INTRODUCTION
Down syndrome (DS) is an autosomal trisomy 21 and is one of the most frequently occurring chromosomal abnormalities. DS occurs once in every 600 to 800 live births and is frequently associated with congenital heart disease (CHD). The incidence of CHDs increases from 0.8% in the general population to approximately 40%-65% in patients with DS. At the same time, children with DS comprise approximately 10% of all children with CHD. Such malformations include all structural and functional cardiac defects present at birth, even if discovered later in life. These malformations can be single or multiple and usually lead to significant implications for the children and their families. These children may develop congestive heart failure, pulmonary vascular disease, pneumonia, or failure to thrive. CHDs are the most common cause of death in children with DS during the first two years of life[1,2].
Atrioventricular septal defects (AVSD; with or without other CHD) and ventricular septal defects (VSD; with or without other CHD) have both been reported as the most common congenital heart defects and make up approximately 45% and 35% of CHD associated with DS, respectively. Additionally, isolated secundum atrial septal defect (ASD), isolated persistent patent ductus arteriosus (PDA), and an isolated Fallout of Tetralogy (TOF) individually comprise approximately 8%, 7%, and 4% of CHD associated with DS, respectively. The remaining 1% of CHDs found in patients with DS includes arch abnormalities (aortic coarctation, right aortic arch, aberrant right subclavian artery). DS tends to be associated with the more severe forms of endocardial cushion defect, while the inlet VSD is common in trisomy 21. Several cardiac lesions seen in the non-DS population are rarely if ever found in individuals with DS, e.g., heterotaxy, aortic coarctation or transposition of the great arteries. These differences between the types of CHD in DS and non-DS populations could suggest the impact of a third copy of a gene or genes on chromosome 21 on only specific developmental points[3,4].
There is an increased prevalence of persistent pulmonary hypertension of the neonate (PPHN) in children with DS. These children have an increased risk of developing PPHN even in the absence of structural heart disease and should be followed until resolution of the pulmonary hypertension[5]. Weijerman et al[6], 2010 observed a significantly increased and elevated incidence of PPHN in neonates with DS (5.2%) compared to the general population. Another rare presentation in neonates with DS is the association of hypertrophic cardiomyopathy with complete atrioventricular canal defect in an infant with trisomy 21[7].
While a defect of the atrioventricular canal remains the most common heart malformation in children with DS, the type of associated CHD may be affected by various factors. For example, the parents’ consanguinity status could affect the pattern of CHDs. Al-Jarallah[8], 2009 documented a slightly higher frequency of CHD in a sample of DS children from a Saudi population with a high consanguineous marriage rate. That study found that VSD was the most frequently detected CHD with the predominance of left-right shunt lesions and the relative rarity of cyanotic and complex CHD in this DS population. However, Chéhab et al[9] previously showed that the risk of congenital cardiac anomalies in children with DS was not associated with the parents’ consanguinity; instead, having a maternal age above 32 years was more associated with a lesser occurrence of congenital cardiac anomalies in children with DS.
Ethnicity and sex are additional factors that appear related to the type and frequency of CHD in the DS population. In a study conducted in the United States of America, Freeman et al[10] showed that atrioventricular septal defects had the most significant sex and ethnic differences, with twice as many females affected and with twice as many blacks and half as many Hispanics affected compared to whites. In the Saudi population with DS, VSD was the most common (33.3%) followed by AVSD (22.8%), ASD (21.1%), patent ductus arteriosus (14%) and tetralogy of Fallout (11%). In a Turkish sample, the most common single defect was AVSD (34.2%), followed by second ASD (16.7%) and VSD (16.5%)[11,12]. On the other hand, PDA was the most common cardiac malformation observed in Guatemalan children with DS, followed by VSD, ASD and then AVSD[13]. The most common cardiac malformations in Mexican children with DS were ASD, VSD and PDA, while the AVSDs were less common than the other malformations[14].
Children with DS may also have dysfunctional autonomic cardiac regulation even in the absence of concomitant congenital heart disease, which may be manifested mainly in a reduced heart rate response to excitatory stimuli, including arousal from sleep. This is especially noted if accompanied by sleep-disordered breathing (SDB). O’Driscoll et al[15] described a compromised acute cardio-respiratory response and dampened sympathetic response to SDB in children with DS and SDB compared to typically developed children with SDB. This altered response may be due to inadequate sympathetic activation or blunted vagal withdrawal and could reflect autonomic dysfunction in children with DS that may place them at increased risk for cardiovascular complications, such as pulmonary hypertension.
DS children with CHD have a greater predisposition to develop irreversible pulmonary arterial hypertension (PAH). The increased incidence of pulmonary hypertension in DS children could be a result of additional related problems, such as an upper airway obstruction, pulmonary hypoplasia, structural lung disease, thinner media of the pulmonary arterioles, abnormal pulmonary vasculature growth, alveolar hypoventilation, recurrent pulmonary infection or gastro-esophageal reflux[16]. It has been suggested that PAH may develop earlier and may have a more violent course in patients with Down’s syndrome, carrying with it a high risk of morbidity in a relatively young patient[17,18].
MECHANISM OF CHD IN CHILDREN WITH DS
Heart development in humans is complex and starts very early, from the third to eight weeks of gestation. Development begins with a primitive tube that beats at 25 d of gestation and ends in the four-chamber heart. Many steps occur after the formation of the primitive heart tube, including looping, cell migration, cell transition, and septation events[19]. The development of CHD is a multi-factorial condition and is affected by a series of molecular signaling pathways and morphological events. In children with DS, it is postulated that variations in gene dosage of chromosome 21, environmental factors and genetic modifications not linked to chromosome 21 contribute to the development of CHD[20].
Down’s syndrome is most commonly caused by the presence of an extra copy of all or part of human chromosome 21 (Hsa21). The extra set of the approximately 200-300 genes on the chromosome leads to the many abnormalities associated with this condition. Due to triplication of Hsa21, there is a 1.5-fold increase in the expression of some, if not all, of these genes present in the extra copy. However, all of these genes do not necessarily exhibit a straightforward 1.5-fold increase in expression, and only 30% of Hsa21 genes are significantly over-expressed. Gene expression may be regulated by dosage compensation, which means that only a subset of these genes will exhibit the expected 50% increase in expression[21]. Genetic imbalance caused by the presence of an extra copy of chromosome 21 will seriously disrupt one or more developmental pathways. This imbalance could also induce also an interaction between Hsa21 genes and other disomic genes with relatively subtle or massively disruptive effects on genes located on chromosomes other than 21[22]. These effects could be mediated through modulation of transcription factors, chromatin remodeling proteins, and related molecules or other targets. Thus, the imbalance-induced dysregulation of pathways involved in heart development may cause the cardiac defects observed in DS[23].
The DS critical region (DSCR) is located on the long (q) arm of chromosome 21 and contains a number of genes that are thought to be responsible for some, if not all, of the features of DS. These genes may include the DS critical region 1 gene, or DSCR1, on chromosome 21q11.2; DSCR2 on chromosome 21q22.3; DSCR3 on chromosome 21q22.2; DSCR4 on chromosome 21q22.2; and DSCR5 in the chromosome region 21q22.1-q22.2. DS critical region 1, also known as Calcipressin-1, Adapt78, myocyte-enriched calcineurin-interacting protein 1 or regulator of calcineurin 1, is a 252 amino acid protein that belongs to the RCAN family (regulators of calcineurin) and exists as 4 alternatively spliced isoforms. DSCR1 is abundantly expressed in skeletal muscle, as well as in the brain and heart, and is thought to influence cardiac and nervous system development[24]. DSCR1 is possibly part of a signal transduction pathway involving both the heart and brain and is implicated in cardiac valve formation and in the inhibition of cardiac hypertrophy[25]. Overexpression of DSCR1 may be involved in the pathogenesis of DS, in particular mental retardation or cardiac defects[26]. Barlow et al[27] had previously mapped the DS-CHD region in humans to a 5.27-Mb chromosomal segment containing 82 genes, and Korbel et al[28] had narrowed this segment to a 2.82-Mb critical region likely involved in a DS-CHD endocardial cushion defect.
The presence of specific gene variants or modifiers could, in addition to trisomy 21, further increase the susceptibility to cardiac defects. One such genetic modifier is cysteine-rich with epidermal growth factor (EGF)-like domains (CRELD1), initially identified as a candidate for the AVSD2 locus. CRELD1 belongs to an epidermal growth factor-like family and encodes a cell surface protein that likely functions as a cell adhesion molecule. CRELD1 encodes a novel cell adhesion molecule that is expressed during cardiac cushion development. Missense mutations in CRELD1 have been found in DS patients with AVSD. CRELD1 (3p25.1) is one of the well-studied non-chromosome 21 loci variations that may predispose one to a heart defect. Other genetic modifiers have also been shown to affect heart development. For example, somatic mutations in basic helix-loop-helix (bHLH) transcription factor HEY2 (gridlock) have been identified in CHD in people with DS but not in euploid populations with heart defects[29-32].
Environmental factors interact with the trisomic genome and may modify the occurrence of associated CHD in children with DS. Maternal cigarette smoking, for instance, has been associated with ASVD, TOF, and ASD but not with VSD[33]. However, Khoury and Erickson observed an inverse relationship between maternal alcohol use and the presence of VSD in children with DS. Maternal folic acid supplementation may be associated with a reduced risk for CHD. Individuals with DS are at a high risk for CHD and have been shown to have abnormal folate metabolism[34]. Bean et al[35] found that a lack of maternal folic acid supplementation was more frequent among infants with DS and AVSD or ASD than among infants with DS and no heart defect or with VSD.
FETAL ECHOCARDIOGRAPHY
Fetal echocardiography is considered in cases of suspected DS. Such instances include the observation of a fetal nuchal translucency measurement of 3.5 mm or greater in the first trimester, the presence of a single umbilical artery or following an abnormal or incomplete cardiac evaluation on an anatomic scan, 4-chamber study. Fetal echocardiography can identify fetal cardiac structures as early as 10-12 wk of gestation using vaginal probes with high-resolution transducers, while conventional trans-abdominal echocardiography can detect fetal cardiac anomalies by 16-18 wk of gestation. The optimum period in which to perform a screening examination is at 20-22 wk because, at that time, the fetal cardiac structures can be defined more clearly with ultrasound screening in more than 90% of cases. Fetal echocardiographic examination can be difficult because of fetal physiology, the effect on flow across defects and valves, the inability to see the fetus for orientation reference, and an inability to examine the fetus for clinical findings. Likewise, a detailed heart evaluation can be very difficult during the third trimester because of acoustic shadowing, as in cases of maternal obesity or prone fetal position[36].
Fetal echocardiography can assist in the early recognition of DS by detecting soft syndrome markers, but its main role is to define the exact nature of the cardiac anomaly suspected in the fetus. Such an assessment helps both the parents and the treating physician make the most informed decisions. Fetal echocardiography can provide the possibility of pregnancy termination in cases of severe malformations and of treating in utero the potentially treatable and less severe disorders, e.g., fetal supraventricular tachycardia[37]. In addition, fetal echocardiography can identify babies with complex congenital heart diseases that need delivery in a special tertiary care level center equipped with a Neonatal Intensive Care Unit so that during the transition from pre- to post-natal life, the baby does not face periods of hypoxia or acidosis and can be given immediate care[38].
Soft syndrome markers are ultrasound findings that are considered abnormal and whose presence increases the risk for underlying fetal aneuploidy and congenital heart diseases. These markers are nonspecific and could also present in fetuses without abnormalities. They are often transient and can be readily detected during the second trimester[39].
Echogenic intracardiac foci (EICF) are examples of these soft markers that have been associated with DS, as well as with trisomy 13. They are a common finding seen in approximately 4% of obstetric sonograms, and their incidence can vary with ethnicity. The lowest rates of EICF are seen in black populations, while the highest rates occur among Asian patients. These foci are of no hemodynamic or other short or long term clinical or functional significance, but their importance arises from being a possible marker of a chromosomal abnormality[40]. These foci are discrete areas of increased echogenicity in the region of papillary muscles. They may be single or multiple and usually present in the left ventricle (88% of cases) but occasionally present in the right ventricle (5%) and may be bilateral in approximately 7% of cases. The right-sided, biventricular, multiple, or significantly obvious EICF are associated with a higher risk for fetal aneuploidy compared with the more common single, left ventricular EICF[41,42].
These foci may result from the aggregation of chordal tissues that have failed to fenestrate completely, the enhancement of abnormal tissue, or from a collection of fibrous tissue with increased echogenicity. They may also represent true microcalcifications within the cardiac muscle[43]. The larger size of the left ventricular papillary muscle and the larger masses of chordae tissue, the more likely is to see echogenic foci in this area[44].
A finding of EICF is subjective. Its detection depends on a variety of factors, including the resolution of the sonographic equipment, technique, thoroughness of the examination, and the sonographer’s experience[45]. For proper visualization and grading of EICF, an appropriate transducer frequency (≤ 5 MHz) and an appropriate gain setting should be used. These foci can be diagnosed on the standard 4-chamber view of the fetal heart. Fetal position is also important because intracardiac foci are best visualized when the cardiac apex is oriented toward the transducer. The foci echogenicities are graded according to their comparison to the surrounding bones, especially the thoracic spine. In grade 1, the echogenicity is less than that of the thoracic spine and the EICF image will be lost before that of the thoracic spine. Grade 2 suggests that the echogenicity is representative of bone and that the EICF and thoracic spine images disappear at the same gain setting. In grade 3, the echogenicity of the EICF is greater than that of bone, and the thoracic spine image will be lost before that of the EICF[46].
The foci should be differentiated from other cardiac conditions that can be misdiagnosed with these foci. These conditions include intra-cardiac tumors (rhabdomyomas, teratomas, fibromas, hemangiomas), ventricular thrombi (especially if adherent to the papillary muscles in the left ventricle), dystrophic valves, air in the chambers from fetal demise, endocardial fibroelastosis that is usually multiple and along the endocardial surface, idiopathic infantile arterial calcification, viral infections or metabolic disorders[47].
The presence of an aberrant right subclavian artery (ARSA) is another potential new soft marker that is more commonly seen in fetuses with trisomy 21 and other chromosomal defects than in normal fetuses. An ARSA has been found postnatally in 1%-2% of normal individuals (from neonates to adulthood) at autopsy, but its incidence is increased in cases of DS, with figures ranging between 2.9% and 37.0%[48]. Although it can be considered a weak marker, the second trimester diagnosis of an ARSA should prompt a detailed search for additional “soft markers” and potential structural defects[49].
Once DS is suspected, fetal echocardiography should be performed to detect associated structural cardiac abnormalities. A cardiac anomaly can be classified according to its detectability by fetal echocardiography and its severity. With regard to detectability, the structural cardiac abnormalities are classified as detectable or undetectable. Detectable cardiac anomalies are those that can be identified during routine antenatal assessment incorporating a four-chamber view of the heart at approximately eighteen week’ gestation. These abnormalities usually produce marked cardiomegaly, an obvious abnormality of the atrioventricular connection, or a disparity between the sizes of the atria or ventricles or both. In undetectable abnormalities, the four-chamber view fails to identify them as types of major malformations affecting the left or right heart outflows. Ventricular septal defect, pulmonary stenosis, aortic stenosis, and coarctation of the aorta are considered among the possible undetectable abnormalities[37].
With regard to severity, structural cardiac abnormalities are classified into “complex,”“significant,” and “minor” heart disease. Complex heart disease occurs when there is an atretic or hypoplastic valve or cardiac chamber, which may include a hypoplastic left heart, Truncus arteriosus, pulmonary atresia with ventricular septal defect, or a double outlet ventricle. Heart diseases are considered significant when four chambers of normal or increased size and four valves are present but require intervention, e.g., Ebstein/tricuspid valve dysplasia, significant complete AVSD, large VSD, partial AVSD, ASD, PDA, severe aortic stenosis, severe pulmonary stenosis (PS), transposition of the great arteries (TGA), coarctation of the aorta, total anomalous pulmonary venous connection, or TOF. Cardiac abnormalities are considered minor when no treatment is required, such as in cases with small VSD or relatively mild aortic or pulmonary valve stenosis. If there are multiple cardiac abnormalities, the disorder is classified according to the most severe diagnosis. There are two exceptions from this classification: the endocardial fibroelastosis, which is classified as “complex”, and complete AVSD, which is classified as “significant”[50].
There is a further classification according to severity suggested by Choi et al[51], who identified 5 classes of fetal echocardiography results: normal, minor abnormalities, simple cardiac anomalies, moderate cardiac anomalies, and complex cardiac anomalies. Minor abnormalities are those that do not require any interference, such as PFO, isolate peripheral PS, abnormally looking aortic arch, simple right aortic arch, tortuous ductus without obstruction, and mild right ventricular dilation with or without tricuspid regurgitation (at most mild regurgitation). Simple cardiac anomalies are defined as a simple defect or a defect completely correctable by medical treatment, such as VSD, ASD, and possibly CoA. Moderate cardiac anomalies include defects that are surgically correctable with a low risk for reoperation, such as TOF, CoA, AVSD, and complete TGA. Complex cardiac anomalies are defined as defects correctable with surgery but that carry a high risk for sequelae or defects potentially suitable for a Fontan procedure, such as a double outlet right ventricle, TGA with PS, critical PS, and other Fontan candidates (pulmonary atresia with intact ventricular septum, functional single ventricle, hypoplastic left heart syndrome). There is an additional classification according to surgical risk that has the disadvantages of having great variability between institutions and the need to change the risk stratification in accordance with future advancements in surgical treatment[51,52].
For optimal views of the heart, it is best to direct the fetal cardiac apex toward the anterior maternal wall. Optimization of the sonographic images could be achieved by appropriate adjustment of technical settings, such as acoustic focus, frequency selection, signal gain, image magnification, temporal resolution, harmonic imaging, and Doppler-related parameters. Accurate interpretation of obtained echocardiographic images will depend on a firm understanding of the anatomy of the fetal heart, either to diagnose a congenital abnormality or to confirm normality. According to guidelines from the American Institute for Ultrasound in Medicine, fetal echocardiography should include imaging of the aortic arch, ductal arch, four-chamber view, inferior vena cava, left ventricular outflow tract (LVOT), right ventricular outflow tract (RVOT), short-axis views (“low” for ventricles and “high” for outflow tracts), superior vena cava, and three-vessel and trachea views[53].
For fetuses with major CHD, a full diagnosis requires a sequential segmental approach, similar to postnatal practice[54]. The first step taken in studying the fetal heart is to definitely recognize the “Situs.”In situs solitus, the inferior vena cava (IVC) is anterior and to the right of the aorta, the abdominal aorta is posterior and to the left of the spinal cord, the gastric air bubble is on the left side and the liver is on the right. In situs inversus, there is a mirror image pattern, with the aorta to the right of the IVC, and in situs ambiguous, the aorta and IVC are located on the same side of the spine in right isomerism, while the aorta is centrally located with an interrupted IVC in left isomerism[55]. For determination of the cardiac position and axis, the heart is normally located within the left chest, with the apex pointing to the left (levocardia). In dextrocardia, the heart is located within the right chest with the apex pointing to the right, while in mesocardia the heart is centrally located with the apex pointing anteriorly. Dextroposition should be distinguished from dextrocardia by the normal left-sided axis of the heart despite being displaced to the right due to extracardiac reasons (for example, diaphragmatic hernia, congenital pulmonary cystic adenomatoid malformation, pleural effusion, etc.)[38,56].
The four-chamber view (Figure 1A and B) is a key view of the fetal heart. It is effective in prenatal cardiac screening and can detect 43%-96% of fetal anomalies. It has the advantage of using fetal ribs as external reference points to ensure that the sonographer has “cut” the thorax in the appropriate plane. In a correct four-chamber view, there should be the appearance of a single rib around the fetal thorax. The four-chamber view is situated in a more horizontal plane than in the postnatal period because of the large liver. It can show the two atria and ventricles along with atrioventricular (AV) valves (mitral and tricuspid) and septa (interventricular and interatrial)[57]. The detectability of CHDs by fetal echocardiography increases if the outflow tracts are examined as well as the four-chamber view, but appreciation of abnormalities in the outflow tracts is more challenging than in the four-chamber view[58]. “Extended basic views” of LVOT and RVOT increase the sensitivity of anomaly detection. Alternatively, a comprehensive set of five short-axis projections can be acquired. The LVOT view can be obtained by tilting the transducer 45° from the four-chamber view perpendicular to the septum. This view can show the aorta originating from the LVOT with its main great vessels of the head and neck appearing distally. The RVOT view can be obtained by further rotation in the same direction and by gentle rocking the transducer from the LVOT view. In this view, the pulmonary artery can be seen rising from the RV and dividing into its main branches. The left pulmonary artery and ductus arteriosus can be observed, and the ascending aorta is seen centrally, wrapped by the RV and PA. A comprehensive set of five short-axis projections can also be obtained by cranial or caudal angulation of the ultrasound probe from the four-chamber view with the ventricular septum parallel to the ultrasound beam and the fetal spine[54]. The pulmonary artery and RVOT can be visualized in the most cephalad short-axis view as they course around the aorta. Despite being small, the fetal pulmonary branches bifurcation can still be identified. The ductus arteriosus can likewise be identified and traced into the descending aorta. With caudal and more horizontal angulation from this plane, the ventricles and their respective AV valves can be observed. These views are better for the detection of conotruncal abnormalities that could otherwise be missed in more routine views. However, a specific diagnosis should not be made from a single plane.
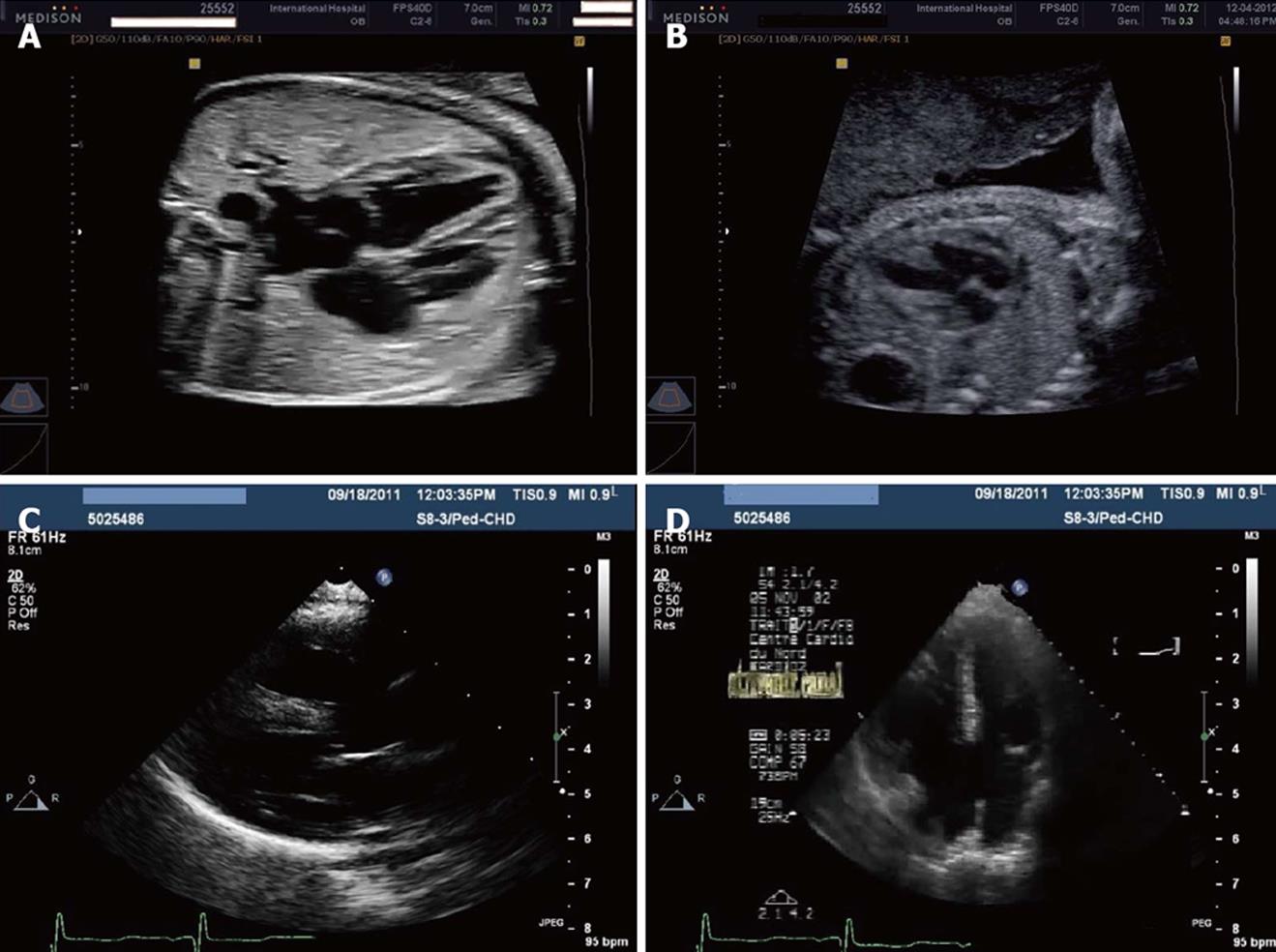
Figure 1 Ultrasonography.
A: Normal 4-chamber view by fetal echocardiography at 26 wk gestation; B: Four-chamber view by fetal echocardiography at 22 wk gestation showing ventricular septal defects; C: Left-parasternal long-axis view in an infant with Down syndrome and complete atrioventricular (AV) canal and pulmonary hypertension; D: Apical 4-chamber view in an infant with Down syndrome and complete AV canal and pulmonary hypertension.
The left and right fetal ventricles are nearly the same size, and the diameter of the pulmonary artery is typically larger than the aorta by approximately 10%. Ventricular volumes can be measured in 2-D mode using the Simpson rule. Other important measures include the RV/LV ratio, LV wall thickness, septal wall thickness, left atrial dimension, PA diameter, and aortic root diameter. These measures should be plotted against gestational age, which can be determined by measurement of the biparietal diameter or fetal length. These measures are helpful in the diagnosis of ventricular hypoplasia (either left or right) and cardiomegaly due to various congenital abnormalities, including pericardial effusion, aneurysms, cardiomyopathies, or tumors. Fetal heart motion, heart rate, wall thickness, chamber size, and motion of the valves or myocardium can be easily traced by M-mode, which can provide information on wall thickness and ventricular shortening fraction. The fetal long axis function may provide additional insight into endocardial function, which is most usefully in the detection of early ischemic changes before the development of sonographically detectable endocardial fibroelastosis. Moreover, color Doppler can be used to detect vascular flow through cardiac chambers, vascular structures, and septal defects[59-63].
Clur et al[64] showed that cardiac functions in trisomy 21 fetuses were abnormal irrespective of the presence of CHD. Evidence for cardiac loading (increased preload and afterload) and LV systolic (in the first trimester) and later diastolic dysfunction was observed. The authors showed significant reduction of tricuspid valve (TV) A-wave velocity and aortic valve peak velocity in trisomy 21 compared with normal fetuses. In addition, they also identified significant increases in the TV-E/A ratio and the ductus venosus-pulsatility index for veins and decreased pulmonary valve peak velocity. Moreover, the authors observed some evidence of left ventricular (LV) systolic dysfunction, such as a reduction of stroke volume (SV) and an increased myocardial performance index. They also found significant reduction of the mitral valve A-wave peak velocity and E/TVI ratio after 14 wk of gestation in the trisomy 21 fetuses with normal hearts compared to the controls with increased nuchal translucency thickness.
A complete AV canal can be more easily visualized in a 4-chamber view than an ASD primum, so that diagnosis of ASD primum type should be considered whenever a defect is noted in the portion of the atrial septum near the AV valves (septum primum). Opening the AV valve during diastole makes a large complete AV canal more obvious on the 4-chamber view, while during systole, the atrial and ventricular septal defects can be clearly seen above and below the closed AV valves, rendering its diagnosis possible as early as the late first trimester. Color flow Doppler can show mixing of flow in the area of the septum primum defect, the dysplastic AV valves, and the ventricular septal defect. It can also show a lack of separation between the right and left ventricular inflow tracts in diastole, producing a classic “H” configuration. Color flow Doppler is also helpful in detecting and evaluating the degree of dysplastic AV insufficiency that may be severe enough to produce fetal heart failure with ascites[65].
TRANSTHORACIC ECHOCARDIOGRAPHY
Although it has been recommended that infants with trisomy should have an echocardiogram in the first month of life, the value of routine neonatal echocardiography in this population is still in debate. Nevertheless, physical examination alone is not sufficient to identify cardiovascular anomalies in neonates with DS. In the newborn with DS, the potential benefits of early diagnosis, in the context of physical examination findings, should be considered in determining whether an echocardiogram should be performed during the neonatal period. Echocardiography should be obtained in all children with DS before proceeding with surgery[66,67].
Echocardiographic examination can provide thorough real-time, non-invasive anatomic and functional information at relatively low cost. In neonates, the echocardiographic windows are more easily obtained and clearer than at any other age because of the reduced interference by lung tissue, which is impenetrable to ultrasound, and because the heart and the great vessels are nearer the probe. Echocardiographic examination should be conducted systematically with the classic standard views [left parasternal, apical, subcostal and suprasternal (Figure 1C and D)] and completed with Doppler ultrasound (color Doppler, pulsed Doppler and continuous wave Doppler). Trans-thoracic echocardiographic examination can usually detect cardiac defects in most cases. It can also determine the level of intra-cardiac shunting, along with its degree and direction, which can be confirmed by saline contrast injection. One mL of saline/blood mixture (which provides a greater intensity and more prolonged effect than the use of agitated saline only) is rapidly injected into a peripheral vein while capturing a four-chamber view of the heart. The simultaneous appearance of bright echoes from air bubbles in the fluid in the right ventricle and left atrium documents right-to-left atrial shunting as the air bubbles produced by hand agitation are too large to cross the pulmonary vascular bed, thereby predominantly aiding visualization of the right heart. The injection of the fluids into a vein that drains into the inferior vena cava could produce better results[68,69].
Echocardiography can also evaluate cardiac functions. For example, left ventricular systolic function can be evaluated by measuring left ventricular ejection fraction, fraction shortening, SV, stroke index, cardiac output and cardiac index. Left ventricular diastolic function can be evaluated by measuring the left ventricular inflow velocity pattern or trans-mitral flow velocity pattern [the early diastolic filling velocity (E-wave) is normally higher than the peak atrial filling velocity (A-wave)], the pulmonary venous flow velocity pattern (pulmonary venous flow velocity pattern usually consists of the antegrade flow during ventricular systole (S-waves: S and S ), antegrade flow during early ventricular diastole (D wave), and retrograde flow), the flow propagation velocity (Vp) during the rapid filling period and the peak early diastolic velocity of the mitral annulus (Ea, E’). The global function of LV can be measured using the Tei index. The Tei index is the first comprehensive index of cardiac functions that covers both systolic and diastolic functions, and it deteriorates and improves with either systolic or diastolic dysfunction[70].
The quantitative assessment of RV size and function is often difficult because of the complex geometric anatomy, anatomical position under the sternum and the heavily trabeculated chamber with poor endocardial definition. However, 2-D echocardiography can easily obtain valuable information about RV size and function. Right ventricular dilatation, compared to the LV size, is a sign of RV dysfunction. Additionally, abnormal motion of the interventricular septum and the eccentricity index estimate RV pressure overload. The Tei index and the tricuspid annular plane systolic excursion both correlate well with RV function[71].
Echocardiography can also evaluate the presence and severity of pulmonary arterial hypertension, which is relatively more common in DS neonates than in non-DS neonates. Doppler echocardiography allows estimation of both systolic and diastolic pulmonary artery pressure (PAP). The systolic PAP can be estimated by measuring the tricuspid regurgitation jet that represents the right ventricular-right atrial pressure gradient. Both PAP and RV systolic pressure are nearly equal in the absence of stenosis of the RV outflow tract, which is the reason that systolic PAP is commonly estimated by techniques that measure RV systolic pressure[72].
Additionally, echocardiography is a useful tool for following up cases and for evaluating treatment outcome. Likewise, it has a further role even in the absence of congenital heart disease. Echocardiography can detect the presence of pericardial effusion in children with DS and hypothyroidism. A number of case studies have described the presence of pericardial effusion in this group of patients. Anah et al[73], for instance, described the presence of a complex association of DS-hypothyroidism-pericardial effusion.
Echocardiography may also reveal impaired cardiac function even in the absence of congenital or structural cardiac defects. A number of studies have documented impaired cardiac functions in patients with DS. They recommended echocardiographic examination before involving patients with DS in surgery or physical exercise, even in the absence of structural cardiac diseases[74,75].
Recommendation
Echocardiographic examination is recommended in children with DS in the following situations: (1) in the first month of life for all neonates with DS; (2) before any cardiac surgery; (3) follow-up after cardiac surgery; (4) serial evaluation of pulmonary hypertension; (5) before involvement in major non-cardiac surgery; and (6) before involvement in physical exercise.