Published online May 28, 2016. doi: 10.5319/wjo.v6.i2.23
Peer-review started: August 27, 2015
First decision: September 28, 2015
Revised: November 9, 2015
Accepted: March 17, 2016
Article in press: March 18, 2016
Published online: May 28, 2016
Cancers of the head and neck account for more than half a million cases worldwide annually, with a significant majority diagnosed as squamous cell carcinoma (HNSCC). Imaging studies such as contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI) and 18F-2-fluoro-2-deoxy-D-glucose positron-emission tomography/computed tomography (18F-FDG PET/CT) are widely used to determine the presence and extent of tumors and metastatic disease, both before and after treatment. Advances in PET/CT imaging have allowed it to emerge as a superior imaging modality compared to both CT and MRI, especially in detection of carcinoma of unknown primary, cervical lymph node metastasis, distant metastasis, residual/recurrent cancer and second primary tumors, often leading to alteration in management. PET/CT biomarker may further provide an overall assessment of tumor aggressiveness with prognostic implications. As new developments emerged leading to better understanding and use of PET/CT in head and neck oncology, the aim of this article is to review the roles of PET/CT in both pre- and post-treatment management of HNSCC and PET-derived parameters as prognostic indicators.
Core tip: In the pre-treatment phase, positron-emission tomography/computed tomography (PET/CT) is valuable in the evaluation of patients with carcinoma of unknown primary origin, detection of synchronous second primary tumor, staging of cervical lymph node metastasis and assessment for distant metastases. In the post-treatment phase, PET/CT is helpful in evaluating treatment response, detecting residual or recurrent tumor and excluding distant metastases. Prognostic factors derived from PET/CT metabolic and functional data are useful in predicting tumor aggressiveness with implication on patient’s survivability, and facilitate selection of treatment modality and personalized treatment options.
- Citation: Nguyen VD, Tantiwongkosi B, Weinheimer WJ, Miller FR. Positron-emission tomography/computed tomography imaging in head and neck oncology: An update. World J Otorhinolaryngol 2016; 6(2): 23-32
- URL: https://www.wjgnet.com/2218-6247/full/v6/i2/23.htm
- DOI: https://dx.doi.org/10.5319/wjo.v6.i2.23
Cancers of the head and neck account for more than half a million cases worldwide annually, with a significant majority diagnosed as squamous cell carcinoma (HNSCC). The incidence of head and neck cancer in the United States is approximately 3% of all new cancer cases, accounting for almost 60000 cases each year and 12000 deaths from the disease[1]. Tobacco and alcohol abuse, human papillomavirus (for oropharyngeal cancers), and Esptein-Barr virus infection (for nasopharyngeal cancers) are important risk factors for the development of head and neck cancers. Patient’s presentation and clinical findings are occasionally nonspecific and can vary depending on the tumor location in the head and neck. Some of these cancers may escape detection despite detailed physical examination, endoscopy and conventional cross sectional imaging, and pose significant challenges in disease diagnosis and management. Imaging studies such as contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI) and 18F-2-fluoro-2-deoxy-D-glucose positron-emission tomography/computed tomography (18F-FDG PET/CT) are widely used to determine the presence and extent of tumors and metastatic disease, both before and after treatment. Advances in PET/CT imaging have allowed it to emerge as a superior imaging modality compared to both CT and MRI in select situations, such as detection of carcinoma of unknown primary (CUP), cervical lymph node metastasis, distant metastasis, residual/recurrent cancer and second primary tumors, often leading to alteration in management[2-5]. Furthermore, PET/CT as an imaging biomarker may provide an overall assessment of tumor aggressiveness with prognostic implications.
With PET/CT imaging, injected positron-emitting radionuclide 18F-FDG is taken up by metabolically active cells, particularly cancers, in different concentrations depending on their relative metabolic rates. The radionuclide is initially transported into cells through glucose transporters with the same mechanism as for glucose but cannot be further metabolized. PET images are then created by detecting emissions from 18F-FDG and reconstructed into a three-dimensional image. CT images are also generated sequentially and coregistered with PET images using fusion software, enabling functional data obtained on PET to be coupled with anatomical CT images. Quantification of FDG uptake is simplified by measurement of the standardized uptake value (SUV), which represents the activity of 18F-FDG measured over a certain interval after radionuclide injection and normalized to its dose and the patient’s body weight[6].
Since the implementation of PET/CT in head and neck oncology over a decade ago with its approval for reimbursement by the Centers for Medicare and Medicaid Services[7], PET/CT has provided high diagnostic accuracy. PET/CT remains especially valuable in detection of regional and distant metastases and evaluation of treatment response. As more patients are cured of their cancers, acute and long-term complications of multimodality approaches including surgery, radiation, and chemotherapy may alter the anatomy and physiology of the head and neck, posing significant challenge in assessing treatment response and detecting residual or recurrent tumor by clinical evaluation and conventional imaging techniques such as CT or MRI. PET/CT may prove helpful with treatment strategy in these patients as well as those with metastatic cervical lymphadenopathy of unknown primary site despite thorough workup.
As new developments emerged leading to better understanding and use of PET/CT in head and neck oncology, this article addresses the roles of PET/CT in both pre- and post-treatment management of HNSCC. PET-derived parameters as prognostic indicators are also discussed.
Proper staging of the head and neck cancer, regional lymph nodes and detection of distant metastasis is critical for developing optimal treatment and determining prognosis. The tumor node metastasis (TNM) staging system of the American Joint Committee on Cancer, 7th edition is used to stage HNSCC[8]. The extent of the primary tumor (T stage) is site specific, while there is considerable overlap in classifying regional lymph node involvement (N0 to N3 stage) with the exception of thyroid and nasopharyngeal cancers. Metastasis outside head neck regions (e.g., mediastinal and axillary lymph nodes) represents distant metastasis (M stage). Initial evaluation and staging include a combination of physical examination, imaging studies, and direct endoscopy with tissue biopsy or fine needle aspiration.
Imaging exams such as contrast-enhanced CT, MRI and PET/CT are important to assess the extent of local extension, involvement of lymph nodes, and presence of distant metastasis. Multiple studies suggest that PET/CT is superior to conventional imaging (CT or MRI) in initial staging and may alter management, especially when unexpected cervical lymph node or distant metastasis is discovered[2-5]. A multicenter prospective study found that PET/CT improved the TNM staging of the primary cancer and subsequently altered the management in 13.7% of the patients, mainly due to the ability of PET/CT to detect metastatic or additional disease[9]. Furthermore, PET/CT can provide accurate tumor localization with precise metabolic tumor volumetric measurements, cervical lymph node staging, detection of metastases, and finding of synchronous second primary tumors that may alter radiation fields and doses for patients undergoing radiation therapy. The National Comprehensive Cancer Network issued an update in clinical practice guidelines in head and neck cancer and PET/CT imaging in 2013, recommending the use of PET/CT in initial staging of the oral cavity, oropharyngeal, hypopharyngeal, glottic, and supraglottic cancers for stage III-IV disease as well as mucosal melanoma and nasopharyngeal carcinoma (World Health Organization class 2-3 and N2-3 diseases)[10].
The CT portion of the PET/CT examination provides the superior contrast and spatial resolution to detect malignant tumor using morphology (such as ill-defined, infiltrative, ulcerative features), enhancement, and interval growth. The PET portion demonstrates semiquantitative assessment with SUV of malignant tumor typically greater than 2.5-3.0[11]. Similarly, a maximum SUV greater than 2.5 is 100% sensitive and a maximum SUV greater than 5.5 is 100% specific for malignant lymphadenopathy[12]. However, SUV assessment should be used in conjunction with other clinical data given the overlap between a malignant lesion (high SUV) and a benign inflammatory uptake (low SUV).
Despite the proven efficacy of PET/CT, false negatives of PET/CT may be seen in patients with occult nodal metastases less than 5 mm or metastatic lymph nodes with necrosis[13-15]. Cancers with low metabolic activity or decreased FDG uptake may also limit PET/CT sensitivity. Therefore, PET/CT does not have the sensitivity to replace neck dissection and its usefulness is uncertain in evaluating patients with clinically negative (N0) neck[16]. In addition, the utility of PET/CT in determining the resectability of head and neck cancers has not been fully explored to date; CT or MRI remains the mainstay in these patients[17].
Additional limitations unique to PET/CT include imaging artifacts, lower osseous and soft tissue contrast/resolution (when performed without intravenous contrast) as compared to contrast-enhanced CT and MRI, respectively. PET typically has a resolution of 5 mm[11], while unenhanced CT and MRI have submillimeter resolution[18]. The addition of intravenous contrast to CT and MR enhances visibility of the lesions and enable separation of the lesions from adjacent vessels. In this regard, contrast-enhanced CT and MRI are superior imaging modalities for evaluating T stage of HNSCC. There is currently no clear recommendation for routine use of PET/CT in initial T staging, as several studies demonstrated 5.5%-8.5% of patients had T staging upstaged on PET/CT[2,5].
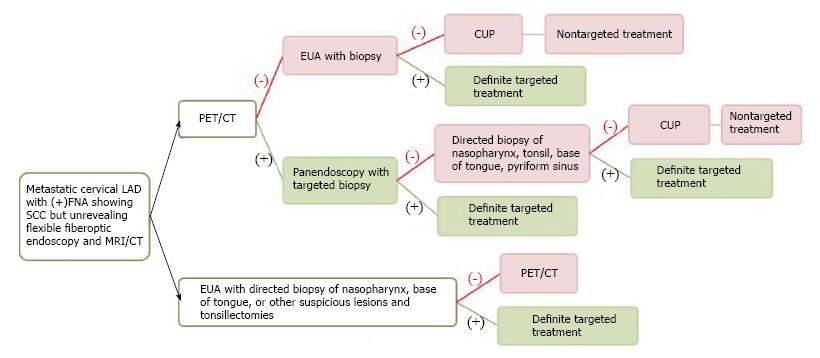
Three to five percent of HNSCC patients present with metastatic cervical lymphadenopathy without definite primary site detected[2,19,20] despite a thorough history (often with nonspecific symptoms or no symptoms), combination of physical examination with office flexible fiberoptic endoscopy (for small submucosal lesion), or conventional contrast-enhanced CT/MRI performed. The work up algorithm to search for the primary tumor is shown in Figure 1, adapted from Tantiwongkosi et al[21] and Schmalbach et al[22].
