Revised: January 1, 2012
Accepted: February 13, 2012
Published online: February 20, 2012
AIM: To contrast the effects of various modifications of body fluid volumes on thirst as reported by healthy volunteers.
METHODS: Ten male volunteers aged between 19 and 37 years (mean 22 years) underwent four experiments each, which comprised infusion of 400-800 mL of acetated Ringer’s solution and intake of 600 mL of tap water. Half of the experiments were preceded by volume depletion (median 1.7 L) with furosemide. A visual analogue scale (0-100 mm) was used to assess perceived thirst during each experiment.
RESULTS: Volume depletion (P < 0.001) and tap water (P < 0.03) both affected thirst by 13 mm per L of fluid, whereas spontaneous diuresis and infusion of Ringer’s acetate did not significantly change the thirst rating (multiple regressions). More detailed analyses showed that the volume depletion increased the median (25th-75th percentiles) thirst rating from 28 mm (21-43) to 59 mm (46-72, P < 0.001) while no change occurred in those who were only slightly thirsty (< 30 mm) before the volume depletion began. Ringer’s solution alleviated thirst in those who were very thirsty, but tended to increase thirst in the volunteers who were not thirsty before the infusion. Similarly, hydration with tap water decreased thirst (by 24 mm, P < 0.04) in those who were thirsty (> 60 mm) while the others reported no change.
CONCLUSION: The change in thirst rating during volume depletion, administration of Ringer’s acetate, and ingestion of tap water were all dependent on the thirst rating obtained when the manipulation of the body fluid volume was initiated.
- Citation: Li YH, Waldréus N, Zdolsek J, Hahn RG. Effects of tap water, electrolyte solution, and spontaneous and furosemide-stimulated urinary excretion on thirst. World J Exp Med 2012; 2(1): 1-6
- URL: https://www.wjgnet.com/2220-315X/full/v2/i1/1.htm
- DOI: https://dx.doi.org/10.5493/wjem.v2.i1.1
Thirst can be defined as a subjective perception and a deep-seated sensation or desire that causes a powerful behavioral drive to drink water. Stimuli that increase thirst include dryness of mouth, increased angiotensin II concentration and osmolality, and decreased blood volume and arterial pressure. Changes of these factors in the opposite direction decrease thirst, which does also distention of the stomach[1]. However, how these factors interact when combined is poorly known.
The present study quantifies and contrasts the effects of various modifications of body fluid volumes on thirst as reported by healthy volunteers. Such knowledge is of value for further studies of thirst in disease, which is apparently changed in, for example, congestive heart failure[2]. Therefore, this study examined combinations of treatments commonly given to modify fluid balance, such as the administration of electrolyte solution and tap water as well as spontaneous and furosemide-induced urinary excretion.
Ten healthy male volunteers with a mean age of 22 years (range 19-37 years) and with a body weight of 80 kg (75-100 kg) underwent 4 experiments each. The protocol was approved by the Ethics Committee of Linköping University (Ref. M114-09, ClinicalTrials.gov Indentifier NCT01062776). Each volunteer gave his consent for participation after being informed about the study both orally and in writing.
On the days of the experiments, the volunteers fasted after midnight but drank 800 mL of water at 6:00 AM. By allowing 2 h to excrete excess fluid we regarded that the volunteer would have arrived at their optimal degree of hydration when the experiments started at 8 AM in the Department of Intensive Care at Linköping University Hospital.
The study was designed to challenge whether changes in thirst during administration of tap water and various volumes of electrolyte-containing fluid would be different if performed in a setting of euhydration or volume depletion.
All 10 volunteers underwent the 4 experiments in random order, determined by the sealed envelope method, and the experiments were separated by at least 2 d.
The layout consisted of 2 arms, one being determined by whether the infused volume of Ringer’s was 5 mL/kg or 10 mL/kg body weight and the other whether or not the infusion was preceded by a volume depletion procedure. Hence, the 4 experiments included: (1) 5 mL/kg of acetated Ringer’s followed by 600 mL of tap water; (2) volume depletion, 5 mL/kg of acetated Ringer’s followed by 600 mL tap water; (3) 10 mL/kg of acetated Ringer’s solution followed by 600 mL of tap water; and (4) volume depletion, 10 mL/kg of acetated Ringer’s followed by 600 mL of tap water.
The volunteers were placed in the lying position on bed and a cannula was placed in the cubital vein. Thirty minutes of rest was allowed before the experiments started.
In the half of the experiments where volume depletion was to be induced, repeated intravenous doses of 5 mg of furosemide (Furix 10 mg/mL, Nycomed, Stockholm, Sweden) were given until diuresis of between 1.5 and 2.0 L had been achieved. For this purpose, 25 (range 15-35) mg of furosemide was required. Sixty minutes elapsed between the last dose of furosemide and the subsequent infusion of the Ringer’s solution, and the complete volume depletion procedure required approximately 2 h.
Plasma volume expansion was achieved by infusing acetated Ringer’s solution (Baxter Medical AB, Kista, Sweden; sodium 130, chloride 110, acetate 30, potassium 4, calcium 2, magnesium 1 mmol/L) over 15 min with the aid of an infusion pump. The fluid bag was kept 1 m above the bed on a radiant warmer to have a temperature of 27-30 °C when entering the volunteer. During and for 90 min after the infusion, venous blood samples up to a total of 40 mL were withdrawn and analyzed as presented elsewhere[3].
The urinary excretion resulting from the infusion of acetated Ringer’s was collected and measured 2 h after the infusion started.
Thereafter, the volunteer drank 600 mL of tap water.
In the half of the experiments that did not include a volume depletion procedure, the infusion of Ringer’s acetate solution was initiated after the initial resting period.
A visual analogue scale (VAS) was used to assess perceived thirst. For this purpose, the study participants were asked to mark with a cross on a 100 mm line to rate their thirst from none (0 mm) to worst possible thirst (100 mm). The VAS scale is commonly used to estimate pain and quality of life[4], and to evaluate thirst in patients with cancer, with renal or heart failure, and in patients during trauma resuscitation[5-8]. However, the scale has not been used previously to grade thirst in volunteers subjected to controlled alterations of the fluid balance.
The rating of thirst on the VAS scale was done in the beginning of each experiment, approximately 2 h after the volume depletion procedure was started (if any), 2 h after the infusion of Ringer’s had been initiated, and 30 min after rehydration with tap water.
In total, 3 thirst ratings were performed in Experiments 1 and 3 (without volume depletion), and 4 thirst ratings were recorded in Experiments 2 and 4 (with volume depletion). Data were missing or rendered invalid on 7 of these 140 occasions, which was usually due to difficulties in obtaining a urine collection in close connection with the thirst rating.
The volunteers were asked to void just before each thirst rating was made, but they were allowed to void freely in a plastic urine bag without rising up from the bed. The volume of the collected urine was recorded on admission to the hospital, after the 2-h volume depletion period, 2 h after the infusion of Ringer’s had been initiated, and exactly 30 min after the tap water was ingested.
The urine collection made at admission to the hospital was tested for color according to a standardized eight-graded scale as described elsewhere[2,9]. Urine colors 1-3 indicate that an otherwise healthy human is well hydrated, 4-5 the existence of moderate volume depletion, and 6-8 severe volume depletion[9].
The results are given as medians (25th-75th percentiles) due to the frequent occurrence skewed distributions. Changes were examined by the Wilcoxon matched-pair test and differences between groups by the Mann-Whitney U test. Simple and multiple linear regression analysis evaluated correlations. P < 0.05 was considered statistically significant.
The thirst rating at baseline for all 40 experiments was 27 (17-39). The systolic arterial pressure was 120 (112-122) mmHg and the diastolic pressure 65 (55-67) mmHg. The urine collections made at baseline all showed color grades 1, 2 or 3. Hence, all volunteers could be considered to be “well hydrated” when the experiments begun[9].
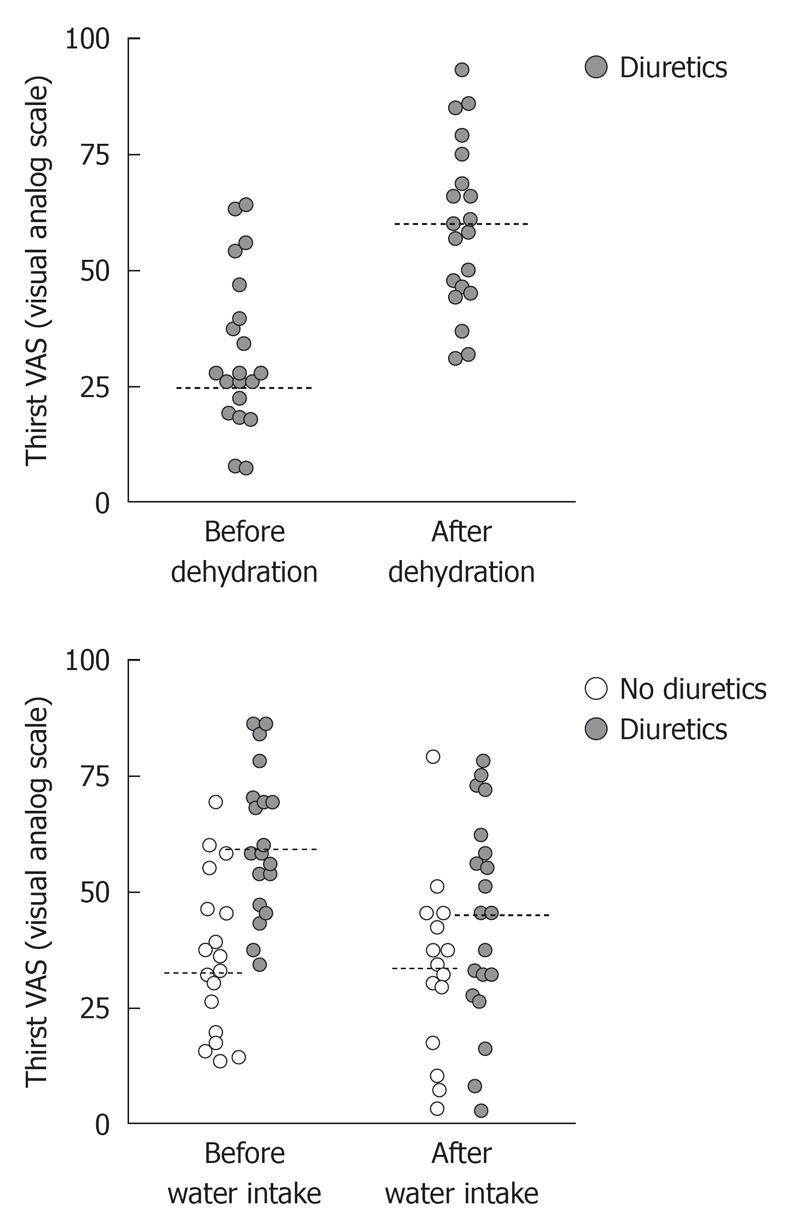
In the 50% of the experiments that started with volume depletion, the furosemide induced diuresis amounting to 1.7 (1.5-1.8) L, which increased the thirst rating from 28 (21-43) to 59 (46-72, P < 0.001; n = 20; Figure 1 top). During the volume depletion process, the diastolic pressure increased by 9% (P < 0.02) whereas the systolic pressure did not change.
There was no overall statistically significant change in thirst due to the infusion of Ringer’s solution.
Before drinking tap water, overall thirst rating was 45 (32-60) and after drinking, it was 37 (27-55, P < 0.10; Figure 1, bottom).
Spontaneous diuresis during the experiments amounted to 0.7 (0.4-1.0) L.
A single multiple regression analysis was made based on all 133 valid thirst ratings obtained during the experiments. The result showed that both furosemide-induced diuresis and ingestion of tap water significantly and independently modified thirst intensity (P < 0.001 and P < 0.03, respectively) whereas Ringer’s acetate and spontaneous diuresis did not. The degree of change was quite similar - one liter of furosemide-induced urinary excretion increased the thirst rating by 13% and one liter of tap water decreased thirst by 13%. In this analysis, the urinary excretion was set to zero for the baseline thirst rating. The furosemide-induced diuresis was set to the voided volume when volume depletion was performed and to zero when volume depletion was not performed.
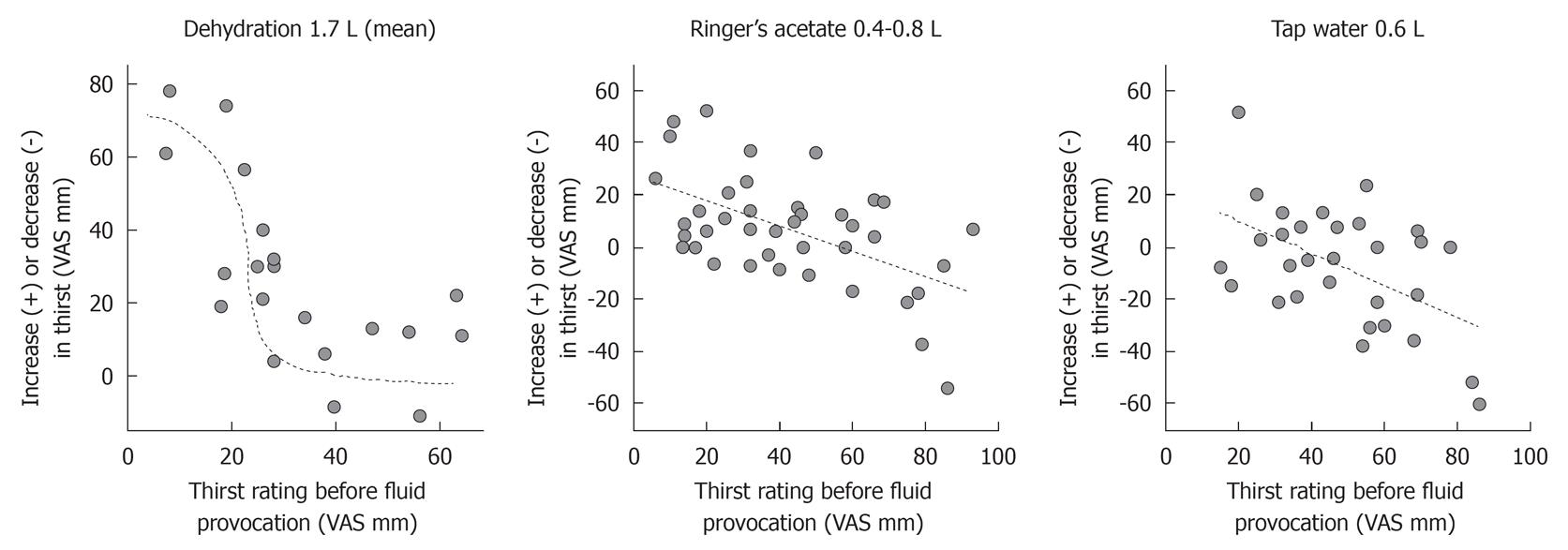
The change in thirst rating in the individual volunteers during volume depletion, administration of Ringer’s acetate, and ingestion of tap water were all dependent on the thirst rating obtained when the change in body fluid volume was initiated (Figure 2).
Hence, there was a statistically significant linear relationship between the change in thirst rating and the value recorded before furosemide was given (r = -0.68, P < 0.002). A plot of the data rather revealed a sigmoid curve (Figure 2, left). Volunteers scoring more than 30 mm at baseline reported an 11 mm (-2 to 15) higher thirst rate after volume depletion, whereas the others reported a change of 31 (25-59) (group difference, P < 0.002). The change in thirst also correlated independently and significantly with the furosemide-induced voiding volume (P < 0.02).
Although there was no overall significant change in thirst due to Ringer’s solution, volunteers with a low thirst score were likely to have an increase in thirst (Figure 2, middle). Hence, when thirst had not been induced by furosemide, the thirst rating changed from 21 (14-35) to 37 (22-54, P < 0.02). When furosemide had been given, the rating hardly changed at all - from 59 (45-72) to 57 (45-74).
Finally, there was a statistically significant relationship between the change in thirst rating and the value recorded before tap water was ingested (r = -0.52, P < 0.004; Figure 2, right) whereas the previous use of furosemide (from which residual volume depletion was still present) had no statistically significant influence (Figure 1, bottom). Hence, water reduced thirst only in those who were already thirsty; volunteers scoring less than 60 had their thirst changed by -2 mm (-15 to +9) whereas those with a thirst rating of 60 or more reported a change by -24 (-44 to +1) (group difference, P < 0.046).
Being a component of the regulatory mechanism that maintains body fluid homeostasis, thirst might arise from deficits in either intracellular or extracellular fluid volume[10]. High osmolality is a well-known signal for thirst[11,12] while the relative contribution of other mechanisms, such as hypovolemia and angiotensin II release, are less well quantified.
How these factors interact when combined is even less well known. The present study illustrates that their contributions to the thirst experience as reported on a VAS scale are dependent on the starting point. It was difficult to aggravate thirst in those who were already thirsty, while those who were not thirsty indicated a markedly increased thirst from the same stimulus. Therefore, thirst experience seems to be saturable and only a relative measure of fluid balance disturbances. The signal may become blunted when adding a fluid balance alteration upon another, although that alteration would be expected aggravate or alleviate pre-existing thirst. Therefore, the responses expected from the medical textbooks cannot always be taken for granted when stimuli are combined.
General observations include that furosemide but not spontaneous diuresis induced thirst. Furosemide dehydrated the volunteers by 1.7 L, which doubled the intensity of the thirst sensation (Figure 1, top). Hence, only the urinary excretion that makes the body deviate from its optimal volume status seemed to stimulate this signal. Moreover, tap water decreased thirst whereas an intravenous infusion of an electrolyte solution did not.
The strength of the overall influence of furosemide-induced diuresis and ingestion of tap water on thirst was further quantified by means of a multiple regression analysis. Their influence appeared to be of quite similar magnitude, although acting in opposite directions. However, the effect of volume depletion was easier to discern since the starting point, being the first thirst rating in the experiment, showed less scattering. Moreover, the furosemide-induced diuresis was almost 3 times greater than the intake of tap water.
The relative nature of the changes of thirst intensity to manipulations of the fluid balance become apparent when considering whether the volunteers reported none, little or severe thirst before the manipulation was initiated. Volume depletion induced thirst primarily in those volunteers who had a low thirst rating before the diuretic was given. Infusion of Ringer’s acetate induced thirst in the volunteers who were not thirsty. On the other hand, the Ringer’s solution did alleviate thirst in those who were very thirsty before the infusion (Figure 2, middle). Finally, tap water, which is grossly hypo-osmotic due to lack of electrolytes, only relieved thirst in those who reported intense thirst (ratings ≥ 60) before they drank the water.
The thirst response to volume depletion described a sigmoid function, showing the greatest change when the starting point was 30-45 mm, while the corresponding relationships for Ringer’s and tap water described zero-order linear relationships (Figure 2).
Shirreffs et al[13] found a gradually increased thirst when dehydration was induced by water deprivation amounting to 1%, 2% and 3% of the body weight in volunteers. The mechanism inducing such thirst is thought to be hyperosmolality[14]. Loss of cell water is detected by osmoreceptors located in the hypothalamus and this gives rise to thirst[15]. However, furosemide induces diuresis by inhibiting sodium reabsorption, but the urinary sodium concentration is still lower than in plasma. The thirst sensation recorded in our study might therefore be the result of hyperosmolality but also of mild hypovolemia, which activates components of the endocrine and autonomic nervous systems. Such activation is evidenced by the rise in diastolic arterial pressure that followed upon volume depletion. Circulating angiotensin II, which is a part of the endocrine response to reduced blood flow, might be the direct mediator of hypovolemic thirst[16]. The reason why spontaneous diuresis did not stimulate thirst is probably that hypovolemia was absent in that situation. However, these mechanisms are speculative as no measurements of the sodium concentration or of the osmolality were made.
Infusion of hypertonic saline stimulates thirst[12] while rehydration with 0.45% saline decreased thirst significantly in young athletes[17]. In the present study, we infused Ringer’s acetate solution that has a sodium concentration and osmolality slightly below that of extracellular body fluid. Despite its composition, increasing the extracellular fluid volume by an electrolyte solution induced thirst in those who were not thirsty. This response might seem unphysiological, but normovolemic volunteers show a rise in serum sodium after having received Ringer’s acetate due to excretion of hypotonic urine[18]. Moreover, blood samples amounting to 40 mL were withdrawn during the period between the fluid infusion and its distribution to the extracellular fluid volume. Finally, no water was allowed by mouth during the 2-h period between the thirst ratings. Still, when entering all fluid volume changes in an overall regression analysis, the conclusion is that Ringer’s acetate did not influence the thirst intensity.
Oral intake of water reduces thirst[11,19] which differs in composition from Ringer’s solution by being almost completely devoid of electrolytes. However, an effect on thirst can be discerned even before plasma osmolality decreases[12]. Further, gargling with tap water for 2 min relieves thirst[19]. In the present study, the overall effect of tap water on thirst was statistically significant and had the same strength as furosemide-induced diuresis. Again, the effectiveness of tap water was greatly dependent on the thirst grading reported when drinking began.
The thirst response to volume depletion followed the sigmoid form that is typical of saturable receptor mechanisms. On the other hand, the linear relationship between the change in thirst rating resulting from the intake of tap suggests that the mechanism here is a different one. The authors hypothesize that the first one is the result of angiotensin II stimulation while the second is an effect of osmolality.
Limitations of the present study include the use of various time periods between the induction of the fluid balance change and the assessment of thirst. With regard to volume depletion, 2 h passed until the volume depletion procedure was completed. Ringer’s solution requires 25-30 min to distribute in the extracellular fluid volume[20], but an additional 75 min elapsed before the assessment of thirst because urine is usually not passed until that time. Finally, thirst was assessed 30 min after the ingestion of tap water because the water was expected to have a rapid turnover and perhaps to be voided early.
In conclusion, thirst is stimulated by volume depletion and relieved by tap water but the response is greatly dependent on whether thirst is present before the fluid challenge. Only those individuals who reported intermediately severe thirst, the range being from approximately 30 to 60 mm, were clearly susceptible to stimuli that either increased or decreased thirst. Spontaneous diuresis and infusion of Ringer’s acetate solution did not significantly affect thirst.
Research nurses Susanne Öster and Helén Didriksson assisted during the experiments.
Treatments commonly given to modify the fluid balance are insufficiently investigated with regard to their effect on thirst.
Thirst visual analogue scale ratings have rarely been used in clinical studies. Therefore, relative changes in thirst intensity when different stimuli have been combined are poorly known.
The thirst sensation can apparently be saturated which should be considered if used as a measure of fluid balance disturbances. Hence, the thirst response is weaker if fluid balance alterations are added to previous changes that would alone be sufficient to aggravate pre-existing high or low thirst ratings. Thirst apparently shows the most predictable response when a subject has close to a normal fluid status.
By understanding that the thirst signal might become blunted investigators and clinicians cannot rely on thirst intensity as an index of the seriousness of fluid balance disorders.
Thirst: a component of the regulatory mechanism that maintains body fluid homeostasis; Volume depletion: reduction of the extracellular fluid volume; Hypovolemia: reduction of the blood volume; Dehydration: loss of total body water; Euhydration: the body water volume is normal.
In this study, Li et al contrast the effects of various modifications of body fluid volumes on thirst as reported by healthy volunteers.
Peer reviewer: Moses Elisaf, Professor, Department of Internal Medicine, University of Ioannina, Medical School, 451 10 Ioannina, Greece
S- Editor Wang JL L- Editor A E- Editor Zheng XM
| 1. | Guyton AC, Hall JE. Textbook of Medical Physiology. 9th ed. Philadelphia, PA: WB Saunders Company 1996; 370. [Cited in This Article: ] |
| 2. | Waldréus N, Sjöstrand F, Hahn RG. Thirst in the elderly with and without heart failure. Arch Gerontol Geriatr. 2011;53:174-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Hahn RG, Li Y, Zdolsek J. Non-invasive monitoring of blood haemoglobin for analysis of fluid volume kinetics. Acta Anaesthesiol Scand. 2010;54:1233-1240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Flaherty SA. Pain measurement tools for clinical practice and research. AANA J. 1996;64:133-140. [PubMed] [Cited in This Article: ] |
| 5. | Morita T, Tei Y, Tsunoda J, Inoue S, Chihara S. Determinants of the sensation of thirst in terminally ill cancer patients. Support Care Cancer. 2001;9:177-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Wirth JB, Folstein MF. Thirst and weight gain during maintenance hemodialysis. Psychosomatics. 1982;23:1125-117, 1125-117, 1134. [PubMed] [Cited in This Article: ] |
| 7. | Holst M, Strömberg A, Lindholm M, Uden G, Willenheimer R. Fluid restriction in heart failure patients: is it useful? The design of a prospective, randomised study. Eur J Cardiovasc Nurs. 2003;2:237-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Gerhardt RT, Shaffer BM, Dixon P, Pfaff JA, Liker J, Ward J, Mueller GM. Diagnostic and predictive values of thirst, angiotensin II, and vasopressin during trauma resuscitation. Prehosp Emerg Care. 2010;14:317-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Armstrong LE, Maresh CM, Castellani JW, Bergeron MF, Kenefick RW, LaGasse KE, Riebe D. Urinary indices of hydration status. Int J Sport Nutr. 1994;4:265-279. [PubMed] [Cited in This Article: ] |
| 10. | McKinley MJ, Cairns MJ, Denton DA, Egan G, Mathai ML, Uschakov A, Wade JD, Weisinger RS, Oldfield BJ. Physiological and pathophysiological influences on thirst. Physiol Behav. 2004;81:795-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Phillips PA, Rolls BJ, Ledingham JG, Forsling ML, Morton JJ, Crowe MJ, Wollner L. Reduced thirst after water deprivation in healthy elderly men. N Engl J Med. 1984;311:753-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 565] [Cited by in F6Publishing: 441] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Thompson CJ, White MC, Baylis PH. Osmoregulation of thirst and vasopressin secretion in Kallmann's syndrome. Clin Endocrinol (Oxf). 1989;30:539-547. [PubMed] [Cited in This Article: ] |
| 13. | Shirreffs SM, Merson SJ, Fraser SM, Archer DT. The effects of fluid restriction on hydration status and subjective feelings in man. Br J Nutr. 2004;91:951-958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Mack GW, Weseman CA, Langhans GW, Scherzer H, Gillen CM, Nadel ER. Body fluid balance in dehydrated healthy older men: thirst and renal osmoregulation. J Appl Physiol. 1994;76:1615-1623. [PubMed] [Cited in This Article: ] |
| 15. | Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583-686. [PubMed] [Cited in This Article: ] |
| 16. | McKinley MJ, Johnson AK. The physiological regulation of thirst and fluid intake. News Physiol Sci. 2004;19:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Riebe D, Maresh CM, Armstrong LE, Kenefick RW, Castellani JW, Echegaray ME, Clark BA, Camaione DN. Effects of oral and intravenous rehydration on ratings of perceived exertion and thirst. Med Sci Sports Exerc. 1997;29:117-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Hahn RG, Drobin D. Rapid water and slow sodium excretion of acetated Ringer's solution dehydrates cells. Anesth Analg. 2003;97:1590-1594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Seckl JR, Williams TD, Lightman SL. Oral hypertonic saline causes transient fall of vasopressin in humans. Am J Physiol. 1986;251:R214-R217. [PubMed] [Cited in This Article: ] |
| 20. | Hahn RG. Volume kinetics for infusion fluids. Anesthesiology. 2010;113:470-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |