Published online Sep 18, 2020. doi: 10.5495/wjcid.v10.i3.33
Peer-review started: April 19, 2020
First decision: July 8, 2020
Revised: July 8, 2020
Accepted: August 1, 2020
Article in press: August 1, 2020
Published online: September 18, 2020
The increasing rates of antibiotic-resistance in recent years have supported emergence of multiple drug-resistant bacteria. Therefore, antibiotics that are recommended by the current clinical guidelines may not be effective for the treatment of complicated urinary tract infection (UTI) and acute pyelonephritis.
To determine the clinical efficacy and safety of antibiotics for the treatment of complicated UTI and acute pyelonephritis.
A search of PubMed, EMBASE, and Google Scholar was conducted for eligible articles describing the use of antibiotics in managing complicated UTI and acute pyelonephritis. The following keywords were used to perform the literature search: “urinary tract infection”, “complicated UTI”, “pyelonephritis”, “treatment”, and “antibiotics”. Additional articles of interest were retrieved from the reference lists of selected papers. Eligibility criteria for this systematic review were diagnosis of either complicated UTI or acute pyelonephritis and use of antibiotics in management. Clinical trials and observational studies were included, while case reports and reviews were excluded. The methodological quality of clinical trials and observational studies was assessed. A descriptive approach was adopted to analyze the data, due to the variation of methodology and interventions.
A total of 183 studies were screened, and 8 matched all the eligibility criteria and were included in this review. The antibiotics used included ceftazidime-avibactam, doripenem, levofloxacin, meropenem-vaborbactam, piperacillin-tazobactam, plazomicin, tazobactam-ceftolozane, and gentamicin. Two clinical trials reported that shorter-duration levofloxacin or non-fluoroquinolone antibiotic treatment was as effective as the duration of antibiotic therapy recommended by the current guidelines in treating complicated UTI and pyelonephritis. Besides that, ceftazidime-avibactam, piperacillin-tazobactam and tazobactam-ceftolozane can be used as alternatives to carbapenem in treating extended-spectrum -lactamase-producing Escherichia coli. The cure rates of complicated UTI and pyelonephritis by meropenem-vaborbactam, piperacillin-tazobactam and tazobactam-ceftolozane was comparable (95.6%-98.4%). Furthermore, levofloxacin had a relatively high rate of adverse events (33.1% and 47.7% in two clinical trials respectively), while tazobactam-ceftolozane had a relatively low rate of adverse events (17.5%). All studies have limitations and a potential for bias.
Core Tip: There is an increasing resistance rate to the antibiotics recommended by current guidelines for the treatment of complicated urinary tract infection (UTI) and acute pyelonephritis. Therefore, alternative antibiotics need to be explored to increase the cure rate and improve the outcomes of patients. The aim of this systematic review is to investigate the efficacy and safety of different antibiotic therapy in treating complicated UTI and acute pyelonephritis. The use of novel antibiotics and combination antibiotic therapy can be considered in treating complicated UTI and acute pyelonephritis when resistance to recommended antibiotics occurs.
- Citation: Ong LT. Antibiotics for complicated urinary tract infection and acute pyelonephritis: A systematic review. World J Clin Infect Dis 2020; 10(3): 33-41
- URL: https://www.wjgnet.com/2220-3176/full/v10/i3/33.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v10.i3.33
A complicated urinary tract infection (UTI) is associated with structural or functional abnormalities of the genitourinary tract or presence of any underlying disease[1]. Patients who have complicated UTI may experience relapse with an organism similar to the pretherapy isolate or reinfection with a new organism[1]. Complicated UTI may be associated with severe morbidity, such as septic shock, renal failure or even death[1]. Acute pyelonephritis is a bacterial infection causing inflammation of the kidney and renal pelvis, which occurs due to the spread of bacteria from the bladder to the kidneys in ascending UTI[2]. The rates of acute pyelonephritis in the United States are about 15 to 17 cases per 10000 females and 3 to 4 cases per 10000 males annually[2].