The choice of treatment depends on staging and histology[23]. With locoregionally advanced cervical lymphadenopathy, the goal of treatment is generally directed at cure; whereas, cervical lymphadenopathy from unknown primary originating below the clavicles may represent incurable disease with distant metastasis. Failure to identify the primary tumor leads to nontargeted treatment (bilateral tonsillectomies, bilateral neck dissection, radiation to cover the whole pharyngeal mucosa and neck)[24] resulting in increased complications, morbidity and mortality.
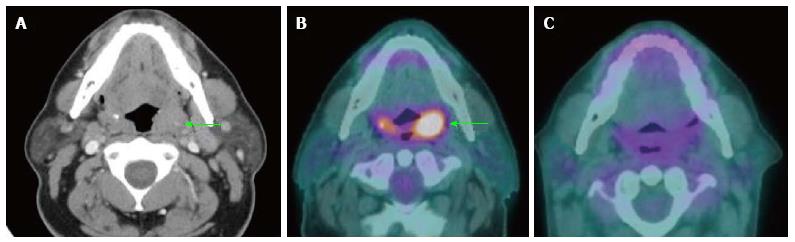
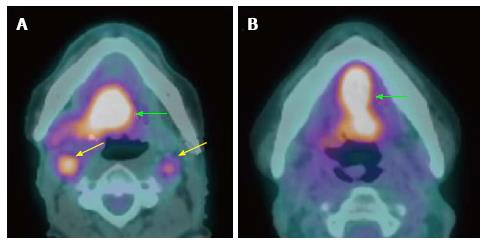
Several studies support the efficacy of PET/CT in detection of primary cancers in patients with CUP (Figure 2). PET/CT is able to identify the primary cancer in approximately 29% to 54% of cases (62%-93% sensitivity, 33%-93% specificity, 56%-89% positive predictive value and 25%-96% negative predictive value)[2,25-31]. A high detection rate of up to 54% can be achieved when the combination of CT, MRI, endoscopy under anesthesia and PET/CT are used. Generally, PET/CT is sensitive and superior for characterizing deep or metastatic cancers, while panendoscopy is more accurate for evaluating smaller or superficial mucosal lesions.
PET/CT is typically performed before panendoscopy to guide the selection of biopsy sites and to avoid erroneous interpretation due to high false positivity (as much as 50%)[29] of FDG uptake at sites manipulated during endoscopy. It is still uncertain when PET/CT should be performed after biopsy; therefore, if carcinoma of unknown primary is suspected, it is best to obtain PET/CT prior to endoscopy and biopsy/tonsillectomy. Over 90% of the unknown primary cancers are squamous cell carcinoma found in Waldeyer’s ring (lymphoid tissue of the nasopharynx, palatine tonsils or base of tongue)[22,32]. Due to variable negative predictive value (25%-96%) of PET/CT, panendoscopy with directed biopsies and bilateral tonsillectomies are considered when PET/CT yields negative result[3]. Pattani et al[33] suggest careful selection of patients for panendoscopy after a negative PET/CT since primary cancer was only found in 9% of CUP cases (1 out of 11 patients).
HNSCC patients are at increased risk for the development of second primary malignancy (SPM), with synchronous SPM occurring within 6 mo of the index primary cancer or metachronous SPM diagnosed > 6 mo of the index cancer. Approximately 1.4% to 18% of head neck cancer patients have SPMs[34], especially when the index cancers are laryngeal carcinomas. The risk for SPMs remains elevated for at least 10 years[35] and are mostly found in the head and neck, lung and esophagus[36] with the vast majority being squamous cell carcinoma[37]. Since SPM is the second leading cause of non-HNSCC death[38], early detection and treatment of SPM may alter management and improve patient survival[34]. A meta-analysis revealed 87.5% sensitivity and 95% specificity of PET/CT in detection of SPM or distant metastasis, while a negative PET/CT study does not completely exclude the presence of SPM[39]. Given the low incidence of synchronous SPMs at initial evaluation of HNSCC patient, several research studies question the cost-effectiveness of panendoscopy[8]. Therefore, PET/CT may complement or replace panendoscopy in detecting synchronous SPMs. For patients with localized disease (stage I or II) being treated with either primary surgery or definitive chemoradiation therapy, a thorough physical examination combined with PET/CT may be adequate, obviating the need for panendoscopy unless tissue biopsy under general anesthesia is deemed necessary.
Localized disease (stage I or II) comprising approximately 30% to 40% of HNSCC is generally treated with either primary surgery or definitive radiation therapy[40]. Locoregionally advanced disease (stage III, IVA, or IVB) associated with high risk of local recurrence and distant metastasis requires a multidisciplinary approach, given the complexity and complications of combined treatment modality that includes surgery, radiation therapy and chemotherapy[41]. In select cases, radical concurrent chemoradiation can be used as a definite therapy in preference to surgery to achieve similar cure rates with preserved functional outcome and less morbidity[42].
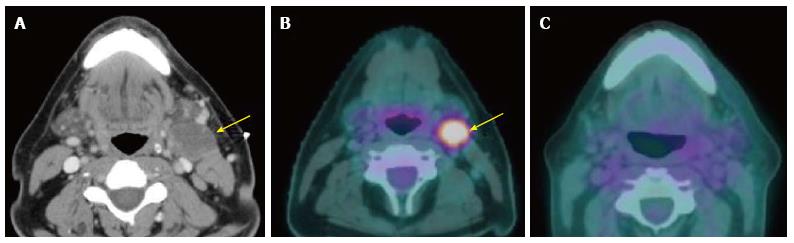
For patients with locoregionally advanced disease who have undergone treatment, management of residual abnormalities can pose significant challenge. Both surgery and radiation may cause inflammation, fibrosis and distortion of the head and neck anatomy leading to difficulties of interpretation with conventional imaging, especially differentiating between residual cancer and complete response[43,44]. Inaccurate post-treatment assessment may result in delayed or unnecessary treatment and increased mortality and morbidity. In this regard, multiple studies have shown that PET/CT is superior to conventional anatomic imaging in assessment of tumor response and detection of residual tumor[3,45-47].
The sensitivity, specificity, positive predictive value, and negative predictive value of PET/CT for detection of residual primary tumor have been reported as high as 94%, 82%, 75% and 95%, respectively[3]. It is important to note the very high negative predictive value of PET/CT: A negative study highly suggests absence of viable residual disease in both primary site and neck (Figures 3 and 4). The low positive predictive value is due to treatment-related FDG-avid inflammation or infection. A positive PET/CT study in the post-treatment phase needs careful correlation with clinical information and corresponding CT/MRI findings[48]. It is suggested that PET/CT should be performed no sooner than 2 mo after completion of treatment to evaluate for residual tumor while avoiding false positive results and to establish a baseline; however, it may be performed sooner if there is clinically suspected recurrent disease[49]. We generally recommend performing PET/CT around 3 mo after completion of treatment at our institution (Figure 5).
Patients with complete treatment response, as documented clinically and by structural imaging (CT, MRI, and PET/CT), are generally observed. With advanced chemoradiation therapy, and improving surgical techniques, many patients may have distant metastasis as the first and only sign of treatment failure. PET/CT as a surveillance tool serves the purpose of detecting early recurrent disease, assessing for a metachronous second primary tumor, and excluding interval development of distant metastases. Close interval follow up in the first two to four years following treatment is necessary since 80% to 90% of all recurrences occur within this timeframe[50,51]; while the risk of SPM is higher than recurrence beyond three years[52,53].
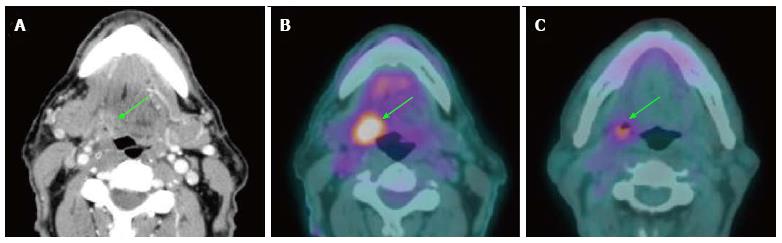
PET/CT has 93%-100% sensitivity and 63%-94% specificity in detection of recurrent tumor in both primary site and the neck, respectively[48,54,55] (Figure 6). The negative predictive value of a single PET/CT and double PET/CT (obtained within 6-mo period) are 91% and 98%, respectively. Negative results of two consecutive PET/CT studies could potentially eliminate the need for routine post-treatment imaging if there is no clinical suspicion of tumor recurrence[56]. In addition, there are no differences in survival between PET/CT detected and clinically detected recurrence[57]. Although there is an appreciable radiation dose and lifetime cancer risk associated with PET/CT, the use of this examination is warranted when utilized in the appropriate clinical setting[58].
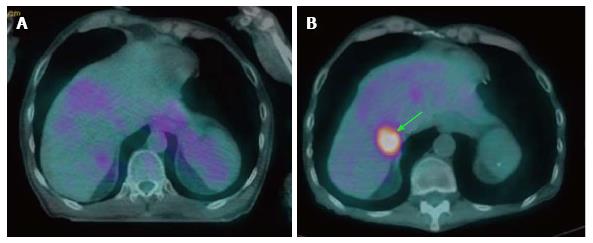
Metachronous second primary tumor may occur after 6 mo of the index primary tumor with 2.8% annual rate[59]. The incidence of distant metastasis following definitive treatment is 9% with the risk increased in patients with locally advanced stages[59,60] (Figure 7). Overall 17.9% of HNSCC patients develop second primary cancers or distant metastasis, especially in patients with recurrent disease[39,60]. The identification of distant metastatic lesions at the time of restaging recurrent tumors may obviate aggressive surgery while focusing on palliative chemoradiation options[60]. Therefore, PET/CT has strong utility in detecting second primary tumors or distant metastases with high sensitivity and specificity[39].
As an invaluable tool in staging cancers of the head and neck, PET/CT imaging provides metabolic and functional data that may serve as quantifiable prognostic factors. The PET-derived parameters, SUV and its various forms, have been shown correlating well with glucose metabolism rate in various cancers, including HNSCC[61], and are useful in predicting tumor aggressiveness and long-term survival of patients[62]. They have also been used in selecting treatment modality and personalizing treatments. In a study comparing resectable, advanced HNSCC patients treated with surgery followed by chemoradiation therapy vs those with chemoradiation and salvage surgery, Roh et al[63] found that patients with high FDG uptake and treated with surgery first had better disease-free survival (DFS). In addition, Inokuchi et al[64] found that high FDG uptake in HNSCC patients treated with definitive chemoradiation predicted a decrease DFS, nodal progression-free survival, and distant metastasis-free survival. The investigators also suggested using pre-treatment FDG uptake of cervical lymph nodes to select patients for planned neck dissection. They found that patients with high FDG uptake and treated with planned neck dissection had better nodal progression-free survival.
Metabolic tumor volume (MTV), a SUV-based parameter representing the tumor volume that has SUV above a specific threshold, has been suggested in the literature as a robust measure in predicting treatment outcomes. For example, clinical trials are underway to explore if patients with human papillomavirus (HPV)-associated oropharyngeal cancers, which are known to have better prognosis than those not associated with HPV, would have similar cancer control with less intensified and therefore less toxic treatment options[65]. It has been suggested that this patient population could be stratified further based on MTV. Patients with more aggressive HPV-related HNSCC, as suggested by the increased MTV, had significantly poorer outcomes in one study conducted by Tang et al[66]. In addition to MTV, total lesion glycolysis (TLG) was first introduced by Larson et al[67]. It is the product of mean SUV and MTV, combining the volumetric and metabolic information of PET/CT to evaluate treatment response. Recent studies demonstrate the usefulness of TLG for evaluating head and neck cancers, with high TLG correlating to increased risk of adverse events or death[62,68,69].
The PET-derived parameters are also currently used in combination with other prognostic factors. N-stage, T-stage, and pre-treatment SUV of lymph node when used in combination have been shown better at predicting distant metastasis-free survival than individual factors[70]. Recently, there has been increased interest in identifying prognostic molecular biomarkers. Moeller et al[71] incorporated HPV status in addition to post-treatment FDG uptake in their mortality risk assessment. In addition conventional parameters (SUV, MTV, TLG, tumor volume, and diameter) in PET/CT, textural parameters to assess tumor heterogeneity such as coefficient of variation, skewness, and kurtosis may also provide prognostic information but are not fully explored in head and neck oncology.
With wide-spread availability and use, PET/CT imaging maintains an important role in head and neck oncology. In the pre-treatment phase, PET/CT is valuable in the evaluation of patients with carcinoma of unknown primary origin before panendoscopy and biopsy, detection of synchronous second primary tumor, staging of cervical lymph node metastasis and assessment for distant metastases. In the post-treatment phase, PET/CT is helpful in evaluating treatment response, detecting residual or recurrent tumor and excluding distant metastases. Prognostic factors derived from PET/CT metabolic and functional data are useful in predicting tumor aggressiveness with implication on patient’s survivability, and facilitate selection of treatment modality and personalized treatment options.
P- Reviewer: Dhiwakar M, Gross M, Jeong HS, Kawamata H S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9172] [Cited by in F6Publishing: 9816] [Article Influence: 1090.7] [Reference Citation Analysis (0)] |
| 2. | Ha PK, Hdeib A, Goldenberg D, Jacene H, Patel P, Koch W, Califano J, Cummings CW, Flint PW, Wahl R. The role of positron emission tomography and computed tomography fusion in the management of early-stage and advanced-stage primary head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:12-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Miller FR, Hussey D, Beeram M, Eng T, McGuff HS, Otto RA. Positron emission tomography in the management of unknown primary head and neck carcinoma. Arch Otolaryngol Head Neck Surg. 2005;131:626-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Gupta T, Master Z, Kannan S, Agarwal JP, Ghsoh-Laskar S, Rangarajan V, Murthy V, Budrukkar A. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2011;38:2083-2095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 5. | Scott AM, Gunawardana DH, Bartholomeusz D, Ramshaw JE, Lin P. PET changes management and improves prognostic stratification in patients with head and neck cancer: results of a multicenter prospective study. J Nucl Med. 2008;49:1593-1600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Tomasi G, Turkheimer F, Aboagye E. Importance of quantification for the analysis of PET data in oncology: review of current methods and trends for the future. Mol Imaging Biol. 2012;14:131-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Griffeth LK. Use of PET/CT scanning in cancer patients: technical and practical considerations. Proc (Bayl Univ Med Cent). 2005;18:321-330. [PubMed] [Cited in This Article: ] |
| 8. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5537] [Cited by in F6Publishing: 6112] [Article Influence: 436.6] [Reference Citation Analysis (0)] |
| 9. | Lonneux M, Hamoir M, Reychler H, Maingon P, Duvillard C, Calais G, Bridji B, Digue L, Toubeau M, Grégoire V. Positron emission tomography with [18F]fluorodeoxyglucose improves staging and patient management in patients with head and neck squamous cell carcinoma: a multicenter prospective study. J Clin Oncol. 2010;28:1190-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Network NCC. Head and Neck Cancers. NCCN Clinical Practice Guidelines in Oncology 2013. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#head-and-neck. [Cited in This Article: ] |
| 11. | Kapoor V, McCook BM, Torok FS. An introduction to PET-CT imaging. Radiographics. 2004;24:523-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 205] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 12. | Payabvash S, Meric K, Cayci Z. Differentiation of benign from malignant cervical lymph nodes in patients with head and neck cancer using PET/CT imaging. Clin Imaging. 2016;40:101-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Stoeckli SJ, Steinert H, Pfaltz M, Schmid S. Is there a role for positron emission tomography with 18F-fluorodeoxyglucose in the initial staging of nodal negative oral and oropharyngeal squamous cell carcinoma. Head Neck. 2002;24:345-349. [PubMed] [Cited in This Article: ] |
| 14. | Wensing BM, Vogel WV, Marres HA, Merkx MA, Postema EJ, Oyen WJ, van den Hoogen FJ. FDG-PET in the clinically negative neck in oral squamous cell carcinoma. Laryngoscope. 2006;116:809-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Ng SH, Yen TC, Chang JT, Chan SC, Ko SF, Wang HM, Lee LY, Kang CJ, Wong AM, Liao CT. Prospective study of [18F]fluorodeoxyglucose positron emission tomography and computed tomography and magnetic resonance imaging in oral cavity squamous cell carcinoma with palpably negative neck. J Clin Oncol. 2006;24:4371-4376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Nahmias C, Carlson ER, Duncan LD, Blodgett TM, Kennedy J, Long MJ, Carr C, Hubner KF, Townsend DW. Positron emission tomography/computerized tomography (PET/CT) scanning for preoperative staging of patients with oral/head and neck cancer. J Oral Maxillofac Surg. 2007;65:2524-2535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Yousem DM, Gad K, Tufano RP. Resectability issues with head and neck cancer. AJNR Am J Neuroradiol. 2006;27:2024-2036. [PubMed] [Cited in This Article: ] |
| 18. | Joshi VM, Navlekar SK, Kishore GR, Reddy KJ, Kumar EC. CT and MR imaging of the inner ear and brain in children with congenital sensorineural hearing loss. Radiographics. 2012;32:683-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Jereczek-Fossa BA, Jassem J, Orecchia R. Cervical lymph node metastases of squamous cell carcinoma from an unknown primary. Cancer Treat Rev. 2004;30:153-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Miller FR, Karnad AB, Eng T, Hussey DH, Stan McGuff H, Otto RA. Management of the unknown primary carcinoma: long-term follow-up on a negative PET scan and negative panendoscopy. Head Neck. 2008;30:28-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Tantiwongkosi B, Yu F, Kanard A, Miller FR. Role of (18)F-FDG PET/CT in pre and post treatment evaluation in head and neck carcinoma. World J Radiol. 2014;6:177-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 42] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 22. | Schmalbach CE, Miller FR. Occult primary head and neck carcinoma. Curr Oncol Rep. 2007;9:139-146. [PubMed] [Cited in This Article: ] |
| 23. | Calabrese L, Jereczek-Fossa BA, Jassem J, Rocca A, Bruschini R, Orecchia R, Chiesa F. Diagnosis and management of neck metastases from an unknown primary. Acta Otorhinolaryngol Ital. 2005;25:2-12. [PubMed] [Cited in This Article: ] |
| 24. | Issing WJ, Taleban B, Tauber S. Diagnosis and management of carcinoma of unknown primary in the head and neck. Eur Arch Otorhinolaryngol. 2003;260:436-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Rudmik L, Lau HY, Matthews TW, Bosch JD, Kloiber R, Molnar CP, Dort JC. Clinical utility of PET/CT in the evaluation of head and neck squamous cell carcinoma with an unknown primary: a prospective clinical trial. Head Neck. 2011;33:935-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Yabuki K, Tsukuda M, Horiuchi C, Taguchi T, Nishimura G. Role of 18F-FDG PET in detecting primary site in the patient with primary unknown carcinoma. Eur Arch Otorhinolaryngol. 2010;267:1785-1792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Dandekar MR, Kannan S, Rangarajan V, Purandare NC, Chaukar DA, Deshmukh A, D’cruz AK. Utility of PET in unknown primary with cervical metastasis: a retrospective study. Indian J Cancer. 2011;48:181-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Padovani D, Aimoni C, Zucchetta P, Paluzzi A, Pastore A. 18-FDG PET in the diagnosis of laterocervical metastases from occult carcinoma. Eur Arch Otorhinolaryngol. 2009;266:267-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Johansen J, Buus S, Loft A, Keiding S, Overgaard M, Hansen HS, Grau C, Bundgaard T, Kirkegaard J, Overgaard J. Prospective study of 18FDG-PET in the detection and management of patients with lymph node metastases to the neck from an unknown primary tumor. Results from the DAHANCA-13 study. Head Neck. 2008;30:471-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | Wong WL, Sonoda LI, Gharpurhy A, Gollub F, Wellsted D, Goodchild K, Lemon C, Farrell R, Saunders M. 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the assessment of occult primary head and neck cancers--an audit and review of published studies. Clin Oncol (R Coll Radiol). 2012;24:190-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Kwee TC, Kwee RM. Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Radiol. 2009;19:731-744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 32. | Cianchetti M, Mancuso AA, Amdur RJ, Werning JW, Kirwan J, Morris CG, Mendenhall WM. Diagnostic evaluation of squamous cell carcinoma metastatic to cervical lymph nodes from an unknown head and neck primary site. Laryngoscope. 2009;119:2348-2354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 33. | Pattani KM, Goodier M, Lilien D, Kupferman T, Caldito G, Nathan CO. Utility of panendoscopy for the detection of unknown primary head and neck cancer in patients with a negative PET/CT scan. Ear Nose Throat J. 2011;90:E16-E20. [PubMed] [Cited in This Article: ] |
| 34. | Kim SY, Roh JL, Yeo NK, Kim JS, Lee JH, Choi SH, Nam SY. Combined 18F-fluorodeoxyglucose-positron emission tomography and computed tomography as a primary screening method for detecting second primary cancers and distant metastases in patients with head and neck cancer. Ann Oncol. 2007;18:1698-1703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Gan SJ, Dahlstrom KR, Peck BW, Caywood W, Li G, Wei Q, Zafereo ME, Sturgis EM. Incidence and pattern of second primary malignancies in patients with index oropharyngeal cancers versus index nonoropharyngeal head and neck cancers. Cancer. 2013;119:2593-2601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Morris LG, Sikora AG, Hayes RB, Patel SG, Ganly I. Anatomic sites at elevated risk of second primary cancer after an index head and neck cancer. Cancer Causes Control. 2011;22:671-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Jones AS, Morar P, Phillips DE, Field JK, Husband D, Helliwell TR. Second primary tumors in patients with head and neck squamous cell carcinoma. Cancer. 1995;75:1343-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 38. | Baxi SS, Pinheiro LC, Patil SM, Pfister DG, Oeffinger KC, Elkin EB. Causes of death in long-term survivors of head and neck cancer. Cancer. 2014;120:1507-1513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 39. | Xu GZ, Guan DJ, He ZY. (18)FDG-PET/CT for detecting distant metastases and second primary cancers in patients with head and neck cancer. A meta-analysis. Oral Oncol. 2011;47:560-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Spector JG, Sessions DG, Chao KS, Haughey BH, Hanson JM, Simpson JR, Perez CA. Stage I (T1 N0 M0) squamous cell carcinoma of the laryngeal glottis: therapeutic results and voice preservation. Head Neck. 1999;21:707-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 41. | Price KA, Cohen EE. Current treatment options for metastatic head and neck cancer. Curr Treat Options Oncol. 2012;13:35-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 42. | Sherriff JM, Ogunremi B, Colley S, Sanghera P, Hartley A. The role of positron emission tomography/CT imaging in head and neck cancer patients after radical chemoradiotherapy. Br J Radiol. 2012;85:e1120-e1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Bronstein AD, Nyberg DA, Schwartz AN, Shuman WP, Griffin BR. Soft-tissue changes after head and neck radiation: CT findings. AJNR Am J Neuroradiol. 1989;10:171-175. [PubMed] [Cited in This Article: ] |
| 44. | Laubenbacher C, Saumweber D, Wagner-Manslau C, Kau RJ, Herz M, Avril N, Ziegler S, Kruschke C, Arnold W, Schwaiger M. Comparison of fluorine-18-fluorodeoxyglucose PET, MRI and endoscopy for staging head and neck squamous-cell carcinomas. J Nucl Med. 1995;36:1747-1757. [PubMed] [Cited in This Article: ] |
| 45. | Andrade RS, Heron DE, Degirmenci B, Filho PA, Branstetter BF, Seethala RR, Ferris RL, Avril N. Posttreatment assessment of response using FDG-PET/CT for patients treated with definitive radiation therapy for head and neck cancers. Int J Radiat Oncol Biol Phys. 2006;65:1315-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 46. | Kitagawa Y, Nishizawa S, Sano K, Ogasawara T, Nakamura M, Sadato N, Yoshida M, Yonekura Y. Prospective comparison of 18F-FDG PET with conventional imaging modalities (MRI, CT, and 67Ga scintigraphy) in assessment of combined intraarterial chemotherapy and radiotherapy for head and neck carcinoma. J Nucl Med. 2003;44:198-206. [PubMed] [Cited in This Article: ] |
| 47. | Lell M, Baum U, Greess H, Nömayr A, Nkenke E, Koester M, Lenz M, Bautz W. Head and neck tumors: imaging recurrent tumor and post-therapeutic changes with CT and MRI. Eur J Radiol. 2000;33:239-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 135] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Mosci C, Davidzon GA, Quon A. FDG-PET/CT Initial and Subsequent Therapy Evaluation: Progressing to PET/MR Imaging. PET Clinics. 2012;7:369-380. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Nakamura S, Toriihara A, Okochi K, Watanabe H, Shibuya H, Kurabayashi T. Optimal timing of post-treatment [18F]fluorodeoxyglucose-PET/CT for patients with head and neck malignancy. Nucl Med Commun. 2013;34:162-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Boysen M, Lövdal O, Tausjö J, Winther F. The value of follow-up in patients treated for squamous cell carcinoma of the head and neck. Eur J Cancer. 1992;28:426-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 153] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | Ritoe SC, Krabbe PF, Kaanders JH, van den Hoogen FJ, Verbeek AL, Marres HA. Value of routine follow-up for patients cured of laryngeal carcinoma. Cancer. 2004;101:1382-1389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Chen AM, Garcia J, Granchi PJ, Johnson J, Eisele DW. Late recurrence from salivary gland cancer: when does “cure” mean cure? Cancer. 2008;112:340-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Spector JG, Sessions DG, Haughey BH, Chao KS, Simpson J, El Mofty S, Perez CA. Delayed regional metastases, distant metastases, and second primary malignancies in squamous cell carcinomas of the larynx and hypopharynx. Laryngoscope. 2001;111:1079-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 54. | Li P, Zhuang H, Mozley PD, Denittis A, Yeh D, Machtay M, Smith R, Alavi A. Evaluation of recurrent squamous cell carcinoma of the head and neck with FDG positron emission tomography. Clin Nucl Med. 2001;26:131-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Fischbein NJ, AAssar OS, Caputo GR, Kaplan MJ, Singer MI, Price DC, Dillon WP, Hawkins RA. Clinical utility of positron emission tomography with 18F-fluorodeoxyglucose in detecting residual/recurrent squamous cell carcinoma of the head and neck. AJNR Am J Neuroradiol. 1998;19:1189-1196. [PubMed] [Cited in This Article: ] |
| 56. | McDermott M, Hughes M, Rath T, Johnson JT, Heron DE, Kubicek GJ, Kim SW, Ferris RL, Duvvuri U, Ohr JP. Negative predictive value of surveillance PET/CT in head and neck squamous cell cancer. AJNR Am J Neuroradiol. 2013;34:1632-1636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Ho AS, Tsao GJ, Chen FW, Shen T, Kaplan MJ, Colevas AD, Fischbein NJ, Quon A, Le QT, Pinto HA. Impact of positron emission tomography/computed tomography surveillance at 12 and 24 months for detecting head and neck cancer recurrence. Cancer. 2013;119:1349-1356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 58. | Huang B, Law MW, Khong PL. Whole-body PET/CT scanning: estimation of radiation dose and cancer risk. Radiology. 2009;251:166-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 333] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 59. | Jovanovic A, van der Tol IG, Kostense PJ, Schulten EA, de Vries N, Snow GB, van der Waal I. Second respiratory and upper digestive tract cancer following oral squamous cell carcinoma. Eur J Cancer B Oral Oncol. 1994;30B:225-229. [PubMed] [Cited in This Article: ] |
| 60. | Gourin CG, Watts T, Williams HT, Patel VS, Bilodeau PA, Coleman TA. Identification of distant metastases with PET-CT in patients with suspected recurrent head and neck cancer. Laryngoscope. 2009;119:703-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer--a PET study. J Nucl Med. 1993;34:1-6. [PubMed] [Cited in This Article: ] |
| 62. | Xie P, Li M, Zhao H, Sun X, Fu Z, Yu J. 18F-FDG PET or PET-CT to evaluate prognosis for head and neck cancer: a meta-analysis. J Cancer Res Clin Oncol. 2011;137:1085-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 63. | Roh JL, Pae KH, Choi SH, Kim JS, Lee S, Kim SB, Nam SY, Kim SY. 2-[18F]-Fluoro-2-deoxy-D-glucose positron emission tomography as guidance for primary treatment in patients with advanced-stage resectable squamous cell carcinoma of the larynx and hypopharynx. Eur J Surg Oncol. 2007;33:790-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Inokuchi H, Kodaira T, Tachibana H, Nakamura T, Tomita N, Nakahara R, Takada A, Mizoguchi N, Tamaki T, Fuwa N. Clinical usefulness of [18F] fluoro-2-deoxy-D-glucose uptake in 178 head-and-neck cancer patients with nodal metastasis treated with definitive chemoradiotherapy: consideration of its prognostic value and ability to provide guidance for optimal selection of patients for planned neck dissection. Int J Radiat Oncol Biol Phys. 2011;79:747-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M. Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol. 2012;6 Suppl 1:S104-S120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 66. | Tang C, Murphy JD, Khong B, La TH, Kong C, Fischbein NJ, Colevas AD, Iagaru AH, Graves EE, Loo BW. Validation that metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;83:1514-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 67. | Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, Casilla C, Fazzari M, Srivastava N, Yeung HW. Tumor Treatment Response Based on Visual and Quantitative Changes in Global Tumor Glycolysis Using PET-FDG Imaging. The Visual Response Score and the Change in Total Lesion Glycolysis. Clin Positron Imaging. 1999;2:159-171. [PubMed] [Cited in This Article: ] |
| 68. | Lim R, Eaton A, Lee NY, Setton J, Ohri N, Rao S, Wong R, Fury M, Schöder H. 18F-FDG PET/CT metabolic tumor volume and total lesion glycolysis predict outcome in oropharyngeal squamous cell carcinoma. J Nucl Med. 2012;53:1506-1513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 69. | Pak K, Cheon GJ, Nam HY, Kim SJ, Kang KW, Chung JK, Kim EE, Lee DS. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med. 2014;55:884-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 70. | Yao M, Lu M, Savvides PS, Rezaee R, Zender CA, Lavertu P, Buatti JM, Machtay M. Distant metastases in head-and-neck squamous cell carcinoma treated with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:684-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | Moeller BJ, Rana V, Cannon BA, Williams MD, Sturgis EM, Ginsberg LE, Macapinlac HA, Lee JJ, Ang KK, Chao KS. Prospective imaging assessment of mortality risk after head-and-neck radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:667-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |