Revised: January 8, 2013
Accepted: January 29, 2013
Published online: March 9, 2013
The human body consists of several physiological barriers that express a number of membrane transporters. For an orally absorbed drug the intestinal, hepatic, renal and blood-brain barriers are of the greatest importance. The ATP-binding cassette (ABC) transporters that mediate cellular efflux and the solute carrier transporters that mostly mediate cellular uptake are the two superfamilies responsible for membrane transport of vast majority of drugs and drug metabolites. The total number of human transporters in the two superfamilies exceeds 400, and about 40-50 transporters have been characterized for drug transport. The latest Food and Drug Administration guidance focuses on P-glycoprotein, breast cancer resistance protein, organic anion transporting polypeptide 1B1 (OATP1B1), OATP1B3, organic cation transporter 2 (OCT2), and organic anion transporters 1 (OAT1) and OAT3. The European Medicines Agency’s shortlist additionally contains the bile salt export pump, OCT1, and the multidrug and toxin extrusion transporters, multidrug and toxin extrusion protein 1 (MATE1) and MATE2/MATE2K. A variety of transporter assays are available to test drug-transporter interactions, transporter-mediated drug-drug interactions, and transporter-mediated toxicity. The drug binding site of ABC transporters is accessible from the cytoplasm or the inner leaflet of the plasma membrane. Therefore, vesicular transport assays utilizing inside-out vesicles are commonly used assays, where the directionality of transport results in drugs being transported into the vesicle. Monolayer assays utilizing polarized cells expressing efflux transporters are the test systems suggested by regulatory agencies. However, in some monolayers, uptake transporters must be coexpressed with efflux transporters to assure detectable transport of low passive permeability drugs. For uptake transporters mediating cellular drug uptake, utilization of stable transfectants have been suggested. In vivo animal models complete the testing battery. Some issues, such as in vivo relevance, gender difference, age and ontogeny issues can only be addressed using in vivo models. Transporter specificity is provided by using knock-out or mutant models. Alternatively, chemical knock-outs can be employed. Compensatory changes are less likely when using chemical knock-outs. On the other hand, specific inhibitors for some uptake transporters are not available, limiting the options to genetic knock-outs.
- Citation: Krajcsi P. Drug-transporter interaction testing in drug discovery and development. World J Pharmacol 2013; 2(1): 35-46
- URL: https://www.wjgnet.com/2220-3192/full/v2/i1/35.htm
- DOI: https://dx.doi.org/10.5497/wjp.v2.i1.35
The human body harbors a number of physiological barriers. From an oral drug administration point of view the intestinal, hepatic, renal and blood-brain barriers are considered pivotal.
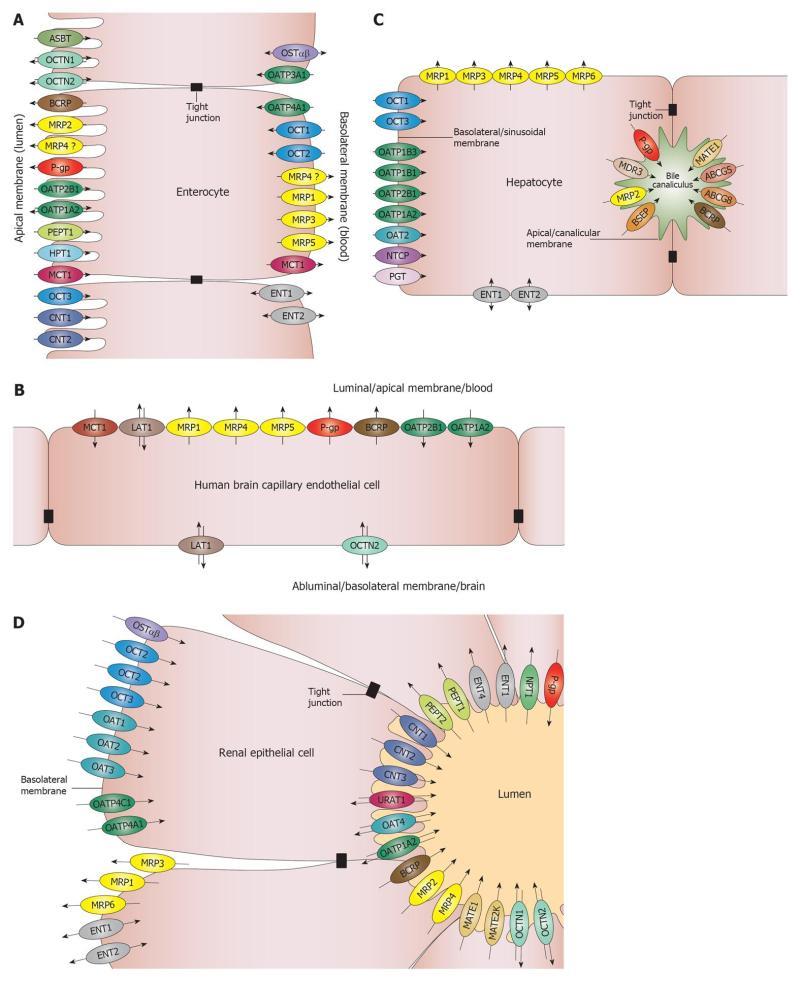
The intestinal barrier is the site of absorption of orally administered drugs. The main cellular components of the intestinal barrier are the enterocytes. Generally, the small intestine is considered of utmost importance. The large surface area and the stomach-proximal position make the small intestine the site of absorption of many oral drugs. With the development of controlled release formulations, more and more studies are concerned with absorption through the colon. The activity of several metabolic enzymes is lower in the colon than in the small intestine[1,2] making the colon an attractive site for absorption. The regional transporter expression data from several papers are inconclusive. The only consensus is that there is significantly higher expression of P-glycoprotein (P-gp)/multidrug resistance protein 1 (ABCB1) in the colon compared to the small intestine, and higher expression of multidrug resistance associated protein 2 (MRP2, ABCC2) in the small intestine compared to the colon[1,3]. Transporters that are expressed in the enterocytes are depicted in Figure 1A. The only transporters that are highly expressed in the intestine and are on the shortlists of both the Food and Drug Administration (FDA)[4] and the European Medicines Agency (EMA)[5] are the apically located P-gp[6] and breast cancer resistance protein (BCRP, ABCG2). These transporters are known to transport many xenobiotics and, therefore, constitute a barrier for drug absorption via the intestines.
Two major interfaces connecting the blood and brain compartments are the blood-brain barrier (BBB) and the blood-cerebrospinal fluid barrier (BCSFB). The BBB is by far the more important barrier, as the surface area of the human BBB is approximately 100-fold larger than the surface area of the BCSFB[7,8]. In addition, the distance between neurons and brain capillaries is less than 20 nm in the BBB while the distance between the brain ventricles and circumventricular organs is in millimeter or centimeter range in the BCSFB[9]. The barrier function in the BBB is provided by the microcapillary endothelial cells that contain no fenestrations. Transporters that are expressed in the brain microcapillary endothelial cells are depicted in Figure 1B. Similar to the intestinal barrier, the two transporters on the list of regulatory agencies are the luminally located P-gp and BCRP, indicating that, from a drug development point of view, the BBB mainly functions as a barrier for drug absorption.
The hepatic barrier is the major site of excretion of drugs and drug metabolites. The transporters that are expressed in the parenchymal cells (hepatocytes) are depicted in Figure 1C. The hepatic transporters on the FDA short list are uptake transporters of the organic anion transporting polypeptide (OATP)/Solute Carrier OATP (SLCO) family members rganic anion transporting polypeptide 1B1 (OATP1B1)/SLCO1B1 and OATP1B3 (SLCO1B3), and efflux transporters P-gp and BCRP. The EMA short list adds three additional hepatic transporters: organic cation transporter 1 (OCT1, SLC22A1), bile salt export pump (BSEP, ABCB11) and multidrug and toxin extrusion protein 1 (MATE1, SLC47A1). BSEP transports bile salts and, therefore, has toxicological significance. Noticeably, missing from both lists is MRP2 (ABCC2), a transporter on the canalicular membrane, which transports many drugs and phase II drug metabolites into the bile. The vectorial summation of the activity of the sinusoidal/basolateral uptake transporters and canalicular/apical efflux transporters drives the secretory function of this barrier.
The renal barrier is the other major site of excretion. The main cellular components of the renal secretory transport are the proximal tubule epithelial cells (PTC). The transporters that are expressed in the PTC are shown in Figure 1D. The renal transporters on the FDA short list are basolateral uptake transporters OCT2 (SLC22A2), OAT1 (SLC22A6), OAT3 (SLC22A8) and apical efflux transporters P-gp and BCRP. The EMA guidance also refers to MATE1 and MATE2/MATE2K (SLC47A2) as transporters that should be considered. This arrangement is similar to the hepatocyte, suggesting that the PTC mainly work in a secretory fashion as well. It should be noted that although significant xenobiotic reuptake occurs through PTC, literature data mainly focus on reuptake of physiological substrates.
In general, the transporters listed above have been shown to play a role in ADMET (Absorption-Distribution-Metabolism-Excretion-Toxicity) of drugs. However, regulatory guidances[4] note that additional transporters (e.g., MRPs) should be considered when relevant for the therapeutic class of drug being studied.
In the pharmaceutical industry transcellular permeation of drugs has been viewed as the combination of passive and/or transporter-mediated processes[10]. Sequencing of the human genome yielded 883 putative transporter genes[11]. The suggested number of two main superfamilies of human membrane transporters, the ATP-binding cassette (ABC) transporters, mediating mainly cellular efflux, and the solute carriers (SLC), mediating mainly cellular uptake of their substrates, is well over 400[12]. It is likely that any particular cell may express dozens of transporters. Because of the large number of transporters and the broad substrate specificity of many of transporters, as well as the energetically unfavorable transbilayer permeation of small charged molecules, it has been suggested that drug transport is essentially carrier mediated[13]. It has been hypothesized that lack of saturation of transcellular permeation of some drugs, which is considered by many as the proof of passive diffusion[14], is the result of transport by a series of transporters with different affinities[13]. It has also been argued that lack of stereospecificity in permeability of some drugs can be attributed to the broad substrate specificity of transporters[15]. Correlation of apparent permeability coefficients (Papp) for the same drug across different cell lines is a focus of the debate[10,13]. However, multiple drugs show identical Papp values in A > B and B > A direction when the known transporters are blocked[16-18]. As in polarized monolayers the transporter expression and activity on the basolateral and apical membranes are likely different these observations require explanation. Even the simplest models used to extract kinetic parameters of transcellular transport of drugs require extensive computation[16,19-23]. Therefore, the consideration of multiple transporters may be a challenging concept to develop into a generally accepted model for use by the pharmaceutical industry.
The vast majority of drugs are effluxed by ABC transporters. Other important transporters include members of the MATE/SLC47 and the equilibrative nucleoside transporter (SLC29) families. In addition, efflux action by SLCO[24,25] and OCT novel (SLC22A4-5) family members[26,27] has been suggested. From a pharmacological point of view the main function of MATE transporters is drug efflux. However, based on their classification as an SLC, as well as the predominant assay format (cellular uptake), these transporters will be discussed among uptake transporters. The list of the ABC and SLC transporters identified by the regulatory agencies as of special importance is shown in Table 1.
| Transporter | Expression (tissue/cell type/localization) | Physiological substrates | Select drug substrates | Guidance |
| P-gp | Brain/endothelial cell/apical | Phospholipids, cytokines, steroids | Aliskiren, ambrisentan, colchicine, dabigatran etexilate, digoxin, everolimus, fexofenadine, imatinib, indinavir, itraconazole, lapatinib, maraviroc, nilotinib, paclitaxel, posaconazole, ranolazine, saxagliptin, sirolimus, sitagliptin, talinolol, tolvaptan, topotecan, vinca alkaloids | FDA/ EMA |
| Kidney/epithelial cell/apical | ||||
| Liver/hepatocyte/canalicular | ||||
| Small intestine/enterocyte/apical | ||||
| (colon) | ||||
| BCRP | Brain/endothelial cell/apical | Vitamins (riboflavin, biotin), porphyrins, estrogen sulfate conjugates | Methotrexate, mitoxantrone, daunorubicin, doxorubicin, imatinib, irrinotecan, lapatinib, rosuvastatin, pitavastatin, provastatin, sulfasalazine, topotecan | FDA/ EMA |
| Liver/hepatocyte/canalicular | ||||
| Small intestine/enterocyte/apical | ||||
| Kidney/epithelial cell/apical | ||||
| Placenta/syncytiotrophoblast/apical (maternal) | ||||
| BSEP | Liver/hepatocyte/canalicular | Taurocholate, glycocholate | Pravastatin, paclitaxel, vinblastine | EMA |
| OATP1B1 | Liver/hepatocyte/basolateral | Bilirubin and its conjugates, thyroxin, triiodothyronine, bile acids, eicosanoids (thromboxane B2, prostaglandin E2, leukotriene C4), dehydroepiandrosterone sulfate, estradiol 17β-glucuronide, estrone 3-sulfate, glycocholate | Atrasentan, atorvastatin, bosentan, ezetimibe, fluvastatin, glyburide, methotrexate, olmesartan, pitavastatin, pravastatin, repaglinide, rifampin, rosuvastatin, simvastatin acid, SN-38 (active metabolite of irinotecan), valsartan | FDA/ EMA |
| OATP1B3 | Liver/hepatocyte/basolateral | Estradiol 17β-glucuronide, taurocholate, estrone 3-sulfate, dehydroepiandrosterone sulfate, thyoxin | Atorvastatin, bosentan, digoxin, methotrexate, olmesartan, paclitaxel, pitavastatin, rosuvastatin, telmisartan, valsartan | FDA/ EMA |
| OAT1 | Kidney/proximal tubular cell/basolateral | Para-aminohippuric acid, homocysteine, Cysteine,dicarboxylates, prostaglandine E2, urate, estrone-3-sulfate | Adefovir, captopril, cidofovir, furosemide, lamivudine, methotrexate, oseltamivir, tenofovir, zalcitabine, zidovudine | FDA/ EMA |
| OAT3 | Kidney/proximal tubular cell/basolateral | Estrone 3-sulfate, estradiol 17β-glucoronide, cAMP, taurocholate, cortisol, dehydroepiandrosterone sulfate, prostaglandine E2, urate, succinate, para-aminohippuric acid | Acyclovir, bumetanide, ciprofloxacin, famotidine, furosemide, methotrexate, oseltamivir acid, (the active metabolite of oseltamivir), penicillin G, pravastatin, rosuvastatin, sitagliptin, valacyclovir, zidovudine | FDA/ EMA |
| 1-Oct | Liver/hepatocyte/basolateral | Corticosterone, β-oestradiol, progesterone, testosterone, choline, creatinine, guanidine, L-carnitine, thiamine, thyramine, acetylcholine, dopamine | Acyclovir, amantadine, gancyclovir, imatinib, lamivudin, metformin, oxaliplatin, quinidine, quinine, ranitidine, zalcitabine | EMA |
| Small intestine/enterocyte/basolateral | ||||
| 2-Oct | Kidney/epithelial cell/ basolateral | β-oestradiol, progesterone, testosterone, choline, creatinine, guanidine, L-carnitine, acetylcholine, dopamine, epinephrine, norepinephrine, histamin, serotonin, choline, dopamine, prostagladine E2 | Amantadine, amilorid, cimetidine, cisplatin, dofetilide, famotidine, lamivudin, metformin, oxaliplatin, pindolol, procainamide, ranitidine, zalcitabine | FDA/ EMA |
| MATE1 | Kidney/epithelial cell/apical | Choline, creatinine, guanidine, corticosterone, estrone 3-sulfate, thiamine | Acyclovir, cimetidine, fexofenadine, gancyclovir, metformin, procainamide, topotecan | EMA |
| Liver/hepatocyte/canalicular | ||||
| MATE2/MATE2K | Kidney/epithelial cell/apical | Choline, creatinine, guanidine, corticosterone, estrone 3-sulfate, thiamine | Acyclovir, cimetidine, gancyclovir, metformin, procainamide, topotecan | EMA |
Both membrane-based assays and cellular assays are widely used to test drug transport and drug-drug interactions by ABC efflux transporters. Membrane assays include ATPase and vesicular transport (VT) assays[28]. ATPase assays are based on coupling of ATPase activity to transport and can be considered as surrogate transport assays. VT assays utilize inside-out vesicles and measure accumulation of substrates into the vesicles. Cell-based assays include monolayer efflux assays, cytotoxicity assays, cellular accumulation and efflux assays as well as dye efflux assays. Monolayer efflux assays monitor transcellular transport of substrates and measure the vectorial contribution of transporters. Monolayer efflux assays can be performed in a bidirectional mode or in a unidirectional mode in the presence and absence of an inhibitor. Cytotoxicity assays are mostly used to measure efflux transporter mediated drug resistance[29,30] which can be reversed by a transporter specific inhibitor. It is assumed that efflux transporters inhibit accumulation, hence, efficacy of substrate chemotherapeutics. Thus, the assay is a surrogate transport assay. Cell accumulation and efflux assays are performed in cells overexpressing the transporter. The most common setup involves accumulation in the presence and absence of a specific inhibitor. In cellular efflux assays, after the initial loading, substrate efflux is measured in the presence and absence of specific inhibitors and cell associated drug content is plotted as the percentage of drug remaining in the cells vs time[31]. With the exception of reversal agent development neither cellular accumulation nor cellular efflux assays are commonly performed in drug ADME studies. Dye efflux assays monitor efflux activity of transporters using fluorescent probe substrates or non-fluorescent precursor probes[29]. The Calcein assay is the prototype of dye efflux assays which use non-fluorescent dyes as probes[29]. The non-fluorescent calcein-AM, which is a substrate for both P-gp[32] and MRP1[33], is cleaved by intracellular esterases to yield the fluorescent calcein, which is a substrate for MRP1, but not for P-gp[34]. Calcein is hydrophilic and will not diffuse out of the cells, therefore it accumulates at a slower rate in P-gp or MRP1 overexpressing cells compared to control cells, unless the transporters are inhibited. The advantage of using a non-fluorescent substrate is that it can be conveniently performed in high throughput without the need of a fluorescence activated cell sorter or extensive washing. Dye efflux assays are commonly performed as inhibition assays[35] applicable to various cell types and, therefore, can be done in a tissue/cell type specific manner[36].
Two large studies correlated P-gp ATPase and P-gp monolayer efflux measurements[37,38]. Both studies found that a group of high passive permeability substrates that were efficacious ATPase activators did not appear to be P-gp substrates in the monolayer assay. The likely explanation is that the contribution of the transporter to the overall permeability of these compounds is negligible. These compounds were then termed as non-transported substrates[37]. However, several of these compounds such as verapamil[37], ketoconazole[37] and itraconazole (Fekete et al: manuscript in preparation) have shown P-gp dependent BBB permeability in humans[39] and mice[40,41]. Due to their high passive permeability, none of the cellular or other vesicular assays would work for these compounds. Therefore, for this group of ABC transporter substrates the ATPase assay is the only assay that predicts a P-gp limited penetration of the BBB.
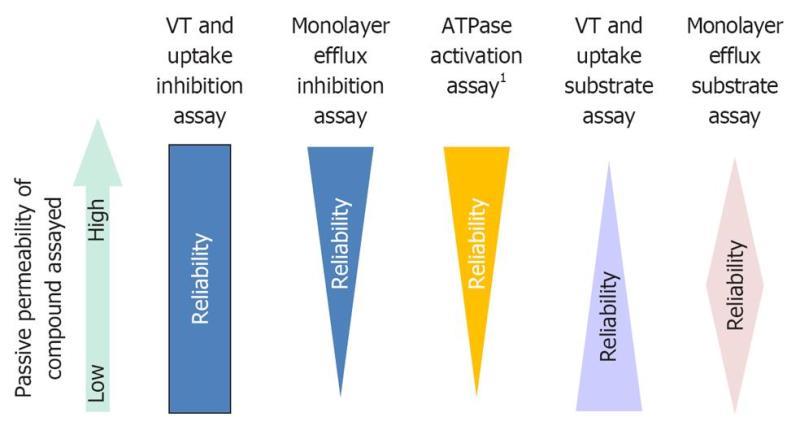
Passive permeability is a key determinant in assay selection. For example, low passive permeability compounds may be false negatives in P-gp ATPase activation assays[37]. VT/uptake assays work best for low passive permeability compounds[28]. For low and intermediate passive permeability compounds monolayer assays work well, although, for some low passive permeability compounds, an uptake transporter is required for significant transcellular transport[42]. Passive permeability does not play a role in membrane assays when used in an inhibition format. However, monolayer assays will not necessarily work for low passive permeability inhibitors. The effect of passive permeability on assay selection is depicted in Figure 2.
Membrane lipid composition is also an important determinant of transporter activity. BCRP[43-45] and BSEP[46,47] activity is significantly greater in mammalian or cholesterol enriched insect cell membranes than in native insect cell membranes, which contain significantly lower amounts of cholesterol[44], and both BCRP[48] and BSEP[49] are localized in cholesterol rich microdomains. Interestingly, perhaps with the exception of the cyclosporin A-BSEP interaction, cholesterol loading did not affect IC50 data[43,44,46]. All in all, these data show that the best and certainly the most relevant expression systems are the mammalian/human cells.
Transporter expression levels may affect apparent ADME parameters. Apparent KM values generated in monolayer assays displayed a linear correlation with P-gp expression[50]. In contrast, the intrinsic KM values that were based on intracellular concentrations showed independence from transporter expression[19,22]. Some IC50 values were also shown to depend on transporter expression with increasing values in higher expressers[36,51]. The phenomenon was predicted by simulations[21] and appears to have in vivo relevance[52,53]. The simulation study also predicted that in a VT system steady-state is established in seconds, as no permeability barriers exist[21].
The monolayer assay system is the suggested assay format for efflux transporter substrate and inhibition assays[4,5]. The advantage of the system is that it shows if the contribution of an efflux transporter is comparable in magnitude to passive permeability and, thus, modulates transcellular permeability of substrate drugs. However, in some aspects VT substrate and inhibition assays offer advantages over the monolayer assays, as data obtained in VT assays are not confounded by permeability barriers. Along this line, in earlier publications VT inhibition assays have been suggested as drug-drug interaction assays for low passive permeability drugs[54].
Digoxin is the consensus substrate for P-gp[4] and PSC833 is a commercially available P-gp specific inhibitor[55]. Dabigatran etexilate[56-58] or fexofenadine[58-60] could also be considered as probes as these are lower bioavailability substrates and are potential probes for clinical drug-drug interaction studies. However, only fexofenadine has been extensively studied in vitro[59,60]. Quinidine is an acceptable alternative to digoxin in microdialysis experiments where application of digoxin is not feasible due to non-specific adherence to tubing as well as toxicity[55,60]. No consensus has been reached on the probe substrates and inhibitors for BCRP. Topotecan[4,61], rosuvastatin[4], prazosin[44,62] and sulfasalazine[63,64] have all been suggested. However, these compounds are substrates of multiple efflux transporters that are co-expressed with BCRP on apical membranes. On the contrary, chlorothiazide, a non-metabolized[65], low bioavailability drug[66] is a specific BCRP substrate[67] and a potential probe. Ko134 and Ko143 have been extensively used in preclinical studies as BCRP-specific inhibitors. For BSEP, taurocholate is the consensus probe[46,68,69] and cyclosporin A, a cholestatic drug[70] is the reference inhibitor used most often[46,68,69,71,72]. Potential ABC transporter probe substrates are listed in Table 1.
Cellular uptake of drugs and endobiotics is mediated via uptake transporters of the SLC superfamily. The list of the uptake transporters identified by the regulatory agencies as important is shown in Table 1. Mechanistically these transporters are uniporters (e.g., OCT1), symporters [e.g., sodium taurocholate cotransporting polypeptide (NTCP, SLC10A1), peptide transporter 1 (PEPT1, SLC15A1)] or antiporters (e.g., OATP1B1, OAT1, MATE1).
Membrane assays are applicable to symporters where the driving force of the transport is known and the assay set-up is straightforward. Na+-taurocholate cotransporting polypeptide (NTCP)-mediated taurocholate transport into right-side out (ROV) rat sinusoidal membrane vesicles has been shown[73]. Proton gradient driven dipeptide transport into ROV prepared from intestinal brush-border membranes has also been published[74]. For exchangers (e.g., OATPs, OATs) a vesicular uptake assay would be cumbersome to perform even if the identity of the exchange ion was known.
The most common assay system for uptake transporters are primary cells [e.g., hepatocytes, brain microcapillary endothelial cells (BME), proximal tubule cells (PTC) of the kidney], cancer cell lines (e.g., Caco-2), immortalized cell lines (e.g., human brain endothelial cell line, hCMEC/D3) or transfectants. Transfectants are the test systems recommended by regulatory agencies[4].
Oocytes microinjected with the mRNA or cDNA of the respective transporter have been used early on. Oocytes offer the option of electrophysiological measurements as the transport of many substrates is electrogenic. However, the system is transient, the quality of the oocytes display seasonal variations, the lipid composition of the plasma membrane is different from physiological and the throughput is low-to-intermediate[75,76].
For uptake transporters brain slices[77], liver slices[78,79] and kidney slices[80,81] are commonly used to compute clearance values.
Uptake transporters have highly overlapping substrate specificities and multiple family members are expressed in the same cell type. Quantification of contribution of the different transporters is a challenge. OATP1B1 and OATP1B3 have very similar substrate specificities and are both expressed in hepatocytes. Estrone-3 sulfate and cholecystokinine octapeptide (CCK-8) are selective substrates of OATP1B1 and OATP1B3, respectively, and can be used as reference substrates to determine activities of these transporters in a hepatocyte preparation[82]. The most notable non-statin drugs are bosentan, a substrate of OATP1B1[83] and OATP1B3[83], valsartan[84] or repaglinide[85], substrates of OATP1B1, and telmisartan[86] or nafcilin[87], substrates of OATP1B3. Fluo-3 is a highly sensitive fluorescent probe of OATP1B3[88]. Rifampin and cyclosporin A are the recommended reference inhibitors[4] however various statins are also commonly used[89]. For clinical drug-drug interaction studies the use of statins as victims/probes has been suggested[4]. OAT1 and OAT3 are co-expressed in the basolateral membrane of PTC. These transporters have overlapping substrate specificities, with OAT3 having a bias for amphiphilic, larger molecular weight compounds[90]. Adefovir can be used as a reference substrate for OAT1 and benzylpenicillin for OAT3[80]. Tenofovir[91], azydothimidine/zidovudine[92], para-amino-hippurate[4] for OAT1 and methotrexate[93], cimetidine[94], furosemide[95], estrone-3-sulfate[4] for OAT3 are also applicable. Probenecid inhibits both transporters[4] but benzylpenicilline is considered an OAT3-specific inhibitor[80,96]. P-aminohippurate has been used as a specific OAT1 inhibitor[80] and also as inhibitor of both transporters[96]. For OCT1[97,98] and OCT2[98-100] metformin is an accepted drug substrate. Alternatively, 1-methyl-4-phenylpyridinium (MPP+) and cimetidine can be used for both OCT1[101,102] and OCT2[4,103]. Cimetidine or verapamil can be used as an OCT1[98,104] and OCT2 inhibitor[4,98,104], although, clinical relevance of cimetidine mediated inhibition of OCT2 has been questioned lately[105]. Metformin is a relevant substrate for both MATE1[106] and MATE2/MATE2K[106] and cimetidine[105] and verapamil[104] are potent inhibitors. Importantly, pyrimethamine has been shown to selectively inhibit MATE1 and MATE2/MATE2K[105,107].
Uptake transporters play a major role in pharmakokinetics of substrate drugs. Inhibition of hepatic[108] and/or renal[109] clearance by co-administered drugs can lead to clinically significant drug-drug interactions. Interactions of the hepatic uptake transporters often result in > 5-fold increase in Cmax values of victim drugs[110]. Nevertheless, most in vitro assays commonly employ either physiological substrates such as estrone-3-sulfate or estradiol-17β-glucuronide for anion transporters or synthetic non-drug substrates, such as tetraethyl-ammonium for cation transporters[89]. Broad-scale application of LC/MS/MS methodology in drug quantification will facilitate revalidation of uptake transporter assays using drug probes.
In vivo studies using knock-out and mutant animals shows the paramount importance of transporters[61,76,111]. Obviously, in vivo significance of a transporter in clearance of a drug can only be addressed by in vivo studies[61]. Other important applications, such as gender difference, as well as age and ontogeny are also preferably studied in vivo[112-114]. With the availability of double and triple knockouts, transporter complementation[115] and transporter-enzyme interplay[116] can now be addressed. Nevertheless, utilization of knockouts is perhaps not as extensive as originally envisioned. Compensatory changes may mask the effect of transporter deletion. P-gp is upregulated in Bsep knockout mice and the metabolism of bile acids is altered as well[117,118]. Cytochrome P450 enzymes which share substrate specificity with P-gp are dramatically up-regulated in P-gp knockout mice in a gender specific manner[119]. Species specificity issues also limit utilization of these models by the pharmaceutical industry. In addition to differences in substrate specificities[120], significant differences have been observed in transporter expression between species. Canalicular expression of MRP2/Mrp2 is about 10-fold greater in rodents than in humans[121] and the ratio of BCRP/P-gp expression in the BBB is about 4-fold greater in humans than it is mice[122]. Chemical knockouts can circumvent the problems stemming from compensatory changes. However, species specificity issues can only be overcome by utilization of humanized models. As the availability of humanized models increases, the relevance of in vivo studies will certainly increase as well[123-125].
In the past decade utilization of transporter assays by the pharmaceutical industry has been rapidly growing. Lower activity pharmacogenomic variants such as BCRP 412G>A[126] and OATP1B1 521 >C[85] make it possible to show the impact of the wild type transporters on human pharmacokinetics of substrate drugs and clearly demonstrate clinical relevance of drug-transporter interactions.
Special thanks to Dr. Katalin Tauberné Jakab and Ms Timea Rosta for their help in preparation of the manuscript.
P- Reviewer Dahan A S- Editor Song XX L- Editor A E- Editor Zheng XM
| 1. | Berggren S, Gall C, Wollnitz N, Ekelund M, Karlbom U, Hoogstraate J, Schrenk D, Lennernäs H. Gene and protein expression of P-glycoprotein, MRP1, MRP2, and CYP3A4 in the small and large human intestine. Mol Pharm. 2007;4:252-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 2. | Lin JH, Chiba M, Baillie TA. Is the role of the small intestine in first-pass metabolism overemphasized. Pharmacol Rev. 1999;51:135-158. [PubMed] [Cited in This Article: ] |
| 3. | Dahan A, Amidon GL. Segmental dependent transport of low permeability compounds along the small intestine due to P-glycoprotein: the role of efflux transport in the oral absorption of BCS class III drugs. Mol Pharm. 2009;6:19-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | U.S. Food and Drug Administration. Guidance for Industry. 2012. Available form: URL: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM292362.pdf. [Cited in This Article: ] |
| 5. | European Medicines Agency. Guideline on the Investigation of Drug Interactions. 2010. Available form: URL: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/05/WC500090112.pdf. [Cited in This Article: ] |
| 6. | Dahan A, Lennernäs H, Amidon GL. The fraction dose absorbed, in humans, and high jejunal human permeability relationship. Mol Pharm. 2012;9:1847-1851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Keep RF, Jones HC. A morphometric study on the development of the lateral ventricle choroid plexus, choroid plexus capillaries and ventricular ependyma in the rat. Brain Res Dev Brain Res. 1990;56:47-53. [PubMed] [Cited in This Article: ] |
| 8. | Pardridge WM. Drug and gene targeting to the brain with molecular Trojan horses. Nat Rev Drug Discov. 2002;1:131-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 299] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 9. | Schlageter KE, Molnar P, Lapin GD, Groothuis DR. Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvasc Res. 1999;58:312-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 195] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Sugano K, Kansy M, Artursson P, Avdeef A, Bendels S, Di L, Ecker GF, Faller B, Fischer H, Gerebtzoff G. Coexistence of passive and carrier-mediated processes in drug transport. Nat Rev Drug Discov. 2010;9:597-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 429] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 11. | Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian AE, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman TJ, Higgins ME, Ji RR, Ke Z, Ketchum KA, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov GV, Milshina N, Moore HM, Naik AK, Narayan VA, Neelam B, Nusskern D, Rusch DB, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C, Yao A, Ye J, Zhan M, Zhang W, Zhang H, Zhao Q, Zheng L, Zhong F, Zhong W, Zhu S, Zhao S, Gilbert D, Baumhueter S, Spier G, Carter C, Cravchik A, Woodage T, Ali F, An H, Awe A, Baldwin D, Baden H, Barnstead M, Barrow I, Beeson K, Busam D, Carver A, Center A, Cheng ML, Curry L, Danaher S, Davenport L, Desilets R, Dietz S, Dodson K, Doup L, Ferriera S, Garg N, Gluecksmann A, Hart B, Haynes J, Haynes C, Heiner C, Hladun S, Hostin D, Houck J, Howland T, Ibegwam C, Johnson J, Kalush F, Kline L, Koduru S, Love A, Mann F, May D, McCawley S, McIntosh T, McMullen I, Moy M, Moy L, Murphy B, Nelson K, Pfannkoch C, Pratts E, Puri V, Qureshi H, Reardon M, Rodriguez R, Rogers YH, Romblad D, Ruhfel B, Scott R, Sitter C, Smallwood M, Stewart E, Strong R, Suh E, Thomas R, Tint NN, Tse S, Vech C, Wang G, Wetter J, Williams S, Williams M, Windsor S, Winn-Deen E, Wolfe K, Zaveri J, Zaveri K, Abril JF, Guigó R, Campbell MJ, Sjolander KV, Karlak B, Kejariwal A, Mi H, Lazareva B, Hatton T, Narechania A, Diemer K, Muruganujan A, Guo N, Sato S, Bafna V, Istrail S, Lippert R, Schwartz R, Walenz B, Yooseph S, Allen D, Basu A, Baxendale J, Blick L, Caminha M, Carnes-Stine J, Caulk P, Chiang YH, Coyne M, Dahlke C, Mays A, Dombroski M, Donnelly M, Ely D, Esparham S, Fosler C, Gire H, Glanowski S, Glasser K, Glodek A, Gorokhov M, Graham K, Gropman B, Harris M, Heil J, Henderson S, Hoover J, Jennings D, Jordan C, Jordan J, Kasha J, Kagan L, Kraft C, Levitsky A, Lewis M, Liu X, Lopez J, Ma D, Majoros W, McDaniel J, Murphy S, Newman M, Nguyen T, Nguyen N, Nodell M, Pan S, Peck J, Peterson M, Rowe W, Sanders R, Scott J, Simpson M, Smith T, Sprague A, Stockwell T, Turner R, Venter E, Wang M, Wen M, Wu D, Wu M, Xia A, Zandieh A, Zhu X. The sequence of the human genome. Science. 2001;291:1304-1351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9175] [Cited by in F6Publishing: 7670] [Article Influence: 333.5] [Reference Citation Analysis (0)] |
| 12. |
|
| 13. | Kell DB, Dobson PD, Oliver SG. Pharmaceutical drug transport: the issues and the implications that it is essentially carrier-mediated only. Drug Discov Today. 2011;16:704-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Camenisch G, Folkers G, van de Waterbeemd H. Review of theoretical passive drug absorption models: historical background, recent developments and limitations. Pharm Acta Helv. 1996;71:309-327. [PubMed] [Cited in This Article: ] |
| 15. | Dobson PD, Lanthaler K, Oliver SG, Kell DB. Implications of the dominant role of transporters in drug uptake by cells. Curr Top Med Chem. 2009;9:163-181. [PubMed] [Cited in This Article: ] |
| 16. | Tran TT, Mittal A, Gales T, Maleeff B, Aldinger T, Polli JW, Ayrton A, Ellens H, Bentz J. Exact kinetic analysis of passive transport across a polarized confluent MDCK cell monolayer modeled as a single barrier. J Pharm Sci. 2004;93:2108-2123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Troutman MD, Thakker DR. Novel experimental parameters to quantify the modulation of absorptive and secretory transport of compounds by P-glycoprotein in cell culture models of intestinal epithelium. Pharm Res. 2003;20:1210-1224. [PubMed] [Cited in This Article: ] |
| 18. | Troutman MD, Thakker DR. Efflux ratio cannot assess P-glycoprotein-mediated attenuation of absorptive transport: asymmetric effect of P-glycoprotein on absorptive and secretory transport across Caco-2 cell monolayers. Pharm Res. 2003;20:1200-1209. [PubMed] [Cited in This Article: ] |
| 19. | Heikkinen AT, Korjamo T, Lepikkö V, Mönkkönen J. Effects of experimental setup on the apparent concentration dependency of active efflux transport in in vitro cell permeation experiments. Mol Pharm. 2010;7:605-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Kalvass JC, Pollack GM. Kinetic considerations for the quantitative assessment of efflux activity and inhibition: implications for understanding and predicting the effects of efflux inhibition. Pharm Res. 2007;24:265-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Lumen AA, Acharya P, Polli JW, Ayrton A, Ellens H, Bentz J. If the KI is defined by the free energy of binding to P-glycoprotein, which kinetic parameters define the IC50 for the Madin-Darby canine kidney II cell line overexpressing human multidrug resistance 1 confluent cell monolayer. Drug Metab Dispos. 2010;38:260-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Tachibana T, Kitamura S, Kato M, Mitsui T, Shirasaka Y, Yamashita S, Sugiyama Y. Model analysis of the concentration-dependent permeability of P-gp substrates. Pharm Res. 2010;27:442-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Tran TT, Mittal A, Aldinger T, Polli JW, Ayrton A, Ellens H, Bentz J. The elementary mass action rate constants of P-gp transport for a confluent monolayer of MDCKII-hMDR1 cells. Biophys J. 2005;88:715-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Westholm DE, Salo DR, Viken KJ, Rumbley JN, Anderson GW. The blood-brain barrier thyroxine transporter organic anion-transporting polypeptide 1c1 displays atypical transport kinetics. Endocrinology. 2009;150:5153-5162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Li L, Meier PJ, Ballatori N. Oatp2 mediates bidirectional organic solute transport: a role for intracellular glutathione. Mol Pharmacol. 2000;58:335-340. [PubMed] [Cited in This Article: ] |
| 26. | Yabuuchi H, Tamai I, Nezu J, Sakamoto K, Oku A, Shimane M, Sai Y, Tsuji A. Novel membrane transporter OCTN1 mediates multispecific, bidirectional, and pH-dependent transport of organic cations. J Pharmacol Exp Ther. 1999;289:768-773. [PubMed] [Cited in This Article: ] |
| 27. | Ohashi R, Tamai I, Nezu Ji J, Nikaido H, Hashimoto N, Oku A, Sai Y, Shimane M, Tsuji A. Molecular and physiological evidence for multifunctionality of carnitine/organic cation transporter OCTN2. Mol Pharmacol. 2001;59:358-366. [PubMed] [Cited in This Article: ] |
| 28. | Glavinas H, Méhn D, Jani M, Oosterhuis B, Herédi-Szabó K, Krajcsi P. Utilization of membrane vesicle preparations to study drug-ABC transporter interactions. Expert Opin Drug Metab Toxicol. 2008;4:721-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Ioja EN, Juhász V, Jánossy J, Herédi-Szabó K, Krajcsi P. Solubility, Delivery and ADME Problems of Drugs and Drug Candidates. Bentham Science. 2011;102-116. [Cited in This Article: ] |
| 30. | Kis E, Nagy T, Jani M, Molnár E, Jánossy J, Ujhellyi O, Német K, Herédi-Szabó K, Krajcsi P. Leflunomide and its metabolite A771726 are high affinity substrates of BCRP: implications for drug resistance. Ann Rheum Dis. 2009;68:1201-1207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Ding PR, Tiwari AK, Ohnuma S, Lee JW, An X, Dai CL, Lu QS, Singh S, Yang DH, Talele TT. The phosphodiesterase-5 inhibitor vardenafil is a potent inhibitor of ABCB1/P-glycoprotein transporter. PLoS One. 2011;6:e19329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Homolya L, Holló Z, Germann UA, Pastan I, Gottesman MM, Sarkadi B. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J Biol Chem. 1993;268:21493-21496. [PubMed] [Cited in This Article: ] |
| 33. | Holló Z, Homolya L, Hegedüs T, Sarkadi B. Transport properties of the multidrug resistance-associated protein (MRP) in human tumour cells. FEBS Lett. 1996;383:99-104. [PubMed] [Cited in This Article: ] |
| 34. | Feller N, Broxterman HJ, Währer DC, Pinedo HM. ATP-dependent efflux of calcein by the multidrug resistance protein (MRP): no inhibition by intracellular glutathione depletion. FEBS Lett. 1995;368:385-388. [PubMed] [Cited in This Article: ] |
| 35. | Cook JA, Feng B, Fenner KS, Kempshall S, Liu R, Rotter C, Smith DA, Troutman MD, Ullah M, Lee CA. Refining the in vitro and in vivo critical parameters for P-glycoprotein, [I]/IC50 and [I2]/IC50, that allow for the exclusion of drug candidates from clinical digoxin interaction studies. Mol Pharm. 2010;7:398-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Glavinas H, von Richter O, Vojnits K, Mehn D, Wilhelm I, Nagy T, Janossy J, Krizbai I, Couraud P, Krajcsi P. Calcein assay: a high-throughput method to assess P-gp inhibition. Xenobiotica. 2011;41:712-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, Serabjit-Singh CS. Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther. 2001;299:620-628. [PubMed] [Cited in This Article: ] |
| 38. | von Richter O, Glavinas H, Krajcsi P, Liehner S, Siewert B, Zech K. A novel screening strategy to identify ABCB1 substrates and inhibitors. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:11-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Takano A, Kusuhara H, Suhara T, Ieiri I, Morimoto T, Lee YJ, Maeda J, Ikoma Y, Ito H, Suzuki K. Evaluation of in vivo P-glycoprotein function at the blood-brain barrier among MDR1 gene polymorphisms by using 11C-verapamil. J Nucl Med. 2006;47:1427-1433. [PubMed] [Cited in This Article: ] |
| 40. | Miyama T, Takanaga H, Matsuo H, Yamano K, Yamamoto K, Iga T, Naito M, Tsuruo T, Ishizuka H, Kawahara Y. P-glycoprotein-mediated transport of itraconazole across the blood-brain barrier. Antimicrob Agents Chemother. 1998;42:1738-1744. [PubMed] [Cited in This Article: ] |
| 41. | von Moltke LL, Granda BW, Grassi JM, Perloff MD, Vishnuvardhan D, Greenblatt DJ. Interaction of triazolam and ketoconazole in P-glycoprotein-deficient mice. Drug Metab Dispos. 2004;32:800-804. [PubMed] [Cited in This Article: ] |
| 42. | Bartholomé K, Rius M, Letschert K, Keller D, Timmer J, Keppler D. Data-based mathematical modeling of vectorial transport across double-transfected polarized cells. Drug Metab Dispos. 2007;35:1476-1481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Glavinas H, Kis E, Pál A, Kovács R, Jani M, Vági E, Molnár E, Bánsághi S, Kele Z, Janáky T. ABCG2 (breast cancer resistance protein/mitoxantrone resistance-associated protein) ATPase assay: a useful tool to detect drug-transporter interactions. Drug Metab Dispos. 2007;35:1533-1542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Pál A, Méhn D, Molnár E, Gedey S, Mészáros P, Nagy T, Glavinas H, Janáky T, von Richter O, Báthori G. Cholesterol potentiates ABCG2 activity in a heterologous expression system: improved in vitro model to study function of human ABCG2. J Pharmacol Exp Ther. 2007;321:1085-1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Telbisz A, Müller M, Ozvegy-Laczka C, Homolya L, Szente L, Váradi A, Sarkadi B. Membrane cholesterol selectively modulates the activity of the human ABCG2 multidrug transporter. Biochim Biophys Acta. 2007;1768:2698-2713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 46. | Kis E, Ioja E, Nagy T, Szente L, Herédi-Szabó K, Krajcsi P. Effect of membrane cholesterol on BSEP/Bsep activity: species specificity studies for substrates and inhibitors. Drug Metab Dispos. 2009;37:1878-1886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 47. | Paulusma CC, de Waart DR, Kunne C, Mok KS, Elferink RP. Activity of the bile salt export pump (ABCB11) is critically dependent on canalicular membrane cholesterol content. J Biol Chem. 2009;284:9947-9954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 48. | Storch CH, Ehehalt R, Haefeli WE, Weiss J. Localization of the human breast cancer resistance protein (BCRP/ABCG2) in lipid rafts/caveolae and modulation of its activity by cholesterol in vitro. J Pharmacol Exp Ther. 2007;323:257-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 49. | Ismair MG, Häusler S, Stuermer CA, Guyot C, Meier PJ, Roth J, Stieger B. ABC-transporters are localized in caveolin-1-positive and reggie-1-negative and reggie-2-negative microdomains of the canalicular membrane in rat hepatocytes. Hepatology. 2009;49:1673-1682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Heikkinen AT, Mönkkönen J. Protein concentration and pH affect the apparent P-glycoprotein-ATPase activation kinetics. Int J Pharm. 2008;346:169-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Litman T, Skovsgaard T, Stein WD. Pumping of drugs by P-glycoprotein: a two-step process. J Pharmacol Exp Ther. 2003;307:846-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Choo EF, Kurnik D, Muszkat M, Ohkubo T, Shay SD, Higginbotham JN, Glaeser H, Kim RB, Wood AJ, Wilkinson GR. Differential in vivo sensitivity to inhibition of P-glycoprotein located in lymphocytes, testes, and the blood-brain barrier. J Pharmacol Exp Ther. 2006;317:1012-1018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Kurnik D, Sofowora GG, Donahue JP, Nair UB, Wilkinson GR, Wood AJ, Muszkat M. Tariquidar, a selective P-glycoprotein inhibitor, does not potentiate loperamide’s opioid brain effects in humans despite full inhibition of lymphocyte P-glycoprotein. Anesthesiology. 2008;109:1092-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Zhang L, Zhang YD, Zhao P, Huang SM. Predicting drug-drug interactions: an FDA perspective. AAPS J. 2009;11:300-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 55. | Sziráki I, Erdo F, Beéry E, Molnár PM, Fazakas C, Wilhelm I, Makai I, Kis E, Herédi-Szabó K, Abonyi T. Quinidine as an ABCB1 probe for testing drug interactions at the blood-brain barrier: an in vitro in vivo correlation study. J Biomol Screen. 2011;16:886-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Härtter S, Koenen-Bergmann M, Sharma A, Nehmiz G, Lemke U, Timmer W, Reilly PA. Decrease in the oral bioavailability of dabigatran etexilate after co-medication with rifampicin. Br J Clin Pharmacol. 2012;74:490-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 57. | Härtter S, Sennewald R, Nehmiz G, Reilly P. Oral bioavailability of dabigatran etexilate (Pradaxa(®) ) after co-medication with verapamil in healthy subjects. Br J Clin Pharmacol. 2012;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 58. | European Medicines Agency. Guideline on the Investigation of Drug Interactions. 2012. Available form: URL: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129606.pdf. [Cited in This Article: ] |
| 59. | Crowe A, Wright C. The impact of P-glycoprotein mediated efflux on absorption of 11 sedating and less-sedating antihistamines using Caco-2 monolayers. Xenobiotica. 2012;42:538-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Ma JD, Tsunoda SM, Bertino JS, Trivedi M, Beale KK, Nafziger AN. Evaluation of in vivo P-glycoprotein phenotyping probes: a need for validation. Clin Pharmacokinet. 2010;49:223-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2418] [Cited by in F6Publishing: 2385] [Article Influence: 170.4] [Reference Citation Analysis (0)] |
| 62. | Wright JA, Haslam IS, Coleman T, Simmons NL. Breast cancer resistance protein BCRP (ABCG2)-mediated transepithelial nitrofurantoin secretion and its regulation in human intestinal epithelial (Caco-2) layers. Eur J Pharmacol. 2011;672:70-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Jani M, Szabó P, Kis E, Molnár E, Glavinas H, Krajcsi P. Kinetic characterization of sulfasalazine transport by human ATP-binding cassette G2. Biol Pharm Bull. 2009;32:497-499. [PubMed] [Cited in This Article: ] |
| 64. | Dahan A, Amidon GL. MRP2 mediated drug-drug interaction: indomethacin increases sulfasalazine absorption in the small intestine, potentially decreasing its colonic targeting. Int J Pharm. 2010;386:216-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 65. | Brettell HR, Aikawa JK, Gordon GS. Studies with chlorothiazide tagged with radioactive carbon (C14) in human beings. Arch Intern Med. 1960;106:57-63. [PubMed] [Cited in This Article: ] |
| 66. | Straughn AB, Melikian AP, Meyer MC. Bioavailability of chlorothiazide tablets in humans. J Pharm Sci. 1979;68:1099-1102. [PubMed] [Cited in This Article: ] |
| 67. | Beéry E, Rajnai Z, Abonyi T, Makai I, Bánsághi S, Erdő F, Sziráki I, Herédi-Szabó K, Kis E, Jani M. ABCG2 modulates chlorothiazide permeability in vitro - characterization of the interaction. Drug Metab Pharmacokinet. 2011;Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 68. | Byrne JA, Strautnieks SS, Mieli-Vergani G, Higgins CF, Linton KJ, Thompson RJ. The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology. 2002;123:1649-1658. [PubMed] [Cited in This Article: ] |
| 69. | Noé J, Stieger B, Meier PJ. Functional expression of the canalicular bile salt export pump of human liver. Gastroenterology. 2002;123:1659-1666. [PubMed] [Cited in This Article: ] |
| 70. | Kassianides C, Nussenblatt R, Palestine AG, Mellow SD, Hoofnagle JH. Liver injury from cyclosporine A. Dig Dis Sci. 1990;35:693-697. [PubMed] [Cited in This Article: ] |
| 71. | Ansede JH, Smith WR, Perry CH, St Claire RL, Brouwer KR. An in vitro assay to assess transporter-based cholestatic hepatotoxicity using sandwich-cultured rat hepatocytes. Drug Metab Dispos. 2010;38:276-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 72. | Jemnitz K, Veres Z, Szabo M, Baranyai Z, Jakab F, Vereczkey L. Differential inhibitory effect of cyclosporin A and bosentan on taurocholate uptake in human and rat hepatocytes as a function of culturing time. Toxicol In Vitro. 2012;26:174-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Koopen NR, Wolters H, Müller M, Schippers IJ, Havinga R, Roelofsen H, Vonk RJ, Stieger B, Meier PJ, Kuipers F. Hepatic bile salt flux does not modulate level and activity of the sinusoidal Na+-taurocholate cotransporter (ntcp) in rats. J Hepatol. 1997;27:699-706. [PubMed] [Cited in This Article: ] |
| 74. | Daniel H, Morse EL, Adibi SA. The high and low affinity transport systems for dipeptides in kidney brush border membrane respond differently to alterations in pH gradient and membrane potential. J Biol Chem. 1991;266:19917-19924. [PubMed] [Cited in This Article: ] |
| 75. | Sigel E. Use of Xenopus oocytes for the functional expression of plasma membrane proteins. J Membr Biol. 1990;117:201-221. [PubMed] [Cited in This Article: ] |
| 76. | Xia CQ, Milton MN, Gan LS. Evaluation of drug-transporter interactions using in vitro and in vivo models. Curr Drug Metab. 2007;8:341-363. [PubMed] [Cited in This Article: ] |
| 77. | Miyajima M, Kusuhara H, Fujishima M, Adachi Y, Sugiyama Y. Organic anion transporter 3 mediates the efflux transport of an amphipathic organic anion, dehydroepiandrosterone sulfate, across the blood-brain barrier in mice. Drug Metab Dispos. 2011;39:814-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J Biol Chem. 2002;277:26934-26943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 231] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 79. | Fork C, Bauer T, Golz S, Geerts A, Weiland J, Del Turco D, Schömig E, Gründemann D. OAT2 catalyses efflux of glutamate and uptake of orotic acid. Biochem J. 2011;436:305-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 80. | Nozaki Y, Kusuhara H, Kondo T, Hasegawa M, Shiroyanagi Y, Nakazawa H, Okano T, Sugiyama Y. Characterization of the uptake of organic anion transporter (OAT) 1 and OAT3 substrates by human kidney slices. J Pharmacol Exp Ther. 2007;321:362-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 81. | Watanabe T, Kusuhara H, Maeda K, Kanamaru H, Saito Y, Hu Z, Sugiyama Y. Investigation of the rate-determining process in the hepatic elimination of HMG-CoA reductase inhibitors in rats and humans. Drug Metab Dispos. 2010;38:215-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 82. | Maeda K, Sugiyama Y. The use of hepatocytes to investigate drug uptake transporters. Methods Mol Biol. 2010;640:327-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Treiber A, Schneiter R, Häusler S, Stieger B. Bosentan is a substrate of human OATP1B1 and OATP1B3: inhibition of hepatic uptake as the common mechanism of its interactions with cyclosporin A, rifampicin, and sildenafil. Drug Metab Dispos. 2007;35:1400-1407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 250] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 84. | Maeda K, Ieiri I, Yasuda K, Fujino A, Fujiwara H, Otsubo K, Hirano M, Watanabe T, Kitamura Y, Kusuhara H. Effects of organic anion transporting polypeptide 1B1 haplotype on pharmacokinetics of pravastatin, valsartan, and temocapril. Clin Pharmacol Ther. 2006;79:427-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 85. | Niemi M, Backman JT, Kajosaari LI, Leathart JB, Neuvonen M, Daly AK, Eichelbaum M, Kivistö KT, Neuvonen PJ. Polymorphic organic anion transporting polypeptide 1B1 is a major determinant of repaglinide pharmacokinetics. Clin Pharmacol Ther. 2005;77:468-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 240] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 86. | Ishiguro N, Maeda K, Kishimoto W, Saito A, Harada A, Ebner T, Roth W, Igarashi T, Sugiyama Y. Predominant contribution of OATP1B3 to the hepatic uptake of telmisartan, an angiotensin II receptor antagonist, in humans. Drug Metab Dispos. 2006;34:1109-1115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 87. | Nakakariya M, Shimada T, Irokawa M, Maeda T, Tamai I. Identification and species similarity of OATP transporters responsible for hepatic uptake of beta-lactam antibiotics. Drug Metab Pharmacokinet. 2008;23:347-355. [PubMed] [Cited in This Article: ] |
| 88. | Baldes C, Koenig P, Neumann D, Lenhof HP, Kohlbacher O, Lehr CM. Development of a fluorescence-based assay for screening of modulators of human organic anion transporter 1B3 (OATP1B3). Eur J Pharm Biopharm. 2006;62:39-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Solvo Biotechnology. Available form: URL: http://www.solvo.com/. [Cited in This Article: ] |
| 90. | Feng B, LaPerle JL, Chang G, Varma MV. Renal clearance in drug discovery and development: molecular descriptors, drug transporters and disease state. Expert Opin Drug Metab Toxicol. 2010;6:939-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 91. | Bleasby K, Hall LA, Perry JL, Mohrenweiser HW, Pritchard JB. Functional consequences of single nucleotide polymorphisms in the human organic anion transporter hOAT1 (SLC22A6). J Pharmacol Exp Ther. 2005;314:923-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 92. | Wada S, Tsuda M, Sekine T, Cha SH, Kimura M, Kanai Y, Endou H. Rat multispecific organic anion transporter 1 (rOAT1) transports zidovudine, acyclovir, and other antiviral nucleoside analogs. J Pharmacol Exp Ther. 2000;294:844-849. [PubMed] [Cited in This Article: ] |
| 93. | Uwai Y, Taniguchi R, Motohashi H, Saito H, Okuda M, Inui K. Methotrexate-loxoprofen interaction: involvement of human organic anion transporters hOAT1 and hOAT3. Drug Metab Pharmacokinet. 2004;19:369-374. [PubMed] [Cited in This Article: ] |
| 94. | Erdman AR, Mangravite LM, Urban TJ, Lagpacan LL, Castro RA, de la Cruz M, Chan W, Huang CC, Johns SJ, Kawamoto M. The human organic anion transporter 3 (OAT3; SLC22A8): genetic variation and functional genomics. Am J Physiol Renal Physiol. 2006;290:F905-F912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 95. | Hasannejad H, Takeda M, Taki K, Shin HJ, Babu E, Jutabha P, Khamdang S, Aleboyeh M, Onozato ML, Tojo A. Interactions of human organic anion transporters with diuretics. J Pharmacol Exp Ther. 2004;308:1021-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 96. | Brown CD, Sayer R, Windass AS, Haslam IS, De Broe ME, D’Haese PC, Verhulst A. Characterisation of human tubular cell monolayers as a model of proximal tubular xenobiotic handling. Toxicol Appl Pharmacol. 2008;233:428-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 97. | Bachmakov I, Glaeser H, Fromm MF, König J. Interaction of oral antidiabetic drugs with hepatic uptake transporters: focus on organic anion transporting polypeptides and organic cation transporter 1. Diabetes. 2008;57:1463-1469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 98. | Kimura N, Masuda S, Tanihara Y, Ueo H, Okuda M, Katsura T, Inui K. Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab Pharmacokinet. 2005;20:379-386. [PubMed] [Cited in This Article: ] |
| 99. | Kimura N, Okuda M, Inui K. Metformin transport by renal basolateral organic cation transporter hOCT2. Pharm Res. 2005;22:255-259. [PubMed] [Cited in This Article: ] |
| 100. | Song IS, Shin HJ, Shin JG. Genetic variants of organic cation transporter 2 (OCT2) significantly reduce metformin uptake in oocytes. Xenobiotica. 2008;38:1252-1262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 101. | Martel F, Vetter T, Russ H, Gründemann D, Azevedo I, Koepsell H, Schömig E. Transport of small organic cations in the rat liver. The role of the organic cation transporter OCT1. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:320-326. [PubMed] [Cited in This Article: ] |
| 102. | Umehara KI, Iwatsubo T, Noguchi K, Kamimura H. Functional involvement of organic cation transporter1 (OCT1/Oct1) in the hepatic uptake of organic cations in humans and rats. Xenobiotica. 2007;37:818-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 103. | Tahara H, Kusuhara H, Endou H, Koepsell H, Imaoka T, Fuse E, Sugiyama Y. A species difference in the transport activities of H2 receptor antagonists by rat and human renal organic anion and cation transporters. J Pharmacol Exp Ther. 2005;315:337-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 104. | U.S. Food and Drug Administration. Drug Development and Drug Interactions: Regulatory Guidance and Manual for Policies and Procedures. 2006. Available form: URL: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093606.htm. [Cited in This Article: ] |
| 105. | Ito S, Kusuhara H, Yokochi M, Toyoshima J, Inoue K, Yuasa H, Sugiyama Y. Competitive inhibition of the luminal efflux by multidrug and toxin extrusions, but not basolateral uptake by organic cation transporter 2, is the likely mechanism underlying the pharmacokinetic drug-drug interactions caused by cimetidine in the kidney. J Pharmacol Exp Ther. 2012;340:393-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 106. | Tanihara Y, Masuda S, Sato T, Katsura T, Ogawa O, Inui K. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol. 2007;74:359-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 287] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 107. | Nakamura T, Yonezawa A, Hashimoto S, Katsura T, Inui K. Disruption of multidrug and toxin extrusion MATE1 potentiates cisplatin-induced nephrotoxicity. Biochem Pharmacol. 2010;80:1762-1767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 108. | Yoshida K, Maeda K, Sugiyama Y. Transporter-mediated drug--drug interactions involving OATP substrates: predictions based on in vitro inhibition studies. Clin Pharmacol Ther. 2012;91:1053-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 109. | Li M, Anderson GD, Wang J. Drug-drug interactions involving membrane transporters in the human kidney. Expert Opin Drug Metab Toxicol. 2006;2:505-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 110. | Zhang L, Huang SM, Lesko LJ. Transporter-mediated drug-drug interactions. Clin Pharmacol Ther. 2011;89:481-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 111. | Glaeser H, Fromm MF. Animal models and intestinal drug transport. Expert Opin Drug Metab Toxicol. 2008;4:347-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 112. | Bebawy M, Chetty M. Gender differences in p-glycoprotein expression and function: effects on drug disposition and outcome. Curr Drug Metab. 2009;10:322-328. [PubMed] [Cited in This Article: ] |
| 113. | Cheng X, Klaassen CD. Tissue distribution, ontogeny, and hormonal regulation of xenobiotic transporters in mouse kidneys. Drug Metab Dispos. 2009;37:2178-2185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 114. | Hatano R, Onoe K, Obara M, Matsubara M, Kanai Y, Muto S, Asano S. Sex hormones induce a gender-related difference in renal expression of a novel prostaglandin transporter, OAT-PG, influencing basal PGE2 concentration. Am J Physiol Renal Physiol. 2012;302:F342-F349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 115. | Robey RW, Massey PR, Amiri-Kordestani L, Bates SE. ABC transporters: unvalidated therapeutic targets in cancer and the CNS. Anticancer Agents Med Chem. 2010;10:625-633. [PubMed] [Cited in This Article: ] |
| 116. | van Waterschoot RA, Schinkel AH. A critical analysis of the interplay between cytochrome P450 3A and P-glycoprotein: recent insights from knockout and transgenic mice. Pharmacol Rev. 2011;63:390-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 117. | Lam P, Wang R, Ling V. Bile acid transport in sister of P-glycoprotein (ABCB11) knockout mice. Biochemistry. 2005;44:12598-12605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 118. | Wang R, Lam P, Liu L, Forrest D, Yousef IM, Mignault D, Phillips MJ, Ling V. Severe cholestasis induced by cholic acid feeding in knockout mice of sister of P-glycoprotein. Hepatology. 2003;38:1489-1499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 119. | Schuetz EG, Umbenhauer DR, Yasuda K, Brimer C, Nguyen L, Relling MV, Schuetz JD, Schinkel AH. Altered expression of hepatic cytochromes P-450 in mice deficient in one or more mdr1 genes. Mol Pharmacol. 2000;57:188-197. [PubMed] [Cited in This Article: ] |
| 120. | Zolnerciks JK, Booth-Genthe CL, Gupta A, Harris J, Unadkat JD. Substrate- and species-dependent inhibition of P-glycoprotein-mediated transport: implications for predicting in vivo drug interactions. J Pharm Sci. 2011;100:3055-3061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 121. | Li M, Yuan H, Li N, Song G, Zheng Y, Baratta M, Hua F, Thurston A, Wang J, Lai Y. Identification of interspecies difference in efflux transporters of hepatocytes from dog, rat, monkey and human. Eur J Pharm Sci. 2008;35:114-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 122. | Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, Terasaki T. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem. 2011;117:333-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 579] [Cited by in F6Publishing: 579] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 123. | Jiang XL, Gonzalez FJ, Yu AM. Drug-metabolizing enzyme, transporter, and nuclear receptor genetically modified mouse models. Drug Metab Rev. 2011;43:27-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 124. | Patterson D, Graham C, Cherian C, Matherly LH. A humanized mouse model for the reduced folate carrier. Mol Genet Metab. 2008;93:95-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 125. | Scheer N, Balimane P, Hayward MD, Buechel S, Kauselmann G, Wolf CR. Generation and characterization of a novel multidrug resistance protein 2 humanized mouse line. Drug Metab Dispos. 2012;40:2212-2218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 126. | Keskitalo JE, Zolk O, Fromm MF, Kurkinen KJ, Neuvonen PJ, Niemi M. ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2009;86:197-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 300] [Article Influence: 20.0] [Reference Citation Analysis (0)] |