Current guidelines (Infectious Diseases Society of America and European Society of Clinical Microbiology and Infectious Diseases) recommend the use of oral fluoroquinolones for treatment of acute pyelonephritis and complicated UTI as an outpatient, because fluoroquinolones are absorbed well from the gastrointestinal tract and can penetrate the kidney[3]. Oral amoxicillin-clavulanate potassium, a cephalosporin, and trimethoprim-sulfamethoxazole can be used as alternatives[3]. One of the following three intravenous therapies is recommended by the Infectious Diseases Society of America for patients hospitalized for acute pyelonephritis: (1) A fluoroquinolone; (2) An aminoglycoside (with or without ampicillin); or (3) An extended-spectrum cephalosporin (with or without an aminoglycoside)[3].
However, there are limitations of the antibiotics currently recommended, such as adverse events associated with the antibiotics, presence of antibiotic-resistant bacteria, or compliance of medication. Therefore, alternative antibiotics must be considered to improve the prognosis and outcome of the patients. Alternative antibiotics, such as novel antibiotics or combination therapy, may be more effective than the antibiotics suggested by the guidelines in treating complicated UTI or acute pyelonephritis.
The aim of this review was to investigate the clinical efficacy and safety of antibiotics for the treatment of complicated UTI and acute pyelonephritis based on the current literature.
A systematic search was conducted to identify studies involving the treatment of complicated UTI or pyelonephritis with antibiotics. Search terms included the following keywords and word combinations: “urinary tract infection”, “complicated UTI”, “pyelonephritis”, “treatment”, and “antibiotics”. The search was conducted using the three major literature databases of PubMed, EMBASE and Google Scholar. Relevant articles published in English from 2010 to 2019 were identified. Additional articles of interest were retrieved from the reference list of selected papers.
Only adults diagnosed with complicated UTI or acute pyelonephritis were included in this review. The eligibility criteria included diagnosis of the complicated UTI or acute pyelonephritis based on clinical or microbiological evaluation and the use of antibiotics in management. Both oral and intravenous antibiotic therapies were included in this review. Case reports, articles without original data, and review articles were excluded from this study.
