Published online Aug 4, 2015. doi: 10.5492/wjccm.v4.i3.213
Peer-review started: November 9, 2014
First decision: December 26, 2014
Revised: March 3, 2015
Accepted: June 4, 2015
Article in press: June 8, 2015
Published online: August 4, 2015
Spontaneous intracerebral hemorrhage is a type of stroke associated with poor outcomes. Mortality is elevated, especially in the acute phase. From a pathophysiological point of view the bleeding must traverse different stages dominated by the possibility of re-bleeding, edema, intracranial hypertension, inflammation and neurotoxicity due to blood degradation products, mainly hemoglobin and thrombin. Neurological deterioration and death are common in early hours, so it is a true neurological-neurosurgical emergency. Time is brain so that action should be taken fast and accurately. The most significant prognostic factors are level of consciousness, location, volume and ventricular extension of the bleeding. Nihilism and early withdrawal of active therapy undoubtedly influence the final result. Although there are no proven therapeutic measures, treatment should be individualized and guided preferably by pathophysiology. The multidisciplinary teamwork is essential. Results of recently completed studies have birth to promising new strategies. For correct management it’s important to establish an orderly and systematic strategy based on clinical stabilization, evaluation and establishment of prognosis, avoiding secondary insults and adoption of specific individualized therapies, including hemostatic therapy and intensive control of elevated blood pressure. Uncertainty continues regarding the role of surgery.
Core tip: Spontaneous intracerebral hemorrhage is associated with poor outcome. Neurological deterioration and death are common in early hours, so it is a true neurological-neurosurgical emergency. Nihilism and early withdrawal of active therapy clearly influence the outcome. Action should be taken fast and accurately. Treatment should be individualized and guided preferably by pathophysiology in a multidisciplinary team work. For correct management it’s important to establish an orderly and systematic strategy based on clinical stabilization, evaluation and establishment of prognosis, avoiding secondary insults and adoption of specific individualized therapies, including hemostatic therapy and intensive control of elevated blood pressure.
- Citation: Godoy DA, Piñero GR, Koller P, Masotti L, Napoli MD. Steps to consider in the approach and management of critically ill patient with spontaneous intracerebral hemorrhage. World J Crit Care Med 2015; 4(3): 213-229
- URL: https://www.wjgnet.com/2220-3141/full/v4/i3/213.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v4.i3.213
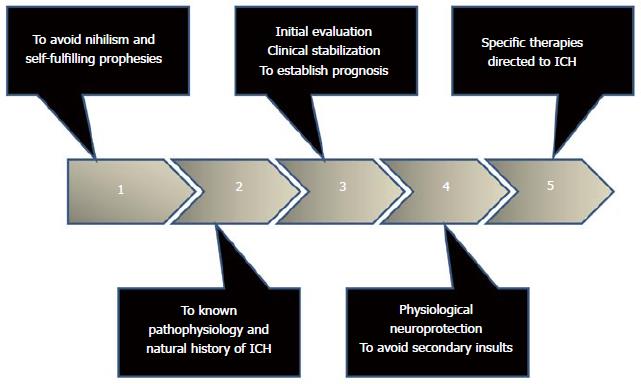
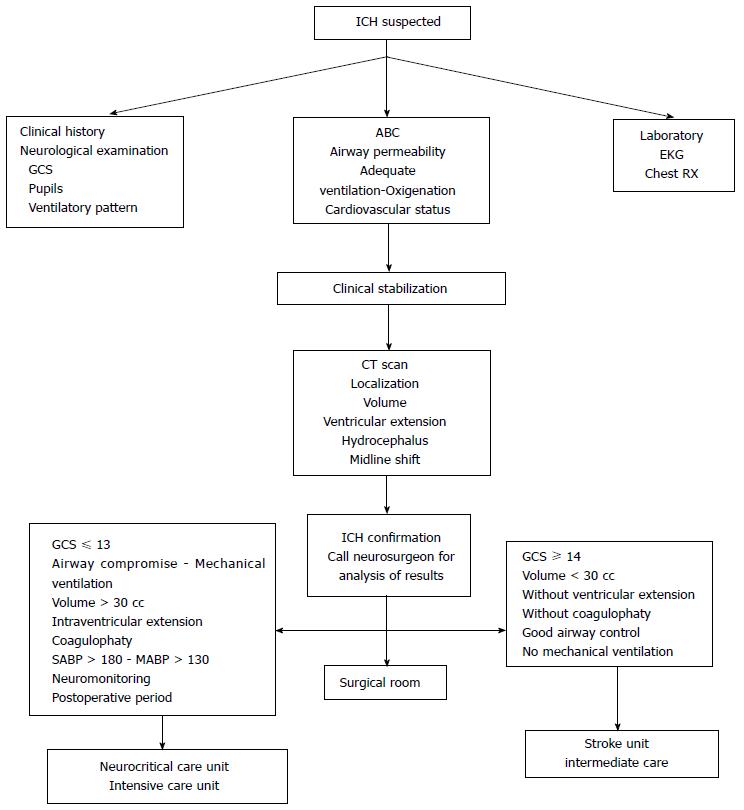
Intracerebral hemorrhage (ICH) is defined as the spontaneous extravasation of blood into the brain parenchyma with or without extension of bleeding into subarachnoid or intraventricular spaces[1-4]. ICH account for 10% to 30% of all stroke-hospital admissions[1-4], and is one of the most devastating forms of stroke. Its estimated incidence is between 12 and 15 cases per 100000 inhabitants per year. Arterial hypertension and oral anticoagulants are the major risk factors[1-4]. The clinical presentation is characterized by a rapidly deteriorating neurological status coupled with signs and symptoms of elevated intracranial pressure[1-5]. The diagnosis is established by the use of neuroimaging [computed tomography (CT) scan or magnetic resonance imaging (MRI)][1-5]. The mortality rate is averaging 50%, most of which occur during the first 5 d[1-5]. Only one-third of the survivors resume his life prior to the event[1-5]. Unfortunately, there is no proven specific treatment; however, a comprehensive and multidisciplinary approach based on pathophysiology helps to achieve favorable results[1-5]. The main objective of this manuscript is to review all aspects of spontaneous ICH, with emphasis on its pathophysiology with the intention to suggest steps to consider for the management of this lethal entity (Figure 1).
Nihilism (from the Latin nihil, “nothing”) is the philosophical principle that is based on the negation of one or more of the supposed meanings of life. Nietzsche indicates that denial or disbelief in anything are the results of doubt and disorientation[6]. The nihilism has dominated the scene of spontaneous ICH for many years, perhaps due to the absence of specific therapies. One of the most important determinants of the outcome of the individual victims of ICH is the level of support provided. If this support is not adequate or suspended based on beliefs of poor prognosis, it can trigger self-fulfilling prophecies[7]. As stated by Robert Merton, self-fulfilled prophesies are based on a false conception or belief that eventually triggers a behavior or conduct false that with the time becomes true[8].
Moreover, in ICH and other brain injuries, orders of do-not-resuscitation (DNR) or withdrawal of support based on self-fulfilled prophesies or nihilism, have a definite influence on mortality[7,9,10]. Delete nihilistic attitude is indispensable in the management of spontaneous ICH.
These philosophical principles have scientific evidence that supports them. Various studies have highlighted the impact of treating this population of patients in specialized, multidisciplinary units, which increase the probability of survival and good outcome[11,12]. The reasons remain uncertain, but several factors seem to influence, such as; the absence of nihilistic attitude, decreased stay in intensive care units, a lower incidence of neurological or systemic complications and early discharge to rehabilitation units[11-13].
Thirty-day mortality of ICH victims is nearly to 50%, most of which occurs during the acute phase[1-4]. The causes of death vary according to the time course of the disease[14-16]. Nearly 80% of cases of early death are of neurological origin[14-16]. About one-fifth of these patients does not reach the hospital and dies due to the magnitude of the primary or initial damage[17]. The rest of the patients dies by withdrawal of support due to brain death secondary to localization of the bleeding (brainstem); intracranial hypertension due to initial bleeding or as a result of the expansion of the hematoma[14-16]. The remaining 20% died by cardiac causes[14-16]. After the first week, death is caused by medical complications, mainly sepsis[14-16].
One-year mortality varies according to different locations: 51% for deep (thalamic or putaminal), 57% for lobar, 42% for cerebellar and 65% for brain stem hemorrhages, respectively[1].
The events that follow bleeding within the brain parenchyma are varied, complex, simultaneous, and interrelated. For teaching purposes, we will divide them, in different phases[18].
Vascular rupture: Arterial hypertension is a common risk factor for ICH[1-5]. Nearly 80% of patients with ICH present arterial hypertension at the admission and most have a history of hypertension[1,5]. Chronic hypertension imposes constant mechanical stress to cerebral arterioles (60-100 μ in diameter), which triggers hyperplasia of smooth muscle cells[1-5,19]. Over time, muscle cells die, are replaced by collagen, weakening the arterial wall, making it prone to stasis, occlusion, and rupture[19].
