NOISE-INDUCED HEARING LOSS
The human ear is an exquisitely sensitive organ, allowing us to perceive and distinguish among the myriad sounds around us, be they pleasurable, informative or damaging. Located within the inner ear is the cochlea, the specialised peripheral end organ of the auditory system, which mediates the transduction of sound waves into electrical nerve impulses that travel to the brain for central processing of auditory information. Unfortunately, this extreme sensitivity of the cochlea comes at a cost as it makes it highly susceptible to injury when exposed to loud sound. The consequence of this injury is the loss of hearing, which can be either temporary or permanent. Noise-induced hearing loss may result from either brief exposure to an intense “impulse” noise or sustained and repeated exposure to excessive sound levels (i.e., continued exposure to high levels of noise over an extended period of time). The hearing loss from noise exposure is typically binaural (symmetric), and the severity of it is related to the intensity, frequency, duration and temporal characteristics (e.g., impulse/impact, intermittent or continuous noise) of the noise exposure[1,2].
Excessive noise is the most common occupational and environmental health hazard. Dangerous levels of noise are generated in a large number of workplaces such as construction sites, mines, saw mills, military bases, and airports, among many others. Although usually associated with occupational exposure, noise-induced hearing loss is becoming increasingly prevalent in recreational settings. Many people, especially children and teenagers, voluntarily expose themselves to potentially injurious noise levels via portable music players, stereos, video games, rock concerts, and nightclubs. Other non-occupational sources of loud noise include firearms, power tools such as chain saws and drills, lawn mowers, and recreational vehicles such as motorcycles.
Noise-induced hearing loss is the second most common sensorineural hearing deficit, after age-related hearing loss (presbyacusis), and is the leading cause of preventable sensorineural hearing loss (SNHL) in the industrialised world[3]. According to recent global estimates released by the World Health Organization (WHO, 2012), there are 360 million people worldwide (over 5% of the world’s population) with disabling hearing loss. Disabling hearing loss, as defined by WHO, is “hearing loss greater than 40 dB in the better hearing ear in adults and a hearing loss greater than 30 dB in the better hearing ear in children”. A significant proportion (16%) of the disabling hearing loss in the adult population in the world is attributed to occupational noise exposure[4]. In the United States, approximately 15% (26 million) of people between 20 to 69 years of age have high frequency hearing loss from overexposure to loud noise at work or during leisure activities. Hearing loss has considerable social and economic implications at both the individual and societal levels. This devastating sensory disability and the serious communication difficulties has a negative impact on the quality of life of the affected individual and can lead to feelings of loneliness, social isolation and depression.
Although it can be permanent and not fully treatable, noise-induced hearing loss is virtually 100% preventable. Obviously, the best preventive measure against noise-induced hearing loss is to completely avoid or minimise exposure to excessively noisy environments. When this is not possible, the only preventative measure available is the consistent and proper use of hearing protection devices such as earplugs and earmuffs. When used correctly, these protective devices can provide 20 to 40 dB of attenuation, however their use is often impractical in many settings and they are not completely effective in harsh environments, or because of incorrect use.
Avoiding or reducing modifiable risk factors associated with noise-induced hearing loss such as voluntary exposure to loud noise, non-use of hearing protection, cigarette smoking, lack of exercise, poor diet (low dietary intake of antioxidant-rich food), and poor oral health (tooth loss) may reduce the risk or delay the onset of this debilitating condition[5,6]. The presence of cardiovascular disease and diabetes are also major risk factors. In addition to these, several non-modifiable risk factors related to noise-induced hearing loss exist, particularly age and genetics[5]. Age plays the most significant role, with the risk typically increasing with advancing age. Furthermore, great genetic variability in the susceptibility to noise-induced hearing loss has been documented in both humans and mice[7].
The association between noise exposure and hearing loss was first recognised by the physician Sir Francis Bacon (1561-1626)[8]. In 1890, Habermann was the first to describe the cochlear histopathological features of noise-induced hearing loss from examining the temporal bones of an elderly ex-boilermaker[9]. However, it was not until 1907 that Wittmaack conducted the first experimental research of noise-induced deafness in animals[9,10]. Substantial insights into the pathophysiology of noise-induced cochlear injury were gained by Wittmaack’s experiments and the many others that followed, including Hallowell Davis’s systemic studies on guinea pigs and humans at Harvard University in 1943.
The cochlea sustains dramatic cellular injury following noise overexposure. The pathological consequences (pattern and extent) depend on the acoustic characteristics of the noise (i.e., sound intensity, frequency and duration), age and genetics[10]. The two types of hearing loss from noise exposure - temporary and permanent hearing loss (also known as temporary and permanent threshold shift) - also vary in their mechanisms[11]. Noise exposure is known to produce a variety of structural changes to the various cells within the cochlea. The most vulnerable are sensory hair cells, particularly the outer hair cells, which have traditionally been the focus of most hearing loss studies. A major impact is on sensory hair cell stereocilia which can undergo mechanical damage during noise exposure. Other changes include the loss of outer hair cells, damage to the inner hair cell - auditory nerve synapse, swelling of the primary auditory neurones in the spiral ganglion, damage to the supporting cells, acute swelling of the stria vascularis, reduced cochlear blood flow and the loss of fibrocytes in the spiral ligament[2,11-15]. In addition, direct mechanical disruption of the cochlea can be induced by impulse noise exposure, e.g., rupturing of the organ of Corti and its separation from the basilar membrane.
COCHLEAR INFLAMMATION
Cochlear inflammation has been implicated as a major etiologic factor in a range of conditions that cause hearing loss. These include acoustic trauma (noise-induced cochlear damage), otitis media (middle ear infection), meningitis, autoimmune inner ear disease, and ototoxicity (drug-induced inner ear damage, e.g., aminoglycoside antibiotics, platinum-based chemotherapeutic agents)[16-23]. Labyrinthitis can also be evoked by cochlear surgery and the insertion of cochlear implants[24,25]. Pathogen-induced labyrinthitis as a consequence of otitis media or meningitis is usually associated with bacterial and viral infections. Labyrinthitis secondary to otitis media (tympanogenic labyrinthitis) primarily occurs by the spread of the infection from the middle ear into the inner ear through the three-layered round window membrane[18,22,26,27]. Meningogenic labyrinthitis most likely occurs by the spread of infection from the meninges into the perilymphatic space of the cochlea through the cochlear aqueduct[19,28,29]. Mycotic (fungal) labyrinthitis is rare, and is usually associated with systemic debilitating diseases and occurs by either the tympanogenic, meningogenic or hematogenic route[30].
Labyrinthitis usually affects the cochlea more severely than the vestibular system, resulting in adverse effects on cochlear function[26]. A well-documented complication of cochlear inflammation is partial or complete SNHL. Pathological consequences that have been observed in animal models of cochlear inflammation include degeneration of hair cells of the organ of Corti, disruption of fibrocytes in the spiral ligament, loss of interdental cells of the spiral limbus, swelling of the stria vascularis, and vascular damage[26,31-33]. The disruption of the spiral ligament fibrocytes has been suggested as a major contributor to the inflammation-induced cochlear dysfunction[32,34]. Decreased immunostaining for gap junction protein connexin 26 in type I and type II fibrocytes and decreased Na+-K+-ATPase staining in type II fibrocytes, both of which are critical in the maintenance of cochlear homeostasis, were observed in a guinea pig model of labyrinthitis induced by inoculation of the protein antigen keyhole limpet hemocyanin (KLH) into the scala tympani[35]. In addition, reduced connexin 26 immunostaining in the spiral ligament was also demonstrated in a mouse model of otitis media induced by the transtympanic inoculation of viable Streptococcus pneumoniae[36].
Analogous to the central nervous system and the retina of the eye, the cochlea is separated from the systemic circulation by a blood-labyrinth barrier, which has similar physiological characteristics as the blood-brain barrier and the blood-retinal barrier. This barrier is important in maintaining the ionic composition of the cochlear fluid compartments, and is essential for the functional integrity of the cochlea[37]. Because of the existence of this blood-labyrinth barrier and the relative absence of resident tissue macrophages, the inner ear was originally considered an immunologically privileged organ, isolated from the immune system and protected from immune surveillance. However, this hypothesis has been refuted by research demonstrating that the inner ear is capable of rapidly generating an active inflammatory/immune response in the presence of antigens or pathogens. In addition, connections exist between the inner ear and the systemic lymphatic system through cervical lymph nodes[38].
Although the intended purpose of the immune response in the inner ear is to defend the hearing organ against invading pathogens and to clear cellular debris, the inflammatory response can also cause significant bystander injury to the delicate structures of the cochlea[37,39]. Because mammalian inner ear tissues have limited abilities of repair and regeneration (unlike avian auditory hair cells which have the capacity to regenerate), this damage is irreversible, leading to permanent hearing loss. Immune-related cochlear inflammation is increasingly recognised as a potential mechanism of inner ear disease and associated hearing loss. Systemic administration of immunosuppressive drugs (e.g., corticosteroids) has been shown to effectively ameliorate some cases of idiopathic, rapidly progressive bilateral SNHL, implicating inner ear inflammation as an underlying mechanism of the hearing loss[40]. Histopathological studies of human temporal bones also support the hypothesis that a number of otological disorders are linked with inflammatory responses[41]. The severity of hearing impairment and the potential for recovery correlate with the extent of inflammation-induced tissue damage. Animal studies have demonstrated that the development of inflammation and hearing loss following an immunological challenge can be rapid, with the onset of hearing loss occurring at 12 to 15 h, and peaking at 24 to 48 h[42,43].
Regardless of the cause, the cochlear inflammatory response follows a similar course with three characteristic stages: an initial acute stage, a fibrotic stage, and an ossification stage[44]. The acute phase of cochlear inflammation, which lasts approximately 3 to 7 d, is characterised by the production of proinflammatory mediators such as cytokines and chemokines, an increased expression of adhesion molecules, the recruitment and infiltration of inflammatory cells such as polymorphonuclear leukocytes (mostly neutrophils), monocytes, macrophages and lymphocytes, and the breakdown of the blood-labyrinth barrier[31,44]. In the chronic stage of the cochlear inflammatory response, a fibrotic matrix is formed in the perilymphatic spaces, which later becomes calcified. This bony occlusion of the fluid-filled cochlear scalae, known as labyrinthitis ossificans, is most extensive in post-meningitis cases[45].
The cochlea itself can mount an immune response. Resident cells in the cochlea can express a range of inflammatory mediators, which are thought to play critical roles in the inflammatory response[46,47]. The cochlea communicates with the immune system via the systemic circulation. Entry of inflammatory cells occurs primarily through the spiral modiolar vein and its tributaries (collecting venules) situated at the base of the scala tympani[48]. Inflammatory cells accumulate in the perivascular space surrounding the spiral modiolar vein, and then stream into the scala tympani along the extravascular space of the collecting venules. Other areas where circulating inflammatory cells enter the cochlea include the blood vessels of the spiral ligament and the spiral ganglion. The lateral wall of the cochlea and the spiral ganglion represent the most permeable parts of the blood-labyrinth barrier, partly due to their high vascularisation[49,50].
The mammalian cochlea contains resident macrophages at normal/steady state[16,25,49,51]. These macrophages are phenotypically similar to the tissue macrophages in other organs of the body (e.g., microglia of the central nervous system) and are found in small numbers predominantly in the spiral ligament and the spiral ganglion. Moreover, it was recently reported that a large number of perivascular resident macrophages (PVMs) are present in the stria vascularis surrounding the endothelial cells of the capillaries[52]. Data from radiation chimeras have shown that these resident macrophages in the cochlea form an exchanging and migratory population, supplied continuously from haematopoietic precursors in the bone marrow, and exhibiting slow turnover during steady-state conditions[25,49,52]. These haematopoietic precursors migrate into the cochlea and differentiate into tissue macrophages. Bromodeoxyuridine (BrdU) labelling has demonstrated that the marked increase in macrophage numbers in the cochlea following an insult such as noise exposure is not due to the proliferation of these resident cochlear macrophages, but rather occurs by the migration of macrophages from the vascular system[16,53].
The signals that initiate the recruitment and infiltration of inflammatory cells into the cochlea are still under scrutiny, and a wide range of soluble mediators (e.g., cytokines, chemokines) may be involved. The sources of proinflammatory mediators in the cochlea include various resident cochlear cells types (e.g., spiral ligament fibrocytes, supporting cells) and infiltrating leukocytes migrating from the cochlear vasculature. In vitro studies using cultured murine spiral ligament fibrocytes have shown that upon stimulation with proinflammatory cytokines, fibrocytes secrete a variety of inflammatory mediators such as tumor necrosis factor α (TNF-α), interleukin (IL)-1β, IL-6, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2), keratinocyte-derived chemokine, soluble intercellular adhesion molecule-1 (sICAM-1) and vascular endothelial growth factor, which play important roles in the recruitment of inflammatory cells into the cochlea[32,33,47,54]. The secretion of sICAM-1 is compatible with an earlier study that reported strong intercellular adhesion molecule-1 (ICAM-1) expression in the spiral ligament and spiral modiolar vein in the early phase of labyrinthitis induced by the inoculation of KLH into the scala tympani[55]. It is speculated that chemokines produced by the fibrocytes are presented to the surface of vascular endothelial cells via the process of transcytosis, which consequently attracts inflammatory cells. Fibrocytes, vascular endothelial cells, and inflammatory cells together may form networks interconnected by cytokines, chemokines and various other inflammatory mediators[32,47].
It is well documented that inhibition of TNF-α with the soluble TNF-α receptor-FC fusion protein Etanercept, given either systemically or directly into the cochlea, significantly attenuates the cochlear inflammatory response[56]. This suggests that TNF-α plays a major role in the development of cochlear inflammation. Studies on organ of Corti explants have shown that TNF-α alone, in the absence of antigens or pathogens, has the ability to induce the recruitment of inflammatory cells into the cochlea from the systemic circulation[57]. TNF-α is also expressed by infiltrating leukocytes, suggesting that it is likely involved in a positive feedback loop that further amplifies the recruitment of inflammatory cells. This is supported by the evidence that TNF-α inhibition can prevent the recruitment of inflammatory cells into the cochlea[56]. TNF-α can also induce nitric oxide synthesis by stimulating the expression of inducible nitric oxide synthase (iNOS), which can further aggravate inflammation and degeneration in the cochlea[58].
The expression of many proinflammatory mediators is mostly regulated by nuclear factor κB (NF-κB)[23]. NF-κB comprises a family of inducible transcription factors that play a pivotal role in immune and inflammatory responses. Activation of NF-κB induces the transcription of cytokines such as TNF-α, IL-1β and IL-6, as well as iNOS, and the adhesion molecules, ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1). NF-κB activation in the cochlea has been demonstrated following intraperitoneal injection of lipopolysaccharide (LPS)[59], suggesting that the cochlea can become immunologically active even after systemic administration of bacterial toxins. Cochlear activation of NF-κB has also been reported to occur following acoustic trauma (see the following section) and in cisplatin-induced ototoxicity[21].
At present, it is technically impossible to positively identify inflammatory processes within the human inner ear. There are no well-defined detection methods available and diagnostic biopsy of the human cochlea is not feasible. To overcome this limitation, high field magnetic resonance imaging (MRI) techniques were recently developed by our group to quantitatively evaluate the development of cochlear inflammatory processes in a guinea pig model induced by the intratympanic injection of LPS[60]. For the first time, dynamic changes in cochlear vascular permeability following cochlear inflammation was quantified using dynamic contrast enhanced-MRI and ultrasmall superparamagnetic iron oxide particles were used to characterise the recruitment of macrophages into the cochlea. These methodologies therefore hold considerable potential as diagnostic tools for human inner ear diseases such as labyrinthitis and could also be used to quantitatively assess the efficacy of treatments for cochlear inflammation.
NOISE-INDUCED COCHLEAR INFLAMMATION
Recent years have advanced our understanding of the underlying mechanisms of noise-induced cochlear damage. One of the most compelling hypotheses postulates oxidative stress (the excessive formation of reactive oxygen species or free radicals) in the cochlea as a key mechanism of noise-induced hearing loss[10,14,61]. An increase in reactive oxygen species is also thought to be involved in age-related and drug-induced hearing loss (ototoxicity). Oxidative stress alters the redox balance of the cells, leading to the activation of cell death pathways (apoptosis and necrosis) in the cochlea and hearing loss.
Other studies, however, have implied the intrinsic involvement of inflammation in noise-induced cochlear tissue damage. Early ultrastructural studies in the noise-exposed mammalian cochlea have identified macrophage-like cells in the damaged organ of Corti, mainly in the tunnel of Corti and in the outer hair cell region, appearing 5 d after acoustic overstimulation[62,63]. These macrophages are likely involved in mopping up cell debris. The presence of transforming monocytes in the area and mononuclear leukocytes within the spiral lamina blood vessels suggested that these dendritic macrophages originated from blood-borne monocytes[63].
Several studies have demonstrated that after acoustic trauma, a large influx of inflammatory cells from the vasculature can be observed in the cochlea, generally peaking between 2 and 7 d after exposure to traumatic noise, and diminishing thereafter[16,17,64-66]. Inflammatory cells within the cochlea were identified immunohistochemically using their cell surface markers CD45, a receptor tyrosine phosphatase present on all hematopoietic/bone marrow-derived leukocytes or F4/80, a marker of activated macrophages and monocytes. The study by Tornabene et al[17] showed that CD45-positive cells increased from an average of 0.3 cells/section in the non-exposed cochlea to a maximum of 88 cells/section at 2 and 4 d after noise exposure. These infiltrating cells were localised predominantly in the spiral ligament, particularly in the inferior region among type I and type IV fibrocytes and in the region adjacent to the bony cochlear capsule among type III fibrocytes, and in the perilymph-filled spaces of the scala tympani and scala vestibuli[16,17,64,65]. Leukocytes were also observed within the spiral limbus, another region known to be susceptible to acoustic injury, and in the spiral ganglion[16,64,65]. A few cells were also found in the stria vascularis and the perivascular spaces of the modiolus[17,67]. This recruitment of macrophages to the cochlea following excessive stimuli is similar to what occurs in other sensory organs, such as the retina of the eye. Thus, exposure to damaging light causes an infiltration of inflammatory cells to the light-damaged region of the retina[68].
BrdU labelling has demonstrated that these inflammatory cells migrate from the vasculature, and it appears that most of these cells enter the cochlea through the blood vessels of the lateral wall[16]. The lateral wall is highly vascularized, and the spiral ligament is the site where the large majority of inflammatory cells can be found. Immunostaining with other monocyte/macrophage markers (CD68, CX3CR1, Iba-1) demonstrated that the vast majority of these infiltrating cells are derived from the monocyte/macrophage lineage, with a small number representing other leukocytes such as T and B lymphocytes[16,25]. Hirose et al[16] coined the term “cochlear macrophage” for those inflammatory cells, to indicate an inducible exchanging population of phagocytic cells that respond to acoustic injury.
The recruitment and extravasation of these inflammatory cells into the cochlea is mediated by cytokines (e.g., TNF-α, IL-1β, IL-6), chemokines (e.g., MCP-1, MCP-5, MIP-1β) and cell adhesion molecules [e.g., ICAM-1, platelet-endothelial cell-adhesion molecule-1 (PECAM-1)], which are upregulated immediately after noise exposure[17,46,69-71]. Fujioka et al[46] demonstrated an upregulation of the proinflammatory cytokines TNF-α, IL-1β and IL-6 in the noise-damaged cochlea as early as 3 h after noise exposure. IL-6 immunoreactive cells were observed initially in the lower and lateral regions of the spiral ligament, specifically in the cytoplasm of type IV and III fibrocytes, then throughout the spiral ligament and even in the stria vascularis[46]. Double labelling with NeuN, a neuronal marker, showed IL-6 expression in the spiral ganglion neurons 12-24 h after noise exposure. IL-6 upregulation in the noise-exposed cochlea likely contributes to cochlear injury, as the inhibition of IL-6 suppressed cochlear inflammation and mitigated the hearing loss[64]. Chemokines that are chemotactic for macrophages such as MCP-1/CCL2, MCP-5/CCL12, and MIP-1β/CCL4 are upregulated in the noise-exposed cochlea 2 h following acoustic trauma[17]. The early expression of chemokines suggests that resident cochlear cells may be responsible for this upregulation.
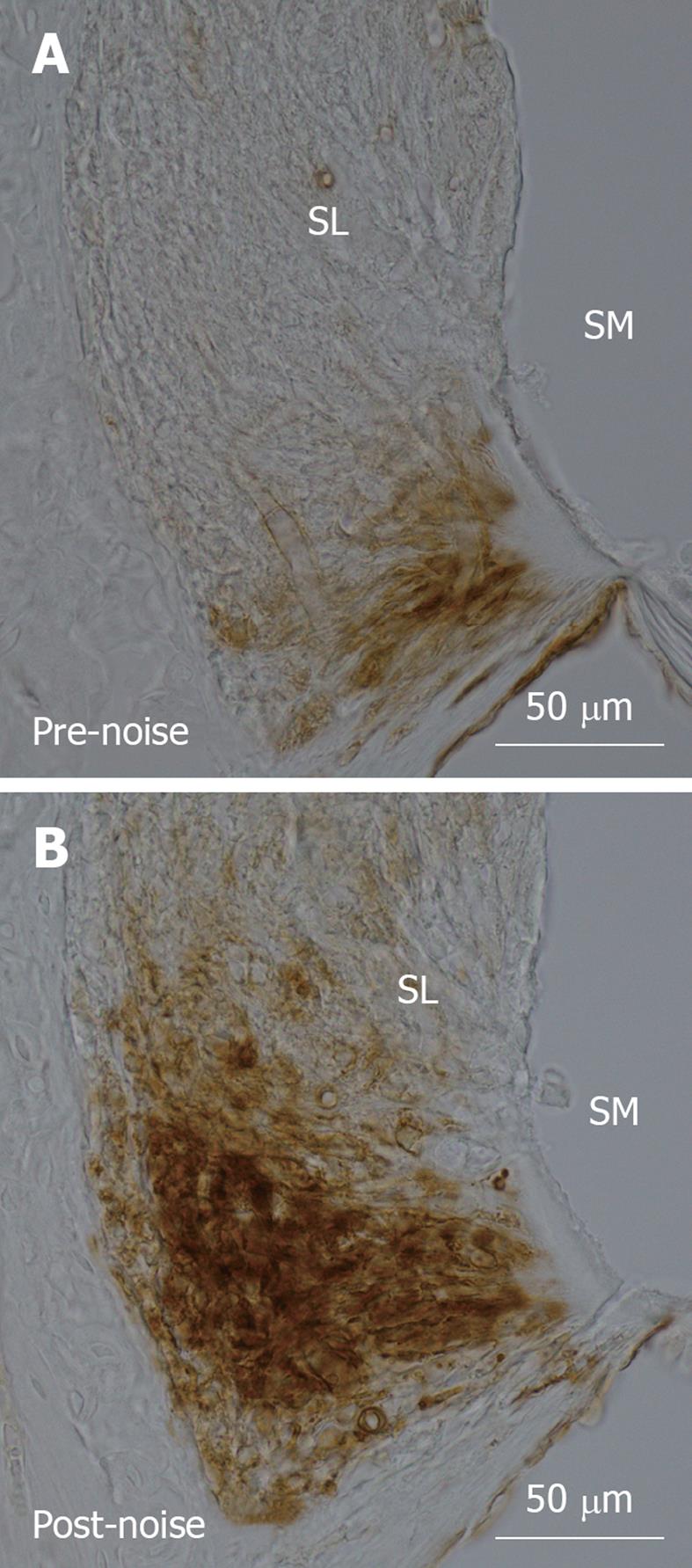
ICAM-1/CD54 is a vascular adhesion molecule that plays a critical role in mediating temporary adhesion/immobilisation of leukocytes to vascular endothelial cells in preparation for extravasation. Increased expression of ICAM-1 at the protein level is seen 24 h after noise exposure, reaching a maximum at 2 and 4 d, and returning to basal levels by 14 d[17]. This elevated expression is seen chiefly in the vascular endothelial cells and fibrocytes occupying the root region of the spiral ligament, and less intensely in the region of the spiral ligament adjacent to the cochlear bony capsule. The endosteal cells lining the scala tympani and scala vestibuli and capillaries of the stria vascularis also show increased ICAM-1 immunolabelling. Upregulation of ICAM-1 at the mRNA level is first observed 2 h after noise exposure. The increased ICAM-1 expression in these cells regulates and directs the extravasation and cellular infiltration of inflammatory leukocytes. Results from our recent study on ICAM-1 expression following acute noise exposure in mice are compatible with these findings (Figure 1). Other adhesion molecules that show increased expression following noise exposure include P-selectin, PECAM-1 and VCAM-1[72,73]. Shi et al[72] demonstrated that the expression of these adhesion molecules is modulated by poly(ADP-ribose) polymerase-1 (PARP-1), a DNA repair enzyme. They suggested that noise activates PARP-1 in capillary endothelial cells of the spiral ligament and stria vascularis, which may act through NF-κB to regulate the expression of adhesion proteins in the lateral wall.
Figure 1 Intercellular adhesion molecule-1 immunolabelling in the spiral ligament of the cochlear basal turn in C57BL/6 mice.
A: In the non-noise exposed cochlea, intercellular adhesion molecule-1 (ICAM-1) was expressed by type IV fibrocytes and vascular endothelial cells in the lowest region of the spiral ligament; B: Mice exposed to traumatic noise (100 dB SPL, 8-16 kHz) for 24 h showed increased expression of ICAM-1, peaking at 24 h following acoustic trauma. ICAM-1 immunolabelling became more intense and expanded to cover a much greater area in the inferior region of the spiral ligament. ICAM-1 immunoexpression was determined by immunoperoxidase histochemistry and photomicrographs of mid-modiolar cochlear sections were taken with a digital light microscope (Nikon Eclipse 80i) at 40 × magnification. SL: Spiral ligament; SM: Scala media.
The expression of many proinflammatory mediators that participate in the acute inflammatory response is broadly regulated by the transcription factor NF-κB. Apart from its pivotal role in immune and inflammatory responses, NF-κB is also implicated in a range of processes such as cell survival, apoptosis, development, differentiation and cell growth[74]. NF-κB comprises a family of five inducible transcription factors, p50/p105 (NF-κB1), p52/p100 (NF-κB2), p65 (RelA), RelB, and c-Rel[75]. They exist as hetero- or homo-dimeric complexes, with the p50/p65 hetero-dimer being the predominant form. In quiescent cells, NF-κB is expressed in the cytoplasm in a latent form, with an inhibitory protein (IκB) bound to the dimer. Upon stimulation, the inhibitory protein is degraded, activating the NF-κB dimer, which then translocates to the nucleus where it binds to the promoters of its target genes. NF-κB activation in the cochlea has been demonstrated following noise exposure[73,76,77]. Following a 2 h exposure of mice to traumatic noise (124 dB SPL), translocation of p65 and p50 to the nucleus of fibrocytes in the lateral wall was observed, indicating NF-κB activation[76]. Prominent nuclear localisation of NF-κB occurred 2 h after noise exposure, but the nuclear immunostaining subsided after 72 h, suggesting an early response of NF-κB to acoustic overstimulation.
As mentioned earlier, a large population of PVMs exist in the stria vascularis, however, these cells are not found elsewhere in the cochlea, including the spiral ligament[52]. The PVMs play an important role in regulating the integrity of the intrastrial fluid-blood barrier by modulating the expression of tight- and adherens-junction proteins between the endothelial cells via the secretion of pigment epithelium growth factor (PEDF)[78,79]. The integrity of the barrier is critical for establishing and maintaining the endocochlear potential and preventing the entry of toxic substances into the cochlea[80]. Exposure to excessive noise leads to breakdown and increased permeability of the blood-labyrinth barrier by causing PVMs to change morphology and detach from strial capillaries and also by causing a significant downregulation of PEDF production and tight junction protein expression[81]. Similar to the cochlea, the retina of the eye contains perivascular macrophages, which also contribute to the maintenance of the blood-retinal barrier[82]. Recent evidence has demonstrated that bone marrow-derived cells (BMDCs) are recruited to the stria vascularis during the first week after acoustic injury to repair and restore the noise-damaged blood vessels[83]. These cells promote angiogenesis and neovascularization, differentiating into PVMs, pericytes and endothelial cells and integrating into the strial blood vessels by 4 wk after noise exposure. This recruitment is mediated by an intrinsic (iNOS)-dependent stromal cell-derived factor-1α (SDF-1α) signalling pathway. Blocking the activity of iNOS or SDF-1α significantly reduced both the number of infiltrating BMDCs and the capillary density (vascular repair) in the stria vascularis of the noise-exposed cochlea.
Similar to noise-induced hearing loss, oxidative stress and inflammation are major contributing factors to cisplatin-induced ototoxicity. Cisplatin has been shown to increase the expression of inflammatory mediators such as iNOS, cyclo-oxygenase-2 and TNF-α, which are downstream targets of the transcription factor, signal transducer and activator of transcription-1 (STAT1)[84]. Cisplatin-induced activation of STAT1 is dependent on ROS generation through NOX3, a member of the NOX family of superoxide-generating nicotinamide adenine dinucleotide phosphate oxidases. NOX3 is expressed almost exclusively in the inner ear and serves as the primary source of ROS generation in the cochlea[85]. siRNA-mediated gene silencing of NOX3 mitigates cisplatin-induced hearing loss, demonstrating a key role of NOX3 in the development of cisplatin-mediated ototoxicity[86]. In contrast to these findings, recent data from our group showed that exposure to noise results in a significant down-regulation of NOX3 in the cochlea[87]. We propose that the reduction in NOX3 may represent an endogenous protective mechanism to reduce oxidative stress in the noise-exposed cochlea. These studies provide evidence that NOX3 is involved in the development of noise- and cisplatin-induced cochlear injury, albeit in a different way.
The exact role inflammatory cells play once recruited to the noise-damaged cochlea remains unclear. It is possible that the inflammatory response exacerbates the cellular damage in the cochlea by causing bystander tissue injury. It has also been suggested that the recruitment of inflammatory cells following acoustic injury is part of a wound healing response, given that infiltrating cells are largely observed in the region of the spiral ligament where noise-induced fibrocyte loss is most evident[16,17,49,69]. Leukocytes may play a critical role in promoting repair by removing cellular debris created by the primary insult. These cells may contribute to the repair process by changing the local environment via the secretion of chemical mediators such as cytokines and growth factors. Inflammatory leukocytes could function along with resident fibrocytes of the spiral ligament to regulate repair of the noise-damaged cochlear structures. It has been speculated that the fibrocytes initiate the local inflammatory process[65]. These cells express similar cytokines, chemokines and adhesion molecules, and also respond to signals used by leukocytes for cell-cell signalling. Cochlear fibrocytes can perhaps be considered facultative resident macrophages, serving some functions normally performed by circulating macrophages.
TREATMENT STRATEGIES FOR MITIGATING NOISE-INDUCED COCHLEAR INFLAMMATION
At the present time, there is no cure for noise-induced hearing loss, or any other types of hearing loss. The only therapeutic intervention for the hearing impaired is the use of hearing devices such as hearing aids that amplify sound or cochlear implants. A cochlear implant is a neural prosthesis that functions by electrically stimulating residual spiral ganglion neurons, the primary auditory neurons of the cochlea.
Corticosteroids (glucocorticoids) are widely used in the treatment of numerous acute and chronic inflammatory diseases, and have also long been used in the management of SNHL of various causes, including noise-induced hearing loss. Corticosteroids are typically administered systemically, either intravenously or orally. Appropriate doses of steroids supress excessive inflammation, but are unable to completely recover the associated hearing loss. Higher doses, on the other hand, can be deleterious to cochlear function in the long term and are often accompanied by a wide range of adverse side effects[88]. Glucocorticoids exert their actions by binding to and activating soluble cytoplasmic glucocorticoid receptors, which translocate to the nucleus and bind to specific DNA sites, culminating in the downregulation of proinflammatory cytokines and adhesion molecules[89]. Experiments have demonstrated that dexamethasone, a popular glucocorticoid, suppresses TNF-α-induced inflammatory mediator release from cultured spiral ligament fibrocytes[54]. The otoprotective effects of steroids may be mediated through the actions of NF-κB, as glucocorticoids are shown to be potent inhibitors of NF-κB activation via the induction of the IκBα inhibitory protein[90]. Local routes of steroid delivery have been developed without the unfavourable side effects. Direct infusion of dexamethasone into the perilymphatic space using osmotic mini-pumps has been reported to show protective effects against noise-induced injury in the guinea pig cochlea[91]. Intratympanic administration of steroids have also shown good therapeutic efficacy[92].
From our existing knowledge of the underlying mechanisms and pathways of the cochlear inflammatory response, rational therapeutic approaches can be devised to supress the inflammation and reduce cochlear injury. It is speculated that there are networks in the cochlea among inflammatory cells, fibrocytes and vascular endothelial cells, which are interconnected by various proinflammatory mediators (chemokines, cytokines, and adhesion molecules)[47]. Appropriate control of these networks could potentially attenuate the inflammatory reaction in the cochlea. Because of their early expression in the inflammatory response and their role in recruiting inflammatory cells into the cochlea, targeting chemokines/cytokines through direct inhibition may represent an effective novel therapeutic strategy.
Satoh et al[56] examined the therapeutic potential of anti-TNF-α therapy and showed that blocking the activity of TNF-α using Etanercept, a soluble TNF-α receptor-FC fusion protein, significantly attenuated the cochlear inflammatory response (reduced inflammatory cell infiltration and cochlear fibrosis) in an animal model of immune-mediated labyrinthitis induced by immunisation with KLH. A further study showed that neutralisation of TNF-α using Etanercept markedly decreased the expression and secretion of proinflammatory cytokines (TNF-α, IL-1β and IL-6) in the cochlea after cisplatin injection[21].
Another potential treatment strategy would be to block IL-6 signalling in the cochlea. It is interesting in this regard that specific humanised neutralising antibodies against IL-6 have recently been used clinically with promising effects in patients with rheumatoid arthritis and inflammatory bowel disease. In fact, a recent study by Wakabayashi et al[64] showed that inhibition of IL-6 with IL-6 receptor neutralising antibody (MR16-1) resulted in a dramatic suppression of the cochlear inflammatory response (reduced infiltration of inflammatory cells) and significantly improved hearing function in noise-exposed mice.
Recently, Nakamoto et al[70] showed that administration of geranylgeranylacetone (GGA), an anti-ulcer drug, suppressed the expression of proinflammatory cytokines (IL-6 and IL-1β) in the noise-exposed cochlea and also improved auditory function. GGA activates heat shock transcription factor 1 (HSF1), which induces the expression of heat shock proteins. HSF1 is also known to directly or indirectly regulate cytokine expression, such as inhibiting the expression of IL-6 and IL-1β. GGA can also reduce inflammation in other organs (e.g., liver) without apparent side effects even at large doses. GGA may therefore provide a novel beneficial strategy for the prevention of noise-induced hearing loss.
The role of antioxidants in noise-induced hearing loss has been the subject of extensive research. Antioxidants have been demonstrated to provide a protective effect in the cochlea by restoring the redox balance. A recent study examined the effects of antioxidant treatment on the inflammatory response in the cochlea following noise exposure[67]. This study reported that antioxidant treatment not only reduced markers of oxidative stress, but also significantly reduced the infiltration of inflammatory cells into the cochlea. This finding suggests an anti-inflammatory role of antioxidants in the cochlea.
Extensive evidence from in vitro and in vivo studies has demonstrated the strong anti-inflammatory potential of adenosine, a ubiquitous signalling molecule and neuromodulator, in a range of tissues[93-98]. Adenosine exerts its anti-inflammatory action by influencing almost all aspects of the immune response[99]. The A2A receptor, reported to be the crucial receptor involved in the suppression of inflammation, is a promising target for the treatment of inflammatory conditions. Selective A2A receptor agonists have been used successfully in the therapy of sepsis, inflammatory bowel disease, skin inflammation and arthritis[98], and a similar effect could be postulated in the cochlea. In addition, A2A receptor agonists have been reported to suppress neuroinflammation in animal models[99]. In the mammalian (rat) cochlea, A2A receptors are expressed in the inner hair cells and supporting Deiters’ cells of the organ of Corti, spiral ligament, spiral ganglion neurons, and blood vessels[100]. This broad distribution suggests an important role of A2A receptors in the cochlea. The systemic administration of exogenous adenosine is limited by its peripheral side effects[97]. An alternative approach for augmenting the availability and actions of endogenous adenosine that has received increasing attention in recent years is the inhibition of adenosine kinase[94,101,102]. Adenosine kinase inhibitors, such as ABT-702, have demonstrated excellent efficacy in animal models of acute and chronic inflammation[101,103,104], and may have considerable therapeutic potential in cochlear inflammation. Adenosine kinase is extensively distributed in the adult cochlea[105,106], and may have a critical role in the regulation of adenosine signalling under physiological and pathological conditions.
CONCLUSION
The cochlea responds to trauma and infection like organs elsewhere in the body by eliciting an inflammatory response. Exposure to excessive noise triggers a cochlear inflammatory response that is characterised by an initial upregulation of numerous proinflammatory mediators and adhesion molecules by various resident cochlear cell types, followed by the rapid recruitment and infiltration of inflammatory cells into the cochlea from the systemic circulation. Much has been learned over the years of the noise-induced inflammatory process in the cochlea from animal models, but the exact mechanisms by which noise elicits this response is still unclear. The noise-induced inflammatory response may be involved in propagating cellular damage in the cochlea, but there is also a possibility that it may be involved in reparative processes. The mechanism and importance of this response in the noise-injured cochlea requires further exploration. With deeper knowledge of the underlying cochlear inflammatory response, we can explore and develop novel therapeutic interventions to protect cochlear tissues from inflammation-induced injury and noise-induced hearing loss.
ACKNOWLEDGMENTS
The authors acknowledge the financial research support to work on cochlear inflammation from the Health Research Council of New Zealand, Auckland Medical Research Foundation, Deafness Research Foundation (NZ) and the Freemasons Lodge Discovery 501.