The titles and abstracts of all studies were screened for their eligibility for inclusion. The full-text manuscript was used to assess eligibility when a decision could not be made based on title and abstract solely. Data on population, study design, intervention, clinical outcomes, and adverse events were collected using a standardized electronic database within Microsoft Word. Outcome of the patients was defined as one of the following: Clinical failure rate; microbiological eradication; cure rate; duration of treatment; or length of hospital stay. Due to variation among the interventions and study designs, a descriptive approach was used to report the data (instead of a meta-analysis). The methodological quality of the studies was assessed using Cochrane risk of bias assessment for randomized control trials (RCTs)[4], The Newcastle-Ottawa scale for non-randomized control trial[5] and Downs and Black Checklist for Study Quality for observational studies[6] (author LTO) were used. PRISMA guidelines were used as a basis for reporting the results of this systematic review.
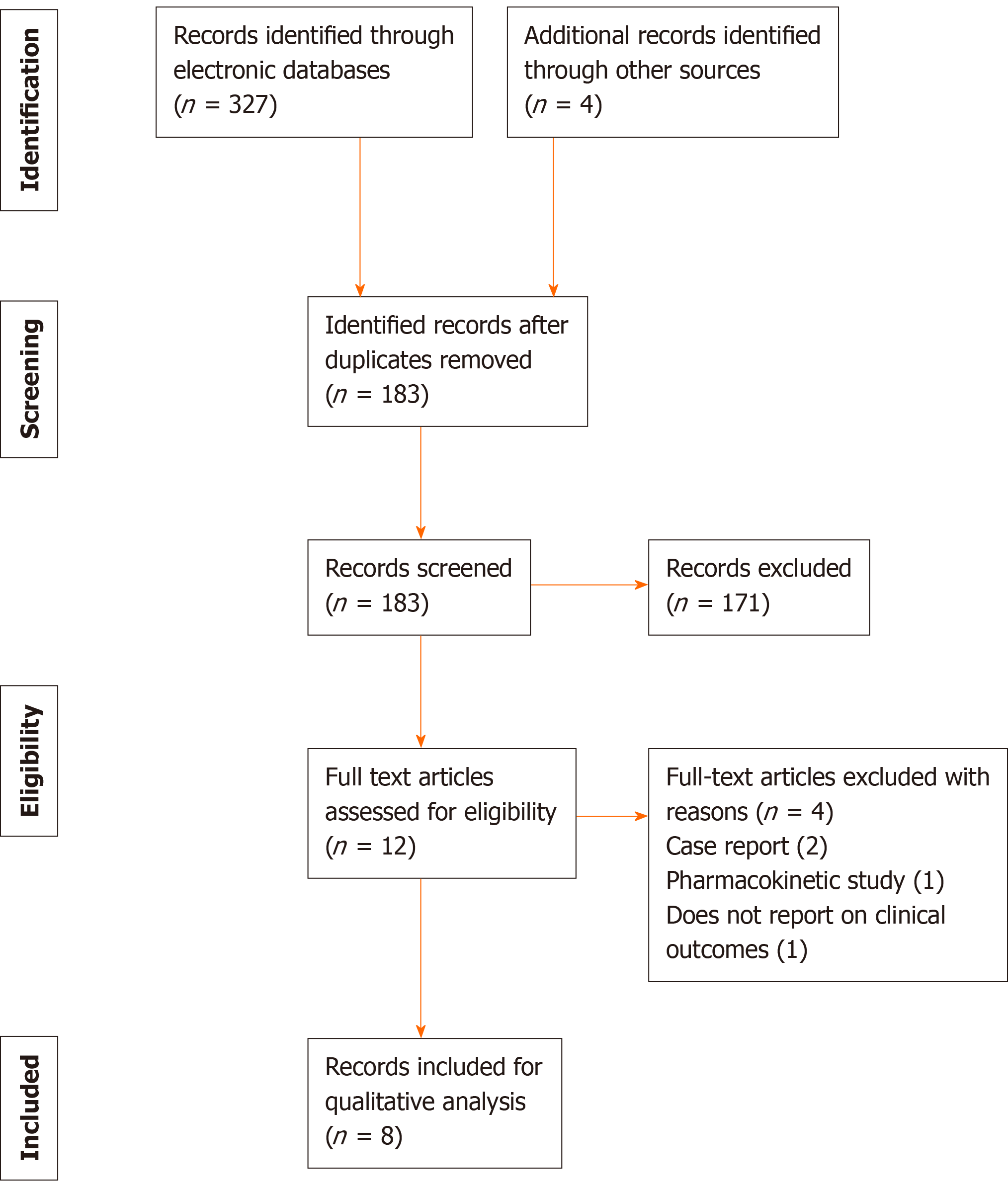
A total of 331 articles were retrieved by the search strategy, of which 183 studies were screened and 12 studies were assessed for eligibility based on the full manuscript. After exclusion, 8 studies matched the eligibility criteria and were included in the review for analyses[7-14]. Among them, 5 studies were RCTs, 2 studies were observational studies, and 1 study was a non-randomized trial (Figure 1). A total of 2531 participants were enrolled in all the studies identified. The antibiotics included in the studies were ceftazidime-avibactam, doripenem, levofloxacin, meropenem-vaborbactam, piperacillin-tazobactam, plazomicin, tazobactam-ceftolozane and gentamicin. Escherichia coli (E. coli) was the most common causative pathogen of the cases of complicated UTI and pyelonephritis, but other Gram-negative and Gram-positive species had been isolated from patients.
Two observational studies included in this review were retrospective cohort studies. Park et al[7] compared the efficacy of carbapenem and non-carbapenem antibiotics in treating patients with acute pyelonephritis due to extended-spectrum -lactamase (ESBL)-producing E. coli[7]. The non-carbapenem antibiotics used in the treatment were aminoglycosides, -lactam/-lactamase inhibitors, fluoroquinolones, and trimethoprim/sulfamethoxazole. The risk of microbiological failure (weighted hazard ratio: 0.99) and clinical failure rate (weighted hazard ratio: 1.05) were similar for the two groups. The aim of the study was to determine if the initial dosing of gentamicin improved patient’s outcomes in pyelonephritis[8]. Initial dosing of gentamicin decreased the intravenous (IV) antibiotic treatment length and length of hospital stay. Patients who were given gentamicin, in general, showed an association with better outcomes.