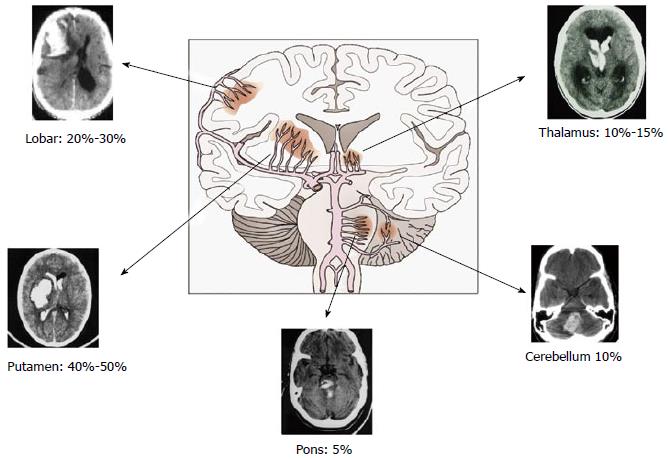
The sites at higher risk for these changes are the bifurcations or branches of penetrating arteries, such as lenticulostriate, thalamus and brainstem perforating arteries, thus explaining the most common hematoma locations[1-5] (Figure 2).
The extent of bleeding is mainly determined by the size of the gap in the arteriolar wall, systemic blood pressure, and hemostatic mechanisms[19].
Sometimes, the arterioles invaded by collagen develop microscopic dilatations, known as “Charcot-Bouchard aneurysms”. These changes can be found in autopsy specimens, but they are not always associated with bleeding sites; therefore, their clinical significance is controversial[1-5,19].
In non-hypertensive individuals, particularly the elderly, amyloid angiopathy is the substrate of arterial bleeding. It results from the deposition of amyloid protein in the tunica media and adventitia of capillaries, arterioles, cortical and leptomeningeal arteries causing fragility of the vessel wall. These vessels could break spontaneously or for sudden and abrupt blood pressure changes. Distinctive features of this entity are the predilection for lobar regions (especially in the posterior areas of the brain), multifocality, and recurrence[1-5,19].
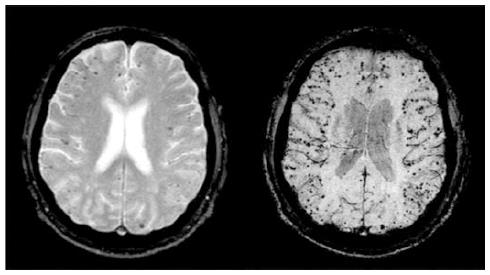
Today, attention is directed towards early detection of micro hemorrhages by MRI, because these have been shown to predict higher risk of lobar ICH[19,20] (Figure 3).
Following vascular rupture, the phase of hematoma formation begins, which develops within 60 min of the initial bleeding[18]. The sudden bleeding into the brain causes mechanical destruction of the parenchyma and may produce mass effect with increased intracranial pressure, distortions and tissue shift with potential herniation and cerebral ischemia[1-5,19,20]. The bleeding also triggers cell death through necrosis and apoptosis[18,21]; inflammation[18,21,22]; and vasogenic edem[18,21-23].
A substantial proportion of patients has enlargement of the hematoma after the initial event. This expansion is often associated with deterioration of neurological status and poor clinical outcomes[1-5,24-26].
An increase in the volume of the hematoma is seen in 38% of patients during the first three hours post-stroke. In two-thirds of this population, expansion of the hemorrhage is evident in the first hour[24-26]. Hematoma growth may occur despite the absence of coagulopathy and although knowledge of the mechanisms of expansion remain inconclusive, they seem to involve continuous bleeding from the initial site or additional bleeding from damage to adjacent small vessels causing satellite hemorrhages at the periphery of the clot[19].
Various risk factors have been associated with hematoma enlargement. Alcohol abuse, irregularly shaped hematomas, low levels of fibrinogen and prothrombin, diabetes mellitus, liver disease, are frequently reporters factors. However, the most consistent is the time elapsed between symptom onset and first CT scan[18,24,27,28]. Longer is the time until the first imaging study, lower the probability of detecting this complication.
After the initial 24 h, the next phase is dominated by the development of edema around the hemorrhage. This period reaches its peak on the third day after the first bleeding, and then declines slowly[18,21,23].
The most severe form of edema is localized around the clot, mainly spread through the white matter. This edema is primarily vasogenic due to alteration of the blood-brain barrier (BBB). Physical destruction damages the BBB and for the synthesis of substances that contributes to damage, such as thrombin and extracellular matrix metalloproteinases[18,21,23].
After ICH, cerebral blood flow (CBF) changes with a characteristic temporal profile[29]. Three phases have been described[29]: (1) phase I: first 48 h. Metabolism and CBF are reduced in a coupled manner. This period is known as “hibernation phase”; (2) phase II or reperfusion phase: between days 2 to 14. CBF and metabolism vary in the whole cerebral parenchyma, with areas of hypo normal and high CBF; and (3) phase III - normalization: starts in the second week after hemorrhage. CBF and metabolism return to normal values, except in the hemorrhagic site.
Multiple factors contribute to CBF alterations: mechanical compression of microvasculature, intracranial hypertension, disruption of cerebral autoregulation, vasoactive substances and inflammation[29]. Following ICH, CBF decrease, with lowest values in the perihematomal region[30,31], however in this zone, metabolic activity also decrease, indicating the absence of ischemia[32-34].
In summary, the available data allow us to confirm that the area around ICH is characterized by a slight decrease in regional cerebral blood flow but this occurs as a result of the concomitant decrease in metabolic demands. Mitochondrial dysfunction might be responsible for the metabolic depression[35].
Recent studies of metabolism in perihematomal zone have revealed a remarkable metabolic distress characterized by an increase in the uptake and glucose utilization especially in the first 4 d after hemorrhage[36]. This metabolic crisis may persist for about one week, and it is not a consequence of ischemia; therefore, we should speak of metabolic penumbra rather than ischemic penumbra[36].
The other phenomena taking place around the hematoma are inflammation and neurotoxicity[18,21,22]. Bleeding activates astrocytes and microglia, which in turn stimulate the release of pro-inflammatory mediators, such as cytokines, intercellular adhesion molecules, and matrix metalloproteinases[18,21,22].
Neurotoxicity occurs through extravasation of proteins and osmotically active solutes that promoting the development of edema and stimulation of proteinases such as thrombin, fibrinogen, and tissue plasminogen activator. The coagulation cascade is activated in conjunction with lysis of red blood cells, which releases potent neurotoxic substances, such as iron, bilirubin, and hemin[18,21,22].
Prognostication is essential for a correct approach. From a practical point of view, the severity of ICH can be established accurately with clinical examination and neuroimaging[1-5,37]. Glasgow coma scale (GCS) is the most commonly used tool to assess the level of consciousness. Deficits can be established with NIHS scale[1-5,37].
Non-contrasted CT scan is the imaging of choice in the acute phase (Recommendation I, Level A). It confirms the bleeding with excellent sensitivity, determines its location, size, ventricular or subarachnoid extension, degree of distortion or displacement structures and the presence of complications such as hydrocephalus or edema[2,3,37]. It also helps to establish prognosis, monitoring the evolution and the response to different therapeutic modalities.
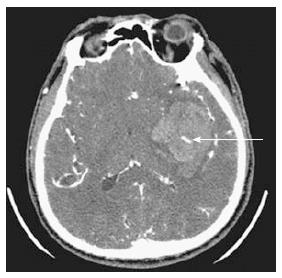
Recent studies have indicated that CT angiography with contrast can be very useful[38-40]. Extravasation of contrast within or in adjacent areas of hematoma indicates active bleeding. It has been called “spot sign” and predicts hematoma expansion[38-40] (Figure 4).
Multiple and varied factors (clinical, biochemical, images) have been described as independent predictors of mortality, however, only GCS score and hematoma volume have shown the most predictive power[1-5,37].
Unlike other neurocritical entities, there is no universally accepted and validated scale for ICH.
Hemphill, basing on multivariable model of their population, detected five independent factors associated with 30-d mortality, developing a risk stratification scale, which e called ICH score[41] (Table 1). In this scale, mortality increased as the punctuation increased. No patient with an Score of 0 died, whereas all patients with 5 points died[41]. This scale has been validated externally[42,43]. Since the original description of ICH score, several scales have been developed, each with their strengths and weaknesses[44-46].
| Components | Points |
| GCS score | |
| 3-4 | 2 |
| 5-12 | 1 |
| 13-15 | 0 |
| ICH volume (cm3) | |
| ≥30 | 1 |
| < 30 | 0 |
| IVH | |
| Yes | 1 |
| No | 0 |
| Infratentorial origin of ICH | |
| Yes | 1 |
| No | 0 |
| Age (yr) | |
| ≥ 80 | 1 |
| < 80 | 0 |
| Total ICH score | 0-6 |
It is important to note here that any prediction model lacks validity in centers with nihilism, self-fulfilling prophesies, withdrawal support or DNR politics[9,10].
The main objective should be directed to ensure the ABC (patent airway, adequate breathing, oxygenation, and circulation), achieve clinical stability and then, transfer to imaging study. The neurosurgeon should be actively involved in decision-making[1-5,37] (Figure 5). It is very important to develop a strategy to prevent, detect and correct secondary insults[2-5,37,47,48] (Table 2).
| Systemic | Intracraneal |
| Arterial Hypotension Hypoxia Hypercapnia - Hypocapnia Hyperthermia Hyperglycemia - Hypoglycemia Hyponatremia - Hypernatremia Anemia SIRS DIC | Intracranial hypertension Cerebral hematoma Edema Seizures Vasospasm Hydrocephalus Infections |
This strategy has a significant impact on the outcome[2,3,7,47,48].
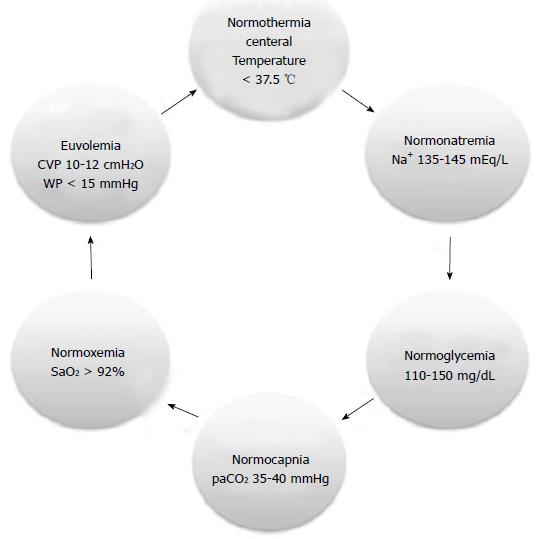
The basis of therapeutic of any neurological injury is to achieve organic homeostasis, which we call “physiological neuroprotection”[47,48]. From a practical point of view and easy to remember is to maintain healthy 6 principal clinical variables (6 N rule), such as euvolemia, paO2 and paCO2 levels, temperature, glycemia, and natremia[47,48]. Target to achieve for each variable are depicted in Figure 6.
ICH patients are susceptible to develop ventilation and oxygenation alterations[1-5,37,47-51] due to compromise of defense reflexes of the upper airways such as cough and swallowing, increasing the risk of aspiration of gastric contents[1-5,47-51]. Pons or supratentorial hemorrhages with mass effect can compromise respiratory rhythm. This population is also at risk for neurogenic or cardiogenic pulmonary edema. We recommend keeping low threshold for intubation and as a general rule all patients in coma should be intubated[1-5,37,47-51]. Therapeutic targets will be directed to maintain SaO2 greater than 92% while maintaining normal levels of CO2, since hypercapnia causes cerebral vasodilatation and ICP increased, whereas that hypocapnia causes vasoconstriction triggering cerebral ischemia[1-5,37,47-51].
During resuscitation is essential to avoid systemic hypotension, ensuring blood pressure levels that allow adequate cerebral perfusion pressure (CPP)[1-5,37,47-51]. For this reason, it is necessary to normalize blood volume. The first therapeutic step is infusion of fluids, preferably isotonic saline[1-5,37,47-51], avoiding hypotonic fluids (0.45% saline, 5% dextrose, Ringer’s lactate) that exacerbate brain swelling. Hypertonic saline solutions are an option especially for individuals with signs of herniation, intracranial hypertension or severe hyponatremia. If fluids are not sufficient to ensure adequate blood pressure, vasopressors (noradrenaline) or inotropes (dopamine) should be started[1-5,37,47-51].
Hyperthermia is highly prevalent in neurointensive care[52]. Initially, elevated temperature is attributable to acute phase response[52,53], during which inflammatory mechanisms are triggered, and sympathetic activity is increased[52,53]. However, directly or indirectly damage of hypothalamus and thermoregulatory centers cannot be excluded[52,53]. The brain is more warmer than the rest of the body[52,53]. Hyperthermia exerts its deleterious effects through various mechanisms: it increases levels of excitatory amino acids, cytokines, and reactive oxygen species, inhibits proteolytic enzymes, damages BBB, increases intracranial pressure and triggers apoptotic mechanisms[53].
Clinical studies have shown a close association between hyperthermia on admission or during the first 24 h and outcome. Moreover, hyperthermia has demonstrated its independent predictive power of poor outcome[53-55]. Hyperthermia can be controlled with the use of external cooling methods (ice, thermal blankets), internal (intravascular cooling devices) or pharmacological (acetaminophen, aspirin)[56,57]. Until now, there is no a study that prospectively evaluated the impact of fever control on the outcome nor that is the most suitable method to control fever[56,57], and due to ethical concerns is very unlikely to be performed ever.
Disorders of sodium and water metabolism are common in neurocritical ill patients[58]. Imbalances in the metabolism of sodium produce changes in osmolarity and in water distribution, which in turn, trigger changes in the volume cerebral[58].
In neurocritical care patients, hyponatremia (serum Na+ < 135 mEq/L) occurs in 15% to 20% of patients, increasing the likelihood of unfavorable outcomes[58]. The elderly population is very susceptible to this disorder. The causes are varied, highlighting the syndrome of inappropriate antidiuretic hormone secretion (SIADH) and cerebral salt-wasting syndrome (CSWS)[58,59].
The treatment of hyponatremia, depend on the presence or absence of symptoms and the underlying cause[58-60]. In the presence of SIADH, fluid restriction is indicated while in the presence of CSWS volume expansion is necessary. In symptomatic cases, hyponatremia should be corrected with hypertonic saline solutions at a slow rate, preferably not more than 10 mmol/liter per day to avoid severe complications such as pontine myelinolysis. Sometimes, fludrocortisone can be used as an adjunct at 0.1-0.4 mg/d[58-61].
Hypernatremia (serum Na+ > 145 mEq/L) is less frequent[58]. Its incidence is about 10%, and it is considered a marker of severity of injury with negative predictive power[58,61]. The most common causes are iatrogenic due to excessive sodium intake or water loss secondary to mannitol infusion[58,61]. Diabetes insipidus is another disorder to take in mind[58,61]. The cornerstone of treatment are reposition and retention of water[58,61]. The replacement should be performed with hypotonic solutions like 5% dextrose or ringer lactate because isotonic saline can exacerbate losses. To avoid loss of water desmopressin at 0.4 mg IV or 100-200 μg via nasal route should be utilized. Such doses may be repeated if necessary. Sharp corrections should be avoided[58,61].
Blood glucose levels should be kept within a narrow range, avoiding extreme variations since the brain is very vulnerable to such situations[62,63]. Hypoglycemia should not be allowed in any way and must be corrected immediately[62,63]. The brain does not tolerate episodes of hypoglycemia as their compensatory mechanisms are exhausted quickly and easily[62,63].
During injury, the brain increases susceptibility to acute derangements of blood glucose[62,63]. After injury, the brain increased glucose demand.
Hyperglycemia is common during the acute phase of ICH[62,64]. Its incidence averages 40% and is independently associated with worse outcome[61,62,64].
Its etiology is variable, not being clear whether it is a marker of severity or only one component of the metabolic response to injury[62-64]. Hyperglycemia contributes to brain damage through various mechanisms that provoke edema and cerebral ischemia[63]. IV regular insulin is the drug of choice to correct high blood glucose levels but still not yet well determined when starting therapy[62-65]. Intensive insulin therapy (glucose levels between 80-110 mg/dL) is contraindicated because at these levels starts cellular metabolic distress[66,67]. The current trend is to maintain the lower limit of about 150 mg/dL and not higher than 200 mg/dL[2,3,37,62,65].
The gastrointestinal tract is of vital importance in patients with brain injury[68]. Multiple hormones and neuropeptides are released by the brain and intestine in response to injury, establishing an interaction finely regulated by enteric nerve plexus and the autonomic nervous system[68]. In ICH patients, a number of factors combine to break the normal physiology, including hypothalamic damage, intracranial hypertension, prolonged fasting, mechanical ventilation, drugs (vasopressors, anticonvulsants, opioids, antibiotics, corticosteroids), inflammation (cytokines), hypoalbuminemia, electrolyte imbalances[68,69]. The most important complications are gastrointestinal bleeding, diarrhea, gastroparesis and ileus, which favor bacterial translocation and malnutrition, sepsis and multiorgan dysfunction[68,69].
The incidence of clinically significant gastrointestinal bleeding (erosive gastritis or stress ulcer) ranges from 0.6% to 6%[69]. The main substrate for gastric mucosal damage is the presence of inadequate splanchnic perfusion[69]. Risk factors are mechanical ventilation (> 48 h), coagulopathy, severe traumatic brain and spinal cord injury[69]. Some principles are relevant for management, to avoid arterial hypotension and some drugs (steroids, noradrenaline); early nutrition and protection with proton pump inhibitors or local agents[69]. H2 receptors blockers are not recommended because they are associated with encephalopathy, interaction with anticonvulsants and modify the local pH favoring bacterial colonization and pneumonia[69].
Constipation is common after neuroinjury with a negative impact upon outcome[68]. Incidence rates are between 30% to 60%[68]. Predisposing factors are immobility, fasting, electrolyte disturbances, and drugs (opioids, sedatives, dopamine). Its prevention is based on adequate fluid and electrolyte balance, rich-fiber diet and laxatives[68].
Diarrhea is a complication with a prevalence of 8% to 21%[68]. Fever, hypothermia, hypoalbuminemia, sepsis, multiple organ dysfunction, broad spectrum antibiotics, enteral nutrition, and clostridium difficile (CD) colonization are predisposing factors[68].
Brain injury, determines a hypermetabolic state, with exaggerated protein catabolism[68,70]. During injury, the brain increases its metabolic requirements[62,70]. Nutrition should become one of the key goals of therapy. Malnourished patients are more prone to developing infectious complications, bedsores, gastrointestinal bleeding, all associated with poor outcomes[68]. Enteral feeding must be supplied early with low calories (25-30 kcal/kg per day), 40% of which in the form of lipids and 15%-20% as protein (1.5-2 g/kg per day) accompanied by a regimen of glycemic control and the contribution of fiber, vitamins, oligoelements and pharmaconutrients (glutamine, arginine)[70].
The incidence of seizures after ICH varied between 4.6% and 8.2%[71]. Acute seizures should be treated following classical algorithms since they are associated with increased cerebral metabolism, ICP and midline shift contributing to secondary injury[71-76]. Lobar location and small hematomas are independent predictors of early seizures[72]. Although antiepileptic drugs (AEDs) may reduce the incidence of seizures in cortical and subcortical hemorrhages[72], their prophylactic use is not recommended because it is unclear their efficacy and impact over final outcome[73,74]. Phenytoin use was associated with more fever burden and worse outcomes after ICH[74]. Electroencephalographic seizures without clinical manifestations occur in around 30% of patients after ICH[75]. Nonconvulsive seizures are associated with early hematoma growth and a trend toward poor outcome[76]. Continuous EEG monitoring should be considered in all patients with a decreased level of consciousness without clear reason to justify[2]. Current Guidelines recommended anti-epileptic treatment for up to one month, after which therapy should be discontinued in the absence of seizures[2,37].
There are two mechanisms involved in the genesis of acute hydrocephalus: extrinsic compression of ventricular system by proximity (thalamic, cerebellar hematomas); displacing midline structures (putaminal hematomas); or obstruction of CSF circulation by clots[1,2,4,37,77]. Hydrocephalus causes impairment of consciousness, intracranial hypertension and cerebral ischemia, being an independent predictor of mortality and poor outcome[77].
The extent of bleeding to the ventricular space complicates about 40% of spontaneous ICHs[2,4,37,77-80].
Intraventricular blood is a poor prognostic factor[2,4,37,77-80]. Its volume determines the predictive power, being lethal when exceeding 20 cc, due to hydrocephalus, intracranial hypertension and ischemia of the cerebral cortex[2,4,37,77-80]. External ventricular drainage is a therapeutic option but insufficient and ineffective when used as a single measure[2,4,37,77-81].
Patency of the ventriculostomy is difficult to maintain due to frequent plugging clots. Thrombolytic drugs were tested with different protocols and doses[2,4,37,78-82]. Studies with small numbers of patients showed a trend to reduce need for definitive ventricle peritoneal shunts, and decrease mortality rates with acceptable functional outcomes; however, there is an increased risk of infectious or hemorrhagic complications[2,4,37,78-82].
CLEAR-IVH study evaluated the strategy of external ventricular drainage more rtPA instillation[82]. Resolution rates of clots were significantly higher with shorter permanence time of ventriculostomy in rtPA group[82]. By contrast, symptomatic bleeding rate was higher in the group rtPA. Mortality rates not changed significantly[82]. The study had several methodological limitations, for example; selection criteria for study inclusion, did not include location of bleeding or extension of intraventricular hemorrhage; management of known factors that influence rates of bleeding such as blood pressure levels or coagulation state not were considerate and the study was not designed to assess long-term functional outcome[82], a situation that is being evaluated in CLEAR III study[83].
Endoscopically removal of the clot and controlled lumbar drainage are promising therapeutic alternatives that need large-scale validation[79-81]. Preliminary results indicate that lumbar drainage after radiological permeation of third and four ventricles was associated with a reduction in the need for permanent ventricular shunting[78,80].
Although ICH causes structural changes in brain parenchyma and intracranial hemodynamics than potentially increase ICP, is unclear its prevalence, temporal profile and the impact that intracranial hypertension have on the outcome.
Intracranial hypertension is more common immediately after bleeding[84]. Elevated ICP only have an impact on the outcome only in comatose patients[85]. There was not relationship between ICP values at any time and outcome at 6 mo[86].
An observational study of ICP recordings in patients with IVH and ICH of less than 30 mL found that the percentage of readings above 30 mmHg was an independent predictor of mortality (P < 0.001) and disability at 30 d (P = 0.01)[87]. Kamel and Hemphill analyzed ICH patients with ICP monitoring. Seventy percent of them presented at least one episode of ICP above 20 mmHg while, in 63%, ICP exceeded 25 mmHg. Intracranial hypertension was less frequent in older and infratentorial hemorrhages and was not related to poor outcome[88].
Recently, a prospective, randomized controlled study assessed the impact of ICP monitoring in the management of supratentorial ICH. The risk of herniation was lower in ICP group (10.9% vs 20.5%, P = 0.04). At 6 mo, mortality and disability were lower in ICP group (6.5% vs 9.1%, P < 0.05)[89].
Current recommendations are based on low level of evidence (Class IIb C). However, they suggest ICP monitoring in comatose patients with signs of herniation, hydrocephalus or widespread ventricular hemorrhage[2,3,37].
The treatment of intracranial hypertension has been extrapolated from severe head trauma[2,3,37]. Briefly, after evacuating hemorrhage when were indicated, we follow a staggered, step by step, phased, sequential pathway[1-4,37,90]. CT scans are performed periodically[1-4,37,90].
We begin with general measures (sedation, analgesia, prevention and correction of secondary insults) positioning the head in a neutral position at 30 degrees of horizontal[1-4,37,90]. If ICP remains high, we continue with CSF drainage at not more than 20 mL per hour. If we don’t have ventricular drainage or if it resulted ineffective, we start osmotherapy with hypertonic saline or mannitol until the limit of sodium or serum osmolality of 155 mEq/L or 320 mosm/kg respectively[1-4,37,90]. After this measures, if ICP remains increased, we hyperventilate slightly, maintaining paCO2 levels between 30 and 35 mmHg. At this point, we indicate monitoring of cerebral oxygenation. We do not utilize neuromuscular paralysis unless strictly necessary for ICP normalization. Mean arterial pressure would be titled to a CPP target between 55-70 mmHg[1-4,37,90].
The non-response to initial therapy, define a state of “refractory intracranial hypertension”. Prior to the adoption of “second level’’ measures (barbiturates at high doses, hypothermia, decompressive craniectomy) we performed indomethacin test[90].
Elevated arterial blood pressure (ABP) levels are common in the acute phase of ICH[2,3,37,91]. Etiology is multifactorial[2,3,37,91]. There are arguments for and against their control. Those who are in favor of lowering the pressure levels are based on that hypertension is associated with poor outcomes[92] and may cause expansion of the hematoma[28,93]. INTERACT study, randomized patients to intensive BP control (target SBP 140 mmHg) vs traditional management (SBP 180 mmHg) within 6 h of ICH onset, showed a trend towards reduction in hematoma growth in the intensive treatment group, without increase the rate of neurological deterioration or other adverse events[94].
ATACH I study demonstrated safety of nicardipine for acute reduction of BP in acute ICH[95], while ADAPT trial showed that control arterial hypertension to a target of SBP of lower than 150 mmHg within 24 h of onset did not produce clinically or CBF changes in perihematomal region[96].
INTERACT II trial[97], randomized patients with spontaneous ICH and elevated SBP (≥ 150 and ≤ 220 mmHg) to a strategy of intensive control (SBP < 140 mmHg) vs guideline-recommendations (SBP < 180 mmHg) within 6 h of symptoms onset, showed a borderline decrease in poor outcome at 90 d (OR = 0.87, 95%CI: 0.75-1.01; P = 0.06)[97].
ATACH II trial[98] is an ongoing multi-center, randomized phase III trial to determine the efficacy of early, intensive, BP control initiated within 4.5 h of symptom onset[98]. The expansion of window from 3 to 4.5 h was based on ATACH-I that suggests a reduction of hematoma expansion, death and disability in patients treated within 4.5 h after symptom onset[98].
SCORE-IT is an ancillary study of ATACH II that tests the hypothesis that patients with a Spot Sign will receive clinical benefit from intensive ABP reduction[99].
With regard to pharmacological management, its preferably use agents that do not cause cerebral vasodilation and sudden hypotension, so labetalol (loading dose of 10-20 mg in 1-2 min, repeated every 1-20 min until the desired level of blood pressure were reached or until a maximum dose of 200 mg) or nicardipine (5-15 mg/h) are good options[2,3,37].
Venous thromboembolism (VTE) is one of the most feared complications of ICH. The incidence varies between 2%-17%, with a mortality rate of 5%[2-4,37,100,101].
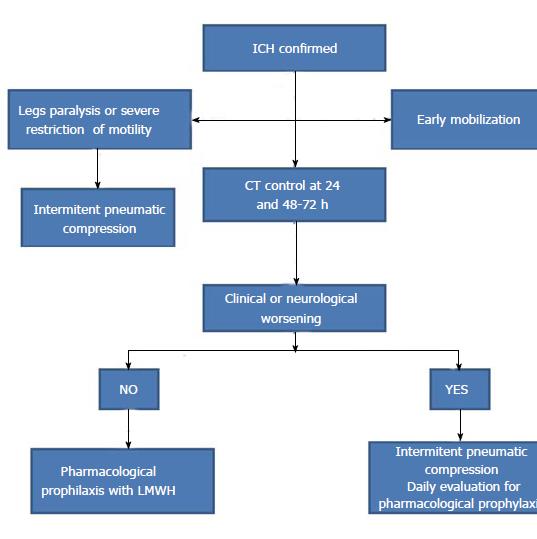
Risk factors for VTE are: older age, female gender, obesity, prolonged bed-rest, legs paralysis, lobar hematoma, great volume, NIHSS score ≥ 12, withdrawal of antithrombotic treatment, and pro-hemostatic agents such as prothrombin complex or recombinant activated factor VII[102]. For optimal selection of strategy for VTE prevention is crucial for maintaining the balance between risk of hematoma enlargement and VTE. Strategies to prevent VTE in ICH patients are pharmacological and nonpharmacological[103]. Nonpharmacological agents are graduated compression stockings (CS), intermittent pneumatic compression (IPC) plantar venous pump, vena cava filters and early mobilization[103].
VICTORIA study compared the combination of IPC with CS vs CS alone. The combination of the two strategies was significantly superior in reducing the risk of VTE[104].
CLOTS II study[105], showed that CS positioned to the root of the thighs are superior to the CS positioned below the knees. In CLOTS III, IPC was associated with a significant reduction in the risk of VTE[106].
The main indication for vena cava filters is represented by the absolute contraindication to anticoagulant therapy[107], so, it is reasonable to reserve filters for patients with very high risk of VTE[107]. The role of early mobilization for prevention of VTE is controversial and unclear[108].
Systematic reviews and meta-analysis in terms of efficacy and safety of pharmacological prophylaxis for prevention of VTE balanced with the risk of hematoma expansion showed that unfractionated heparin or low molecular weight heparins significantly reduces the risk of pulmonary embolism, whereas not reduced the risk of DVT or death from all causes[109,110]. No increase in the risk of hematoma expansion was observed[110]. Based on actual recommendations[2,3,37,110] a possible flow chart for VTE prevention in ICH is depicted in Figure 7.
The urgent reversal therapy represents the cornerstone of management of antithrombotic-related ICH. It aims is to restore adequate hemostasis by neutralizing the anticoagulant or antiplatelet activity with specific antidotes, avoiding hematoma growth and devastating consequences of drugs induced coagulopathy[102].
Specific antidotes are available only for few anticoagulants, such as vitamin K antagonists (VKAs), unfractionated heparin (UFH) and idrabiotaparinux, not marketed yet.
Protamine sulfate is the recognized specific antidote for unfractionated heparin[111]. The goal of protamine for the reversal of unfractionated heparin is the normalization of activated partial thromboplastin time (aPTT). Protamine has a partial effect on LMWH reversal. Therefore higher dose may be necessary[112].
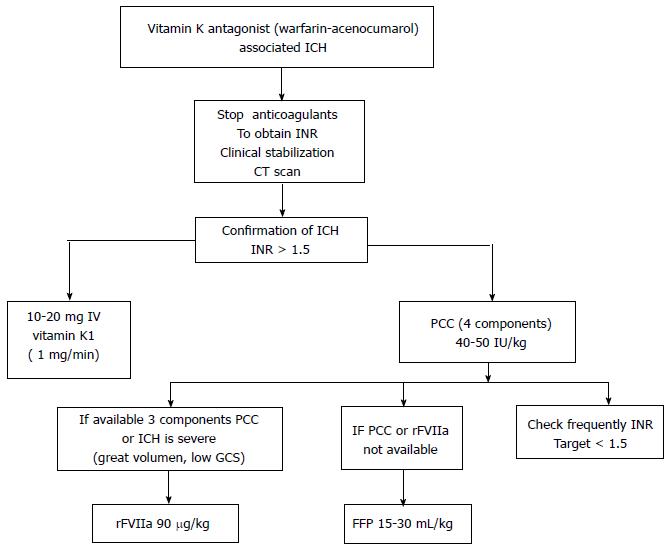
Despite intravenous administration of vitamin K1 (VK1) represents the most used for VKAs reverse (recommendation IA), it is not the only strategy because it’s slow onset of action and because need between 12-16 h to complete its action[111-113]. VK1 should be always administered together with prothrombin complex concentrates (PCCs), rFVII or fresh frozen plasma (FFP) because all these agents prove VK dependent coagulation factors[102].
PCCs, rFVII and FFP have short half time, therefore, the missed dose of VK1 could determine the rebound of International Normalized Ratio (INR) values after their pharmacological action[102-111].
The goal of urgent VKAs reversal in ICH is to bring the INR values ≤ 1.4 within 2-4 h[102,114-116]. At the end of pro-hemostatic infusion, INR should be re-checked, and the adjunctive dose should be infused if its values continue to be ≥ 1.5[102,116] (Figure 8).
PCCs a derivate of plasma contain three or four non-activated vitamin K depending coagulation factors (II, VII, IX, and X). Three factors PCCs lack for Factor VII[117]. PPCs restore INR and reduce hematoma enlargement rates, but it is controversial if it’s associated with mortality reduction or better functional outcome[117,118].
PCCs are considered the first choice for VKAs urgent reversal from many scientific Societies[2,37]. Limitation of PCCs derived from thromboembolic risk. Thromboembolic burden of PCCs is lower than 2%[119].
FFP is another effective strategy that leads to VKAs neutralization in 4-6 h but has it certain limitations, such as volume overload, especially in elderly or patients with limited cardiac reserve; delays in time due to thawing and blood group typing; infectious risk and TRALI (transfusion acute lung injury)[102].
Many reports have demonstrated that rFVII is effective for prompt VKAs reversal in few minutes without volume overload, but its use in this context is not recommended due to high risk of arterial and venous thromboembolic complications[119,120].
Recent trials have demonstrated that new oral anticoagulants, dabigatran, apixaban, edoxaban, rivaroxaban, reduce the risk of ICH in comparison with warfarin, however this effect is not negligible, ranging from 0.2% to 0.4% per year. Case-fatality rate of new oral anticoagulants related ICH is not significantly different compared with warfarin ranged between 50%-70%[121-123].
After urgent reversal, coagulation parameters should be performed but, again, it is unclear if adjunctive dose should be administered if coagulation parameters remain abnormal[102]. Therefore the proposed coagulation assays, such as aPTT, aPTT ratio, dTT, PT, PT ratio and anti-Xa, are suboptimal tools for predicting the response to pro-hemostatic agents, whereas methods aimed at global evaluation of hemostasis, such as thromboelastogram, platelet reactivity, and thrombin generation might be more useful[102,124].
Which is the optimal strategy for urgent reversal of antiplatelet activity in antiplatelet-related ICH remain unclear Despite platelets transfusion or intravenous desmopressin have been proposed, literature failed to demonstrate their beneficial effect in ICH[125,126]. Desmopressin has been proposed as a nonspecific strategy in antiplatelet related bleeding. However, its role in antiplatelet-related ICH is uncertain[127].
Despite the time elapsed the debate continues, with an open end. The removal of the hematoma reduces its volume, corrects distortions and displacements, reduces ICP and improves CPP. Furthermore, abort the continuation of neurotoxic and inflammatory cascades[2,4,37,128-131]. However, these theoretical advantages must be weighed against parenchyma damage required to access to the hematoma[128-131].
Most neurosurgeons agree to operate lobar or cerebellar hematomas in patients who deteriorate clinically, however, uncertainty remains regarding deep hemorrhages[128-131].
Current guidelines, recommend surgical treatment in the following situations[2,3,37]: (1) cerebellar hematomas of more than 3 cm in diameter in patients who deteriorate clinically with secondary hydrocephalus or compression of brainstem or fourth ventricle (grade C); (2) hemorrhages secondary to arteriovenous malformations, angiomas, cavernous malformations, aneurysms, etc. (grade C); and (3) lobar hematomas of moderate or larger volume in young patients with neurological impairment (grade B).
The STICH study, enrolled patients with supratentorial hemorrhages within the initial 72 h of symptoms onset, and then randomized them to medical vs surgical treatment based on the principle of uncertainty about the usefulness of surgery[129]. Mortality and functional outcomes were the same for both groups[129]. A small subgroup of patients was identified as able to evolve better. They are individuals aware (GCS between 9 and 12 points) with superficial hematomas, located at 1 cm or less in the cerebral cortex[129].
The STICH II study[130], compare surgery (within 12 h of randomization), with conservative medical treatment in patients with spontaneous supratentorial hemorrhage, lobar, superficial (≤ 1 cm from the cortex), with a volume between 10 and 100 mL, without ventricular extension of bleeding within 48 h of onset of symptoms[130]. There were no differences between groups in terms of mortality or disability rates at 6 mo. The subgroup of patients with worse initial prognosis evidenced a favorable trend if they were operated early[130].
A recent meta-analysis found that surgery seemed effective in patients with a higher consciousness level (GCS score 9-12) operated within eight hours of symptom onset[131].
Recently, the European Stroke Organization declaims that there is no evidence to support surgical intervention on a routine basis to improve outcome after supratentorial ICH, but early surgery may be of value for patients with a GCS score 9-12[37].
There is a worldwide tendency to operate these patients with minimally invasive techniques, either by endoscopy or stereotaxic with or without the combination of a catheter into the hematoma to instill fibrinolytic in order to accelerate the resolution of hematoma[2-5,37,131-133].
MISTIE II study randomized patients to a control group or clot aspiration with rtPA in putaminal (58%) or lobar (42%) hemorrhages above 25 mL and GCS < 14 and NIHSS > 6. Higher rates of clot removal and lower mortality were observed in the treated group[132]. MISTIE II and other trials of minimally invasive surgery (MIS) have shown encouraging results, so a phase III trial started in 2013[133].
Zhou’s meta-analysis concluded that patients who would most benefit from minimally invasive surgery are those between 30 and 80 years with superficial supratentorial hematomas, with volumes between 25 and 40 mL, admitted within 72 h in a good level of consciousness (GCS ≥ 9)[132].
The spontaneous ICH is a neurological-neurosurgical emergency far from diminishing its prevalence will increase in the coming years. Despite being one of the most devastating forms of stroke, a light on the horizon looms as a result of advances in knowledge and the results of recent trials. It is extremely important and essential to remove nihilism and self-fulfilling prophesies. A multidisciplinary approach is essential. Set the prognosis helps us in the process of decision-making and communication with the patient or their relatives. The therapy should be individualized and follows a deep pathophysiologic analysis. The cornerstones of therapy are correct evaluation, avoiding secondary insults through neuroprotection physiological measures, intensive control of blood pressure especially in acute and rapid reversal of antithrombotic and anticoagulant drugs. The role of surgery is still open to debate especially in deep bleeding.
P- Reviewer: Cattermole G, Lin J, Llompart-Pou J, Nayci A S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450-1460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1182] [Cited by in F6Publishing: 1090] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 2. | Morgenstern LB, Hemphill JC, Anderson C, Becker K, Broderick JP, Connolly ES, Greenberg SM, Huang JN, MacDonald RL, Messé SR. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1080] [Cited by in F6Publishing: 993] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 3. | Rodríguez-Yáñez M, Castellanos M, Freijo MM, López Fernández JC, Martí-Fàbregas J, Nombela F, Simal P, Castillo J, Díez-Tejedor E, Fuentes B. Clinical practice guidelines in intracerebral haemorrhage. Neurologia. 2013;28:236-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632-1644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1159] [Cited by in F6Publishing: 1082] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 5. | Staykov D, Huttner HB, Köhrmann M, Bardutzky J, Schellinger PD. Novel approaches to the treatment of intracerebral haemorrhage. Int J Stroke. 2010;5:457-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Nietzsche F. Obras completas de Nietzsche. Aguilar: Buenos Aires 1963; . [Cited in This Article: ] |
| 7. | Becker KJ, Baxter AB, Cohen WA, Bybee HM, Tirschwell DL, Newell DW, Winn HR, Longstreth WT. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology. 2001;56:766-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 375] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 8. | Merton RK. Teoría y estructura sociales. México: FCE 1980; . [Cited in This Article: ] |
| 9. | Hemphill JC, Newman J, Zhao S, Johnston SC. Hospital usage of early do-not-resuscitate orders and outcome after intracerebral hemorrhage. Stroke. 2004;35:1130-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 229] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Zahuranec DB, Morgenstern LB, Sánchez BN, Resnicow K, White DB, Hemphill JC. Do-not-resuscitate orders and predictive models after intracerebral hemorrhage. Neurology. 2010;75:626-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Diringer MN, Edwards DF. Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med. 2001;29:635-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 445] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 12. | Terént A, Asplund K, Farahmand B, Henriksson KM, Norrving B, Stegmayr B, Wester PO, Asberg KH, Asberg S. Stroke unit care revisited: who benefits the most A cohort study of 105,043 patients in Riks-Stroke, the Swedish Stroke Register. J Neurol Neurosurg Psychiatry. 2009;80:881-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Kurtz P, Fitts V, Sumer Z, Jalon H, Cooke J, Kvetan V, Mayer SA. How does care differ for neurological patients admitted to a neurocritical care unit versus a general ICU. Neurocrit Care. 2011;15:477-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Zurasky JA, Aiyagari V, Zazulia AR, Shackelford A, Diringer MN. Early mortality following spontaneous intracerebral hemorrhage. Neurology. 2005;64:725-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Fan JS, Huang HH, Chen YC, Yen DH, Kao WF, Huang MS, Huang CI, Lee CH. Emergency department neurologic deterioration in patients with spontaneous intracerebral hemorrhage: incidence, predictors, and prognostic significance. Acad Emerg Med. 2012;19:133-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Naidech AM, Bernstein RA, Bassin SL, Garg RK, Liebling S, Bendok BR, Batjer HH, Bleck TP. How patients die after intracerebral hemorrhage. Neurocrit Care. 2009;11:45-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Moon JS, Janjua N, Ahmed S, Kirmani JF, Harris-Lane P, Jacob M, Ezzeddine MA, Qureshi AI. Prehospital neurologic deterioration in patients with intracerebral hemorrhage. Crit Care Med. 2008;36:172-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Rincon F, Mayer SA. Novel therapies for intracerebral hemorrhage. Curr Opin Crit Care. 2004;10:94-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Sutherland GR, Auer RN. Primary intracerebral hemorrhage. J Clin Neurosci. 2006;13:511-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Vernooij MW, Heeringa J, de Jong GJ, van der Lugt A, Breteler MM. Cerebral microbleed preceding symptomatic intracerebral hemorrhage in a stroke-free person. Neurology. 2009;72:763-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 977] [Cited by in F6Publishing: 1032] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 22. | Wang J, Doré S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:894-908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 493] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 23. | Gebel JM, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, Spilker J, Tomsick TA, Duldner J, Broderick JP. Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2631-2635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 180] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Mayer SA. Ultra-early hemostatic therapy for intracerebral hemorrhage. Stroke. 2003;34:224-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 419] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 26. | Steiner T, Bösel J. Options to restrict hematoma expansion after spontaneous intracerebral hemorrhage. Stroke. 2010;41:402-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 27. | Kazui S, Minematsu K, Yamamoto H, Sawada T, Yamaguchi T. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke. 1997;28:2370-2375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 237] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Ohwaki K, Yano E, Nagashima H, Hirata M, Nakagomi T, Tamura A. Blood pressure management in acute intracerebral hemorrhage: relationship between elevated blood pressure and hematoma enlargement. Stroke. 2004;35:1364-1367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 275] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 29. | Qureshi AI, Hanel RA, Kirmani JF, Yahia AM, Hopkins LN. Cerebral blood flow changes associated with intracerebral hemorrhage. Neurosurg Clin N Am. 2002;13:355-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Nath FP, Kelly PT, Jenkins A, Mendelow AD, Graham DI, Teasdale GM. Effects of experimental intracerebral hemorrhage on blood flow, capillary permeability, and histochemistry. J Neurosurg. 1987;66:555-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 115] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Bullock R, Brock-Utne J, van Dellen J, Blake G. Intracerebral hemorrhage in a primate model: effect on regional cerebral blood flow. Surg Neurol. 1988;29:101-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 93] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Qureshi AI, Wilson DA, Hanley DF, Traystman RJ. No evidence for an ischemic penumbra in massive experimental intracerebral hemorrhage. Neurology. 1999;52:266-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 144] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Zazulia AR, Diringer MN, Videen TO, Adams RE, Yundt K, Aiyagari V, Grubb RL, Powers WJ. Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. J Cereb Blood Flow Metab. 2001;21:804-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 297] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 34. | Herweh C, Jüttler E, Schellinger PD, Klotz E, Jenetzky E, Orakcioglu B, Sartor K, Schramm P. Evidence against a perihemorrhagic penumbra provided by perfusion computed tomography. Stroke. 2007;38:2941-2947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Kim-Han JS, Kopp SJ, Dugan LL, Diringer MN. Perihematomal mitochondrial dysfunction after intracerebral hemorrhage. Stroke. 2006;37:2457-2462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 36. | Vespa PM. Metabolic penumbra in intracerebral hemorrhage. Stroke. 2009;40:1547-1548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, Forsting M, Harnof S, Klijn CJ, Krieger D. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014;9:840-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 483] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 38. | Huynh TJ, Demchuk AM, Dowlatshahi D, Gladstone DJ, Krischek O, Kiss A, Hill MD, Molina CA, Rodriguez-Luna D, Dzialowski I. Spot sign number is the most important spot sign characteristic for predicting hematoma expansion using first-pass computed tomography angiography: analysis from the PREDICT study. Stroke. 2013;44:972-977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Romero JM, Brouwers HB, Lu J, Delgado Almandoz JE, Kelly H, Heit J, Goldstein J, Rosand J, Gonzalez RG. Prospective validation of the computed tomographic angiography spot sign score for intracerebral hemorrhage. Stroke. 2013;44:3097-3102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Brouwers HB, Goldstein JN, Romero JM, Rosand J. Clinical applications of the computed tomography angiography spot sign in acute intracerebral hemorrhage: a review. Stroke. 2012;43:3427-3432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1304] [Cited by in F6Publishing: 1319] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 42. | Godoy DA, Boccio A. ICH score in a rural village in the Republic of Argentina. Stroke. 2003;34:e150-e151; author reply e150-e151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Clarke JL, Johnston SC, Farrant M, Bernstein R, Tong D, Hemphill JC. External validation of the ICH score. Neurocrit Care. 2004;1:53-60. [PubMed] [Cited in This Article: ] |
| 44. | Godoy DA, Piñero G, Di Napoli M. Predicting mortality in spontaneous intracerebral hemorrhage: can modification to original score improve the prediction. Stroke. 2006;37:1038-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | Hwang BY, Appelboom G, Kellner CP, Carpenter AM, Kellner MA, Gigante PR, Sander Connolly E. Clinical grading scales in intracerebral hemorrhage. Neurocrit Care. 2010;13:141-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Bruce SS, Appelboom G, Piazza M, Hwang BY, Kellner C, Carpenter AM, Bagiella E, Mayer S, Connolly ES. A comparative evaluation of existing grading scales in intracerebral hemorrhage. Neurocrit Care. 2011;15:498-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Díez-Tejedor E, Fuentes B. Homeostasis as basis of acute stroke treatment: stroke units are the key. Cerebrovasc Dis. 2005;20 Suppl 2:129-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Auer RN. Non-pharmacologic (physiologic) neuroprotection in the treatment of brain ischemia. Ann N Y Acad Sci. 2001;939:271-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Goldstein JN, Gilson AJ. Critical care management of acute intracerebral hemorrhage. Curr Treat Options Neurol. 2011;13:204-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Rincon F, Mayer SA. Clinical review: Critical care management of spontaneous intracerebral hemorrhage. Crit Care. 2008;12:237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Gujjar AR, Deibert E, Manno EM, Duff S, Diringer MN. Mechanical ventilation for ischemic stroke and intracerebral hemorrhage: indications, timing, and outcome. Neurology. 1998;51:447-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 153] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 52. | Kilpatrick MM, Lowry DW, Firlik AD, Yonas H, Marion DW. Hyperthermia in the neurosurgical intensive care unit. Neurosurgery. 2000;47:850-855; discussion 855-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 192] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 53. | Badjatia N. Hyperthermia and fever control in brain injury. Crit Care Med. 2009;37:S250-S257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 54. | Schwarz S, Häfner K, Aschoff A, Schwab S. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology. 2000;54:354-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 272] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 55. | Rincon F, Lyden P, Mayer SA. Relationship between temperature, hematoma growth, and functional outcome after intracerebral hemorrhage. Neurocrit Care. 2013;18:45-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 56. | Badjatia N. Fever control in the neuro-ICU: why, who, and when. Curr Opin Crit Care. 2009;15:79-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Lord AS, Karinja S, Lantigua H, Carpenter A, Schmidt JM, Claassen J, Agarwal S, Connolly ES, Mayer SA, Badjatia N. Therapeutic temperature modulation for fever after intracerebral hemorrhage. Neurocrit Care. 2014;21:200-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Tisdall M, Crocker M, Watkiss J, Smith M. Disturbances of sodium in critically ill adult neurologic patients: a clinical review. J Neurosurg Anesthesiol. 2006;18:57-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 59. | Harrigan MR. Cerebral salt wasting syndrome: a review. Neurosurgery. 1996;38:152-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 181] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 60. | Overgaard-Steensen C. Initial approach to the hyponatremic patient. Acta Anaesthesiol Scand. 2011;55:139-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Stelfox HT, Ahmed SB, Khandwala F, Zygun D, Shahpori R, Laupland K. The epidemiology of intensive care unit-acquired hyponatraemia and hypernatraemia in medical-surgical intensive care units. Crit Care. 2008;12:R162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 62. | Godoy DA, Di Napoli M, Rabinstein AA. Treating hyperglycemia in neurocritical patients: benefits and perils. Neurocrit Care. 2010;13:425-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 63. | Garg R, Chaudhuri A, Munschauer F, Dandona P. Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy. Stroke. 2006;37:267-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 64. | Godoy DA, Piñero GR, Svampa S, Papa F, Di Napoli M. Hyperglycemia and short-term outcome in patients with spontaneous intracerebral hemorrhage. Neurocrit Care. 2008;9:217-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Godoy DA, Di Napoli M, Biestro A, Lenhardt R. Perioperative glucose control in neurosurgical patients. Anesthesiol Res Pract. 2012;2012:690362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 66. | Vespa P, Boonyaputthikul R, McArthur DL, Miller C, Etchepare M, Bergsneider M, Glenn T, Martin N, Hovda D. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med. 2006;34:850-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 212] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 67. | Oddo M, Schmidt JM, Carrera E, Badjatia N, Connolly ES, Presciutti M, Ostapkovich ND, Levine JM, Le Roux P, Mayer SA. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit Care Med. 2008;36:3233-3238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 219] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 68. | Btaiche IF, Chan LN, Pleva M, Kraft MD. Critical illness, gastrointestinal complications, and medication therapy during enteral feeding in critically ill adult patients. Nutr Clin Pract. 2010;25:32-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 69. | Schirmer CM, Kornbluth J, Heilman CB, Bhardwaj A. Gastrointestinal prophylaxis in neurocritical care. Neurocrit Care. 2012;16:184-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | Krenitsky J. Glucose control in the intensive care unit: a nutrition support perspective. Nutr Clin Pract. 2011;26:31-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 71. | Yang TM, Lin WC, Chang WN, Ho JT, Wang HC, Tsai NW, Shih YT, Lu CH. Predictors and outcome of seizures after spontaneous intracerebral hemorrhage. Clinical article. J Neurosurg. 2009;111:87-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 72. | Passero S, Rocchi R, Rossi S, Ulivelli M, Vatti G. Seizures after spontaneous supratentorial intracerebral hemorrhage. Epilepsia. 2002;43:1175-1180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 73. | Messé SR, Sansing LH, Cucchiara BL, Herman ST, Lyden PD, Kasner SE. Prophylactic antiepileptic drug use is associated with poor outcome following ICH. Neurocrit Care. 2009;11:38-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 74. | Naidech AM, Garg RK, Liebling S, Levasseur K, Macken MP, Schuele SU, Batjer HH. Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke. 2009;40:3810-3815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 75. | Claassen J, Jetté N, Chum F, Green R, Schmidt M, Choi H, Jirsch J, Frontera JA, Connolly ES, Emerson RG. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356-1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 276] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 76. | Vespa PM, O’Phelan K, Shah M, Mirabelli J, Starkman S, Kidwell C, Saver J, Nuwer MR, Frazee JG, McArthur DA. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60:1441-1446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 364] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 77. | Phan TG, Koh M, Vierkant RA, Wijdicks EF. Hydrocephalus is a determinant of early mortality in putaminal hemorrhage. Stroke. 2000;31:2157-2162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 78. | Staykov D, Huttner HB, Struffert T, Ganslandt O, Doerfler A, Schwab S, Bardutzky J. Intraventricular fibrinolysis and lumbar drainage for ventricular hemorrhage. Stroke. 2009;40:3275-3280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 79. | Hinson HE, Hanley DF, Ziai WC. Management of intraventricular hemorrhage. Curr Neurol Neurosci Rep. 2010;10:73-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 80. | Gaberel T, Magheru C, Emery E. Management of non-traumatic intraventricular hemorrhage. Neurosurg Rev. 2012;35:485-494; discussion 494-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 81. | Dey M, Jaffe J, Stadnik A, Awad IA. External ventricular drainage for intraventricular hemorrhage. Curr Neurol Neurosci Rep. 2012;12:24-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 82. | Naff N, Williams MA, Keyl PM, Tuhrim S, Bullock MR, Mayer SA, Coplin W, Narayan R, Haines S, Cruz-Flores S. Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: the intraventricular hemorrhage thrombolysis trial. Stroke. 2011;42:3009-3016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 83. | Ziai WC, Tuhrim S, Lane K, McBee N, Lees K, Dawson J, Butcher K, Vespa P, Wright DW, Keyl PM. A multicenter, randomized, double-blinded, placebo-controlled phase III study of Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III). Int J Stroke. 2014;9:536-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 84. | Janny P, Papo I, Chazal J, Colnet G, Barretto LC. Intracranial hypertension and prognosis of spontaneous intracerebral haematomas. A correlative study of 60 patients. Acta Neurochir (Wien). 1982;61:181-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Ropper AH, King RB. Intracranial pressure monitoring in comatose patients with cerebral hemorrhage. Arch Neurol. 1984;41:725-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 86. | Fernandes HM, Siddique S, Banister K, Chambers I, Wooldridge T, Gregson B, Mendelow AD. Continuous monitoring of ICP and CPP following ICH and its relationship to clinical, radiological and surgical parameters. Acta Neurochir Suppl. 2000;76:463-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 87. | Ziai WC, Melnychuk E, Thompson CB, Awad I, Lane K, Hanley DF. Occurrence and impact of intracranial pressure elevation during treatment of severe intraventricular hemorrhage. Crit Care Med. 2012;40:1601-1608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 88. | Kamel H, Hemphill JC. Characteristics and sequelae of intracranial hypertension after intracerebral hemorrhage. Neurocrit Care. 2012;17:172-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 89. | Zeng J, Zheng P, Tong W, Fang W. Decreased risk of secondary brain herniation with intracranial pressure monitoring in patients with haemorrhagic stroke. BMC Anesthesiol. 2014;14:19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 90. | Godoy DA, Rabinstein AA, Biestro A, Ainslie PN, Di Napoli M. Effects of indomethacin test on intracranial pressure and cerebral hemodynamics in patients with refractory intracranial hypertension: a feasibility study. Neurosurgery. 2012;71:245-257; discussion 257-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Qureshi AI, Ezzeddine MA, Nasar A, Suri MF, Kirmani JF, Hussein HM, Divani AA, Reddi AS. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med. 2007;25:32-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 92. | Zhang Y, Reilly KH, Tong W, Xu T, Chen J, Bazzano LA, Qiao D, Ju Z, Chen CS, He J. Blood pressure and clinical outcome among patients with acute stroke in Inner Mongolia, China. J Hypertens. 2008;26:1446-1452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 93. | Rodriguez-Luna D, Piñeiro S, Rubiera M, Ribo M, Coscojuela P, Pagola J, Flores A, Muchada M, Ibarra B, Meler P. Impact of blood pressure changes and course on hematoma growth in acute intracerebral hemorrhage. Eur J Neurol. 2013;20:1277-1283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 94. | Anderson CS, Huang Y, Wang JG, Arima H, Neal B, Peng B, Heeley E, Skulina C, Parsons MW, Kim JS. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7:391-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 575] [Cited by in F6Publishing: 526] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 95. | Qureshi AI. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH): rationale and design. Neurocrit Care. 2007;6:56-66. [PubMed] [Cited in This Article: ] |
| 96. | Butcher KS, Jeerakathil T, Hill M, Demchuk AM, Dowlatshahi D, Coutts SB, Gould B, McCourt R, Asdaghi N, Findlay JM. The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial. Stroke. 2013;44:620-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 97. | Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, Lindley R, Robinson T, Lavados P, Neal B. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355-2365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1051] [Cited by in F6Publishing: 972] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 98. | Qureshi A, Palesch Y. Expansion of recruitment time window in antihypertensive treatment of acute cerebral hemorrhage (ATACH) II trial. J Vasc Interv Neurol. 2012;5:6-9. [PubMed] [Cited in This Article: ] |
| 99. | Goldstein J, Brouwers H, Romero J, McNamara K, Schwab K, Greenberg S, Rosand J. SCORE-IT: the Spot Sign score in restricting ICH growth-an Atach-II ancillary study. J Vasc Interv Neurol. 2012;5:20-25. [PubMed] [Cited in This Article: ] |
| 100. | Kappelle LJ. Preventing deep vein thrombosis after stroke: strategies and recommendations. Curr Treat Options Neurol. 2011;13:629-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 101. | André C, de Freitas GR, Fukujima MM. Prevention of deep venous thrombosis and pulmonary embolism following stroke: a systematic review of published articles. Eur J Neurol. 2007;14:21-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Masotti L, Di Napoli M, Godoy DA, Rafanelli D, Liumbruno G, Koumpouros N, Landini G, Pampana A, Cappelli R, Poli D. The practical management of intracerebral hemorrhage associated with oral anticoagulant therapy. Int J Stroke. 2011;6:228-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 103. | Caprini JA. Mechanical methods for thrombosis prophylaxis. Clin Appl Thromb Hemost. 2010;16:668-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 104. | Lacut K, Bressollette L, Le Gal G, Etienne E, De Tinteniac A, Renault A, Rouhart F, Besson G, Garcia JF, Mottier D. Prevention of venous thrombosis in patients with acute intracerebral hemorrhage. Neurology. 2005;65:865-869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 105. | Dennis M, Sandercock PA, Reid J, Graham C, Murray G, Venables G, Rudd A, Bowler G. Effectiveness of thigh-length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. Lancet. 2009;373:1958-1965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 293] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 106. | CLOTS (Clots in Legs Or sTockings after Stroke) Trial Collaboration. Thigh-length versus below-knee stockings for deep venous thrombosis prophylaxis after stroke: a randomized trial. Ann Intern Med. 2010;153:553-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 107. | Imberti D, Ageno W, Dentali F, Donadini M, Manfredini R, Gallerani M. Retrievable vena cava filters: a clinical review. J Thromb Thrombolysis. 2012;33:258-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 108. | Langhorne P, Stott D, Knight A, Bernhardt J, Barer D, Watkins C. Very early rehabilitation or intensive telemetry after stroke: a pilot randomised trial. Cerebrovasc Dis. 2010;29:352-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 109. | Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007;146:278-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 371] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 110. | Masotti L, Godoy DA, Napoli MD, Rabinstein AA, Paciaroni M, Ageno W. Pharmacological prophylaxis of venous thromboembolism during acute phase of spontaneous intracerebral hemorrhage: what do we know about risks and benefits. Clin Appl Thromb Hemost. 2012;18:393-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 111. | Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e44S-e88S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1106] [Cited by in F6Publishing: 1016] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 112. | van Veen JJ, Maclean RM, Hampton KK, Laidlaw S, Kitchen S, Toth P, Makris M. Protamine reversal of low molecular weight heparin: clinically effective. Blood Coagul Fibrinolysis. 2011;22:565-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 113. | Ageno W, Garcia D, Aguilar MI, Douketis J, Finazzi G, Imberti D, Iorio A, Key NS, Lim W, Marietta M. Prevention and treatment of bleeding complications in patients receiving vitamin K antagonists, part 2: Treatment. Am J Hematol. 2009;84:584-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 114. | Hanger HC, Geddes JA, Wilkinson TJ, Lee M, Baker AE. Warfarin-related intracerebral haemorrhage: better outcomes when reversal includes prothrombin complex concentrates. Intern Med J. 2013;43:308-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 115. | Levi M, Eerenberg E, Kamphuisen PW. Bleeding risk and reversal strategies for old and new anticoagulants and antiplatelet agents. J Thromb Haemost. 2011;9:1705-1712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 116. | Witt DM, Delate T, Hylek EM, Clark NP, Crowther MA, Dentali F, Ageno W, Martinez KD, Garcia DA. Effect of warfarin on intracranial hemorrhage incidence and fatal outcomes. Thromb Res. 2013;132:770-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 117. | Kuwashiro T, Yasaka M, Itabashi R, Nakagaki H, Miyashita F, Naritomi H, Minematsu K. Effect of prothrombin complex concentrate on hematoma enlargement and clinical outcome in patients with anticoagulant-associated intracerebral hemorrhage. Cerebrovasc Dis. 2011;31:170-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 118. | Dowlatshahi D, Butcher KS, Asdaghi N, Nahirniak S, Bernbaum ML, Giulivi A, Wasserman JK, Poon MC, Coutts SB. Poor prognosis in warfarin-associated intracranial hemorrhage despite anticoagulation reversal. Stroke. 2012;43:1812-1817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 119. | Dentali F, Marchesi C, Giorgi Pierfranceschi M, Crowther M, Garcia D, Hylek E, Witt DM, Clark NP, Squizzato A, Imberti D. Safety of prothrombin complex concentrates for rapid anticoagulation reversal of vitamin K antagonists. A meta-analysis. Thromb Haemost. 2011;106:429-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 238] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 120. | Ingerslev J, Vanek T, Culic S. Use of recombinant factor VIIa for emergency reversal of anticoagulation. J Postgrad Med. 2007;53:17-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 121. | Lauer A, Pfeilschifter W, Schaffer CB, Lo EH, Foerch C. Intracerebral haemorrhage associated with antithrombotic treatment: translational insights from experimental studies. Lancet Neurol. 2013;12:394-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 122. | Majeed A, Hwang HG, Connolly SJ, Eikelboom JW, Ezekowitz MD, Wallentin L, Brueckmann M, Fraessdorf M, Yusuf S, Schulman S. Management and outcomes of major bleeding during treatment with dabigatran or warfarin. Circulation. 2013;128:2325-2332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 231] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 123. | Piccini JP, Garg J, Patel MR, Lokhnygina Y, Goodman SG, Becker RC, Berkowitz SD, Breithardt G, Hacke W, Halperin JL. Management of major bleeding events in patients treated with rivaroxaban vs. warfarin: results from the ROCKET AF trial. Eur Heart J. 2014;35:1873-1880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 124. | Hoffman M, Dargaud Y. Mechanisms and monitoring of bypassing agent therapy. J Thromb Haemost. 2012;10:1478-1485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 125. | Creutzfeldt CJ, Weinstein JR, Longstreth WT, Becker KJ, McPharlin TO, Tirschwell DL. Prior antiplatelet therapy, platelet infusion therapy, and outcome after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2009;18:221-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 126. | de Gans K, de Haan RJ, Majoie CB, Koopman MM, Brand A, Dijkgraaf MG, Vermeulen M, Roos YB. PATCH: platelet transfusion in cerebral haemorrhage: study protocol for a multicentre, randomised, controlled trial. BMC Neurol. 2010;10:19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 127. | Naidech AM, Maas MB, Levasseur-Franklin KE, Liotta EM, Guth JC, Berman M, Rosenow JM, Lindholm PF, Bendok BR, Prabhakaran S. Desmopressin improves platelet activity in acute intracerebral hemorrhage. Stroke. 2014;45:2451-2453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 128. | Kreitzer N, Adeoye O. An update on surgical and medical management strategies for intracerebral hemorrhage. Semin Neurol. 2013;33:462-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 129. | Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, Karimi A, Shaw MD, Barer DH. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 619] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 130. | Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382:397-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 935] [Cited by in F6Publishing: 793] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 131. | Gregson BA, Broderick JP, Auer LM, Batjer H, Chen XC, Juvela S, Morgenstern LB, Pantazis GC, Teernstra OP, Wang WZ. Individual patient data subgroup meta-analysis of surgery for spontaneous supratentorial intracerebral hemorrhage. Stroke. 2012;43:1496-1504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 132. | Zhou X, Chen J, Li Q, Ren G, Yao G, Liu M, Dong Q, Guo J, Li L, Guo J. Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: a meta-analysis of randomized controlled trials. Stroke. 2012;43:2923-2930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 133. | Barnes B, Hanley DF, Carhuapoma JR. Minimally invasive surgery for intracerebral haemorrhage. Curr Opin Crit Care. 2014;20:148-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |