INTRODUCTION
A recent population-based cohort study by Wilder et al[1] has shown that early exposure to anesthetics is a significant risk factor for later development of learning disabilities (LD). They found, in particular, that the risk for LD increased with longer cumulative duration of anesthesia exposure and that their data cannot reveal whether anesthesia itself may contribute to LD or whether the need for anesthesia is a marker for other unidentified factors that contribute to LD. Animal studies have also shown that exposure to a variety of commonly used intravenous and volatile anesthetics produces cognitive and social behavioral deficits that last into adulthood[2,3]. Thus, the possibility that general anesthetics might have long-lasting effects on the developing brain has gained traction with anesthesiologists, popular media and parents. Although cause-effect relationships have not been established, these cognitive and behavioral dysfunctions have been attributed to the suppression of neurogenesis or widespread neuroapoptosis after anesthetic exposure in the early postnatal period[2,4]. In addition, anesthetic exposure fundamentally alters dendritic spine formation and therefore synaptic function[5]. Taken together, these results strongly suggest that anesthetics can produce synaptic remodeling in the developing brain that could potentially produce long-term cognitive impairment.
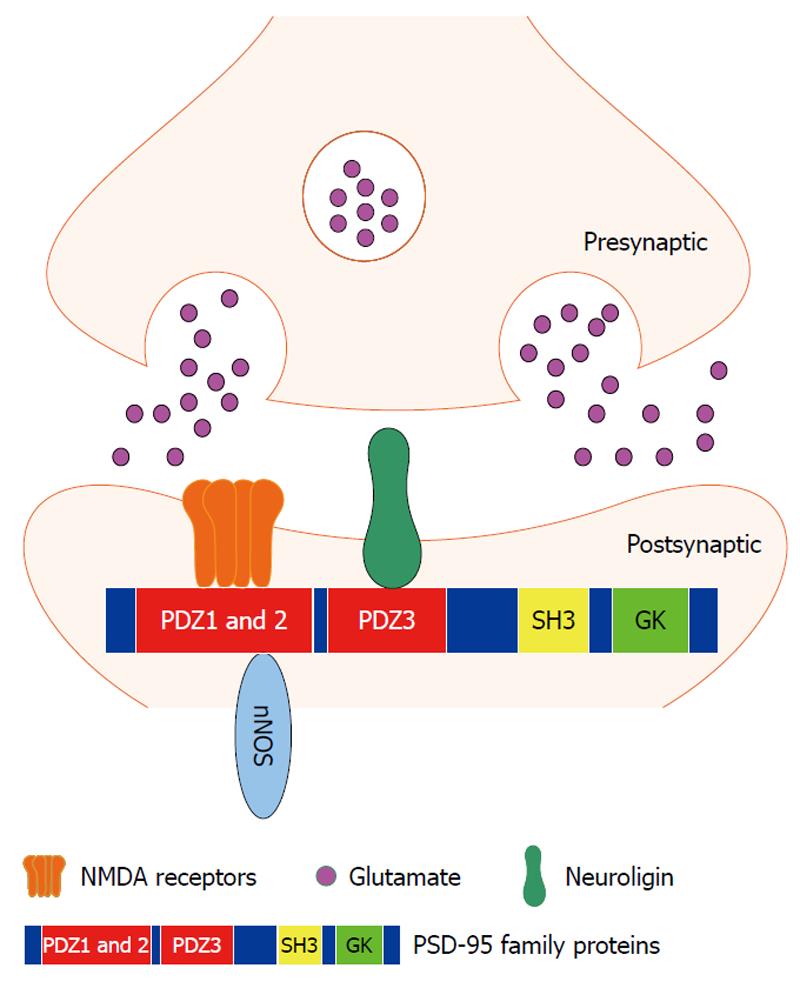
In the central nervous system, the hippocampus is a critical region for learning and memory. N-methyl-D-aspartate (NMDA) receptors are present at the postsynaptic membrane of the hippocampal synapses and are involved in the synaptic mechanism of learning and memory. The functional activities of NMDA receptors are regulated by postsynaptic density-95 (PSD-95) family proteins, which contain three PDZ (PSD 95/Discs large/Zona occludens-1) domains in their N-terminus (Figure 1). Through the first two PDZ domains (mainly PDZ2), PSD-95 interacts with NMDA receptor NR2 subunit and neuronal nitric oxide synthase (nNOS)[6,7] to regulate NMDA receptor-mediated synaptic plasticity[6-8]. PSD-95 PDZ3 domain-mediated PSD-95/neuroligin signaling at the postsynaptic site of the hippocampal synapses modulates the presynaptic release probability of glutamate vesicles in a retrograde manner[9]. In addition to its effect on synaptic function, PSD-95 has also been proposed to affect synapse maturation and stabilization[10-12]. PSD-95 promotes synaptogenesis and deletion of the PSD-95 PDZ2 domain specifically prevents multi-innervated spine formation[13]. Our previous studies have demonstrated that PSD-95 PDZ domain-mediated protein-protein interactions are disrupted by clinically relevant concentrations of inhaled anesthetics and inhaled anesthetic binding results in chemical shift changes of PSD-95 PDZ2[14], and that disrupting PSD-95 PDZ domain-mediated protein interactions reduces the threshold for inhalational anesthesia[15]. Moreover, hippocampal neurogenesis can be regulated by glutamate release and subsequent NMDA receptor activation[16]. Therefore, it is possible that synaptic PDZ interactions are involved in the regulation of neurogenesis and synaptogenesis in the hippocampus and disruption of the PDZ interactions is one of the mechanisms underlying anesthetic-induced cognitive dysfunction.
Figure 1 N-methyl-D-aspartate receptor signaling and protein interactions mediated by PDZ domains of postsynaptic density-95 family proteins at postsynaptic density in the central nervous system.
Through the first two PDZ domains (mainly PDZ2), postsynaptic density-95 (PSD-95) family proteins interact with N-methyl-D-aspartate (NMDA) receptor NR2 subunit and downstream molecule neuronal nitric oxide synthase to regulate NMDA receptor-mediated synaptic plasticity. PSD-95 PDZ3 domain-mediated PSD-95/neuroligin interaction at the postsynaptic site of synapses modulates the presynaptic release probability of glutamate vesicles in a retrograde manner.
HIPPOCAMPAL NEUROGENESIS AND ANESTHETIC-INDUCED COGNITIVE DYSFUNCTION
Neurogenesis in both the developing and adult dentate gyrus is important for hippocampal function, specifically learning and memory[17]. Stratmann et al[4] showed that in postnatal day (PND) 60 rats, 4 h of isoflurane exposure at 1 minimum alveolar concentration increased early neuronal differentiation in the dentate gyrus, decreased progenitor proliferation for 1 d, and subsequently increased progenitor proliferation 5-10 d after anesthesia. Identical isoflurane treatment in PND 7 rats did not induce neuronal lineage selection but decreased progenitor proliferation until at least 5 d after anesthesia[4]. They also showed that isoflurane exposure improved spatial reference memory of PND 60 rats long-term but caused a delayed-onset, progressive, persistent hippocampal deficit in PND 7 rats in fear conditioning and spatial reference memory tasks[4]. Thus, isoflurane differentially affects both neurogenesis and long-term neurocognitive function in PND 60 and PND 7 rats. In another study[18] that subjected PND 14 and PND 60 rats to repeated isoflurane exposure (1.7%, 35 min daily for 4 successive days), object recognition and reversal learning were significantly impaired in isoflurane-treated young rats, whereas adult animals were unaffected, and these deficits became more pronounced as the animals grew older. The memory deficit was paralleled by a decrease in the hippocampal stem cell pool and persistently reduced neurogenesis, subsequently causing a reduction in the number of dentate gyrus granule cell neurons in isoflurane-treated rats[18]. These results suggest that hippocampal neurogenesis might mediate the long-term neurocognitive dysfunction after isoflurane exposure in neonatal rats.
Given that (1) hippocampal neurogenesis can be regulated by glutamate release and subsequent NMDA receptor activation[16] and (2) PSD-95 PDZ domain-mediated protein-protein interactions modulate presynaptic glutamate release[9] and regulate postsynaptic NMDA receptor function[6-8], it is well-founded that PSD-95 PDZ domain-mediated protein-protein interactions may play an important role in hippocampal neurogenesis and then contribute to early anesthetic exposure-produced long-term cognitive impairment.
HIPPOCAMPAL SYNAPTOGENESIS AND ANESTHETIC-INDUCED COGNITIVE DYSFUNCTION
The developing brain is most vulnerable during the period of rapid synaptogenesis[2]. Administration with a combination of drugs commonly used in pediatric anesthesia (midazolam, nitrous oxide and isoflurane) in doses sufficient to maintain a surgical plane of anesthesia for 6 h causes widespread apoptotic neurodegeneration in the developing brain of PND 7 rats, deficits in hippocampal synaptic function and persistent memory/learning impairments[2]. Therefore, synapse development is a critical element in the progress of anesthetic neurotoxicity. Head et al[19] reported a substantial loss of dendritic spines and a reduction in synapses in cultured neurons and the hippocampus of PND 7 rodents exposed to isoflurane (1.4%, 4 h). However, isoflurane (1.5%, 2 h) exposure on PND 16 significantly increased dendritic spine density in the rat medial prefrontal cortex during synaptogenesis[20]. These results suggest that volatile anesthetics with different potencies could rapidly interfere with physiological patterns of synaptogenesis and thus might impair appropriate circuit assembly in the developing cerebral cortex. Increasing age might alter the response of dendritic spines to anesthetics from vulnerable to resistant. It is well established that NMDA receptor signaling plays a crucial role in synaptic development and neuronal survival[21]. During the critical period of synaptogenesis, inhibition of NMDA receptor signaling is detrimental to brain development[22]. NMDA receptor-interacting protein PSD-95 is also an important regulator of synaptic structure and plasticity. By using a combined electron microscopic, genetic and pharmacological approach, Nikonenko et al[13] have uncovered a new mechanism through which PSD-95 regulates synaptogenesis. They found that PSD-95 overexpression affected spine morphology and promoted the formation of multi-innervated spines contacted by up to seven presynaptic terminals. The formation of multiple contacts was specifically prevented by deletion of the PDZ2 domain of PSD-95[13], which interacts with NOS. Similarly, PSD-95 overexpression combined with small interfering RNA-mediated down-regulation or the pharmacological blockade of nNOS prevented axon differentiation into varicosities and multisynapse formation[13]. Conversely, treatment of hippocampal slices with a nitric oxide donor or cyclic guanosine monophosphate analogue induced multiinnervated spines and NOS blockade also reduced spine and synapse density in developing hippocampal cultures[13].
Given that PSD-95 promotes synaptogenesis and multi-innervated spine formation is specifically prevented by deletion of the PSD-95 PDZ2 domain[13], it is presumable that PSD-95 PDZ domain-mediated protein-protein interactions may be involved in hippocampal synaptogenesis and then contribute to synaptic plasticity and early anesthetic exposure-produced long-term cognitive impairment.
CONCLUSION
Approximately 1.5 million fetuses or newborns are exposed to anesthetic agents each year[23]. A recent clinical cohort study has shown that early exposure to anesthetics is a significant risk factor for later development of LD[1]. In animal studies, newborn rats exposed to commonly used anesthetic agents (e.g. isoflurane) develop neurodegenerative changes in multiple areas of the brain that are associated with long-term deficits in learning and memory[2,4,24]. These provocative findings bring into question whether anesthetic drugs can be used safely in pediatric anesthesia and have prompted many to investigate the neurotoxic effect of anesthetic drugs on the developing brain. Although recent studies have indicated that hippocampal neurogenesis and synaptogenesis may be involved in the mechanisms by which early anesthetic exposure produces long-term cognitive impairment[4,5,19], the underlying molecular mechanism remains to be elucidated.
Synaptic PDZ interactions might be potential targets in pathogenesis of neonatal anesthetic neurotoxicity. In the future, we may compare the effects of early anesthetic exposure and synaptic PDZ interaction disruption on hippocampal neurogenesis, hippocampal synaptogenesis, synaptic plasticity and long-term neurocognitive function; we may also define the relationship among PDZ interaction disruption, neurogenesis suppression, synaptogenesis interference and early anesthetic exposure-produced long-term cognitive impairment. By using PDZ interaction inhibitors, we could determine whether disruption of PDZ interactions can mimic the effect of inhaled anesthetics on hippocampal neurogenesis, synaptogenesis and long-term neurocognitive function; by using overexpression of anesthetic-resistant PDZ mutants, we could determine whether synaptic PDZ interactions are involved in the molecular mechanisms that underlie early anesthetic exposure-regulated neurogenesis, synaptogenesis and synaptic plasticity in the hippocampus. Together, evaluating both structural and functional synaptic plasticity will help us demonstrate the role of synaptic PDZ interactions in early anesthetic exposure-produced long-term cognitive impairment.
Peer reviewer: Maria Dalamaga, MD, PhD, MSc, MPH, Assistant Professor, Department of Clinical Biochemistry, University of Athens, School of Medicine, “Attikon” General University Hospital, 28th Octovriou #19, 15341 Athens, Agia Paraskevi, Greece
S- Editor Wang JL L- Editor Roemmele A E- Editor Zheng XM