Based on the RCTs and a non-randomized trial, 1 study used oral antibiotic therapy[8], 6 studies used IV antibiotic therapy[8-13], and 2 studies used a combination of oral and IV antibiotic therapy[9,10]. Two RCTs involved studying the efficacy of antibiotics used in different doses and duration, while three RCTs involved studying the efficacy of different antibiotic therapies. The outcome was most commonly assessed at 5-9 d post-treatment and 1-2 mo post-treatment[8-13]. Most of the patients showed improvement in clinical symptoms, such as fever, dysuria, urinary frequency, and suprapubic pain, after 5-9 d of initiation of antibiotic therapy[8-13]. All of the antibiotic therapy used in the studies had cure rates greater than 60%[8-13]. All the studies described the microbiological etiology in their cases. The infections were caused primarily by E. coli, and Klebsiella pneumoniae was the second most common bacteria identified[8-13]. All of the clinical findings of the studies are shown in Table 1.
| Ref. | Study design | Population | Therapy | Findings |
| Park et al[7], 2014 | Observational study | 152 patients with pyelonephritis caused by ESBL-producing Escherichia coli | Carbapenems for median 12 d vs non-carbapenems for median 8 d | Clinical failure was similar between the two groups (weighted HR: 1.05) |
| Wagenlehner et al[8], 2016 | RCT | 1033 with suspected or confirmed cUTI/APN, randomized 1:1 to each arm | Ceftazidime-avibactam vs doripenem up to 10 d or 14 d for patients with bacteremia | Microbiological eradication rate: 77.4% ceftazidime-avibactam; 71.0% doripenem |
| Ren et al[9], 2017 | TCT | 330 patients diagnosed with cUTI or APN, randomized 1:1 to each arm | IV levofloxacin 750 mg for 5 d vs IV levofloxacin 500 mg and shift to oral levofloxacin 500 mg for 7-14 d | Clinical success rate: 89.87% in IV levofloxacin 750 mg vs 89.31% in IV/oral levofloxacin 50 vs 0 mg |
| Kaye et al[10], 2018 | RCT | 550 patients with cUTI or APN, randomized 1:1 to each arm | Meropenem-vaborbactam vs piperacillin-tazobactam for 10 d | Clinical success rate: 98.4% in the meropenem-vaborbactam group vs 95.6% in the piperacillin-tazobactam group |
| Connolly et al[11], 2018 | RCT | 145 patients diagnosed with cUTI and APN, randomized at 22, 76 and 47 in each arm | Plazomicin at 10 mg/kg vs plazomicin at 15 mg/kg vs levofloxacin 750 mg for 5 d | Microbiological eradication rate in MITT and MIE population: 50.0% and 85.7% (plazomicin at 10 mg/kg) vs 60.8% and 88.6% (plazomicin at 15 mg/kg) vs 58.6% and 81.0% (levofloxacin) |
| Rudrabhatla et al[12], 2018 | RCT | 54 patients diagnosed with APN, randomized 1:1 to each arm | Non-fluoroquinolone antibiotics for 7 d vs 14 d | Patients who received antibiotics for 7 d had shorter hospital stay (8 d vs 14 d) and less antibiotic consumption (8.4 DDs vs 17.4 DDs) No patients required retreatment |
| Arakawa et al[13], 2018 | Non-randomized, trial | 115 patients diagnosed with pyelonephritis or complicated cystitis | IV tazobactam-ceftolozane every 8 h for 7 d | Clinical response rate was 96.6% |
| Ryanto et al[14], 2019 | Observational study | 152 patients diagnosed with severe pyelonephritis/urosepsis | Gentamicin was prescribed for 43.4% patients; 32% of patients were given initial dosing of gentamicin | Duration of IV, time of resolution, and length of stay is short in patients given gentamicin; initial dose of IV gentamicin improved the outcome of patients |
The rates of adverse events associated with the antibiotic therapy in the trials were mostly around 30% to 50%[8-11]. Levofloxacin, in the Connolly et al[11] trial, had a relatively high rate of adverse events (47.7%)[11]. However, this could be due to the small population of patients (n = 7) taking levofloxacin therapy in that trial. The most common adverse effects reported in the trials was headache, which was reported in the use of ceftazidime-avibactam, doripenem, levofloxacin, meropenem-vaborbactam. piperacillin-tazobactam, and plazomicin[8-11]. Both levofloxacin and tazobactam-ceftolozane were frequently associated with gastrointestinal illness and abnormal laboratory findings, which were reduced leukocyte count and increased aminotransferase respectively[11,13]. Tazobactam-ceftolozane had a low rate (at 17.5%) of adverse events reported[13]. All the adverse events associated with antibiotic therapy are shown in Table 2.
| Antibiotics | Ref. | Adverse events reported | Most common adverse effects | Frequency, n/total (%) |
| Ceftazidime-avibactam | Wagenlehneret al[8] | Headache, nausea, diarrhea, constipation | Headache | 185/511 (36.2) |
| Doripenem | Wagenlehneret al[8] | Headache, nausea, diarrhea, constipation | Headache | 158/509 (31.0) |
| Levofloxacin | Ren et al[9] | Reduction in leukocyte count, reduction in neutrophil count, increased ALT, increased AST, increased platelet count, increased blood pressure, gastrointestinal, reaction at injection site, cutaneous/subcutaneous, nervous system/mental, immune, infection, hepatobiliary, metabolic/nutritional, musculoskeletal/connective tissue | Reduction in leukocyte count and gastrointestinal | 109/329 (33.1) |
| Connolly et al[11] | Headache, diarrhea, vomiting, nausea, dizziness | Headache | 21/44 (47.7) | |
| Meropenem-vaborbactam | Kaye et al[10] | Headache, diarrhea, nausea, asymptomatic bacteriuria, catheter site phlebitis, infusion site phlebitis, urinary tract infection, hypokalemia, vaginal infection, ALT increased, anemia, AST increased, pyrexia | Headache | 106/272 (39.0) |
| Piperacillin-tazobactam | Kaye et al[10] | Headache, diarrhea, nausea, asymptomatic bacteriuria, catheter site phlebitis, infusion site phlebitis, urinary tract infection, hypokalemia, vaginal infection, ALT increased, anemia, AST increased, pyrexia, dyspnea | Headache | 97/273 (35.5) |
| Plazomicin | Connolly et al[11] | Headache, diarrhea, vomiting, nausea, dizziness | Headache | 33/96 (34.4) |
| Tazobactam-ceftolozane | Arakawa et al[13] | Diarrhea, ALT increased, constipation, AST increased, insomnia, headache, pyelonephritis, pyelonephritis acute, contusion, viral upper respiratory tract infection | Diarrhea and ALT increased | 20/114 (17.5) |
The clinical studies included in this review varied in study design, eligibility, time to follow-up, and outcomes. The most common diagnostic criteria used in the studies were pyuria, presence of 1-2 uropathogens, or presence of clinical symptoms, such as dysuria, urinary frequency, flank tenderness, or fever. Biases were identified in the RCTs, including selection bias, performance bias, and response bias. Overall, the methodological quality of the studies was moderate. One RCT had good quality and four RCTs had fair quality, based on the thresholds for converting the Cochrane risk of bias tool to agency for healthcare research and quality standards[4]. The total score for methodological quality for the two observational studies based on the Downs and Black Checklist for Study Quality[6] was 12 and 15.
Antibiotic resistance is one of the major reasons for exploration of other antibiotics to manage complicated UTI and acute pyelonephritis[3]. Rates of quinolone resistance among Enterobacteriaceae were 1% in the mid-to-late 1900s and 1% to 3% as late as 2008 but the quinolone resistance rates have increased to 10%-30% in recent years[15]. Besides that, some of the antibiotics recommended by the current clinical guidelines may cause serious adverse drug reactions. For example, cephalosporin may result in rashes, diarrhea, anaphylaxis and haemolytic anaemia, and has shown a frequent association with morbidity from Clostridium difficile infection[16]. Besides that, trimethoprim-sulfamethoxazole therapy has been associated with neurological defect, reduced oxygen-carrying capacity, gastrointestinal illness and drug hypersensitivity, while aminoglycosides have been associated with nephrotoxicity, such as acute tubular necrosis and ototoxicity[17,18].
ESBL-producing E. coli is one of the causative bacteria for acute pyelonephritis and carbapenems are considered first-choice treatment for ESBL producers[19]. However, due to the increasing carbapenem resistance rate in Enterobacteriaceae, carbapenems should be used judiciously[7]. The study by Park et al[7] suggested non-carbapenem antibiotics had the same efficacy against ESBL-producing E. coli as carbapenems; however, insufficient research data and conflicting study results have discouraged the use of non-carbapenem antibiotics[7]. Besides that, amikacin was suggested as an alternative due to low resistance rate but there are insufficient data about the therapeutic efficacy and association of amikacin with nephrotoxicity[7,20].
An RCT showed that ceftazidime-avibactam and doripenem have the same efficacy in treating hospitalized patients with complicated UTI and acute pyelonephritis[8]. Moreover, the clinical cure rate of ceftazidime-avibactam was found to be similar for patients with ceftazidime-nonsusceptible and ceftazidime-susceptible pathogens[8]. Therefore, ceftazidime-avibactam can be used as an alternative to carbapenem to reduce the spread of carbapenem-resistant bacteria.
Dosing of antibiotics is also an important factor in reducing antibiotic resistance; therefore, it is essential to optimize the current regimens. An RCT showed that levofloxacin at 750 mg/d for 5 d is as effective as 500 mg/d plus oral regimen of levofloxacin for 7-14 d in treating complicated UTI and acute pyelonephritis in terms of clinical efficacy, microbiological efficacy, and tolerance[9]. High-dose levofloxacin can have prolonged bactericidal activity against E. coli with minimum inhibitory concentration up to 32 mg/mL, due to increased concentration of the antibiotic in the urine[21]. Therefore, levofloxacin at 750 mg/d is preferred because the duration of treatment is shorter and the total drug dose was 23% less[9]. Another RCT involved patients stopping non-fluoroquinolone antibiotics at day 7 or continuing treatment until day 14[12]. Truncating non-fluoroquinolone antibiotics at day 7 is advised, as this strategy can reduce antibiotic consumption, length of hospital stay and treatment-related adverse events, and generally yield the same outcome as seen in the patients who continued the antibiotic treatment until day 14[12]. Studies have shown that shorter durations of antibiotic therapy are effective for common infections, such as bacteremia and community-acquired pneumonia and can prevent the rise of antimicrobial resistance[22].
Both meropenem-vaborbactam and piperacillin-tazobactam are effective in treating complicated UTI and acute pyelonephritis, with the overall success rates of 98.4% and 95.6% respectively[10]. Piperacillin-tazobactam has been shown to be effective in patients from whom Enterobacteriaceae was isolated, including ESBL-producers[10]. Plazomicin is a aminoglycoside that is effective in treating adult patients with complicated UTI including acute pyelonephritis, with microbiological eradication over 85%[11]. Plazomicin is derived from sisomicin with structural modifications that can prevent degradation from aminoglycoside-modifying enzymes, which is a common mechanism of aminoglycosides resistance[23]. Therefore, plazomicin has the potential to treat complicated UTI and acute pyelonephritis caused by multidrug-resistant Enterobacteriaceae; however, further studies involving larger sample size should be conducted[11].
Tazobactam-ceftolozane is a novel antibiotic therapy that is effective in the treatment of complicated UTI and pyelonephritis, with microbiological response rate and clinical repose rate of 80.7% and 96.6% respectively[13]. Tazobactam-ceftolozane has a favourable safety profile, with a low rate of adverse events (17.5%), and has excellent antibacterial activity against Gram-negative bacteria, which encompass the Enterobacteriaceae spp., including ESBL-producing strains and multidrug-resistant Pseudomonas aeruginosa[13]. Finally, an initial dose of IV gentamicin has been associated with positive patient outcomes, due to its effectiveness in severe cases of suspected Gram-negative sepsis, especially against P. aeruginosa[14]. However, only 54% of E. coli strains found in urine have been reported as sensitive to gentamicin[24]. Duration and dose of gentamicin need to be monitored closely, due to increased risk of adverse effects, such as nephrotoxicity[14].
This systematic review has limitations. It is possible that evidence and clinical studies were missed by the search strategy employed. A comparison of efficacy between different antibiotic therapies is difficult, due to the significant variation in study designs, interventions, and outcome measures. Besides that, some novel antibiotic therapies have limited and incomplete clinical data for comparison.
In conclusion, several novel antibiotics and combination therapies have proven to be effective in treating complicated UTI and pyelonephritis. The clinical data have shown that shorter duration of treatment with lower consumption of antibiotics are effective for treatment and can reduce the development of multiple drug resistance bacteria. Ceftazidime-avibactam, piperacillin-tazobactam and tazobactam-ceftolozane can be used as an alternative to carbapenem to treat ESBL-producing E. coli. Finally, meropenem-vaborbactam, piperacillin-tazobactam and tazobactam-ceftolozane have high cure rates in treating complicated UTI and pyelonephritis. Therefore, the use of novel antibiotics and combination antibiotic therapy can be considered for treating complicated UTI and acute pyelonephritis when resistance to recommended antibiotics occurs. In future trials, standardized diagnostic criteria and outcome measures should be adopted for direct comparison. Moreover, further research is needed to identify the spectrum of patients in whom different antibiotics offer better clinical outcomes and prognosis.
Antibiotics that are recommended by the current clinical guidelines may not be effective for treatment of complicated urinary tract infection (UTI) and acute pyelonephritis, due to the increasing resistance rates to the antibiotics.
This systematic review is intended to provide comprehensive information to help clinicians in determining suitable antibiotics for the management of complicated UTI and acute pyelonephritis.
The aim of this study was to determine the clinical efficacy and safety of antibiotics for the treatment of complicated UTI and pyelonephritis.
A search of three medical literature databases (PubMed, EMBASE and Google Scholar) was conducted for eligible articles describing the use of antibiotics in managing complicated UTI and acute pyelonephritis. The following keywords were used to perform the literature search: “urinary tract infection”, “complicated UTI”, “pyelonephritis”, “treatment”, and “antibiotics”. Eligibility criteria included diagnosis of either complicated UTI or acute pyelonephritis and use of antibiotics in management. Clinical trials and observational studies were included in this review, while case reports and reviews were excluded.
Eight studies matched all the eligibility criteria and were included in this review. The antibiotics included in those studies were ceftazidime-avibactam, doripenem, levofloxacin, meropenem-vaborbactam, piperacillin-tazobactam, plazomicin, tazobactam-ceftolozane, and gentamicin. The clinical data have shown that shorter duration of treatment with lower consumption of antibiotics is effective for treatment and can reduce the development of multiple drug resistance bacteria. Ceftazidime-avibactam, piperacillin-tazobactam and tazobactam-ceftolozane can be used as alternatives to carbapenem to treat ESBL-producing Escherichia coli. Besides that, meropenem-vaborbactam, piperacillin-tazobactam and tazobactam-ceftolozane have high cure rates in treating complicated UTI and pyelonephritis
Novel antibiotics and combination antibiotic therapy regimens are effective in managing complicated UTI and acute pyelonephritis when resistance to recommended antibiotics occurs.
Further research is needed to compare the efficacy of different antibiotic therapies and identify the spectrum of patients in whom different antibiotics offer better clinical outcomes and prognosis.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American College of Physicians, No. 04011459.
Specialty type: Infectious diseases
Country of origin: Malaysia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mesquita J S-Editor: Zhang L L-Editor: Filipodia P-Editor: Xing YX
| 1. | Nicolle LE; AMMI Canada Guidelines Committee*. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005;16:349-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Belyayeva M, Jeong JM. Acute Pyelonephritis. [cited 2019 Feb 28] In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519537. [Cited in This Article: ] |
| 3. | Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambayh PA, Tenke P, Nicolle LE; Infectious Diseases Society of America. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1219] [Cited by in F6Publishing: 1163] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 4. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18487] [Cited by in F6Publishing: 21212] [Article Influence: 1631.7] [Reference Citation Analysis (2)] |
| 5. | Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses, 2012. Available from: http://wwwohrica/programs/clinical_epidemiology/oxfordasp.. [Cited in This Article: ] |
| 6. | Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5177] [Cited by in F6Publishing: 5462] [Article Influence: 210.1] [Reference Citation Analysis (0)] |
| 7. | Park SH, Choi SM, Chang YK, Lee DG, Cho SY, Lee HJ, Choi JH, Yoo JH. The efficacy of non-carbapenem antibiotics for the treatment of community-onset acute pyelonephritis due to extended-spectrum β-lactamase-producing Escherichia coli. J Antimicrob Chemother. 2014;69:2848-2856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Wagenlehner FM, Sobel JD, Newell P, Armstrong J, Huang X, Stone GG, Yates K, Gasink LB. Ceftazidime-avibactam Versus Doripenem for the Treatment of Complicated Urinary Tract Infections, Including Acute Pyelonephritis: RECAPTURE, a Phase 3 Randomized Trial Program. Clin Infect Dis. 2016;63:754-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 245] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 9. | Ren H, Li X, Ni ZH, Niu JY, Cao B, Xu J, Cheng H, Tu XW, Ren AM, Hu Y, Xing CY, Liu YH, Li YF, Cen J, Zhou R, Xu XD, Qiu XH, Chen N. Treatment of complicated urinary tract infection and acute pyelonephritis by short-course intravenous levofloxacin (750 mg/day) or conventional intravenous/oral levofloxacin (500 mg/day): prospective, open-label, randomized, controlled, multicenter, non-inferiority clinical trial. Int Urol Nephrol. 2017;49:499-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Kaye KS, Bhowmick T, Metallidis S, Bleasdale SC, Sagan OS, Stus V, Vazquez J, Zaitsev V, Bidair M, Chorvat E, Dragoescu PO, Fedosiuk E, Horcajada JP, Murta C, Sarychev Y, Stoev V, Morgan E, Fusaro K, Griffith D, Lomovskaya O, Alexander EL, Loutit J, Dudley MN, Giamarellos-Bourboulis EJ. Effect of Meropenem-Vaborbactam vs. Piperacillin-Tazobactam on Clinical Cure or Improvement and Microbial Eradication in Complicated Urinary Tract Infection: The TANGO I Randomized Clinical Trial. JAMA. 2018;319:788-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 207] [Article Influence: 34.5] [Reference Citation Analysis (1)] |
| 11. | Connolly LE, Riddle V, Cebrik D, Armstrong ES, Miller LG. A Multicenter, Randomized, Double-Blind, Phase 2 Study of the Efficacy and Safety of Plazomicin Compared with Levofloxacin in the Treatment of Complicated Urinary Tract Infection and Acute Pyelonephritis. Antimicrob Agents Chemother. 2018;62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Rudrabhatla P, Deepanjali S, Mandal J, Swaminathan RP, Kadhiravan T. Stopping the effective non-fluoroquinolone antibiotics at day 7 vs continuing until day 14 in adults with acute pyelonephritis requiring hospitalization: A randomized non-inferiority trial. PLoS One. 2018;13:e0197302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Arakawa S, Kawahara K, Kawahara M, Yasuda M, Fujimoto G, Sato A, Yokokawa R, Yoshinari T, Rhee EG, Aoyama N. The efficacy and safety of tazobactam/ceftolozane in Japanese patients with uncomplicated pyelonephritis and complicated urinary tract infection. J Infect Chemother. 2019;25:104-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Ryanto S, Wong M, Czarniak P, Parsons R, Travers K, Skinner M, Sunderland B. The use of initial dosing of gentamicin in the management of pyelonephritis/urosepsis: A retrospective study. PLoS One. 2019;14:e0211094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 15. | Spellberg B, Doi Y. The Rise of Fluoroquinolone-Resistant Escherichia coli in the Community: Scarier Than We Thought. J Infect Dis. 2015;212:1853-1855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Macy E, Contreras R. Adverse reactions associated with oral and parenteral use of cephalosporins: A retrospective population-based analysis. J Allergy Clin Immunol 2015; 135: 745-52. e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Ho JM, Juurlink DN. Considerations when prescribing trimethoprim-sulfamethoxazole. CMAJ. 2011;183:1851-1858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 18. | Saleh P, Abbasalizadeh S, Rezaeian S, Naghavi-Behzad M, Piri R, Pourfeizi HH. Gentamicin-mediated ototoxicity and nephrotoxicity: A clinical trial study. Niger Med J. 2016;57:347-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005;18:657-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2039] [Cited by in F6Publishing: 2096] [Article Influence: 110.3] [Reference Citation Analysis (0)] |
| 20. | Cho SY, Choi SM, Park SH, Lee DG, Choi JH, Yoo JH. Amikacin therapy for urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Korean J Intern Med. 2016;31:156-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Stein GE, Schooley SL, Nicolau DP. Urinary bactericidal activity of single doses (250, 500, 750 and 1000 mg) of levofloxacin against fluoroquinolone-resistant strains of Escherichia coli. Int J Antimicrob Agents. 2008;32:320-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care. 2011;15:R267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 23. | Aggen JB, Armstrong ES, Goldblum AA, Dozzo P, Linsell MS, Gliedt MJ, Hildebrandt DJ, Feeney LA, Kubo A, Matias RD, Lopez S, Gomez M, Wlasichuk KB, Diokno R, Miller GH, Moser HE. Synthesis and spectrum of the neoglycoside ACHN-490. Antimicrob Agents Chemother. 2010;54:4636-4642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 24. | Akinbowale OL, Peng H, Barton MD. Antimicrobial resistance in bacteria isolated from aquaculture sources in Australia. J Appl Microbiol. 2006;100:1103-1113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |