is explained on the basis of sizes where MOR/DOR heteromers can be easily transferred to the lysosomal pathway as compared to MOR homomers which are larger in size, through more accessible sites for proteolysis or modifications, such as ubiquitination. Finally, proposing MOR/DOR heteromer as a suitable example to demonstrate the role of GPCR heteromerization causing differences in the cellular responses[34].
The antinociceptive effects of DOR selective agonist SNC80 have clearly depicted the involvement of MOR/DOR heteromers. It was also shown in cells co-expressing a chimeric G-protein with either opioid receptor heteromers or individual receptor homomers that SNC80 induced intracellular calcium release only in cells expressing MOR/DOR heteromers[64]. Additionally MOR knockout animals did not showed the antinociceptive effect of SNC80 which was right shifted by almost 3-fold and with DOR knockouts the right shift was almost 6-fold[64]. Combining these results emphasized on the necessary presence of both MOR and DOR for the antinociceptive activity of SNC80. Further in a study combination of highly selective MOR agonists with DOR antagonists (and vice versa) were used to explore MOR/DOR heteromer mediated signaling.
As for example reversal of the MOR mediated signaling from β-arrestin-mediated into Gαi/o-mediated, in cells co-expressing MOR/DOR heteromers by blocking of the DOR by its selective antagonist which also resulted to enhancement of morphine-mediated antinociception[19,59].
These results highlight the functioning of DOR ligands in allosteric modulation of MOR (protomer) activity among the MOR/DOR heteromer. Moreover, in a report loss of the antidepressant and anxiolytic effects of the DOR agonist, UFP-512, were shown using a fusion of TAT peptide to the peptide corresponding to the distal C-tail of DOR to disrupt MOR/DOR heteromers signifying their potential role in anxiety and depression[65].
Many studies have reported that receptor heteromerization leads to new binding properties[8-10], suggesting that heteromerization induces an alteration in the conformation of the ligand-binding site. The identification of an agonist, 6′-GNTI [6′-guanidinyl-17-(cyclopropylmethyl)-6,7-dehydro-4,5a-epoxy-3,14-dihydroxy-6,7-2′,3′ indolomorphinan], an analgesic showing relative selectivity for DOR/KOR heteromers, supports the notion that receptors within a heteromer are capable of adopting active conformations that are absent in their homomeric counterparts[43]. 6′-guanidinonaltrindole precisely activates only the opioid receptor heteromers and not homomers[43]. Occurrence of heterodimers is tissue-specific as can be seen from the example of 6-GNTI which upon administration to spinal region induces analgesia but not in the brain. This study has given a proof-in-principle for the compounds targeting opioid heterodimers that they could be a better probe with increased analgesia and lesser side effects.
Another example is of a DOR1 selective agonist, 2-methyl-4 alpha alpha-(3-hydroxyphenyl)-1,2,3,4,4a,5,12,12 alpha alpha-octahydro-quinolino[2,3,3-g]isoquinoline (TAN-67), which is known for reducing ethanol consumption in mice. The study reveals that its activity depends on presence of both the DOR and MOR receptors suggesting DOR1 to be a DOR/MOR heteromer which exhibited reduced ethanol consumption without dysphoria production. One more such example is of CYM51010 which is a MOR/DOR heteromer selective agonist[66]. The biasing of CYM51010 towards the MOR/DOR heteromer was determined by the tail-flick antinociception assay which demonstrated analgesic activity comparable to morphine and during chronic administration it resulted in lesser tolerance development in comparison to morphine[66]. Further the MOR/DOR heteromer selective antibody treatment although partially but significantly blocked CYM51010-induced β-arrestin recruitment, GTPγS binding and intrathecal antinociception[66] reinforcing the result that CYM51010 exhibited its effect mainly through activation of MOR/DOR heteromer. Thereby, proposing CYM51010 as potent analgesic with lesser tolerance development as compared to morphine.
In addition, drug “cocktails” targeting homo/heteromers could be therapeutically valuable. As for example an opioid cocktail comprising of morphine and either methadone or DAMGO enhances the morphine induced endocytosis. It is benefitted by the homomeric MOR resulting into reduced development of tolerance and dependence[67].
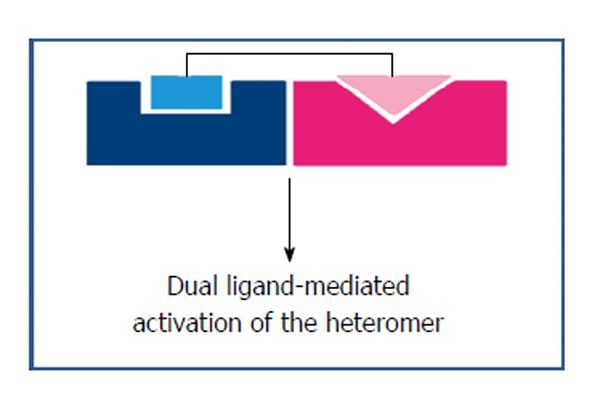
Dualsteric ligands represent a novel mode of targeting GPCRs as they can bind simultaneously to both, the orthosteric and allosteric sites of a receptor protein or two receptors present in the near vicinity or heterodimers[68], as shown in Figure 2. This approach facilitates the exploitation by a single compound, of both the orthosteric and the allosteric sites. The orthosteric interaction on one hand provides high affinity for binding and activation of receptors, the allosteric interaction on the other hand results in receptor subtype-selectivity thereby modulating both the efficacy and the activation of intracellular signaling pathway. With the increase in reports on allosteric interactions for GPCRs and the insight of spatial geometry of ligand/GPCR/complexes, the rational design of dualsteric ligands promises the achievement of finely tuned GPCR modulation.
GPCRs physical interaction is becoming progressively more recognized. The mechanism behind allosteric modulation of receptor function may possibly be provided through these interactions. Such allosteric interactions are expected to occur in a way that when a ligand binds to one protomer, it behaves as an allosteric enhancer of the other protomer[69]. Apart from allosteric modulations, heteromerization also affects the ligand recognition, G protein-coupling and trafficking. One such example is of opioid receptor heteromers having difference in ligand recognition at the receptor heteromer. According to recent models only one G protein binds to two receptor units meaning that in case of a heteromer it will have to select a particular G protein it should bind to as these receptors are usually coupled to different G proteins[70].
Following this rational the attempts of synthesizing heteromer selective ligands has given rise to the generation of dual ligands viz. the mu-delta agonist-antagonist (MDAN) series of ligands linked by a spacer of variable length. Out of these the ligand MDAN21 consists of two pharmacophores, MOR agonist MA19 and DOR antagonist DN20, which are separated by a 21-atom spacer[71]. In comparison to morphine, MDAN21 exhibited 100-times greater potency without significant development of tolerance and dependence[71]. Furthermore, this ligand also immobilized the heteromer at the cell surface possibly by bridging both the protomers and thereby inhibiting the MOR receptor internalization in cells expressing MOR/DOR heteromers[72]. More examples of such ligands are ENTI, which comprises of oxymorphone (high affinity μ-agonist) linked by a spacer arm to a low affinity DOR antagonist and DM-SNC80, containing naltrexone (high affinity μ-antagonist) joined by a spacer arm to a low affinity DOR agonist[73]. Though, the analgesic activity and the side effects of these ligands have not yet been adequately evaluated. These studies altogether, projects dual ligands crucial role in the examination of both the in vitro and in vivo properties of MOR/DOR heteromers.
Certain endogenously occurring amphiactive peptides as MERF, containing overlapping opioid and anti-opioid peptide sequences represent important molecules to study these protein-protein interactions that modulate endogenous opioid function[74]. Also anti-opioid peptides which bind to specific anti-opioid receptors attenuate opioid analgesia and are also involved in tolerance development and physical dependence. Thus in accordance to anti-opioid hypothesis, if the anti-opioid receptors can be blocked by a suitably designed peptide which can block or act as an antagonist at anti-opioid receptors, then the tolerance development and physical dependence might be reduced or attenuated.
In this connection, based on MERF and well known modulation of opioid system by NPFF/FMRFa peptides a chimeric peptide, of Met-enkephalin (YGGFM) and FMRFa, YFa (YGGFMKKKFMRFamide) was previously designed by our group[75]. The tetrapeptide (YGGF) sequence is a common endogenous opioid peptides allosteric (message) sequence that activates the receptor and cascades the intracellular signaling pathways. Whereas, the (FMRFamide) sequence is a positive allosteric modulator of anti-opioid receptors and here is orthosteric (address) sequence having role in opioid effect modulation and development of tolerance. YFa can bind to opioid receptors through its N-terminus Tyr-Gly-Gly-Phe (YGGF), (allosteric, message) sequence and to anti-opioid receptors through its C-terminus Phe-Met-Arg-Phe (FMRFamide), (orthosteric, address) sequence, which are separated by 3-lysine residues based on Schwyzer compartment theory[76].
YFa induced naloxone-reversible antinociception suggesting opioid receptors mediated analgesia it potentiated morphine induced antinociception and attenuated tolerance development to morphine analgesia, upon intraperitoneal administration[77]. Antagonist and protein expression studies revealed that YFa produces tolerance free KOR specific antinociception[77] may be due to its ability to adopt a helical conformation. In addition, it induced after 4 d pretreatment, cross tolerance to 20 mg/kg morphine analgesia with 80 mg/kg YFa[78] and these results have been further substantiated by forskolin-stimulated cAMP inhibition and Eu-GTP-γS binding studies[79]. Moreover, smooth muscle contraction study performed on ileum of guinea pig and vas deferens of mouse revealed role of anti-opioid receptors in normalizing the effects mediated by opioid receptors[80]. These observations proposed the role of this amphiactive peptide in pain modulation.
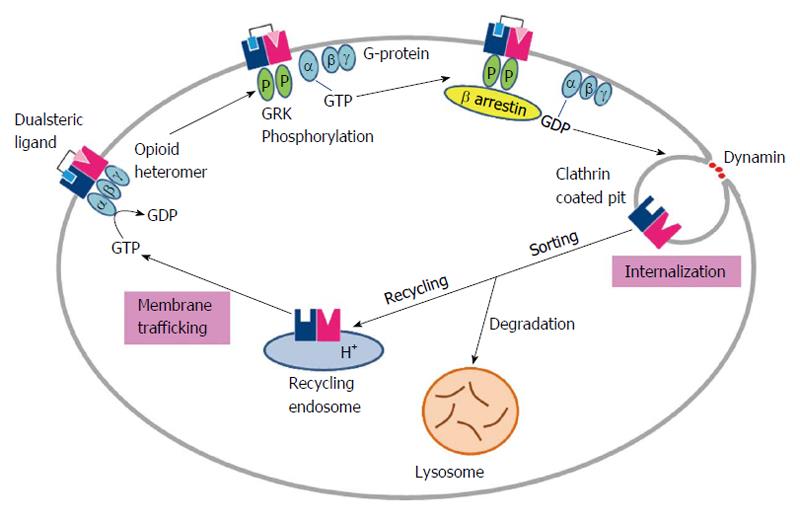
Opioid receptor heteromerization has lead to the alteration of opioid ligand properties and it also affects the trafficking of receptors in the cell culture model systems[19,81-83]. A number of studies have reported the heteromers trafficking from an intracellular compartment to the cell surface. On the other hand a few studies have examined the MOR/DOR heteromers trafficking from the cell surface to an intracellular compartment (endocytosis) among which some conflicts about their presence only at the cell surface while others presume their pre-assembling in the endoplasmic reticulum prior being trafficked to the cell surface. To exemplify this one of study was done with MOR expressing cells in which DOR expression could be induced and it was revealed that the two receptors form heteromers only when present at the cell surface as this required the interactions with G proteins. Another study reported MOR/DOR heteromers presence in the endoplasmic reticulum where they were associated with Gαz protein, using BRET in combination with cell fractionation[84]. These two conflicting results having differences in the detection of heteromerization site is due to the disparity of the experimental conditions. The first study used induction of DOR expression in MOR expressing cells which is staggered receptor expression while the other study specifically picked up co-expressing MOR/luciferase and DOR/GFP.
Another twist arises with the studies claiming heteromer endocytosis to be probe selective, by showing that some of the agonists (damgo, Deltorphin II, SNC80, methadone) induces the MOR/DOR heteromers endocytosis but others (DPDPE, DSLET) do not[42]. Furthermore, studies report blocking of MOR selective agonist induced endocytosis by the DOR selective antagonists[81]. Additionally, endocytosed MOR/DOR heteromers were found to be targeted for degradation whereas the MOR homomers were found to be recycled back to the cell surface[81]. Overall these studies proposed that changes in receptor trafficking properties are due to heteromerization, as shown in Figure 3.
Further studies using a MOR/DOR heteromer selective dual ligand, MDAN21, revealed blocking of heteromer endocytosis, it was successfully induced by the combination of individual monovalent pharmacophores (DN20 and MA19). This observation highlighted the importance of the 21-atom spacer in MDAN21 joining the two pharmacophores, which has effectively bridged the protomers, thereby immobilizing the MOR/DOR heteromer and preventing its internalization. Further studies are still required for the characterization of the underlying mechanisms involved in the differential trafficking of MOR/DOR heteromers.
In one of the study it was shown that chaperone proteins are required for the efficient cell surface expression of MOR/DOR heteromers while examination of their biosynthesis and maturation. Therefore in MOR and DOR co-expressing cells the heteromer was found to localize in the Golgi apparatus significantly, clearly indicating the requirement of presence of receptor transport protein 4 for cell surface expression of the heteromer[42]. The heteromer was protected from ubiquitination and proteasomal degradation by this chaperone during folding and maturation[42]. Although the role of this chaperone in the unique binding and signaling properties of the MOR/DOR heteromer is not clear.
Taking the other side of the story and looking at the antibodies which have been classically used as analytical tools for the identification, localization and quantification of different antigens including hormones and pathogens. Last decade has seen their usage as therapeutic agents in experimental and clinical medicine[85]. They have significant advantage of electivity, potency and efficacy over the conventional chemical drug-based therapies which makes them more effective in the treatment of various conditions viz. cancer and immune disorders. Additionally they have shown direct impact on signaling pathways within the targeted cells either by binding to the cell surface proteins or acting on their intracellular targets. A study on generation of heteromer specific antibody reported a MOR-DOR heteromer selective antibody that enabled examination of up-regulation of the heteromer in endogenous tissue upon chronic morphine treatment. This subtractive immunization strategy could be used for the generation of MOR-DOR heteromer selective antibodies and also to generate other antibodies selective for other GPCR heteromers. This would then help in studying the physiological and pathophysiological conditions and the role of GPCR heteromers in them[30].
The above mentioned studies put forward the MOR/DOR heteromers as potential targets for the development of novel therapeutics in treatment of pain with lesser side-effects due to their unique pharmacological and signaling properties. Since this would require high-throughput screening (HTS) of a large number of compounds leading to the identification of MOR/DOR heteromer selective ligands, suitable screening assays have to be determined[86].
All the observations of this study points towards the anti-analgesic role of the DOR/MOR heteromer in case of thermal nociception. The anti-analgesic effect exhibited by the combined treatment of methadone and naltriben was dependent DOR dependent, as it was not present in the DOR knockout mice. Moreover, reversal of the anti-analgesic effect was demonstrated which may be either due to the increase in degradation of DORs or by selective blocking of signaling only from the DOR/MOR heteromers but not MORs[87]. Thus below we describe a few of the HTS assays suitable for the heteromer selective ligand screening.
Out of the several assays viz. adenylyl cyclase/cAMP, phospholipase C/Ca2+, or Rho, that are used to measure the G-protein mediated signaling which could be used for HTS, the one measuring the intracellular Ca2+ release is commonly used for the screening of ligands to Gαq coupled receptors. Furthermore, measuring the intracellular Ca2+ release could also be used in the screening of ligands for Gαi or Gαs coupled receptors, because of the development of chimeric G-proteins (Gαqs or Gαqi) which provide for these receptors the calcium readout, that do not normally signal via the Gαq pathway[88]. Therefore by monitoring the release of intracellular Ca2+, the activation of opioid receptors co-expressed with chimeric Gαqi protein can be detected.
Recently, a screening assay has been reported which uses the terminal-carboxyl truncated GPCRs fused to chimeric Gαqi proteins for the detection of heteromer-mediated signaling. These fused receptors do not induce the intracellular Ca2+ release upon agonist binding; it is only observed upon their co-expression with the wild-type receptors[88]. The co-expression of wild-type MOR and Gαqi-fused DOR receptor allowed the detection of Ca2+ release for MOR/DOR heteromers[88]. The most important advantage of this method is it detects only the heteromer mediated signaling in heteromers expressing cells. To exemplify, this assay has been used with a compound ADL5859 which exhibited weak signals in case of MOR/DOR heteromers as compared to DOR homomers[88]. Thus proving that Ca2+ signaling based assays could be very useful in the identification of heteromer-selective compounds.
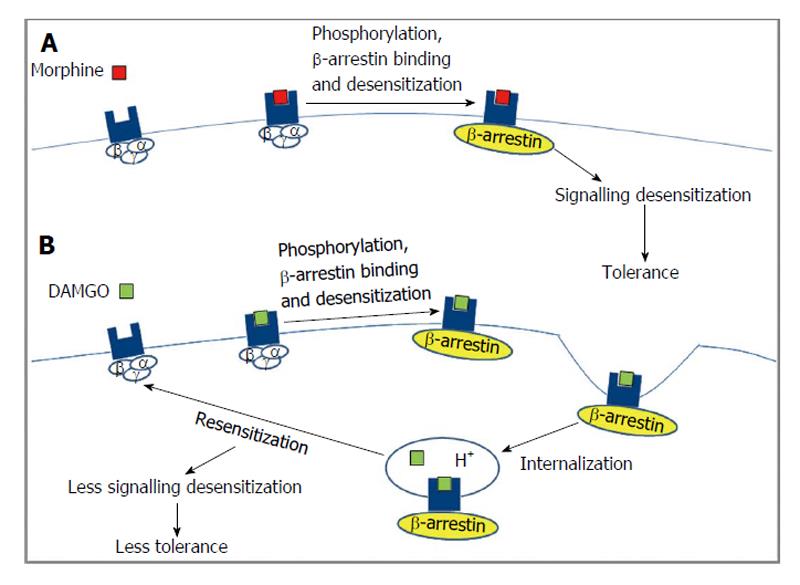
The two subtypes of β-arrestin (1 and 2) exhibit 78% homology and contain binding sites for clathrin and the β2-adaptin subunit in their C-terminal tail which allows them to function as adaptor proteins and target GPCRs to clathrin coated pits for endocytosis[89,90]. The different opioid receptor subtypes exhibit different requirements for binding β-arrestin (type 1 and 2)[91] that could lead to different signaling outcomes[92,93], as shown in Figure 4. Glutathione S-transferase pull down assays show that the third intracellular loop and the C-terminal tail of DOR and only the C-terminal tail of KOR can interact with β-arrestin 1 or β-arrestin 2[91]. These studies did not observe any interaction between β-arrestin 1/2 and MOR. However, studies in HEK-293 cells using β-arrestin 2 tagged to GFP or in striatal neurons using dominant negative β-arrestin 2 show that agonist activated MOR can recruit β-arrestin 2[94,95] although the efficacy of recruitment is agonist dependent[96]. Interestingly, mice lacking β-arrestin 2 potentiated and increased duration of the analgesic effect induced by morphine underscoring the importance of β-arrestin 2 in mediating MOR function[97]. In the case of DOR, a BRET assay suggested that receptor phosphorylation promoted receptor selectivity for β-arrestin 2 over β-arrestin 1 without affecting the stability of the receptor-β-arrestin complex[98]. However, another study used fluorescence and co-immunoprecipitation to show that agonist treated DOR bound and recruited β-arrestin (1 and 2) to the plasma membrane[99]. In addition, it has been shown that over expression of type 1 β-arrestin leads to an attenuation of DOR and KOR but not MOR mediated activation of G-proteins and inhibition of cAMP levels[100].
In addition to being involved in the attenuation of G protein mediated signaling, studies have shown that β-arrestins can induce a sustained extracellular signal-regulated kinases (ERK) phosphorylation that is distinct from the transient G-protein mediated ERK phosphorylation[101]. A recent study showed that MOR ligands such as etorphine and fentanyl, but not morphine or methadone, induced phosphorylation of ERK through a β-arrestin dependent pathway. This led to the translocation of phosphorylated ERK to the nucleus leading to an increase in the activity of Elk-1 and in the transcription of G-protein coupled receptor kinases 2 and β-arrestin 2[102]. More recently, a study showed that heterodimerization between MOR and DOR promotes the recruitment of type 2 β-arrestin onto the plasma membrane thereby changing the spatio-temporal dynamics of ERK mediated signaling that are quite distinct from those observed with the MOR homodimer. Altogether, these studies show that β-arrestins plays crucial role in mediating opioid receptor signaling by serving as a switch between G protein dependent and independent signaling mechanisms[103].
Recent researches have though made somewhat clear the mechanism underlying pain, but still the opioids are the most powerful analgesics, inspite of their limitation of tolerance development and dependence following chronic exposure. To overcome this, a hypothesis has been proposed according to which the chronic administration of opioids is counteracted by anti-opioid system that produces opposite effects[21]. This hypothesis has been supported by a number of experimental and clinical data which clearly shows that opiates activate anti-opioid systems, producing opposite and long-lasting effects which results in opioid induced hyperalgesia (OIH) thereby reducing the analgesic effects (tolerance)[104]. Since the molecular mechanisms that underlie OIH are not clear, this phenomenon is attributed to the sensitization of pronociceptive pathways in response to opioid treatment. Some other adverse effects viz. dependence and abstinence syndrome, may also involve the activation of anti-opioid systems[21]. Therefore it is assumed that compounds that can block the stimulation of anti-opioid systems could lead to the prevention of opioid tolerance development and thus limit the side effects developing due to chronic opioid exposure. This may be due to some sort of physical association between the opioid and anti-opioid receptors[105].
Looking at the other side of the story the discovery of a potent NPFF receptors (anti-opioid) selective antagonist, upon systemic administration prevents the hyperalgesia development which consequently opposes the associated decrease in analgesic effect induced by heroin. This observation reinforces the hypothesis that opioid tolerance development is not only due to a reduction in cellular responsiveness but could also be arising from the secondary up-regulation of the antiopioid systems having pronociceptive properties which renders long-lasting enhancement in pain sensitivity[106].
Looking at the other side of the story suggests that NPFF plays an important role in pain modulation, opioid tolerance development and several other physiological processes[106]. Recent reports proposed the discovery of a potent NPFF receptors (anti-opioid) selective antagonist, RF9 which upon systemic administration prevents the OIH development which consequently opposes the associated decrease in analgesic effect induced by heroin. These observations clearly mark NPFF receptors as part of anti-opioid system and put forward their antagonists as useful therapeutic agents for the improvement of opioids efficacy during chronic pain treatment[23].
Based on certain endogenous amphiactive peptides as MERF, which represent important molecules to study presence of homo/heteromer as well as their interactions that modulate endogenous opioid function[74] a chimeric peptide YFa (YGGFMKKKFMRFamide-YFa) was designed by our group. YFa can bind to opioid receptors through its N-terminus Tyr-Gly-Gly-Phe (YGGF), (allosteric, message) sequence and to anti-opioid receptors through its C-terminus Phe-Met-Arg-Phe (FMRFamide), (orthosteric, address) sequence, separated by 3-lysine residues based on Schwyzer compartment theory.
Following the same lines of scrutiny presently in our group another dualsteric ligand NPYFa (YGGFMKKKPQRFamide) based on YFa, having mammalian anti-opioid sequence in order to achieve a more potent and tolerance free chimeric peptide. NPYFa contains both endogenous opioid (YGGFM; Met-enkephalin) at N-terminus and anti-opioid (NPFF-endogenous mammalian peptide)[107] at C-terminus (C-terminal modified analogue of YFa), separated by 3 lysine residues. Pharmacological profiling of dualsteric ligand NPYFa is in progress.
Decades of research over opioid receptors has recently discovered heteromers existence, opening new ways of investigating the functioning of opioid receptors. This has also given insight to how heteromerization affects the receptor trafficking and vice versa. This review highlighted the dualsteric ligand approach targeting opioid heteromers with improved analgesic efficacy and less tolerance development. Rationally designed dualsteric GPCR agonists targeting opioid receptors heteromers allows exploitation of binding, orthosteric or orthosteric and allosteric sites of two distinct receptors simultaneously. This could be due to orthosteric receptor activation which may be followed by the allosteric subtype-selectivity further leading to intracellular signaling pathway selectivity. Further understanding of such mechanisms enables the selection of ligands with enhanced intrinsic efficacy and lesser side effects.
P- Reviewer: de Francischi JN, Kobayashi T S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379-1412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 600] [Cited by in F6Publishing: 647] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 2. | Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y. Current research on opioid receptor function. Curr Drug Targets. 2012;13:230-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 3. | Filizola M, Devi LA. Grand opening of structure-guided design for novel opioids. Trends Pharmacol Sci. 2013;34:6-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Pradhan AA, Befort K, Nozaki C, Gavériaux-Ruff C, Kieffer BL. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci. 2011;32:581-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 5. | Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36:195-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 345] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 6. | Rozenfeld R, Devi LA. Receptor heteromerization and drug discovery. Trends Pharmacol Sci. 2010;31:124-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Massotte D. In vivo opioid receptor heteromerization: where do we stand? Br J Pharmacol. 2015;172:420-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 858] [Cited by in F6Publishing: 885] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 9. | George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O’Dowd BF. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128-26135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 433] [Cited by in F6Publishing: 457] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 10. | Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110. [PubMed] [Cited in This Article: ] |
| 11. | Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch JL, Koch M, Kessler P, Hentsch D, Birling MC. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct. 2015;220:677-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 197] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 12. | Cahill CM, Holdridge SV, Morinville A. Trafficking of delta-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol Sci. 2007;28:23-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Franco R, Casadó V, Mallol J, Ferrada C, Ferré S, Fuxe K, Cortés A, Ciruela F, Lluis C, Canela EI. The two-state dimer receptor model: a general model for receptor dimers. Mol Pharmacol. 2006;69:1905-1912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Ferré S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, Fuxe K, George SR, Javitch JA, Lohse MJ. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. 2009;5:131-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 15. | Hosohata Y, Vanderah TW, Burkey TH, Ossipov MH, Kovelowski CJ, Sora I, Uhl GR, Zhang X, Rice KC, Roeske WR. delta-Opioid receptor agonists produce antinociception and [35S]GTPgammaS binding in mu receptor knockout mice. Eur J Pharmacol. 2000;388:241-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Casadó V, Cortés A, Mallol J, Pérez-Capote K, Ferré S, Lluis C, Franco R, Canela EI. GPCR homomers and heteromers: a better choice as targets for drug development than GPCR monomers? Pharmacol Ther. 2009;124:248-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Franco R, Casadó V, Cortés A, Mallol J, Ciruela F, Ferré S, Lluis C, Canela EI. G-protein-coupled receptor heteromers: function and ligand pharmacology. Br J Pharmacol. 2008;153 Suppl 1:S90-S98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Franco R, Casadó V, Cortés A, Pérez-Capote K, Mallol J, Canela E, Ferré S, Lluis C. Novel pharmacological targets based on receptor heteromers. Brain Res Rev. 2008;58:475-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci USA. 2004;101:5135-5139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 335] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 20. | van Rijn RM, Whistler JL, Waldhoer M. Opioid-receptor-heteromer-specific trafficking and pharmacology. Curr Opin Pharmacol. 2010;10:73-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Rothman RB. A review of the role of anti-opioid peptides in morphine tolerance and dependence. Synapse. 1992;12:129-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 140] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Allard M, Geoffre S, Legendre P, Vincent JD, Simonnet G. Characterization of rat spinal cord receptors to FLFQPQRFamide, a mammalian morphine modulating peptide: a binding study. Brain Res. 1989;500:169-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 141] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Simonin F, Schmitt M, Laulin JP, Laboureyras E, Jhamandas JH, MacTavish D, Matifas A, Mollereau C, Laurent P, Parmentier M. RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proc Natl Acad Sci USA. 2006;103:466-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 24. | Simonin F. Neuropeptide FF receptors as therapeutic targets. Drugs Future. 2006;31:603-609. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Roumy M, Zajac JM. Neuropeptide FF, pain and analgesia. Eur J Pharmacol. 1998;345:1-11. [PubMed] [Cited in This Article: ] |
| 26. | Kroeze WK, Sheffler DJ, Roth BL. G-protein-coupled receptors at a glance. J Cell Sci. 2003;116:4867-4869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Devi LA. Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol Sci. 2001;22:532-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 28. | Hipser C, Bushlin I, Gupta A, Gomes I, Devi LA. Role of antibodies in developing drugs that target G-protein-coupled receptor dimers. Mt Sinai J Med. 2010;77:374-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci USA. 2010;107:13117-13122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 30. | Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, Lim M, Maillet E, Junek M, Cahill CM. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal. 2010;3:ra54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 31. | Wang D, Sun X, Bohn LM, Sadée W. Opioid receptor homo- and heterodimerization in living cells by quantitative bioluminescence resonance energy transfer. Mol Pharmacol. 2005;67:2173-2184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148-1159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 368] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 33. | He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB, Li Q, Yang H, Luo J, Li ZY. Facilitation of μ-opioid receptor activity by preventing δ-opioid receptor-mediated codegradation. Neuron. 2011;69:120-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 34. | Golebiewska U, Johnston JM, Devi L, Filizola M, Scarlata S. Differential response to morphine of the oligomeric state of μ-opioid in the presence of δ-opioid receptors. Biochemistry. 2011;50:2829-2837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Zheng H, Chu J, Qiu Y, Loh HH, Law PY. Agonist-selective signaling is determined by the receptor location within the membrane domains. Proc Natl Acad Sci USA. 2008;105:9421-9426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Chu P, Murray S, Lissin D, von Zastrow M. Delta and kappa opioid receptors are differentially regulated by dynamin-dependent endocytosis when activated by the same alkaloid agonist. J Biol Chem. 1997;272:27124-27130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 122] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | van Rijn RM, Whistler JL. The only way is up: preventing opioid tolerance by promoting cell surface expression of MOR-DOR heterodimers? Mol Interv. 2008;8:277-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | van Rijn RM, Whistler JL. The delta(1) opioid receptor is a heterodimer that opposes the actions of the delta(2) receptor on alcohol intake. Biol Psychiatry. 2009;66:777-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Clark JA, Liu L, Price M, Hersh B, Edelson M, Pasternak GW. Kappa opiate receptor multiplicity: evidence for two U50,488-sensitive kappa 1 subtypes and a novel kappa 3 subtype. J Pharmacol Exp Ther. 1989;251:461-468. [PubMed] [Cited in This Article: ] |
| 40. | Wolozin BL, Pasternak GW. Classification of multiple morphine and enkephalin binding sites in the central nervous system. Proc Natl Acad Sci USA. 1981;78:6181-6185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 211] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Bhushan RG, Sharma SK, Xie Z, Daniels DJ, Portoghese PS. A bivalent ligand (KDN-21) reveals spinal delta and kappa opioid receptors are organized as heterodimers that give rise to delta(1) and kappa(2) phenotypes. Selective targeting of delta-kappa heterodimers. J Med Chem. 2004;47:2969-2972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Décaillot FM, Rozenfeld R, Gupta A, Devi LA. Cell surface targeting of mu-delta opioid receptor heterodimers by RTP4. Proc Natl Acad Sci USA. 2008;105:16045-16050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 43. | Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, Portoghese PS, Whistler JL. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc Natl Acad Sci USA. 2005;102:9050-9055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 292] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 44. | Poonyachoti S, Portoghese PS, Brown DR. Characterization of opioid receptors modulating neurogenic contractions of circular muscle from porcine ileum and evidence that delta- and kappa-opioid receptors are coexpressed in myenteric neurons. J Pharmacol Exp Ther. 2001;297:69-77. [PubMed] [Cited in This Article: ] |
| 45. | Portoghese PS, Lunzer MM. Identity of the putative delta1-opioid receptor as a delta-kappa heteromer in the mouse spinal cord. Eur J Pharmacol. 2003;467:233-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Fan T, Varghese G, Nguyen T, Tse R, O’Dowd BF, George SR. A role for the distal carboxyl tails in generating the novel pharmacology and G protein activation profile of mu and delta opioid receptor hetero-oligomers. J Biol Chem. 2005;280:38478-38488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | Kabli N, Martin N, Fan T, Nguyen T, Hasbi A, Balboni G, O’Dowd BF, George SR. Agonists at the δ-opioid receptor modify the binding of µ-receptor agonists to the µ-δ receptor hetero-oligomer. Br J Pharmacol. 2010;161:1122-1136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Berg KA, Rowan MP, Gupta A, Sanchez TA, Silva M, Gomes I, McGuire BA, Portoghese PS, Hargreaves KM, Devi LA. Allosteric interactions between δ and κ opioid receptors in peripheral sensory neurons. Mol Pharmacol. 2012;81:264-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 49. | Roussin A, Serre F, Gouardères C, Mazarguil H, Roumy M, Mollereau C, Zajac JM. Anti-analgesia of a selective NPFF2 agonist depends on opioid activity. Biochem Biophys Res Commun. 2005;336:197-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Mauborgne A, Bourgoin S, Poliénor H, Roumy M, Simonnet G, Zajac JM, Cesselin F. The neuropeptide FF analogue, 1DMe, acts as a functional opioid autoreceptor antagonist in the rat spinal cord. Eur J Pharmacol. 2001;430:273-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Zhang X, Bao L, Guan JS. Role of delivery and trafficking of delta-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol Sci. 2006;27:324-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Mollereau C, Mazarguil H, Zajac JM, Roumy M. Neuropeptide FF (NPFF) analogs functionally antagonize opioid activities in NPFF2 receptor-transfected SH-SY5Y neuroblastoma cells. Mol Pharmacol. 2005;67:965-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Moulédous L, Mollereau C, Zajac JM. Opioid-modulating properties of the neuropeptide FF system. Biofactors. 2010;36:423-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Franco R. G-protein-coupled receptor heteromers or how neurons can display differently flavored patterns in response to the same neurotransmitter. Br J Pharmacol. 2009;158:23-31. [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Mohr K, Tränkle C, Kostenis E, Barocelli E, De Amici M, Holzgrabe U. Rational design of dualsteric GPCR ligands: quests and promise. Br J Pharmacol. 2010;159:997-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 56. | Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585-591. [PubMed] [Cited in This Article: ] |
| 57. | Yekkirala AS, Kalyuzhny AE, Portoghese PS. Standard opioid agonists activate heteromeric opioid receptors: evidence for morphine and [d-Ala(2)-MePhe(4)-Glyol(5)]enkephalin as selective μ-δ agonists. ACS Chem Neurosci. 2010;1:146-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 58. | Yekkirala AS, Banks ML, Lunzer MM, Negus SS, Rice KC, Portoghese PS. Clinically employed opioid analgesics produce antinociception via μ-δ opioid receptor heteromers in Rhesus monkeys. ACS Chem Neurosci. 2012;3:720-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J. 2007;21:2455-2465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 60. | Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 675] [Cited by in F6Publishing: 699] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 61. | Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 1991;258:299-303. [PubMed] [Cited in This Article: ] |
| 62. | Nitsche JF, Schuller AG, King MA, Zengh M, Pasternak GW, Pintar JE. Genetic dissociation of opiate tolerance and physical dependence in delta-opioid receptor-1 and preproenkephalin knock-out mice. J Neurosci. 2002;22:10906-10913. [PubMed] [Cited in This Article: ] |
| 63. | Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 356] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 64. | Metcalf MD, Yekkirala AS, Powers MD, Kitto KF, Fairbanks CA, Wilcox GL, Portoghese PS. The δ opioid receptor agonist SNC80 selectively activates heteromeric μ-δ opioid receptors. ACS Chem Neurosci. 2012;3:505-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Kabli N, Nguyen T, Balboni G, O’Dowd BF, George SR. Antidepressant-like and anxiolytic-like effects following activation of the μ-δ opioid receptor heteromer in the nucleus accumbens. Mol Psychiatry. 2014;19:986-994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 66. | Gomes I, Gupta A, Devi LA. G-protein-coupled heteromers: regulation in disease. Methods Enzymol. 2013;521:219-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | He L, Fong J, von Zastrow M, Whistler JL. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271-282. [PubMed] [Cited in This Article: ] |
| 68. | Portoghese PS. From models to molecules: opioid receptor dimers, bivalent ligands, and selective opioid receptor probes. J Med Chem. 2001;44:2259-2269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | Gomes I, Ijzerman AP, Ye K, Maillet EL, Devi LA. G protein-coupled receptor heteromerization: a role in allosteric modulation of ligand binding. Mol Pharmacol. 2011;79:1044-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 70. | Ferré S, Franco R. Oligomerization of G-protein-coupled receptors: a reality. Curr Opin Pharmacol. 2010;10:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci USA. 2005;102:19208-19213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 247] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 72. | Yekkirala AS, Kalyuzhny AE, Portoghese PS. An immunocytochemical-derived correlate for evaluating the bridging of heteromeric mu-delta opioid protomers by bivalent ligands. ACS Chem Biol. 2013;8:1412-1416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 73. | Harvey JH, Long DH, England PM, Whistler JL. Tuned-Affinity Bivalent Ligands for the Characterization of Opioid Receptor Heteromers. ACS Med Chem Lett. 2012;3:640-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Roumy M, Lorenzo C, Mazères S, Bouchet S, Zajac JM, Mollereau C. Physical association between neuropeptide FF and micro-opioid receptors as a possible molecular basis for anti-opioid activity. J Biol Chem. 2007;282:8332-8342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 75. | Gupta S, Pasha S, Gupta YK, Bhardwaj DK. Chimeric peptide of Met-enkephalin and FMRFa induces antinociception and attenuates development of tolerance to morphine antinociception. Peptides. 1999;20:471-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Schwyzer R. Peptide-membrane interactions and a new principle in quantitative structure-activity relationships. Biopolymers. 1991;31:785-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 96] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Vats ID, Dolt KS, Kumar K, Karar J, Nath M, Mohan A, Pasha MA, Pasha S. YFa, a chimeric opioid peptide, induces kappa-specific antinociception with no tolerance development during 6 days of chronic treatment. J Neurosci Res. 2008;86:1599-1607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 78. | Gupta K, Vats ID, Gupta YK, Saleem K, Pasha S. Lack of tolerance and morphine-induced cross-tolerance to the analgesia of chimeric peptide of Met-enkephalin and FMRFa. Peptides. 2008;29:2266-2275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 79. | Kumar K, Kumar S, Kurupati RK, Seth MK, Mohan A, Hussain ME, Pasha S. Intracellular cAMP assay and Eu-GTP-γS binding studies of chimeric opioid peptide YFa. Eur J Pharmacol. 2011;650:28-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 80. | Kumar K, Goyal R, Mudgal A, Mohan A, Pasha S. YFa and analogs: investigation of opioid receptors in smooth muscle contraction. World J Gastroenterol. 2011;17:4523-4531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 81. | Milan-Lobo L, Whistler JL. Heteromerization of the μ- and δ-opioid receptors produces ligand-biased antagonism and alters μ-receptor trafficking. J Pharmacol Exp Ther. 2011;337:868-875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 82. | Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 555] [Cited by in F6Publishing: 530] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 83. | Gupta A, Décaillot FM, Devi LA. Targeting opioid receptor heterodimers: strategies for screening and drug development. AAPS J. 2006;8:E153-E159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Hasbi A, Nguyen T, Fan T, Cheng R, Rashid A, Alijaniaram M, Rasenick MM, O’Dowd BF, George SR. Trafficking of preassembled opioid mu-delta heterooligomer-Gz signaling complexes to the plasma membrane: coregulation by agonists. Biochemistry. 2007;46:12997-13009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 85. | Gupta A, Heimann AS, Gomes I, Devi LA. Antibodies against G-protein coupled receptors: novel uses in screening and drug development. Comb Chem High Throughput Screen. 2008;11:463-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 86. | Fujita W, Gomes I, Devi LA. Heteromers of μ-δ opioid receptors: new pharmacology and novel therapeutic possibilities. Br J Pharmacol. 2015;172:375-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 87. | Milan-Lobo L, Enquist J, van Rijn RM, Whistler JL. Anti-analgesic effect of the mu/delta opioid receptor heteromer revealed by ligand-biased antagonism. PLoS One. 2013;8:e58362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 88. | van Rijn RM, Harvey JH, Brissett DI, DeFriel JN, Whistler JL. Novel screening assay for the selective detection of G-protein-coupled receptor heteromer signaling. J Pharmacol Exp Ther. 2013;344:179-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 89. | Luttrell LM. Regulators of CPCR activity: the arrestins. In: Devi LA, editor. The G-protein coupled receptor handbook 2005; 159-198. [Cited in This Article: ] |
| 90. | DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1077] [Cited by in F6Publishing: 1109] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 91. | Cen B, Xiong Y, Ma L, Pei G. Direct and differential interaction of beta-arrestins with the intracellular domains of different opioid receptors. Mol Pharmacol. 2001;59:758-764. [PubMed] [Cited in This Article: ] |
| 92. | Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201-17210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 667] [Cited by in F6Publishing: 667] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 93. | Tohgo A, Choy EW, Gesty-Palmer D, Pierce KL, Laporte S, Oakley RH, Caron MG, Lefkowitz RJ, Luttrell LM. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem. 2003;278:6258-6267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 273] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 94. | Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS. Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol Pharmacol. 2004;66:106-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 95. | Haberstock-Debic H, Kim KA, Yu YJ, von Zastrow M. Morphine promotes rapid, arrestin-dependent endocytosis of mu-opioid receptors in striatal neurons. J Neurosci. 2005;25:7847-7857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 96. | Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. An opioid agonist that does not induce mu-opioid receptor--arrestin interactions or receptor internalization. Mol Pharmacol. 2007;71:549-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 174] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 97. | Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495-2498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 797] [Cited by in F6Publishing: 782] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 98. | Qiu Y, Loh HH, Law PY. Phosphorylation of the delta-opioid receptor regulates its beta-arrestins selectivity and subsequent receptor internalization and adenylyl cyclase desensitization. J Biol Chem. 2007;282:22315-22323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 99. | Zhang X, Wang F, Chen X, Li J, Xiang B, Zhang YQ, Li BM, Ma L. Beta-arrestin1 and beta-arrestin2 are differentially required for phosphorylation-dependent and -independent internalization of delta-opioid receptors. J Neurochem. 2005;95:169-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Cheng ZJ, Yu QM, Wu YL, Ma L, Pei G. Selective interference of beta-arrestin 1 with kappa and delta but not mu opioid receptor/G protein coupling. J Biol Chem. 1998;273:24328-24333. [PubMed] [Cited in This Article: ] |
| 101. | Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518-35525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 403] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 102. | Zheng H, Loh HH, Law PY. Beta-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) Translocate to Nucleus in Contrast to G protein-dependent ERK activation. Mol Pharmacol. 2008;73:178-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 103. | Alfaras-Melainis K, Gomes I, Rozenfeld R, Zachariou V, Devi L. Modulation of opioid receptor function by protein-protein interactions. Front Biosci (Landmark Ed). 2009;14:3594-3607. [PubMed] [Cited in This Article: ] |
| 104. | Simonnet G, Rivat C. Opioid-induced hyperalgesia: abnormal or normal pain? Neuroreport. 2003;14:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 198] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 105. | Elhabazi K, Trigo JM, Mollereau C, Moulédous L, Zajac JM, Bihel F, Schmitt M, Bourguignon JJ, Meziane H, Petit-demoulière B. Involvement of neuropeptide FF receptors in neuroadaptive responses to acute and chronic opiate treatments. Br J Pharmacol. 2012;165:424-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 106. | Ossipov MH, Lai J, Vanderah TW, Porreca F. Induction of pain facilitation by sustained opioid exposure: relationship to opioid antinociceptive tolerance. Life Sci. 2003;73:783-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 107. | Gouardères C, Jhamandas K, Sutak M, Zajac JM. Role of opioid receptors in the spinal antinociceptive effects of neuropeptide FF analogues. Br J Pharmacol. 1996;117:493-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |