Published online Oct 18, 2020. doi: 10.5492/wjccm.v9.i4.63
Peer-review started: June 16, 2020
First decision: July 21, 2020
Revised: July 31, 2020
Accepted: August 24, 2020
Article in press: August 24, 2020
Published online: October 18, 2020
High mobility group box 1 (HMGB1) has been studied as a molecule associated with severe outcomes in sepsis and thrombomodulin (TM) seems to decrease HMGB1 activity.
To investigate the role of the thrombomodulin/high mobility group box 1 (T/H) ratio in patients with sepsis and their association with their clinic, testing the hypothesis that higher ratios are associated with better outcomes.
Twenty patients diagnosed with sepsis or septic shock, according to the 2016 criteria sepsis and septic shock (Sepsis-3), were studied. Patients were followed until they left the intensive care unit or until they achieved 28 d of hospitalization (D28). The following clinical outcomes were observed: Sequential Organ Failure Assessment (SOFA) score; Need for mechanical pulmonary ventilation; Presence of septic shock; Occurrence of sepsis-induced coagulopathy; Need for renal replacement therapy (RRT); and Death.
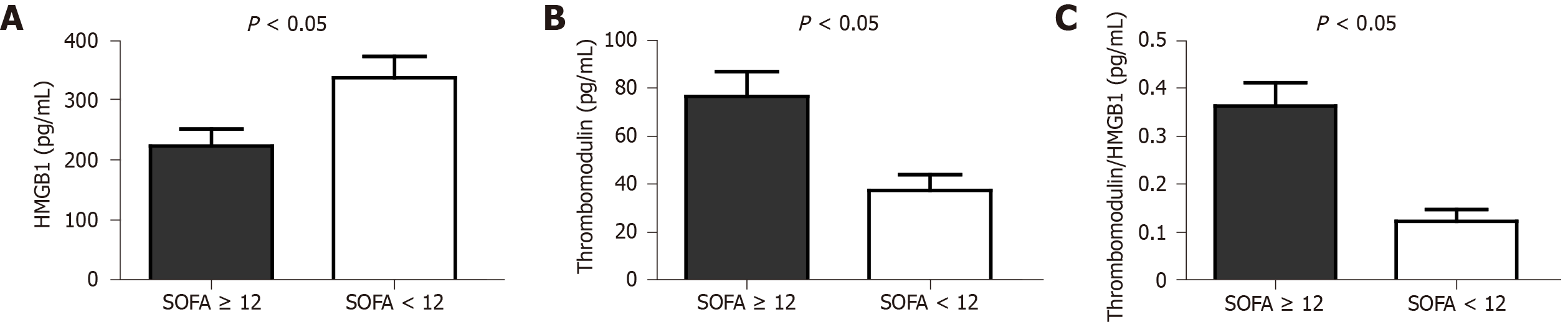
The results showed that patients with SOFA scores greater than or equal to 12 points had higher serum levels of TM: 76.41 ± 29.21 pg/mL vs 37.41 ± 22.55 pg/mL among those whose SOFA scores were less than 12 points, P = 0.003. The T/H ratio was also higher in patients whose SOFA scores were greater than or equal to 12 points, P = 0.001. The T/H ratio was, on average, three times higher in patients in need of RRT (0.38 ± 0.14 vs 0.11 ± 0.09), P < 0.001.
Higher serum levels of TM and, therefore, higher T/H ratio in the first 24 h after the diagnosis of sepsis were associated with more severe disease and the need for renal replacement therapy, while those with better clinical outcomes and those who were discharged before D28 showed a tendency for lower T/H ratio values.
Core Tip: The knowledge of physiological mechanisms that lead an organism to respond to an infectious agent with such intensity is of great importance. It has been described that during sepsis, an organism produces intense inflammatory activity, caused by the action of several inflammatory mediators. High mobility group box 1 (HMGB1) has been the target of recent studies for its proinflammatory actions as well as for the possibility of having its action reduced by thrombomodulin. For this reason, this study proposed to evaluate the relationship between thrombomodulin and HMGB1 in the initial phase of sepsis and its association with clinical outcomes in sepsis patients.
- Citation: Rodrigues AT, Rodrigues JT, Rodrigues CT, Volpe CMO, Rocha-Silva F, Nogueira-Machado JA, Alberti LR. Association between thrombomodulin and high mobility group box 1 in sepsis patients. World J Crit Care Med 2020; 9(4): 63-73
- URL: https://www.wjgnet.com/2220-3141/full/v9/i4/63.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v9.i4.63
Sepsis is a severe syndrome characterized by physiological, pathological and biochemical life-threatening modifications induced by an infection. It is one of the major causes of mortality in intensive care unit (ICU) worldwide. Its treatment is complex and demands the proper use of specific antibiotics, vasoactive amines, and, in certain situations, corticosteroids. In addition, advanced technology, such as mechanical pulmonary ventilators and renal replacement therapy (RRT), can also be required[1-4].
It is known that inflammatory activity is the most evident feature of sepsis and because of that the host immune response has been studied to develop new therapeutic strategies. One potential treatment relied on the modulation of pro-inflammatory mediators, such as tumor necrose factor-α (TNF-α) and interleukin-1 (IL-1). However, even though this strategy had promising results in animal models, the same results could not be replicated in human studies[5,6].
High mobility group box 1 (HMGB1) is a nuclear protein, released by cells during oxidative stress that has proinflammatory activity. It has been studied as a promising therapeutic target because of its delayed increase 12 to 18 h after TNF-α peaks[6-10]. Janeway et al[11], in 1989, described the role of damage-associated molecular patterns (DAMPs) during the early stages of toxemia. HMGB1 seems to act as a DAMP[6], activating macrophages and monocytes, as well as promoting dendritic cell maturation. In its reduced state, HMGB1 exhibits minimum activity. However, in sepsis, as oxidative stress increases, it assumes the role of a proinflammatory molecule and stimulates the release of some cytokines, such as IL-1β and IL-17, TNF-α, macrophage inflammatory protein (MIP)-1α, MIP-1β, MIP-2, granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor[12]. HMBG1 has also been associated with a procoagulant state, promoting the occurrence of sepsis-induced coagulopathy (SIC)[13,14].
On the other hand, thrombomodulin (TM) and antithrombin (AT) seem to have immunomodulating activities in sepsis. TM is a cell membrane glycoprotein expressed on the luminal surface of endothelial cells, where it modulates thrombin procoagulant effects. Thrombin and the TM-thrombin complex can cleave HMBG1, reducing its activity and, hence, its proinflammatory action[15,16]. In animal models, the sepsis mortality rate decreases with the coadministration of AT and TM[8,16]. Xie et al[17] (2010) demonstrated the role of oxidative stress in animal models. They observed that the use of hydrogen gas, by reducing oxidative products, led to the decreased release of HMGB1 and proinflammatory activity. The efficacy of TM-α in the management of intravascular coagulation associated with sepsis has been evaluated in clinical trials[18], but its effects are still being evaluated.
This study had the objective of evaluating the TM/HMGB1 ratio among sepsis cases and their associated outcomes: Sequential Organ Failure Assessment (SOFA) score; Mechanical ventilation; Shock; Coagulopathy; Severe acute kidney injury (AKI); and Death.
This was a case-control study. Twenty patients diagnosed with sepsis or septic shock, were selected according to the 2016 criteria sepsis and septic shock (Sepsis-3) and followed until they left the ICU or until they achieved 28 d of hospitalization (D28). The following clinical outcomes were observed: SOFA score; Need for pulmonary mechanical ventilation (MV); Presence of septic shock; occurrence of SIC; Need for RRT; and Death. Their association with HMGB1 and thrombomodulin levels and thrombomodulin/high mobility group box 1 (T/H) ratio were analyzed.
This study was carried out in the ICU of Santa Casa de Belo Horizonte (SCBH) between October 2018 and March 2019.
Twenty adult patients diagnosed with sepsis (cases) were consecutively selected according to the criteria presented in 2016 by the third international consensus definitions for Sepsis-3[1]. Sepsis was confirmed by the presence of fever and/or leukocytosis or leukopenia and/or elevated C-reactive protein level associated with the presence of an infection focus and an increase in the SOFA score greater than or equal to 2 points compared to baseline scores. Sepsis patients (cases) were followed for up to 28 d in the ICU or until discharge from the unit.
The control group was formed by 20 patients without sepsis or acute severe life-threatening disease. They were invited to be included in the control group, and blood samples for the measurement of HMGB1 and TM were collected from those who had signed the informed consent form.
Among the samples collected in the first 24 h of diagnosis to determine the patient clinical state and proper patient attendance, 10 mL of blood was reserved in VacutainerTM tubes containing saline solution to dose TM and HMGB1. The method applied for examination was sandwich ELISA. The quantification of TM and HMGB1 was performed using the ELISA kit for HMGB1 protein commercial kits, Lot: L160322647 e DOU SETR Human Thrombomodulin/BDCA-3, Catalog No. DY3947 (Lot P 168874), following the manufacturer’s guidelines.
For the diagnosis and clinical management of patients with sepsis (cases), the following exams were performed and data collected, as requested by the assistant medical team: Hemogram; Determination of international normalized ratio (INR) and activated prothrombin time; Dosage of C-reactive protein, urea and creatinine as well as the arterial blood gas and lactate dosage in arterial blood; Determination of serum levels of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and gamma-glutamyl-transferase; Serum bilirubin measurements; Blood cultures, urine cultures; and secretion cultures.
The controls and cases were compared according to their demographic, clinical and laboratory characteristics and levels of HMGB1 and thrombomodulin and the T/H ratio. The association of serum levels of HMGB1 and thrombomodulin and the T/H ratio between cases and the following clinical outcomes was assessed: SOFA score greater than or equal to 12; Need for and time of pulmonary MV; PaO2/FiO2 ratio[19]; Presence of shock; Presence of SIC; Presence of severe AKI with the need for RRT; and Death until D28.
The clinical evaluation of the patients with sepsis was made through prospective analysis of their medical records from the time of sepsis diagnosis until their discharge from the ICU or until D28. The diagnosis of sepsis followed the third international consensus definitions for Sepsis-3 recommendations[1]. The parameters evaluated included age; sex; SOFA score greater than 12 points[20,21]; need for and duration of mechanical pulmonary ventilation; presence of septic shock (according to third international consensus definitions for Sepsis-3)[1]; presence of AKI according to the criteria established by the Kidney Disease Improving Global Outcomes Group[22,23] and need for RRT; presence of coagulation disorders, such as thrombocytopenia and elevated INR values; time in ICU therapy; and death. The score for the diagnosis of SIC score proposed by Iba et al[24] in 2017 and validated by Yamakawa et al[25] (2019) was used to define the presence of SIC. It considers three parameters: The INR, platelet count, and SOFA score.
The sepsis management protocol in the ICU of the SCBH recommends the use of low-molecular-weight heparin in patients with septic shock. Additionally, if there is a contraindication to its use, mechanical prophylaxis should be considered.
The SOFA score with scores equal to or greater than 12 points was chosen as a cohort point because it has been associated with higher mortality rates by some authors[20,21].
These outcomes were correlated with the TM and HMGB1 serum levels in the peripheral blood of patients diagnosed with sepsis (case group), testing the hypothesis that higher T/H ratios could be associated with better outcomes.
The sample size was calculated using Open Epi, open source epidemiological statistics for public health, version 3.01, updated in 2013 (available at https://www.openepi.com/SampleSize/SSPropor.htm), admitting alpha error of 0.05, beta error of 0.20 (80% statistical power). Considering the number of beds in the ICU (110 beds) and the frequency of sepsis patients in Brazilian ICUs (16.7%)[2] the sample size found was 13 patients/group. In order address potential bias an analysis of the power to compare two means was also performed using the normal comparison method considering a 95% confidence interval, 80% comparative power and sample size ratio (group2/group1).
The statistical analysis was performed using Epi Info, version 3.5.4 for Windows, Atlanta: Centers for Disease Control and Prevention[26]. The ANOVA test was used to compare parametric continuous numerical variables, and the Mann-Whitney/Wilcoxon and Kruskal-Wallis test when ANOVA was not indicated. The results were expressed as the mean ± SD, when they were parametric, or the median and variation between the first and third quartiles, when nonparametric. The comparison of the distribution of categorical variables was analyzed through Fisher’s test, two-sided Student’s, t-tests and yates corrected chi-squared (χ2) test. The significance of probability was considered expressive when its value was less than 0.05 (P < 0.05).
The demographic characteristics of the cases and controls are shown in Table 1. The patients demonstrated a higher average age than the controls. The control group displayed higher weight and BMI values than the patients (Table 1).
The comorbidities found more frequently among cases were heart disease; high blood pressure or heart valve disease (40%); oncologic or hematologic diseases (45%); compensated chronic liver disease (40%); post-liver transplantation (5%); non-dialysis chronic kidney disease (15%); dialysis CKD (5%); chronic obstructive pulmonary disease (10%); and diabetes mellitus (15%).
Patients diagnosed with sepsis (cases) had a mean SOFA score of 9.6 ± 4.8 points with the following average per system, as shown in Table 2. The cases that evolved to death had mean SOFA scores equal to 12.83 ± 2.64 points.
| System | Points ± SD |
| Overall mean score | 9.6 ± 4.8 |
| Respiratory function | 2.1 ± 1.28 |
| Scoring in the coagulation system | 1.1 ± 1.56 |
| Scoring in circulatory function | 2.7 ± 1.63 |
| Liver function score | 0.1 ± 0.22 |
| Neurological function | 1.61 ± 0.85 |
| Renal function | 2.3 ± 1.87 |
There was no significant difference between the cases and controls in terms of the global evaluation of the serum dosage of HMGB1 (291.11 ± 119.49 pg/mL vs 328.14 ± 164.04 pg/mL), TM (52.9 ± 31.49 pg/mL vs 53.31 ± 37.69 pg/mL) and the T/H ratio (0.22 ± 0.17 vs 0.21 ± 0.18), P = 0.419, 0.970 and 0.857 (t-test) respectively. However, when sepsis patients (case group) with SOFA scores ≥ 12 points were compared to those with SOFA scores < 12 points, there was a significant difference between those groups in terms of both the TM level and the T/H ratio (Figure 1).
Among the 20 patients with sepsis (cases), 14 of them (70%) needed MV. The mean MV time was 9.25 ± 9.8 d. Among the case group, the level of TM and HMGB1 had no association with the need of VM nor the time (d) of mechanical ventilation P = 0.509 and 0.888, respectively (Mann-Whitney test). The mean T/H ratio among the case group was not associated with the mean time in MV either, P = 0.760 (ANOVA).
Regarding hemodynamic alterations, fourteen patients in the case group (70%) required the use of vasoactive amines to maintain a mean arterial pressure (MAP) above 65 mmHg, and eleven (55%) met the septic shock criteria according to the Sepsis-3[1]. The study showed a mean TM serum level of 54.48 ± 36.34 pg/mL for those patients diagnosed with septic shock and 50.79 ± 23.97 pg/mL for those patients without septic shock, P = 0.797 (t-test) and the T/H ratio was 0.21 ± 0.17 and 0.23 ± 0.16 for those with and without shock, respectively, P = 0.791 (t-test). The HMGB1 serum levels were 313.39 pg/mL ± 119.13 pg/mL and 263.96 ± 121.26 pg/mL for those with and without shock, respectively, P = 0.227 (t-test).
Concerning coagulation disorders, a median platelet count of 177 × 109/L (QR 63 × 109/L-312 × 109/L) was found in patients with sepsis (cases) and of 185 × 109/L (QR 164 × 109/L-213 × 109/L) in the control group, P = 0.807 (Mann-Whitney test). Eight patients in the case group (40%) demonstrated platelets values lower than 150 × 109/L. The mean INR was 1.3 ± 0.43. The difference between cases with RNI ≤ 1.2 or > 1.2 is showed in Table 3.
| Cases (n = 20) | |||
| INR ≤ 1.2 (n = 13) | INR > 1.2 (n = 7) | P value1 | |
| HMGB1 (pg/mL) | 300.28 ± 133.21 | 261.81 ± 133.21 | 0.431 |
| TM (pg/mL) | 46.67 ± 26.71 | 64.77 ± 38.33 | 0.875 |
| T/H | 0.19 ± 0.15 | 0.28 ± 0.18 | 0.257 |
Among the sepsis patients (case group), eight (40%) met the criteria of SIC[24] as shown in Table 4. Concerning renal function, the median serum level of creatinine was 2.5 (QR 0.87-4.19) mg/dL among the case group and 1.03 (QR 0.89-1.12) mg/dL among the control group, P = 0.09 (Mann-Whitney test). Twelve patients in the case group (60%) had acute kidney insufficiency secondary to sepsis, and 10 (50%) required RRT.
The presence of severe acute kidney failure with the need for RRT revealed a significant association with serum levels of TM and the T/H ratio, as shown in Table 5. The patients stayed in the ICU for an average of 15.05 ± 10.2 d. Nine (45%) were discharged from the ICU before D28, five (25%) stayed for more than 28 d, and six (30%) died. In terms of the cases’ evolution (discharge, ICU stay on D28 or death), the HMGB1 levels were 305.47 ± 103.15 pg/mL, 311.5 ± 188.41 pg/mL and 252.575 ± 79.21 pg/mL respectively, P = 0.662, and TM: 40.78 ± 24.34 pg/mL, 55.74 ± 32.12 pg/mL and 68.96 ± 37.59 pg/mL, P = 0.240. Nevertheless, patients who were discharged before D28 displayed had a lower T/H ratio (0.14 ± 0.09) compared to those who died or remained hospitalized after D28 (0.28 ± 0.18), P = 0.039 (t-test).
The aim of the study was to test the hypothesis that higher T/H ratios would be associated with better outcomes, considering the anti-inflammatory activity of TM. However, the study had several limitations, including the small sample size, its observational characteristic and its being conducted in one single center.
Sepsis is currently the leading cause of death in ICUs[1,2,6,19,27,28], affecting more frequently patients with extreme ages and patients with chronic diseases[1,29]. Regarding the serum level of HMGB1, elevated values are not expected in the initial phase of sepsis. Gibot et al[30] (2007) demonstrated that serum HMGB1 levels greater than 4000 pg/mL (4 ng/mL) on the third evaluation day patients with septic shock were associated with a higher risk of death, with an odds ratio equal to 5.5 and ranging from 1.3-23.6 considering the 95% confidence interval. However, as occurred in the current study, the authors found no significant association between HMBG1 and the clinical and laboratory parameters that make up the SOFA score when these were assessed separately[30,31]. Other authors have failed to demonstrate an association between serum HMGB1 levels and patient survival in other clinical situations[32,33].
However, the aim of this study was to assess the relationship between the two molecules TM and HMGB1 in the first 24 h and their association with the evolution of patients with sepsis (cases). Some experimental studies suggest that TM is able to reduce the signaling action of HMGB1 in sepsis, and clinical trials are underway to evaluate the effect of TM administration on patients with sepsis[8,13,34].
Regarding hemodynamic conditions, two-thirds of the patients’ case group needed vasoactive drugs to maintain a MAP greater than or equal to 65 mmHg. Hemodynamic changes in sepsis result from the association among complex mechanisms, both cellular and humoral, which lead to endothelial lesions, promote greater vascular permeability and, hence, cause organ damage[35]. Among humoral reactions, the cytokines released by macrophages play an important role in the inflammatory response to infection[36]. This set of responses to an offending agent can lead to the activation of the coagulation cascade and, consequently, to disseminated intravascular coagulation with impaired tissue perfusion of organs[22,37].
The hemodynamic changes observed in patients with septic shock can also cause acute renal dysfunction, which appears as a consequence of immunological, toxic and inflammatory mechanisms that are involved in kidney damage. Fortunately, better outcomes have been observed among patients with AKI who require RRT in recent years. This change in prognosis is probably due to improvements in the sensitivity of the diagnosis of AKI, and, consequently, to the onset of RRT at a more appropriate time[22,38].
In this study, it was observed that 50% of the cases’ patients required RRT. Levy et al[39], in 2010 reported that 85.6% of patients with sepsis had cardiovascular dysfunction, 30.8% had respiratory dysfunction, 39.5% had renal dysfunction, 10.2% had hepatic impairment, and 25.7% had hematological abnormalities. Okamoto et al[40], 2012, studying acute renal failure in patients with sepsis, observed that, although the presence of acute renal failure was not associated with a longer hospital stay, mortality was twice as high in septic patients with acute renal failure.
Although hemodynamic changes were not associated with changes in serum TM and HMGB1 nor T/H ratio in the present study, cases who needed RRT presented higher levels of TM in peripheral blood and higher T/H ratios when compared to controls and cases without RRT. TM is also a marker of endothelial injury, and its increase in patients with severe AKI could be secondary to higher production rates or reduced clearance by the kidney, as noted by Małyszko et al[41] in 2004.
Regarding sepsis coagulation disorders, there is the possibility of confounding factors such as decompensated chronic liver diseases or the use of oral anticoagulants. In the current study only one patient had chronic liver disease, but this patient had no changes in clotting factors or platelet counts. The sepsis management protocol in the ICU of the SCBH recommends the use of low-molecular-weight heparin instead of oral anticoagulants in patients with septic shock or mechanical prophylaxis when low-molecular-weight heparin is not indicated.
Activation of the coagulation system in sepsis occurs through a multifactorial mechanism and involves the activation of PRRs by PAMPs and DAMPs, including HMGB1, which has been associated with a procoagulant state and the presence of disseminated intravascular coagulation[13,14,42]. In sepsis, platelet activation can be triggered by the action of thrombin and by inflammatory mediators that promote thrombocytopenia, thrombin generation and increased inflammation[43]. Platelets are also capable of releasing HMGB1, which plays a proinflammatory and an important procoagulant role[42,44]. Another molecule whose role in SIC has been studied is TM[8,15,45]. TM and the TM-antithrombin complex assist in the degradation of HMGB1 and, therefore, reduce its proinflammatory effect[13]. Although this effect has been observed in animal models[8,15,45], in the current study, a positive association was observed between patients with higher TM levels in the first 24 h and the presence of SIC[24] compared to controls and cases without SIC.
The clinical use of recombinant TM has been tested, and although theoretically promising, it has not been associated with a significant reduction in mortality or other secondary outcomes when compared to placebo to date[2,34,46,47]. Rhodes et al[48] recommended not using antithrombin due to a lack of evidence of an effect. Regarding TM, the authors reported that they would not make recommendations until its effects were further studied. A recently published randomized clinical trial (the SCARLET randomized clinical trial) also failed to demonstrate a significant reduction in mortality on D28 in patients with SIC as a consequence of the use of human recombinant thrombomodulin[34]. Some authors suggested that the start of administration of recombinant TM could have been delayed in relation to the onset of inflammatory reactions and the activation of the coagulation cascade, and these authors question whether there is a profile of sepsis presentation that would benefit more from its use[49].
Sepsis is a serious clinical syndrome that requires advanced life support, and a diagnosis should be made as early as possible since mortality increases in patients with greater hemodynamic impairment, as shown by the evaluation of these patients. With the growing knowledge on sepsis, a new challenge has become evident: improving post-sepsis quality of life. Despite the severity of sepsis and the difficulties related to its diagnosis and treatment, survival has improved. However, the risk of reinfection is greater in patients who have sepsis, in addition to the greater propensity to exhibit serious injuries or enough to compromise the patients' ability to maintain self-care[50,51]. The work by Westphal et al[52] analyzed 217 inpatients with sepsis, with only 63 out of 112 patients experiencing high survival of more than 2 years after discharge. Among the survivors, 36 answered a quality of life questionnaire, and the following was observed from the answers: A significant reduction in functional capacity, vitality, and mental health; The presence of pain; Worse general health status; and Main physical and emotional aspects.
High mobility group box 1 (HMGB1) has been studied as a molecule associated with severe outcomes in sepsis and thrombomodulin (TM) seems to decrease HMGB1 proinflammatory activity.
We aimed to investigate the role of the thrombomodulin/high mobility group box 1 (T/H) ratio, in the first 24 h, in patients with sepsis.
To test the hypothesis that higher ratios would be associated with better outcomes.
We studied twenty patients diagnosed with sepsis. They were followed until they left the intensive care unit or until they achieved 28 d of hospitalization. The following clinical outcomes were observed: Sequential Organ Failure Assessment (SOFA) score; Need for mechanical ventilation; Presence of septic shock; Occurrence of sepsis-induced coagulopathy; Need for renal replacement therapy (RRT); and Death.
The results showed that patients with SOFA scores greater than or equal to 12 points and those who need RRT had higher serum levels of TM and therefore higher T/H ratio.
The authors concluded that higher serum levels of TM and, therefore, higher T/H ratio in the first 24 h after the diagnosis of sepsis were associated with a more severe disease.
As this was a single center study, we cannot extrapolate the results to the general population. Further studies with bigger samples and at different centers are needed.
The authors thank the ICU staff for being so kind with all their patients.
Manuscript source: Unsolicited manuscript
Specialty type: Critical care medicine
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pota V S-Editor: Yan JP L-Editor: A P-Editor: Li JH
| 1. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13771] [Cited by in F6Publishing: 13742] [Article Influence: 1717.8] [Reference Citation Analysis (2)] |
| 2. | Sales Júnior JA, David CM, Hatum R, Souza PC, Japiassú A, Pinheiro CT, Friedman G, Silva OB, Dias MD, Koterba E, Dias FS, Piras C, Luiz RR, Grupo de Estudo de Sepse do Fundo AMIB. [An epidemiological study of sepsis in Intensive Care Units: Sepsis Brazil study]. Rev Bras Ter Intensiva. 2006;18:9-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Rochwerg B, Oczkowski SJ, Siemieniuk RAC, Agoritsas T, Belley-Cote E, D'Aragon F, Duan E, English S, Gossack-Keenan K, Alghuroba M, Szczeklik W, Menon K, Alhazzani W, Sevransky J, Vandvik PO, Annane D, Guyatt G. Corticosteroids in Sepsis: An Updated Systematic Review and Meta-Analysis. Crit Care Med. 2018;46:1411-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 4. | Lamontagne F, Rochwerg B, Lytvyn L, Guyatt GH, Møller MH, Annane D, Kho ME, Adhikari NKJ, Machado F, Vandvik PO, Dodek P, Leboeuf R, Briel M, Hashmi M, Camsooksai J, Shankar-Hari M, Baraki MK, Fugate K, Chua S, Marti C, Cohen D, Botton E, Agoritsas T, Siemieniuk RAC. Corticosteroid therapy for sepsis: a clinical practice guideline. BMJ. 2018;362:k3284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Fisher CJ, Agosti JM, Opal SM, Lowry SF, Balk RA, Sadoff JC, Abraham E, Schein RM, Benjamin E. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334:1697-1702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 873] [Cited by in F6Publishing: 799] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 6. | Shimaoka M, Park EJ. Advances in understanding sepsis. Eur J Anaesthesiol Suppl. 2008;42:146-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Takehara K, Murakami T, Kuwahara-Arai K, Iba T, Nagaoka I, Sakamoto K. Evaluation of the effect of recombinant thrombomodulin on a lipopolysaccharide-induced murine sepsis model. Exp Ther Med. 2017;13:2969-2974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Iba T, Miki T, Hashiguchi N, Yamada A, Nagaoka I. Combination of antithrombin and recombinant thrombomodulin attenuates leukocyte-endothelial interaction and suppresses the increase of intrinsic damage-associated molecular patterns in endotoxemic rats. J Surg Res. 2014;187:581-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Matzinger P. Essay 1: the Danger model in its historical context. Scand J Immunol. 2001;54:4-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Abdulmahdi W, Patel D, Rabadi MM, Azar T, Jules E, Lipphardt M, Hashemiyoon R, Ratliff BB. HMGB1 redox during sepsis. Redox Biol. 2017;13:600-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Janeway C. Immunogenicity signals 1,2,3 ... and 0. Immunol Today. 1989;10:283-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 105] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Tang D, Kang R, Zeh HJ, Lotze MT. High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal. 2011;14:1315-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 363] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 13. | Ito T, Kawahara K, Nakamura T, Yamada S, Nakamura T, Abeyama K, Hashiguchi T, Maruyama I. High-mobility group box 1 protein promotes development of microvascular thrombosis in rats. J Thromb Haemost. 2007;5:109-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Okamoto K, Tamura T, Sawatsubashi Y. Sepsis and disseminated intravascular coagulation. J Intensive Care. 2016;4:23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Ito T, Kawahara K, Okamoto K, Yamada S, Yasuda M, Imaizumi H, Nawa Y, Meng X, Shrestha B, Hashiguchi T, Maruyama I. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler Thromb Vasc Biol. 2008;28:1825-1830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 16. | Iba T, Nakarai E, Takayama T, Nakajima K, Sasaoka T, Ohno Y. Combination effect of antithrombin and recombinant human soluble thrombomodulin in a lipopolysaccharide induced rat sepsis model. Crit Care. 2009;13:R203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Xie K, Yu Y, Pei Y, Hou L, Chen S, Xiong L, Wang G. Protective effects of hydrogen gas on murine polymicrobial sepsis via reducing oxidative stress and HMGB1 release. Shock. 2010;34:90-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Aikawa N, Shimazaki S, Yamamoto Y, Saito H, Maruyama I, Ohno R, Hirayama A, Aoki Y, Aoki N. Thrombomodulin alfa in the treatment of infectious patients complicated by disseminated intravascular coagulation: subanalysis from the phase 3 trial. Shock. 2011;35:349-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526-2533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1812] [Cited by in F6Publishing: 4025] [Article Influence: 335.4] [Reference Citation Analysis (0)] |
| 20. | Rodrigues-Filho EM, Fernandes R, Garcez A. SOFA in the first 24 hours as an outcome predictor of acute liver failure. Rev Bras Ter Intensiva. 2018;30:64-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Hissa PNG, Hissa MRN, Araújo PSR. Análise comparativa entre dois escores na previsão de mortalidade em unidade terapia intensiva. Revista da Sociedade Brasileira de Clínica Médica. 2013;11:21-26. [Cited in This Article: ] |
| 22. | Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, Bagshaw SM, Glassford NJ, Lankadeva Y, Vaara ST, Schneider A. Acute kidney injury in sepsis. Intensive Care Med. 2017;43:816-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 402] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 23. | Reference Keys. Kidney Int Suppl (2011). 2012;2:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7:e017046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 223] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 25. | Yamakawa K, Yoshimura J, Ito T, Hayakawa M, Hamasaki T, Fujimi S. External Validation of the Two Newly Proposed Criteria for Assessing Coagulopathy in Sepsis. Thromb Haemost. 2019;119:203-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Centers for Disease Control and Prevention. Epi Info™, version 3.5.4. for windows, Division of Health Informatics & Surveillance (DHIS), Center for Surveillance, Epidemiology & Laboratory Services (CSELS). Available from: https://www.cdc.gov/epiinfo/. [Cited in This Article: ] |
| 27. | Morello LG, Dalla-Costa LM, Fontana RM, Netto ACSO, Petterle RR, Conte D, Pereira LA, Krieger MA, Raboni SM. Assessment of clinical and epidemiological characteristics of patients with and without sepsis in intensive care units of a tertiary hospital. Einstein (Sao Paulo). 2019;17:eAO4476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Laterre PF, Levy MM, Wittebole X, Dugernier T, Francois B, Opal SM. Should we continue to test soluble thrombomodulin, or other systemic anticoagulants, as a life-saving therapy for sepsis-induced coagulopathy? Anaesth Crit Care Pain Med. 2019;38:419-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Rhee C, Jones TM, Hamad Y, Pande A, Varon J, O'Brien C, Anderson DJ, Warren DK, Dantes RB, Epstein L, Klompas M; Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program. Prevalence, Underlying Causes, and Preventability of Sepsis-Associated Mortality in US Acute Care Hospitals. JAMA Netw Open. 2019;2:e187571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 277] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 30. | Gibot S, Massin F, Cravoisy A, Barraud D, Nace L, Levy B, Bollaert PE. High-mobility group box 1 protein plasma concentrations during septic shock. Intensive Care Med. 2007;33:1347-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Zheng S, Weng Q, Wu W, Ding G. Blood purification treatment initiated at the time of sepsis diagnosis effectively attenuates serum HMGB1 upregulation and improves patient prognosis. Exp Ther Med. 2017;14:3029-3035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Malig MS, Jenne CN, Ball CG, Roberts DJ, Xiao Z, Kirkpatrick AW. High Mobility Group Box-1 Protein and Outcomes in Critically Ill Surgical Patients Requiring Open Abdominal Management. Mediators Inflamm. 2017;2017:6305387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Alpkvist H, Athlin S, Mölling P, Norrby-Teglund A, Strålin K. High HMGB1 levels in sputum are related to pneumococcal bacteraemia but not to disease severity in community-acquired pneumonia. Sci Rep. 2018;8:13428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Vincent JL, Francois B, Zabolotskikh I, Daga MK, Lascarrou JB, Kirov MY, Pettilä V, Wittebole X, Meziani F, Mercier E, Lobo SM, Barie PS, Crowther M, Esmon CT, Fareed J, Gando S, Gorelick KJ, Levi M, Mira JP, Opal SM, Parrillo J, Russell JA, Saito H, Tsuruta K, Sakai T, Fineberg D; SCARLET Trial Group. Effect of a Recombinant Human Soluble Thrombomodulin on Mortality in Patients With Sepsis-Associated Coagulopathy: The SCARLET Randomized Clinical Trial. JAMA. 2019;321:1993-2002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 35. | Russell JA, Rush B, Boyd J. Pathophysiology of Septic Shock. Crit Care Clin. 2018;34:43-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 36. | Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 620] [Cited by in F6Publishing: 554] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 37. | Siqueira-Batista R, Gomes AP, Calixto-Lima L, Vitorino RR, Perez MC, Mendonça EG, Oliveira MG, Geller M. Sepsis: an update. Rev Bras Ter Intensiva. 2011;23:207-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Wan L, Bagshaw SM, Langenberg C, Saotome T, May C, Bellomo R. Pathophysiology of septic acute kidney injury: what do we really know? Crit Care Med. 2008;36 Suppl 4:S198-S203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 258] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 39. | Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, Parker MM, Gerlach H, Reinhart K, Silva E, Harvey M, Regan S, Angus DC; Surviving Sepsis Campaign. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38:367-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 643] [Cited by in F6Publishing: 635] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 40. | Okamoto TY, Dias JCY, Taguti P, Sacon MF, Kauss IAM, Carrilho CMD de M, Cardoso LTQ, Grion CMC, Matsuo T. Acute renal injury in patients with severe sepsis: prognostic factors. Scientia Medica. 2012;22:138-141. [Cited in This Article: ] |
| 41. | Małyszko J, Małyszko JS, Myśliwiec M. Endothelial cell injury markers in chronic renal failure on conservative treatment and continuous ambulatory peritoneal dialysis. Kidney Blood Press Res. 2004;27:71-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Iba T, Levy JH, Raj A, Warkentin TE. Advance in the Management of Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. J Clin Med. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 43. | Delabranche X, Helms J, Meziani F. Immunohaemostasis: a new view on haemostasis during sepsis. Ann Intensive Care. 2017;7:117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 44. | Timmermans K, Kox M, Scheffer GJ, Pickkers P. DANGER IN THE INTENSIVE CARE UNIT: DAMPS IN CRITICALLY ILL PATIENTS. Shock. 2016;45:108-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 45. | Iba T, Saitoh D. Efficacy of antithrombin in preclinical and clinical applications for sepsis-associated disseminated intravascular coagulation. J Intensive Care. 2014;2:66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | van der Poll T. Recombinant Human Soluble Thrombomodulin in Patients With Sepsis-Associated Coagulopathy: Another Negative Sepsis Trial? JAMA. 2019;321:1978-1980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Ito T, Thachil J, Asakura H, Levy JH, Iba T. Thrombomodulin in disseminated intravascular coagulation and other critical conditions-a multi-faceted anticoagulant protein with therapeutic potential. Crit Care. 2019;23:280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 48. | Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45:486-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1784] [Cited by in F6Publishing: 1862] [Article Influence: 266.0] [Reference Citation Analysis (1)] |
| 49. | Hasegawa D, Nishida O. Individualized recombinant human thrombomodulin (ART-123) administration in sepsis patients based on predicted phenotypes. Crit Care. 2019;23:231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Prescott HC, Angus DC. Enhancing Recovery From Sepsis: A Review. JAMA. 2018;319:62-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 437] [Cited by in F6Publishing: 534] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 51. | Prescott HC, Langa KM, Iwashyna TJ. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA. 2015;313:1055-1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 52. | Westphal GA, Vieira KD, Orzechowski R, Kaefer KM, Zaclikevis VR, Mastroeni MF. [Analysis of quality of life following hospital discharge among survivors of severe sepsis and septic shock]. Rev Panam Salud Publica. 2012;31:499-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |