Published online Feb 12, 2015. doi: 10.5318/wjo.v5.i1.1
Peer-review started: June 21, 2014
First decision: August 14, 2014
Revised: November 27, 2014
Accepted: December 29, 2014
Article in press: December 31, 2014
Published online: February 12, 2015
Pseudopemphigoid can cause a chronic cicatricial conjunctivitis that is clinically identical to the manifestations seen in mucous membrane pemphigoid, a disorder with a common clinical phenotype and multiple autoimmune links. For the purpose of this review, we will describe pseudopemphigoid as caused by topical drugs, the most common etiology with ocular manifestations, and as caused by the pemphigus disease, a more rare etiology. Specifically, we will discuss the ophthalmological features of drug-induced cicatricial conjunctivitis, pemphigus vulgaris, and paraneoplastic pemphigus. Other etiologies of pseudopemphigoid exist that will not be described in this review including autoimmune or inflammatory conditions such as lichen planus, sarcoidosis, granulomatosis with polyangiitis (Wegener’s granulomatosis), erythema multiforme (minor, major, and Stevens-Johnson syndrome), bullous pemphigoid, skin-dominated linear IgA bullous dermatosis, and skin-dominated epidermolysis bullosa acquisita. Prompt diagnosis of the underlying etiology in pseudopemphigoid is paramount to the patient’s outcome as certain diseases are associated with a more severe clinical course, increased ocular involvement, and differential response to treatment. A complete history and ocular examination may find early cicatricial changes in the conjunctiva that are important to note and evaluate to avoid progression to more severe disease manifestations. When such cicatricial changes are noted, proper diagnostic techniques are needed to help elucidate a diagnosis. Lastly, collaboration between ophthalmologists and subspecialists such as dermatologists, pathologists, immunologists, and others involved in the care of the patient is needed to ensure optimal management of disease.
Core tip: Pseudopemphigoid in the context of chronic cicatricial conjunctivitis mimicking mucous membrane pemphigoid is a disease with terminology that has continuously evolved since its inception. Recent understanding of the ophthalmological and systemic manifestations of pseudopemphigoid as caused by topical drugs and the pemphigus disease demonstrates that significantly decreased vision and/or increased mortality due to paraneoplastic associations may result. Proper diagnosis and treatment of the underlying disease is therefore critical in order to provide maximal care to the patient.
- Citation: Huang LC, Wong JR, Alonso-Llamazares J, Nousari CH, Perez VL, Amescua G, Karp CL, Galor A. Pseudopemphigoid as caused by topical drugs and pemphigus disease. World J Ophthalmol 2015; 5(1): 1-15
- URL: https://www.wjgnet.com/2218-6239/full/v5/i1/1.htm
- DOI: https://dx.doi.org/10.5318/wjo.v5.i1.1
The first use of the term pseudopemphigoid referred to a non-progressive, unilateral cicatricial conjunctivitis that developed in response to certain aggravating topical medications[1]. Pseudopemphigoid was originally named due to its clinical similarity to mucous membrane pemphigoid (MMP) - an autoimmune blistering disease characterized by subepithelial deposition of antigen-antibody complexes at the basement membrane zone. Subepithelial deposition of autoantibody complexes seen in pemphigoid disease differentiates it from pemphigus that is characterized by intraepithelial deposition of autoantibody complexes. If clinical manifestations of pemphigus produce conjunctival cicatrization identical to MMP, then pemphigus may therefore be characterized as “pseudopemphigoid” and the modern terminology of pseudopemphigoid now includes any etiology that mimics MMP in clinical presentation.
The purpose of this review is to elaborate on the epidemiology, clinical features, diagnosis, and treatment options available for patients with pseudopemphigoid. This paper will review the ocular manifestations associated with three etiologies of pseudopemphigoid including the most common cause, drug-induced cicatricial conjunctivitis[2], and two rare causes from the pemphigus family, pemphigus vulgaris and paraneoplastic pemphigus. Pemphigus foliaceus, a third subset of the pemphigus family, does not involve the conjunctiva and will not be discussed in this review.
Pseudopemphigoid may be caused by a variety of other conditions not included in this review article such as sarcoidosis, granulomatosis with polyangiitis (Wegener’s granulomatosis), bullous pemphigoid, skin-dominated linear IgA bullous dermatosis, and skin-dominated epidermolysis bullosa acquisita[3]. Additionally, inflammatory and/or autoimmune disease associated cicatricial conjunctivitis characterized by an interface/lichenoid lymphocytic infiltrate such as lichen planus[4], graft vs host disease, erythema multiforme spectrum, and discoid lupus erythematosis[5] are not included in this review article.
Historically, pseudopemphigoid referred to a unilateral drug-induced cicatricial reaction identical to MMP that did not progress upon removal of the inciting drug. The term has since evolved and for the purposes of this review, pseudopemphigoid will be characterized according to the criteria proposed by Thorne et al[2]: (1) Chronic cicatricial conjunctivitis; (2) A biopsy that rules out MMP; and (3) The existence of an alternate cause for cicatrization.
MMP refers to a group of autoimmune, subepithelial, blistering diseases that predominantly affect the mucous membranes and are notable for linear deposition of autoantibodies (IgG, IgA, or C3) along the epithelial basement membrane zone on biopsy. The primary distinction between MMP and pseudopemphigoid is that MMP consists exclusively of autoimmune blistering diseases with subepidermal deposition of autoantibodies as opposed to pseudopemphigoid that simply mimics MMP in clinical presentation but does not involve subepidermal deposition of autoantibodies.
Clinical features: Pseudopemphigoid, similarly to MMP, may produce a chronic cicatricial conjunctivitis in patients characterized by the presence of scarring. The clinical presentation of a patient with cicatricial conjunctivitis includes irritation, burning, a foreign body sensation, photophobia, tearing, dryness, redness or blurry vision and hyperemic conjunctiva, misalignment of eyelashes, cicatricial entropion, and trichiasis[6].
The Foster[7] staging system developed for MMP may be utilized to characterize the severity of chronic cicatricial conjunctivitis secondary to pseudopemphigoid as well. In Stage I, conjunctival inflammation develops with mucous discharge, subepithelial fibrosis, and areas of degenerated cells in the conjunctival epithelium. Abnormal connective tissue and small white striae develop around the superficial vessels of the substantia propria, producing conjunctival “shrinkage.” In Stage II, inferior conjunctival foreshortening occurs. In Stage III, subepithelial bands of connective tissue create symblepharon (conjunctival adhesions), corneal neovascularization, trichiasis (misdirected eyelash growth), dystichiasis (eyelash growth arising from meibomian glands), and keratopathy due to scarring of the conjunctival goblet cells, lacrimal gland ducts, and meibomian gland orifices. In Stage IV, severe sicca syndrome, keratinization, and ankyloblepharon (lid adhesions) develop (Table 1).
| Staging for severity of cicatricial conjunctivitis | |
| Foster Staging[7] | |
| I | Subepithelial fibrosis, positive rose-bengal staining in conjunctiva, conjunctival “shrinkage” from abnormal connective tissue due to small white striae that form around the superficial vessels in substantia propria |
| II | Marked foreshortening of inferior conjunctiva described by (1) 0%-25%; (2) 25%-50%; (3) 50%-75%; and (4) 75%-100% |
| III | Corneal neovascularization, trichiasis, dystichiasis, keratopathy, subepithelial bands of connective tissue resulting in symblepharon (conjunctival adhesions) formation that is described by (1) 0%-25%; (2) 25%-50%; (3) 50%-75%; and (4) 75%-100% |
| IV | Severe sicca syndrome, keratinization, ankyloblepharon |
| Mondino Staging[134] | |
| I | 0%-25% loss of inferior conjunctival fornix depth |
| II | 25%-50% loss of inferior conjunctival fornix depth |
| III | 50%-75% loss of inferior conjunctival fornix depth |
| IV | 75%-100% loss of inferior conjunctival fornix depth |
Despite a severe end-stage presentation, early cicatrization secondary to pseudopemphigoid and MMP is often nonspecific and subtle which causes patients to present with disease that is already erosive and scarring[8]. Additionally, more than 65% of patients may have cicatricial conjunctivitis develop without any symptoms[9]. In a prospective study of 163 eyes with cicatricial change, a diagnostic delay of a median 225 d after symptom onset was noted causing 59% of patients to present as Stage III at diagnosis[10]. Therefore, it is paramount to have etiologies such as MMP and pseudopemphigoid on the differential diagnosis for any patient who presents with cicatrization to best optimize management.
Diagnosis: The first step to discovering the etiology of chronic cicatricial conjunctivitis involves the history. The patient should be asked about any past medical history of chemical or thermal burns; membranous conjunctivitis caused by infectious organisms such as adenovirus; mucocutaneous disorders such as erythema multiforme (minor, major, and Stevens-Johnson syndrome), Sjogren’s syndrome; systemic allergic disease such as chronic atopic conjunctivitis or rosacea; chronic graft-vs-host disease following organ transplantation; history of trachoma if from endemic areas; previous eyelid surgery; or previous use of any aggravating topical or systemic medications (Table 2). Additionally, because many etiologies of pseudopemphigoid include systemic autoimmune bullous disease, the physician should inquire about new onset cutaneous or oral mucosal lesions. Most patients presenting with cicatricial conjunctivitis will have a history that makes the diagnosis simple. If inconclusive evidence is found from the history, then other methods of diagnosis are needed including a conjunctival biopsy.
| Trauma |
| Physical trauma |
| Chemical burn |
| Thermal burn |
| Radiation burn |
| Infection |
| Trachoma |
| Membranous conjunctivitis |
| Allergic |
| Chronic atopic keratoconjunctivitis |
| Mucocutaneous disease |
| Erythema multiforme |
| Stevens-Johnson Syndrome |
| Toxic epidermal necrolysis |
| Immunobullous disorders |
| Mucous membrane pemphigoid |
| Bullous pemphigoid |
| Pemphigus vulgaris |
| Paraneoplastic pemphigus |
| Lichen planus |
| Dermatitis herpetiformis |
| Systemic lupus erythematosus |
| Systemic disorders |
| Rosacea |
| Sjogren's syndrome |
| Graft-vs-host disease |
| Sarcoidosis |
| Ectodermal dysplasia |
| Erythroderma ichthyosiform congenital |
| Drug-induced |
| Systemic |
| Topical |
Epidemiology: Drug-induced conjunctival cicatrization (DICC), also known as drug-induced ocular pseudopemphigoid, produces clinical findings identical to MMP in response to varying offending topical and systemic drugs[11]. The incidence of DICC remains unknown, but has been documented to occur most often in patients who require long-term use of glaucoma medications[11]. In a retrospective cohort study of 145 pseudopemphigoid patients, DICC was the most common cause of pseudopemphigoid in this population occurring in 28.3% of patients[2].
Pathophysiology: DICC can develop as a non-progressive, self-limiting “toxic” reaction to an offending topical drug or as a progressive, immunological process that continues despite cessation of the offending drug[12-14]. Although increased activity of fibroblasts has been implicated as a possible effect on the local immune system, the exact mechanism by which offending topical drugs directly induce cicatricial conjunctivitis remains unknown[15].
When IgG localized to the ocular epithelial basement membrane zone are found, then autoimmune phenomenon are suggested[15,16]. Practolol, an oral beta-blocker, and its derivative metipranolol, a topical beta-blocker that treats glaucoma, have been implicated to induce immunologically mediated DICC[17-20]. This is related to the chemical structure and pharmacologic metabolism in the body - both compounds require deacetylation for metabolic activation, which produces a toxic aniline derivative in practolol and a slightly less toxic phenol derivative in metipranolol[17]. When oxidized, these derivatives become highly reactive and are normally neutralized in the body by the addition of glucuronic acid or sulfate. However, this mechanism is insufficient in patients that have a lower capacity for enzymatic detoxification[17]. When this occurs, proteins can bind these reactive oxidative products to create antigens[17]. Therefore, the toxicity potential of practolol and metipranolol to produce immunologically mediated cicatricial conjunctivitis occurs in patients who are susceptible to these reactions required for metabolic activation of the drug due to its pharmacologic structure. Drug chemical structure has not been implicated in the mechanism of cicatricial conjunctivitis induced by other offending topical drugs and in many cases of DICC, a toxic or immune-mediated reaction cannot be further defined.
Epitope spreading is one possible theory that may elucidate the mechanism behind autoimmune phenomenon as induced by topical drugs. Epitope spreading[21,22] refers to the phenomenon of autoimmune reactivity not only against one protein, but also against other epitopes on the same protein or other proteins in the same tissue. Intramolecular epitope spreading that occurs between different epitopes on the same protein is often used to explain the molecular pathogenesis and severity of disease in bullous pemphigoid[23]. Additionally, epitope spreading may occur due to tissue damage that causes certain antigens to become newly exposed to autoreactive T or B cells, thus producing an autoimmune disease in predisposed individuals[21,24]. This mechanism of epitope spreading can be promoted by injury that exposes previously sequestered antigens, causing activation of antigen presenting cells that attract autoreactive lymphocytes in these individuals[22]. Intermolecular epitope spreading that occurs between two different proteins has been cited to explain the conversion of one autoimmune disease into another. Pemphigus autoimmune disease converting into pemphigoid disease, or conversions between other autoimmune blistering diseases either simultaneously or separated by a few years, is hypothesized to occur when tissue damage exposes protein parts that are normally undetected by the immune system[25,26]. In a similar manner, ocular mucosal injury due to Stevens-Johnson syndrome, Lyell Syndrome, or direct chemical injury from drugs may be implicated to expose normally hidden antigens to processing and presentation by activated T-cells, resulting in the formation of MMP[12,21,24].
Incidences of MMP developing in uninvolved eyes of patients that did not receive the inciting drug may indicate an immunological etiology[12]. On the other hand, instances of unilateral changes histologically and immunologically identical to MMP that occur in only the eye that received an offending drug is considered to be drug-induced[16]. The absence of bilateral ocular involvement, other mucosal or cutaneous manifestations, and disease that is non-progressive after cessation of the offending drug suggests a drug-induced reaction. Therefore, DICC may involve either a toxic mechanism of damage or an autoimmune etiology where inciting topical medications sensitize predisposed individuals to developing a more rapid onset of ocular MMP.
Clinical findings: DICC produces symptoms of cicatrization clinically identical to MMP. Two distinguishing factors that differentiate DICC from MMP include unilaterality of symptoms localized to the eye that received the topical therapy as well as non-progression of disease after cessation of the drug. However, reports of progressive DICC have occurred in the literature[13,14].
A total of 7 studies comprising 63 cases of drug-induced conjunctival cicatrization were found in the literature[1,2,14,16,27-29]. The most commonly used inciting topical drugs and the average duration of utilization before onset of DICC symptoms consisted of: timolol (73% or 46/63) for an average 10.5 years, pilocarpine (51% or 32/63) for an average 9 years, dipivefrin (49% or 31/63) for an average 7 years, latanoprost (13% or 8/63) for an average 8 years, echothiophate iodide (11% or 7/63) for an average 8 years, epinephrine (10% or 6/63) for an average 2 years, acetazolamide (6% or 4/63) for an average 9 years, betaxolol (3% or 2/63) for an average 6 years, idoxuridine (6% or 4/63) for an average 2 years, dichlorphenamide (5% or 3/63) for an average 3 years, and bromonidine (8% or 5/63) and other beta blocker antiglaucomatous medications for an unknown duration of time (48% or 30/63).
Of 7 studies comprising 23 patients with drug-induced conjunctival cicatrization found in the literature, the most common clinical findings included: forniceal foreshortening (57% or 13/23), symblepharon formation (48% or 11/23), trichiasis (48% or 11/23), corneal epithelial defects (35% or 8/23), entropion (30% or 7/23), corneal pannus (30% or 7/23), pseudopterygium formation (4% or 1/23), and corneal perforation (4% or 1/23)[1,14,16,27-30].
Diagnostic studies: There are no specific changes associated with medications that induce cicatrization nor is there a favored location of conjunctival involvement to distinguish DICC from idiopathic MMP[11]. Histopathological features seen on conjunctival biopsy can vary according to whether cicatrization is mild or severe[31]. When histopathological changes are seen, biopsy specimens can be identical to that of MMP and include subepithelial fibrosis, subepithelial infiltration with inflammatory cells, reduction or loss of goblet cells, and basement membrane thickening[1,12,19,28]. Conjunctival biopsy with the use of direct immunofluorescence (DIF) is often not helpful as the findings are usually absent or nonspecific. However, IgG and complement staining to the epithelial basement membrane zone have been reported[1,15]. Although it is more common for immunofluorescent testing to lack positive findings, patients who present with both DICC and positive basement membrane zone autoantibody deposition should be considered to have MMP[3]. Otherwise, if the patient presents with a unilateral, non-progressive cicatrization of the conjunctiva, lacks other cutaneous or oral mucosal lesions, has a history of topical medication use for a prolonged amount of time, and other causes of conjunctival shrinkage have been excluded, then DICC should be considered.
Treatment: Management of DICC involves withdrawing the causative drug as early as possible and monitoring the patient carefully for the progressive type of disease. As topical intraocular lowering pressure therapies are commonly implicated in the pathogenesis of DICC, therapy involves a dual approach that includes controlling intraocular pressure and treating the signs of cicatrization. The primary management to resolve or inhibit the progression of fibrosis is cessation of intraocular lowering pressure medication[12]. The treatment to control intraocular pressure includes systemic carbonic anhydrase inhibitors followed by early surgical trabeculectomy[14,16]. If there are no other treatment options for the topical preparation suspected to be the offending drug, then re-introducing the medication in an unpreserved preparation may help. The patient should be followed closely for progressive disease and if progression occurs, then one must consider that the patient has developed ocular MMP and begin the patient on therapy.
A total of 7 studies comprising 63 cases of DICC or drug-induced pseudopemphigoid were found in the literature[1,2,14,16,27-29]. Aside from cessation of the inciting drug, management to control signs of cicatrization included medical treatment involving dapsone (10% or 2/21) or steroids (10% or 2/21). Procedural treatments to control sequelae of cicatrization include electrolysis and cryotherapy. Surgical treatments to control sequelae of cicatrization included anterior lamellar repositioning, tarsectomy, mucous membrane grafting, lower lid retractor tightening, lamellar keratoplasty, conjunctival transplant, terminal tarsal rotation procedure, and everting sutures. When switching to a different anti-glaucomatous medication, acetazolamide (14% or 3/21) or methazolamide (14% or 3/21) were utilized. Surgical treatment to manage uncontrolled intraocular pressure included trabeculectomy.
Prognosis: Clinical outcomes after procedural and/or medical treatment included persistence of ocular lesions without progression (48% or 10/21), remission of ocular lesions defined as regression (33% or 7/21), progression of ocular lesions (10% or 2/21), and recurrence of ocular lesions (5% or 1/21). Overall, average follow up time was 25 mo. If cicatrization is non-progressive upon withdrawal of inciting drug, then prognosis is favorable and management should treat the signs of scarring. If cicatrization is progressive upon withdrawal of inciting drug, then management and prognosis should be according to that of MMP.
Epidemiology: Pemphigus vulgaris (PV) is an intraepithelial blistering disease with a reported incidence of 4 to 4.7 cases per one million individuals[32,33]. PV most often affects patients in the fourth to fifth decade of life with equal occurrence in both sexes[34,35]. This differs from MMP that occurs less commonly at an incidence of 1.13 cases per one million individuals and presents in older individuals with a female predominance[8,36-41]. PV affects all races, but a higher predilection is associated with certain HLA subtypes such as the HLA-DRB1*0402 in Ashkenazi Jews and DRB1*1401/04 and DQB1*0503 in patients of European or Asian origin[42-44].
Pathophysiology: PV is an autoimmune disease characterized by suprabasal acantholysis (loss of cell-to-cell adhesions in epidermal cells that occurs just above the basal layer) induced by IgG binding to target antigens desmoglein 1 and 3 of the cadherin family[45-47]. Acantholysis leads to formation of a cleft which subsequently develops into an intraepithelial bulla[48]. This differs from MMP that is characterized by subepithelial lesions due to autoantibodies directed against various target antigens identified in the basement membrane zone.
PV antigens, desmoglein 1 and 3, are part of desmosome complexes that anchor intermediate filaments for adhesion between adjacent cells. These complexes consist of plakoglobin, plakophilin, desmoplakin, and desmosomal cadherins[45]. Desmoglein 1 (160 kDa) is located more superficially just below the stratum corneum whereas desmoglein 3 (130 kDa) is confined to the lower levels just above the basal cell layer[45,48].
Ocular involvement in PV is rare and its low incidence in the literature may be related to the course of disease or due to underreporting. Desmoglein 3 is heavily expressed in the basal layer of conjunctival epithelium along with strong expression of desmocollin 3, and desmoplakin 1 and 2, throughout the conjunctiva[49,50]. The mechanism on why ocular involvement in PV is rare despite the presence of anti-desmoglein 3 autoantibodies in disease is unclear. Suggestions include that the ocular surface is less exposed to trauma than other tissues normally affected by PV[51]; that there is inactivation of desmoglein 3 in ocular epithelium that is readily compensated by other desmosomal proteins thereby leaving only a minority of patients susceptible to disease if compensation cannot be attained[49]; or that conjunctival involvement in PV is simply underreported.
Clinical findings: PV is characterized by the development of large, flaccid cutaneous blisters and mucosal surface involvement including the oral mucosa, conjunctiva, esophagus, larynx, and genitalia. Cutaneous lesions are fragile blisters that bleed easily and are characteristic for demonstrating Nikolsky’s sign (rubbing of the perilesional skin with slight pressure produces exfoliation of the outer layer) and the indirect Nikolsky sign (moving an intact blister laterally and enlarging it with pressure)[52].
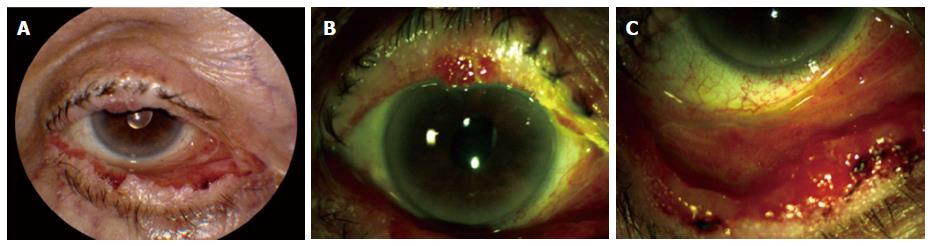
Mucosal involvement is the most common manifestation of PV and painful, chronic erosions of mucus membranes are often the initial presentation[39,53,54]. Ocular involvement in PV is rare and typically benign - ocular lesions do not usually progress to scarring and patients often fully recover without sequelae[51,55]. If ocular involvement occurs, it typically induces bilateral conjunctivitis without fibrosis (Figure 1)[56]. Lid margin erosions in the medial aspect of the lower eyelid can be characteristic of ocular pemphigus vulgaris (OPV)[57]. This differs from MMP that most commonly presents ophthalmologically with signs of overt cicatrization including symblepharon, trichiasis, punctate keratitis, and entropion[10].
A total of 10 studies comprising 36 OPV patients were found in the literature[34,49,51,55,58-63]. The most common ocular symptoms included the following: conjunctival hyperemia (49% or 17/35), conjunctivitis (46% or 16/35), conjunctival ulceration (14% or 5/35), lid margin erosions (14% or 5/35), corneal erosions (6% or 2/35), erosions of the medial canthus (3% or 1/35), and pseudomembrane formation (3% or 1/35). Concomitant systemic manifestations most commonly included oral involvement (80% or 28/35) followed by cutaneous lesions (54% or 19/35). The initial presentation of disease included ocular involvement in 41% (14/34) of cases.
Despite the seemingly benign nature of OPV, other studies suggest that ocular involvement in PV may be a sign of severe or recurrent disease that can occur in conjunction with exacerbation of systemic disease or in patients who have previously failed conventional immunosuppressive therapy[34,51,58]. Although fibrosis is very uncommon in PV, a subset of patients characterized by ocular involvement as the first manifestation of disease can produce a progressive cicatricial conjunctivitis in a similar manner to MMP[35].
In the largest series in the literature regarding OPV patients, Chirinos-Saldaña et al[35] described 15 patients whose presentation included the following: conjunctival hyperemia (100% or 15/15), cicatrization (100% or 15/15), subconjunctival scarring (100% or 15/15), conjunctival cul-de-sac shortening (73% or 11/15), symblepharon formation (40% or 6/15), eyelid involvement including trichiasis or entropion (33% or 5/15), corneal perforation (27% or 4/15), and ankyloblepharon formation (7% or 1/15). Concomitant systemic manifestations included oral (20% or 3/15) and cutaneous involvement (7% or 1/15). The initial presentation of disease included ocular involvement in 100% (15/15) of cases. These results, alongside other reports in the literature involving progressive keratolysis with secondary corneal perforation[64,65], have led authors to conclude that a subset of patients exist with atypical pemphigus characterized by severe ocular involvement as the primary manifestation of disease[35]. Although all patients in this series had immunopathological diagnoses of PV, additional serology studies and/or secondary confirmatory biopsies were not performed to determine the coexistence of MMP. Dual diagnoses of MMP and PV have been previously reported in the literature[66,67] and therefore remain a possibility in this series.
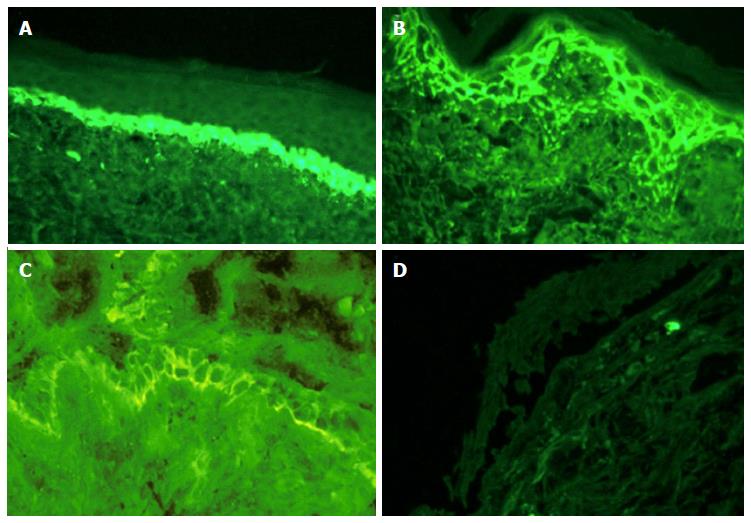
Diagnostic studies: Histopathological studies utilizing hematoxylineosin staining of conjunctival biopsies in OPV demonstrate suprabasal and intraepithelial acantholysis that is characteristic of the pemphigus disease with splitting that occurs above the basal layer[51,61]. This differentiates OPV from MMP where most changes occur at the basement membrane zone with subepithelial conjunctival shrinkage; inflammatory infiltrate involving lymphocytes, macrophages, and plasma cells; and squamous metaplasia progressing to parakeratosis and keratinization of conjunctival epithelium[56,68].
The initial laboratory method to diagnose PV includes a conjunctival biopsy with subsequent DIF to IgG deposits in the intercellular space (Figure 2). This demonstrates antibodies directed against pemphigus antigens including desmosomal proteins desmoglein 3 and 1. This differs from MMP that demonstrates IgG deposits in the subepithelial space with antibodies directed against a variety of antigens not including desmoglein 3 and 1.
Indirect immunofluorescence (IIF) is utilized to detect a titer of circulating autoantibodies through serologic assays. IIF in PV will have serum positive for anti-intercellular substance antibodies in greater than 80%-90% of untreated cases which can be correlated with disease activity[68,69]. This differs from MMP where circulating antibodies are found less commonly than in PV, but may also be utilized to monitor disease activity[3,15,70].
Direct immunoelectron microscopy (IEM) detects peroxidase-labeled antibodies attached to autoantigens in tissue that react with various agents to form electron-dense material[71]. Indirect IEM localizes pemphigus-associated antigens to the extracellular hemidesmosomes in the upper portion of the lamina lucida. This differs from MMP where IEM localizes immune deposits to the lower lamina lucida and lamina densa[56].
The detection of target antigen in OPV can be accomplished through immunoblotting and immunoprecipitation techniques, which identify unknown target antigens bound to autoantibodies. Although immunoprecipitation is less available and more difficult to perform, it is more sensitive than immunoblotting because it utilizes native protein as opposed to denatured protein substrates[52]. A standardized enzyme-linked immunosorbent assay can be utilized to measure autoantibody titers to both desmoglein 3 and desmoglein 1 with higher sensitivity compared to IIF[72]. This differs from MMP that demonstrates immunoprecipitation of various target antigens not including desmoglein 3 and 1.
Treatment: Before the availability of immunosuppressive therapy, mortality for PV reached up to 90% but has now dropped to 3.3% with the use of corticosteroids, cytotoxic drugs, and other biologic agents with immunomodulatory effects[68,73]. Ocular lesions appear to be more responsive to treatment compared to other sites of mucosal involvement[51].
First line therapy for PV includes corticosteroids. Corticosteroids may be used alone or in conjunction with corticosteroid-sparing immunosuppressive agents to allow gradual weaning of steroids to decreased doses or alternate-day therapeutic regimens[60,74,75]. Side effects of corticosteroid treatment most commonly seen include weight gain, cushingoid features, infection, gastrointestinal bleeding, hypertension, hyperglycemia, osteoporosis, and acne[73]. To avoid the occurrence of these side effects, corticosteroids may be used concurrently with sulfone derivatives, immunosuppressive agents, antimetabolites, alkylating agents, and biologic agents.
A total of 10 studies encompassing 39 OPV patients and treatments with multi-drug systemic regimens were found in the literature[34,35,49,55,58-63]. These regimens most commonly consisted of: systemic steroids (82% or 32/39), dapsone (18% or 7/39), azathioprine (13% or 5/39), cyclophosphamide (13% or 5/39), mycophenolate mofetil (10% or 4/39), methotrexate (10% or 4/39), and rituximab (5% or 2/39). Additionally, adjunctive topical drops were utilized in 40% (4/10) studies, of which the most commonly used were topical steroids (8% or 3/39) followed by topical diclofenac, naphazolin, zinc sulphate, chloramphenicol, cyclosporine, and tacrolimus (each 3% or 1/39). Surgical procedures for treatment consisted of penetrating keratoplasty (8% or 3/39) and manual removal of pseudomembranes (3% or 1/39). Overall, the average duration of treatment was 42.1 d.
Prognosis: Factors associated with worse prognosis in PV include ethnicities such as Indo-Asian and Jewish origin, younger age of onset, higher initial intercellular antibody titer, and higher initial desmoglein 3 titer[76,77]. If left untreated, the spread of erosions and bullae leads to severe infection and eventually death with 50% mortality at 2 years and almost 100% mortality at 5 years[78]. If treated, cutaneous lesions heal with re-epithelialization leaving residual hyperpigmentation without scarring.
Of 10 studies encompassing 39 OPV patients treated with multi-drug systemic regimens, outcomes of treatment included remission defined as regression of ocular lesions (54% or 21/39), remission of ocular lesions with persistence of other systemic manifestation of disease (13% or 5/39), persistence of ocular lesions without progression (8% or 3/39), progression of ocular lesions (18% or 7/39), and recurrence of ocular lesions (8% or 3/39). Overall, average follow-up time was 26.6 mo[34,35,49,55,58-63].
Epidemiology: Paraneoplastic pemphigus (PNP), also known as paraneoplastic autoimmune multiorgan syndrome, is a rare intraepithelial blistering disease that occurs less commonly than MMP. PNP occurs at an unknown incidence although approximately 250 cases have been reported in the literature[79]. PNP typically affects patients aged 45-70 years old although cases have occurred in children[80] and males appear to be more commonly affected compared to females[81]. The disease affects all races, but a higher predilection is associated with certain HLA subtypes such as the DRB1*03 allele in Caucasian patients and HLA Cw*14 in Chinese patients[82,83]. Additionally, PNP is strongly associated with underlying malignancy, more often lymphoproliferative neoplasms (chronic lymphocytic leukemia and non-Hodgkin Lymphoma), and the type of malignancy may be related to the ethnic background of the patient. A high prevalence of PNP associated with Castleman’s disease and follicular dendritic cell sarcomas in Chinese and Korean patients has been documented[84].
Pathophysiology: PNP is an intraepithelial blistering disease characterized by autoantibodies that bind desmoglein 3, similarly to the pathogenic mechanism seen in PV. However, in PNP, the autoantibodies bind to epitopes distributed throughout the extracellular domain of desmoglein 3 as opposed to solely the N-terminal extracellular domain in PV[85]. Additionally, PNP has multiple other target antigens including the plakin protein family that connects cytoskeletal networks. These target antigens include desmoplakin I (250 kDa), desmoplakin II (210 kDa), bullous pemphigoid antigen 1 (BPAG1, 230 kDa), envoplakin (210 kDa), periplakin (190 kDa), plectin (500 kDa), desmocollin 2 (105 kDa), desmocollin 3, α2-macroglobulin-like-1 (A2LM1, 170 kDa), desmoglein 1 (160 kDa), and desmoglein 3 (130 kDa)[85-93]. This differs from MMP that is characterized by subepithelial lesions due to autoantibodies directed against various target antigens not including the plakin protein family.
Clinical features: PNP is a systemic autoimmune disease that occurs mostly in the setting of lymphoproliferative malignancies. PNP manifests as persistent painful erosions of mucous membranes and chronic cicatricial conjunctivitis clinically identical to MMP. Anhalt et al[94] termed paraneoplastic pemphigus to refer to a distinct clinical, histopathologic, and immunopathologic condition that included 5 criteria, of which Camisa et al[95] later revised these into major and minor criteria (Table 3)[69,94,95].
| Diagnostic criteria for paraneoplastic pemphigus | |
| Anhalt et al[94] | Camisa et al[95] |
| Painful mucosal and polymorphous skin erosions that involves the trunk, extremities, palms, and soles of a patient with a neoplasm Histological changes including intraepidermal acantholysis, keratinocyte necrosis, and vacuolar interface dermatitis) Direct immunofluorescence findings of IgG and complement localized to the intercellular regions of the epithelium in a linear or granular fashion at the basement membrane zone Circulating autoantibodies that bind to stratified squamous epithelium as well as simple, columnar, and transitional epithelium Immunoprecipitation studies that demonstrate the presence of autoantibodies directed against a complex of five proteins of 250, 230, 210, 190, and 170 kDa | Major criteria Polymorphous mucocutaneous eruption Concurrent internal neoplasia Specific serum immunoprecipitation pattern Minor criteria Histology demonstrating acantholysis Direct immunofluorescence demonstrating intercellular and basement membrane staining Indirect immunofluorescence staining with rat murine epithelium Diagnosis: All three major or two major and two minor required to diagnosis paraneoplastic pemphigus |
The distinguishing clinical manifestations that differentiate PNP include a painful and intractable ulcerating stomatitis that extends to the vermillion surface of the lips[45,96,97] and tense bullae that develop on the palms and/or soles[98]. Cutaneous manifestations of PNP are widely variable and can include superficial vesicles and flaccid blisters (pemphigus-like); scaly erythematous papules with or without tense blisters (bullous pemphigoid-like); polymorphic lesions (erythema multiforme-like); disseminated red scaly papules (graft vs host disease-like); or small violaceous papules with predominant mucosal membrane involvement (lichen planus-like)[99].
A total of 12 studies comprising 23 PNP patients with ocular involvement were found in the literature[84,94,100-109]. The most common ocular symptoms included the following: conjunctival erosions (68% or 15/22), conjunctivitis (45% or 10/22), pseudomembrane formation (27% or 6/22), conjunctival scarring (23% or 5/22), symblepharon formation (18% or 4/22), conjunctival shrinkage (14% or 3/22), forniceal foreshortening (9% or 2/22), corneal epithelial defect (5% or 1/22), and corneal perforation (5% or 1/22). Concomitant systemic manifestations included oral involvement (100% or 23/23) and cutaneous involvement (96% or 22/23). The most common initial presentation of disease was oral involvement (94% or 17/18) followed by ocular (17% or 3/18) and cutaneous (11% or 2/18) lesions.
Additionally, PNP is associated with malignant neoplasms. PNP may be the initial manifestation of a previously undetected malignancy in up to 33% of cases[81,102,110] or PNP may arise years after a patient has already undergone treatment for a previously known malignancy[111]. Of 12 studies comprising 23 PNP patients with ocular involvement, associated malignancies included the following: non-Hodgkin lymphoma (43% or 10/23), Castleman’s disease (22% or 5/23), follicular dendritic cell sarcoma (22% or 5/23), peripheral T cell lymphoma (4% or 1/23), thymoma (4% or 1/23), and squamous cell lung carcinoma (4% or 1/23)[84,94,100-109]. Others have reported the occurrence of chronic lymphocytic leukemia in up to 18%-29% of cases[97,108] as well as adenocarcinoma of various solid organs including the pancreas, colon, breast, prostate, and liver and squamous cell carcinoma of the tongue, cervix, and kidney[107,108,112-116].
Diagnostic studies: Histopathological studies utilizing hematoxylin-eosin staining in conjunctival biopsies from patients with PNP include the characteristic feature of pemphigus - vacuolization of basal cells and suprabasilar intraepithelial acantholysis[109]. Specimens taken from cutaneous biopsy may be widely variable and reflect the clinical polymorphisms present in this disease. Histopathologic features that are unique to PNP and are not found in other pemphigus subsets include vacuolar degeneration of basal keratinocytes with lichenoid or lymphohistiocytic infiltration as well as apoptotic keratinocytes located throughout the epidermis[84,94]. These changes differentiate PNP from MMP where changes occur exclusively at the basement membrane zone with subepithelial conjunctival shrinkage; inflammatory infiltrate involving lymphocytes, macrophages, and plasma cells; and squamous metaplasia progressing to parakeratosis and keratinization of conjunctival epithelium[56,68].
DIF studies show IgG and complement (C3) distributed both intercellularly and at the basement membrane zone in a linear or granular distribution[98]. This differentiates PNP from other types of pemphigus where only intercellular deposits are found and from MMP where only subepithelial deposits are found. Histological examination of biopsy parallels the clinical phenotype categorization and may demonstrate suprabasal acantholysis; keratinocytic dyskeratosis, apoptosis, and necrosis; vacuolization of the basal layer; or a lichenoid appearance seen along the dermal-epidermal junction[117,118].
IIF in PNP demonstrates autoantibodies binding to a variety of epithelium including simple columnar, transitional, respiratory, gastrointestinal, and myocardium[88]. This differentiates PNP from PV where autoantibodies against desmoglein 3 are restricted to stratified squamous epithelial tissues and from MMP where autoantibodies are found only in the epidermal basement membrane zone.
Additionally, IIF using murine bladder and tongue or monkey esophagus distinguishes PNP because these tissues express desmoplakin 1 without desmoglein 3[81,119]. When IIF results are indeterminate, western blotting and immunoprecipitation may provide more sensitive techniques. Detection of autoantibodies to envoplakin and periplakin is most specific followed by desmoplakin I and II[79].
Utilization of IEM enables visualization of autoantibodies binding to desmosomes, hemidesmosomes, and spreading along the keratinocyte cell surface including the lamina lucida[120,121].
Immunoprecipitation demonstrating polyclonal auto-antibodies that target a complex of plakin proteins is best for diagnosis and is a major criterion for diagnosis of PNP[95,122]. The combination of rat bladder IIF and immunoblotting has equally sensitive results that are highly specific; this can be utilized as an alternative approach to immunoprecipitation for serologic diagnosis[122].
Treatment: Treatment for PNP is directed towards relieving symptoms of PNP as well as treating the underlying neoplasm. Resection of benign neoplasms may lead to improvement or remission of cutaneous lesions in 6-11 wk[86,105,118]. However, the disease often progresses despite surgical excision and chemotherapy[118]. Aside from treating the associated neoplasm, management of PNP includes corticosteroids and adjunctive corticosteroid-sparing agents to decrease the incidence of side effects. Concurrent use of corticosteroids with sulfone derivatives, immunosuppressive agents, antimetabolites, alkylating agents, and biologic agents may occur although PNP is much less responsive to therapy compared to other forms of pemphigus[123]. In general, skin lesions are usually more responsive whereas mucosal lesions are highly refractory to treatment and recover more slowly[57,86,124].
A total of 12 studies encompassing 23 PNP patients with ocular involvement were found in the literature[84,94,100-109]. Treatment regimens most commonly consisted of: systemic steroids (81% or 13/16), rituximab (31% or 5/16), cyclophosphamide (31% or 5/16), cyclosporine (25% or 4/16), azathioprine (25% or 4/16), IVIG (25% or 4/16), vincristine (25% or 4/16), chlorambucil (19% or 3/16), and fludarabine, doxorubicin, bleomycin, double filtration membrane plasmapheresis, methotrexate, and daclizumab (each 6% or 1/16). Additionally, adjunctive topical drops were utilized in 27% (3/11) of studies, of which the most commonly used were topical steroids (13% or 2/16) followed by topical tacrolimus (6% or 1/16). Surgical procedures consisting of resection of primary tumor occurred in 48% (11/23) of cases. Discontinuation of treatment due to side effects occurred in 13% of cases (3/23) and included plasmapheresis secondary to hypogammaglobulinemia and hypoalbuminemia; cyclosporine secondary to renal dysfunction; and cyclophosphamide, vincristine, prednisone, and rituximab regimen secondary to chemotherapy related side effects.
Prognosis: Of 12 studies encompassing 23 PNP patients with ocular involvement treated medically and/or surgically, outcomes included remission defined as regression of ocular lesions (30% or 7/23), remission of ocular lesions but persistence of other systemic manifestations (9% or 2/23), persistence of ocular lesions without progression (22% or 5/23), progression of ocular lesions (22%, 5/23), and recurrence of ocular lesions (4% or 1/23). Prognosis including death as final outcome occurred in 61% of cases (14/23) at an average 26 mo after onset of symptoms due to PNP[84,94,100-109]. Although reports of long-term survival have been described in the literature[104,125-128], others indicate that mortality rates may reach up to 90% with a mean survival of less than 1 year[128-130].
Death most commonly occurs secondary to malignancy, sepsis, or respiratory failure due to pulmonary involvement producing bronchiolitis obliterans[131,132]. Pulmonary involvement may occur and can continue to progress despite treatment with immunosuppressants, resection of malignancy, and improvement of other mucocutaneous symptoms[133]. Factors associated with worse prognosis in PNP include presence of erythema multiforme-like lesions, keratinocyte necrosis on biopsy specimens, and non-Hodgkin lymphoma patients with an increased risk of infection due to systemic chemotherapy and corticosteroids[128].
Pseudopemphigoid as caused by topical drugs and pemphigus disease may produce a chronic cicatricial conjunctivitis that can present clinically identical to MMP. In these cases, a vigilant history and examination combined with thorough diagnostic methods are needed to differentiate these diseases (Table 4). Distinguishing between the different causes of pseudopemphigoid that includes but is not limited to drug-induced cicatricial conjunctivitis, pemphigus vulgaris, and paraneoplastic pemphigus is paramount as there may be a poorer prognosis or a more severe clinical course unresponsive to medical management. Collaboration of the ophthalmologist with subspecialists such as the dermatologist, immunologist, and others involved in care of the patient is critical to prevent progression of the disease.
| MMP | Pseudopemphigoid | ||
| PNP | OPV | ||
| Location | Subepidermal | Intraepidermal | Intraepidermal |
| DIF | IgG/IgA/IgM/C3 | IgG/C3 | IgG/C3 |
| IIF on salt-split skin | Dermal, epidermal, or combined depending on antigen | Not applicable | Not applicable |
| IEM: ultrastructural location of antigen | Lamina lucida | Hemidesmosomes | Desmosomes |
| Lamina densa | Desmosomal plaques | ||
| Sublamina densa (anchoring fibrils) | Lamina lucida | ||
| Immunoblot: determination of antigen | Bullous pemphigoid antigen 1 (Bullous Pemphigoid 230) Bullous pemphigoid antigen 2 (Bullous Pemphigoid 180, type XVII collagen) Type VII collagen (290 kDa) Laminin332, epiligrin, or laminin 5 α3β3γ2 (165, 145, 140, 105 kDa) Laminin 6 (α3) Integrin beta 4 45 kDa epithelial protein 130 kDa epithelial protein 140 kDa epithelial protein 205 kDa epithelial protein 168 kDa epithelial protein Uncein LAD-1 (97/120 kDa) | Plakin protein family: Desmoplakin I (250 kDa) Bullous pemphigoid antigen 1 (230 kDa) Desmoplakin II and envoplakin (210 kDa) Periplakin (190 kDa) Plectin (500 kDa) Desmocollin 2 (105 kDa) Desmocollin 3 α2-macroglobulin-like-1 (A2LM1, 170 kDa) Desmoglein 1 (160 kDa) Desmoglein 3 (130 kDa) | Desmoglein 1 (160 kDa) Desmoglein 3 (130 kDa) |
| Increased malignancy | Yes - solid malignancies (laminin 332 subtype) | Yes - lymphoproliferative malignancies | -- |
P- Reviewer: Hashimoto T, Langford MP S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Patten JT, Cavanagh HD, Allansmith MR. Induced ocular pseudopemphigoid. Am J Ophthalmol. 1976;82:272-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 82] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Thorne JE, Anhalt GJ, Jabs DA. Mucous membrane pemphigoid and pseudopemphigoid. Ophthalmology. 2004;111:45-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Chan LS, Ahmed AR, Anhalt GJ, Bernauer W, Cooper KD, Elder MJ, Fine JD, Foster CS, Ghohestani R, Hashimoto T. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138:370-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 437] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 4. | Thorne JE, Jabs DA, Nikolskaia OV, Mimouni D, Anhalt GJ, Nousari HC. Lichen planus and cicatrizing conjunctivitis: characterization of five cases. Am J Ophthalmol. 2003;136:239-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Thorne JE, Jabs DA, Nikolskaia O, Anhalt G, Nousari HC. Discoid lupus erythematosus and cicatrizing conjunctivitis: clinicopathologic study of two cases. Ocul Immunol Inflamm. 2002;10:287-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Kourosh AS, Yancey KB. Pathogenesis of mucous membrane pemphigoid. Dermatol Clin. 2011;29:479-484, x. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Foster CS. Cicatricial pemphigoid. Trans Am Ophthalmol Soc. 1986;84:527-663. [PubMed] [Cited in This Article: ] |
| 8. | Bernard P, Vaillant L, Labeille B, Bedane C, Arbeille B, Denoeux JP, Lorette G, Bonnetblanc JM, Prost C. Incidence and distribution of subepidermal autoimmune bullous skin diseases in three French regions. Bullous Diseases French Study Group. Arch Dermatol. 1995;131:48-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 269] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Leonard JN, Wright P, Haffenden GP, Williams DM, Griffiths CE, Fry L. Skin diseases and the dry eye. Trans Ophthalmol Soc U K. 1985;104:467-476. [PubMed] [Cited in This Article: ] |
| 10. | Radford CF, Rauz S, Williams GP, Saw VP, Dart JK. Incidence, presenting features, and diagnosis of cicatrising conjunctivitis in the United Kingdom. Eye (Lond). 2012;26:1199-1208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Fiore PM. Drug-induced ocular cicatrization. Int Ophthalmol Clin. 1989;29:147-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Pouliquen Y, Patey A, Foster CS, Goichot L, Savoldelli M. Drug-induced cicatricial pemphigoid affecting the conjunctiva. Light and electron microscopic features. Ophthalmology. 1986;93:775-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Tseng SC, Maumenee AE, Stark WJ, Maumenee IH, Jensen AD, Green WR, Kenyon KR. Topical retinoid treatment for various dry-eye disorders. Ophthalmology. 1985;92:717-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 90] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Fiore PM, Jacobs IH, Goldberg DB. Drug-induced pemphigoid. A spectrum of diseases. Arch Ophthalmol. 1987;105:1660-1663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 80] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Leonard JN, Hobday CM, Haffenden GP, Griffiths CE, Powles AV, Wright P, Fry L. Immunofluorescent studies in ocular cicatricial pemphigoid. Br J Dermatol. 1988;118:209-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 88] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Butt Z, Kaufman D, McNab A, McKelvie P. Drug-induced ocular cicatricial pemphigoid: a series of clinico-pathological reports. Eye (Lond). 1998;12:285-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Derous D, de Keizer RJ, de Wolff-Rouendaal D, Soudijn W. Conjunctival keratinisation, an abnormal reaction to an ocular beta-blocker. Acta Ophthalmol (Copenh). 1989;67:333-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Wright P. Untoward effects associated with practolol administration: oculomucocutaneous syndrome. Br Med J. 1975;1:595-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 205] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Wright P. Squamous metaplasia or epidermalization of the conjunctiva as an adverse reaction to topical medication. Trans Ophthalmol Soc U K. 1979;99:244-246. [PubMed] [Cited in This Article: ] |
| 20. | Felix RH, Ive FA, Dahl MG. Cutaneous and ocular reactions to practolol. Br Med J. 1974;4:321-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 170] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Chan LS, Soong HK, Foster CS, Hammerberg C, Cooper KD. Ocular cicatricial pemphigoid occurring as a sequela of Stevens-Johnson syndrome. JAMA. 1991;266:1543-1546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Chan LS, Vanderlugt CJ, Hashimoto T, Nishikawa T, Zone JJ, Black MM, Wojnarowska F, Stevens SR, Chen M, Fairley JA. Epitope spreading: lessons from autoimmune skin diseases. J Invest Dermatol. 1998;110:103-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 240] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Di Zenzo G, Thoma-Uszynski S, Calabresi V, Fontao L, Hofmann SC, Lacour JP, Sera F, Bruckner-Tuderman L, Zambruno G, Borradori L. Demonstration of epitope-spreading phenomena in bullous pemphigoid: results of a prospective multicenter study. J Invest Dermatol. 2011;131:2271-2280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Fania L, Giannico MI, Fasciani R, Zampetti A, Ambrogio S, Balestrazzi E, Feliciani C. Ocular mucous membrane pemphigoid after Lyell syndrome: occasional finding or predisposing event? Ophthalmology. 2012;119:688-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Peterson JD, Chang AJ, Chan LS. Clinical evidence of an intermolecular epitope spreading in a patient with pemphigus foliaceus converting into bullous pemphigoid. Arch Dermatol. 2007;143:272-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Fairley JA, Woodley DT, Chen M, Giudice GJ, Lin MS. A patient with both bullous pemphigoid and epidermolysis bullosa acquisita: an example of intermolecular epitope spreading. J Am Acad Dermatol. 2004;51:118-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Gibran SK. Unilateral drug-induced ocular pseudopemphigoid. Eye (Lond). 2004;18:1270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Hirst LW, Werblin T, Novak M. Drug-induced cicatrizing conjunctivitis simulating ocular pemphigoid. Cornea. 1982;1:121-128. [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Lass JH, Thoft RA, Dohlman CH. Idoxuridine-induced conjunctival cicatrization. Arch Ophthalmol. 1983;101:747-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Kremer I, Rozenbaum D, Aviel E. Immunofluorescence findings in pseudopemphigoid induced by short-term idoxuridine administration. Am J Ophthalmol. 1991;111:375-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Broadway D. Drug-Induced Conjunctival Cicatrisation. Bernauer W, Elder MJ, Dart JKG, editors. Cicatrising Conjunctivitis 28. Vancouver, B.C. Canada: Karger Medical and Scientific Publishers 1997; 86-101. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Baican A, Baican C, Chiriac G, Chiriac MT, Macovei V, Zillikens D, Ciuce D, Sitaru C. Pemphigus vulgaris is the most common autoimmune bullous disease in Northwestern Romania. Int J Dermatol. 2010;49:768-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Huang YH, Kuo CF, Chen YH, Yang YW. Incidence, mortality, and causes of death of patients with pemphigus in Taiwan: a nationwide population-based study. J Invest Dermatol. 2012;132:92-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Brackley R, Pagani JM. Conjunctival erosions associated with pemphigus vulgaris. Optom Vis Sci. 2011;88:1010-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Chirinos-Saldaña P, Zuñiga-Gonzalez I, Hernandez-Camarena JC, Navas A, Ramirez-Luquin T, Robles-Contreras A, Jimenez-Martinez MC, Ramirez-Miranda A, Bautista-de Lucio VM, Graue-Hernandez EO. Cicatricial changes in ocular pemphigus. Eye (Lond). 2014;28:459-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Rauz S, Maddison PG, Dart JK. Evaluation of mucous membrane pemphigoid with ocular involvement in young patients. Ophthalmology. 2005;112:1268-1274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Chan LS, Hammerberg C, Cooper KD. Significantly increased occurrence of HLA-DQB1*0301 allele in patients with ocular cicatricial pemphigoid. J Invest Dermatol. 1997;108:129-132. [PubMed] [Cited in This Article: ] |
| 38. | Zaltas MM, Ahmed R, Foster CS. Association of HLA-DR4 with ocular cicatricial pemphigoid. Curr Eye Res. 1989;8:189-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Elchahal S, Kavosh ER, Chu DS. Ocular manifestations of blistering diseases. Immunol Allergy Clin North Am. 2008;28:119-36, vii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Sobolewska B, Deuter C, Zierhut M. Current medical treatment of ocular mucous membrane pemphigoid. Ocul Surf. 2013;11:259-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Ahmed AR, Foster S, Zaltas M, Notani G, Awdeh Z, Alper CA, Yunis EJ. Association of DQw7 (DQB1*0301) with ocular cicatricial pemphigoid. Proc Natl Acad Sci USA. 1991;88:11579-11582. [PubMed] [Cited in This Article: ] |
| 42. | Ahmed AR, Yunis EJ, Khatri K, Wagner R, Notani G, Awdeh Z, Alper CA. Major histocompatibility complex haplotype studies in Ashkenazi Jewish patients with pemphigus vulgaris. Proc Natl Acad Sci USA. 1990;87:7658-7662. [PubMed] [Cited in This Article: ] |
| 43. | Ahmed AR, Wagner R, Khatri K, Notani G, Awdeh Z, Alper CA, Yunis EJ. Major histocompatibility complex haplotypes and class II genes in non-Jewish patients with pemphigus vulgaris. Proc Natl Acad Sci USA. 1991;88:5056-5060. [PubMed] [Cited in This Article: ] |
| 44. | Loiseau P, Lecleach L, Prost C, Lepage V, Busson M, Bastuji-Garin S, Roujeau JC, Charron D. HLA class II polymorphism contributes to specify desmoglein derived peptides in pemphigus vulgaris and pemphigus foliaceus. J Autoimmun. 2000;15:67-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Kershenovich R, Hodak E, Mimouni D. Diagnosis and classification of pemphigus and bullous pemphigoid. Autoimmun Rev. 2014;13:477-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 46. | Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982;306:1189-1196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 520] [Cited by in F6Publishing: 547] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 47. | Hu CH, Michel B, Schiltz JR. Epidermal acantholysis induced in vitro by pemphigus autoantibody. An ultrastructural study. Am J Pathol. 1978;90:345-362. [PubMed] [Cited in This Article: ] |
| 48. | Bystryn JC, Rudolph JL. Pemphigus. Lancet. 2005;366:61-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 255] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 49. | Olszewska M, Komor M, Mazur M, Rogozinski T. Response of ocular pemphigus vulgaris to therapy. Case report and review of literature. J Dermatol Case Rep. 2008;2:1-3. [PubMed] [Cited in This Article: ] |
| 50. | Messent AJ, Blissett MJ, Smith GL, North AJ, Magee A, Foreman D, Garrod DR, Boulton M. Expression of a single pair of desmosomal glycoproteins renders the corneal epithelium unique amongst stratified epithelia. Invest Ophthalmol Vis Sci. 2000;41:8-15. [PubMed] [Cited in This Article: ] |
| 51. | Daoud YJ, Cervantes R, Foster CS, Ahmed AR. Ocular pemphigus. J Am Acad Dermatol. 2005;53:585-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Baum S, Sakka N, Artsi O, Trau H, Barzilai A. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 53. | Knudson RM, Kalaaji AN, Bruce AJ. The management of mucous membrane pemphigoid and pemphigus. Dermatol Ther. 2010;23:268-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Kneisel A, Hertl M. Autoimmune bullous skin diseases. Part 1: Clinical manifestations. J Dtsch Dermatol Ges. 2011;9:844-856; quiz 857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Palleschi GM, Giomi B, Fabbri P. Ocular involvement in pemphigus. Am J Ophthalmol. 2007;144:149-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Smith RJ, Manche EE, Mondino BJ. Ocular cicatricial pemphigoid and ocular manifestations of pemphigus vulgaris. Int Ophthalmol Clin. 1997;37:63-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Laforest C, Huilgol SC, Casson R, Selva D, Leibovitch I. Autoimmune bullous diseases: ocular manifestations and management. Drugs. 2005;65:1767-1779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Balica S, Bulai Livideanu C, Fournié P, Fortenfant F, Soler V, Barbarot S, Paul C. Is conjunctival mucous involvement a marker of severity in pemphigus vulgaris? J Eur Acad Dermatol Venereol. 2013;27:520-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Merchant S, Weinstein M. Pemphigus vulgaris: the eyes have it. Pediatrics. 2003;112:183-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Lifshitz T, Levy J, Cagnano E, Halevy S. Severe conjunctival and eyelid involvement in pemphigus vulgaris. Int Ophthalmol. 2004;25:73-74. [PubMed] [Cited in This Article: ] |
| 61. | Hodak E, Kremer I, David M, Hazaz B, Rothem A, Feuerman P, Sandbank M. Conjunctival involvement in pemphigus vulgaris: a clinical, histopathological and immunofluorescence study. Br J Dermatol. 1990;123:615-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Hall VC, Liesegang TJ, Kostick DA, Lookingbill DP. Ocular mucous membrane pemphigoid and ocular pemphigus vulgaris treated topically with tacrolimus ointment. Arch Dermatol. 2003;139:1083-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Kozeis N, Tyradellis S, Dragiotis E, Eleftheriadis H. Triamcino-lone acetonide for rare ocular manifestations of pemphigus vulgaris: a case report. Clin Ophthalmol. 2010;4:365-368. [PubMed] [Cited in This Article: ] |
| 64. | Baykal HE, Pleyer U, Sönnichsen K, Thiel HJ, Zierhut M. [Severe eye involvement in pemphigus vulgaris]. Ophthalmologe. 1995;92:854-857. [PubMed] [Cited in This Article: ] |
| 65. | Suami M, Kato M, Koide K, Usami Y, Hata N, Machida H, Hotta Y, Matsumoto K, Takigawa M. Keratolysis in a patient with pemphigus vulgaris. Br J Ophthalmol. 2001;85:1263-1264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Buhac J, Bhol K, Padilla T, Foster CS, Ahmed AR. Coexistence of pemphigus vulgaris and ocular cicatricial pemphigoid. J Am Acad Dermatol. 1996;34:884-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Sami N, Bhol KC, Beutner EH, Plunkett RW, Leiferman KM, Foster CS, Ahmed AR. Simultaneous presence of mucous membrane pemphigoid and pemphigus vulgaris: molecular characterization of both autoantibodies. Clin Immunol. 2001;100:219-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Pleyer U, Niesen U, Mondino B. Clinical and immunological characteristics of oculomucocutaneous disorders. Dev Ophthalmol. 1999;30:62-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 69. | Camisa C, Meisler DM. Immunobullous diseases with ocular involvement. Dermatol Clin. 1992;10:555-570. [PubMed] [Cited in This Article: ] |
| 70. | Letko E, Bhol K, Foster SC, Ahmed RA. Influence of intravenous immunoglobulin therapy on serum levels of anti-beta 4 antibodies in ocular cicatricial pemphigoid. A correlation with disease activity. A preliminary study. Curr Eye Res. 2000;21:646-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Hoang-Xuan T, Robin H, Demers PE, Heller M, Toutblanc M, Dubertret L, Prost C. Pure ocular cicatricial pemphigoid. A distinct immunopathologic subset of cicatricial pemphigoid. Ophthalmology. 1999;106:355-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | Harman KE, Gratian MJ, Seed PT, Bhogal BS, Challacombe SJ, Black MM. Diagnosis of pemphigus by ELISA: a critical evaluation of two ELISAs for the detection of antibodies to the major pemphigus antigens, desmoglein 1 and 3. Clin Exp Dermatol. 2000;25:236-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Zhu X, Pan J, Yu Z, Wang Y, Cai L, Zheng S. Epidemiology of pemphigus vulgaris in the Northeast China: a 10-year retrospective study. J Dermatol. 2014;41:70-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 74. | Huilgol SC, Black MM. Management of the immunobullous disorders. II. Pemphigus. Clin Exp Dermatol. 1995;20:283-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 75. | Korman NJ. New immunomodulating drugs in autoimmune blistering diseases. Dermatol Clin. 2001;19:637-48, viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Carson PJ, Hameed A, Ahmed AR. Influence of treatment on the clinical course of pemphigus vulgaris. J Am Acad Dermatol. 1996;34:645-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 97] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Saha M, Bhogal B, Black MM, Cooper D, Vaughan RW, Groves RW. Prognostic factors in pemphigus vulgaris and pemphigus foliaceus. Br J Dermatol. 2014;170:116-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 78. | Stanley JR. Therapy of pemphigus vulgaris. Arch Dermatol. 1999;135:76-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Zimmermann J, Bahmer F, Rose C, Zillikens D, Schmidt E. Clinical and immunopathological spectrum of paraneoplastic pemphigus. J Dtsch Dermatol Ges. 2010;8:598-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 80. | Mimouni D, Anhalt GJ, Lazarova Z, Aho S, Kazerounian S, Kouba DJ, Mascaro JM, Nousari HC. Paraneoplastic pemphigus in children and adolescents. Br J Dermatol. 2002;147:725-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 81. | Kimyai-Asadi A, Jih MH. Paraneoplastic pemphigus. Int J Dermatol. 2001;40:367-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Martel P, Loiseau P, Joly P, Busson M, Lepage V, Mouquet H, Courville P, Flageul B, Charron D, Musette P. Paraneoplastic pemphigus is associated with the DRB1*03 allele. J Autoimmun. 2003;20:91-95. [PubMed] [Cited in This Article: ] |
| 83. | Liu Q, Bu DF, Li D, Zhu XJ. Genotyping of HLA-I and HLA-II alleles in Chinese patients with paraneoplastic pemphigus. Br J Dermatol. 2008;158:587-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | Choi Y, Nam KH, Lee JB, Lee JY, Ihm CW, Lee SE, Oh SH, Hashimoto T, Kim SC. Retrospective analysis of 12 Korean patients with paraneoplastic pemphigus. J Dermatol. 2012;39:973-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 85. | Maverakis E, Goodarzi H, Wehrli LN, Ono Y, Garcia MS. The etiology of paraneoplastic autoimmunity. Clin Rev Allergy Immunol. 2012;42:135-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | Zhu X, Zhang B. Paraneoplastic pemphigus. J Dermatol. 2007;34:503-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 87. | Numata S, Teye K, Tsuruta D, Sogame R, Ishii N, Koga H, Natsuaki Y, Tsuchisaka A, Hamada T, Karashima T. Anti-α-2-macroglobulin-like-1 autoantibodies are detected frequently and may be pathogenic in paraneoplastic pemphigus. J Invest Dermatol. 2013;133:1785-1793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 88. | Itin PH, Büchner SA, Pittelkow MR. Mucocutaneous paraneoplastic disorders. Dev Ophthalmol. 1997;28:73-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 89. | Hashimoto T, Amagai M, Watanabe K, Chorzelski TP, Bhogal BS, Black MM, Stevens HP, Boorsma DM, Korman NJ, Gamou S. Characterization of paraneoplastic pemphigus autoantigens by immunoblot analysis. J Invest Dermatol. 1995;104:829-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 148] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 90. | Kim SC, Kwon YD, Lee IJ, Chang SN, Lee TG. cDNA cloning of the 210-kDa paraneoplastic pemphigus antigen reveals that envoplakin is a component of the antigen complex. J Invest Dermatol. 1997;109:365-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 91. | Borradori L, Trüeb RM, Jaunin F, Limat A, Favre B, Saurat JH. Autoantibodies from a patient with paraneoplastic pemphigus bind periplakin, a novel member of the plakin family. J Invest Dermatol. 1998;111:338-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Amagai M, Nishikawa T, Nousari HC, Anhalt GJ, Hashimoto T. Antibodies against desmoglein 3 (pemphigus vulgaris antigen) are present in sera from patients with paraneoplastic pemphigus and cause acantholysis in vivo in neonatal mice. J Clin Invest. 1998;102:775-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 253] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 93. | Gallo E, García-Martín P, Fraga J, Teye K, Koga H, Hashimoto T, García-Diez A. Paraneoplastic pemphigus with eosinophilic spongiosis and autoantibodies against desmocollins 2 and 3. Clin Exp Dermatol. 2014;39:323-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 94. | Anhalt GJ, Kim SC, Stanley JR, Korman NJ, Jabs DA, Kory M, Izumi H, Ratrie H, Mutasim D, Ariss-Abdo L. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med. 1990;323:1729-1735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 777] [Cited by in F6Publishing: 666] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 95. | Camisa C, Helm TN. Paraneoplastic pemphigus is a distinct neoplasia-induced autoimmune disease. Arch Dermatol. 1993;129:883-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 90] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Bialy-Golan A, Brenner S, Anhalt GJ. Paraneoplastic pemphigus: oral involvement as the sole manifestation. Acta Derm Venereol. 1996;76:253-254. [PubMed] [Cited in This Article: ] |
| 97. | Kaplan I, Hodak E, Ackerman L, Mimouni D, Anhalt GJ, Calderon S. Neoplasms associated with paraneoplastic pemphigus: a review with emphasis on non-hematologic malignancy and oral mucosal manifestations. Oral Oncol. 2004;40:553-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 98. | Allen CM, Camisa C. Paraneoplastic pemphigus: a review of the literature. Oral Dis. 2000;6:208-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 99. | Nguyen VT, Ndoye A, Bassler KD, Shultz LD, Shields MC, Ruben BS, Webber RJ, Pittelkow MR, Lynch PJ, Grando SA. Classification, clinical manifestations, and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome: a reappraisal of paraneoplastic pemphigus. Arch Dermatol. 2001;137:193-206. [PubMed] [Cited in This Article: ] |
| 100. | Tam PM, Cheng LL, Young AL, Lam PT. Paraneoplastic pemphigus: an uncommon cause of chronic cicatrising conjunctivitis. BMJ Case Rep. 2009;2009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 101. | Lam S, Stone MS, Goeken JA, Massicotte SJ, Smith AC, Folberg R, Krachmer JH. Paraneoplastic pemphigus, cicatricial conjunctivitis, and acanthosis nigricans with pachydermatoglyphy in a patient with bronchogenic squamous cell carcinoma. Ophthalmology. 1992;99:108-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 102. | Ahuero AE, Jakobiec FA, Bhat P, Ciralsky JB, Papaliodis GN. Paraneoplastic conjunctival cicatrization: two different pathogenic types. Ophthalmology. 2010;117:659-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Anan T, Shimizu F, Hatano Y, Okamoto O, Katagiri K, Fujiwara S. Paraneoplastic pemphigus associated with corneal perforation and cutaneous alternariosis: a case report and review of cases treated with rituximab. J Dermatol. 2011;38:1084-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 104. | Camisa C, Helm TN, Liu YC, Valenzuela R, Allen C, Bona S, Larrimer N, Korman NJ. Paraneoplastic pemphigus: a report of three cases including one long-term survivor. J Am Acad Dermatol. 1992;27:547-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 98] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 105. | Fang Y, Zhao L, Yan F, Cui X, Xia Y, Duren A. A critical role of surgery in the treatment for paraneoplastic pemphigus caused by localized Castleman’s disease. Med Oncol. 2010;27:907-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 106. | Lane JE, Woody C, Davis LS, Guill MF, Jerath RS. Paraneoplastic autoimmune multiorgan syndrome (paraneoplastic pemphigus) in a child: case report and review of the literature. Pediatrics. 2004;114:e513-e516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | Tilakaratne W, Dissanayake M. Paraneoplastic pemphigus: a case report and review of literature. Oral Dis. 2005;11:326-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 108. | Wang SH, Chu CY, Chen HH, Chang YL, Chen KY, Chiu HC. Paraneoplastic Pemphigus and Bronchiolitis Obliterans in a Patient with Splenic B-cell Lymphoma. J Formos Med Assoc. 2007;106:768-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 109. | Lee SE, Kim HR, Hashimoto T, Kim SC. Paraneoplastic pemphigus developed shortly after resection of follicular dendritic cell sarcoma. Acta Derm Venereol. 2008;88:410-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 110. | Ostezan LB, Fabré VC, Caughman SW, Swerlick RA, Korman NJ, Callen JP. Paraneoplastic pemphigus in the absence of a known neoplasm. J Am Acad Dermatol. 1995;33:312-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 111. | Fried R, Lynfield Y, Vitale P, Anhalt G. Paraneoplastic pemphigus appearing as bullous pemphigoid-like eruption after palliative radiation therapy. J Am Acad Dermatol. 1993;29:815-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 112. | Matz H, Milner Y, Frusic-Zlotkin M, Brenner S. Paraneoplastic pemphigus associated with pancreatic carcinoma. Acta Derm Venereol. 1997;77:289-291. [PubMed] [Cited in This Article: ] |
| 113. | Chamberland M. Paraneoplastic pemphigus and adenoca-rcinoma of the colon. Union Med Can. 1993;122:201-203. [PubMed] [Cited in This Article: ] |
| 114. | Hinterhuber G, Drach J, Riedl E, Böhler K, Ferenci P, Wolff K, Foedinger D. Paraneoplastic pemphigus in association with hepatocellular carcinoma. J Am Acad Dermatol. 2003;49:538-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 115. | Niimi Y, Kawana S, Hashimoto T, Kusunoki T. Paraneoplastic pemphigus associated with uterine carcinoma. J Am Acad Dermatol. 2003;48:S69-S72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 116. | Wong KC, Ho KK. Pemphigus with pemphigoid-like presentation, associated with squamous cell carcinoma of the tongue. Australas J Dermatol. 2000;41:178-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 117. | Ruocco E, Wolf R, Ruocco V, Brunetti G, Romano F, Lo Schiavo A. Pemphigus: associations and management guidelines: facts and controversies. Clin Dermatol. 2013;31:382-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 118. | Yong AA, Tey HL. Paraneoplastic pemphigus. Australas J Dermatol. 2013;54:241-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 119. | Pizarro A, García-Tobaruela A, Pinilla J. Bronchiolitis obliterans, Castleman’s disease, and a bullous disease: pemphigus vulgaris or paraneoplastic pemphigus? Hum Pathol. 1998;29:657-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 120. | Joly P, Thomine E, Gilbert D, Verdier S, Delpech A, Prost C, Lebbe C, Lauret P, Tron F. Overlapping distribution of autoantibody specificities in paraneoplastic pemphigus and pemphigus vulgaris. J Invest Dermatol. 1994;103:65-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 121. | Mahoney MG, Aho S, Uitto J, Stanley JR. The members of the plakin family of proteins recognized by paraneoplastic pemphigus antibodies include periplakin. J Invest Dermatol. 1998;111:308-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 120] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 122. | Poot AM, Diercks GF, Kramer D, Schepens I, Klunder G, Hashimoto T, Borradori L, Jonkman MF, Pas HH. Laboratory diagnosis of paraneoplastic pemphigus. Br J Dermatol. 2013;169:1016-1024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 123. | Frew JW, Murrell DF. Current management strategies in paraneoplastic pemphigus (paraneoplastic autoimmune multiorgan syndrome). Dermatol Clin. 2011;29:607-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 124. | Mahajan VK, Sharma V, Chauhan PS, Mehta KS, Sharma AL, Abhinav C, Khatri G, Prabha N, Sharma S, Negi M. Paraneoplastic pemphigus: a paraneoplastic autoimmune multiorgan syndrome or autoimmune multiorganopathy? Case Rep Dermatol Med. 2012;2012:207126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 125. | Barbetakis N, Samanidis G, Paliouras D, Boukovinas I, Asteriou C, Stergiou E, Laschos K, Tsilikas C. Paraneoplastic pemphigus regression after thymoma resection. World J Surg Oncol. 2008;6:83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 126. | Perniciaro C, Kuechle MK, Colón-Otero G, Raymond MG, Spear KL, Pittelkow MR. Paraneoplastic pemphigus: a case of prolonged survival. Mayo Clin Proc. 1994;69:851-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 127. | Mascaró JM, Ferrando J, Solé MT, Alsina M, Nousari HC, Anhalt GJ, Font J, Mascaró JM. Paraneoplastic pemphigus: a case of long-term survival associated with systemic lupus erythematosus and polymyositis. Dermatology. 1999;199:63-66. [PubMed] [Cited in This Article: ] |
| 128. | Leger S, Picard D, Ingen-Housz-Oro S, Arnault JP, Aubin F, Carsuzaa F, Chaumentin G, Chevrant-Breton J, Chosidow O, Crickx B. Prognostic factors of paraneoplastic pemphigus. Arch Dermatol. 2012;148:1165-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 129. | Nousari HC, Deterding R, Wojtczack H, Aho S, Uitto J, Hashimoto T, Anhalt GJ. The mechanism of respiratory failure in paraneoplastic pemphigus. N Engl J Med. 1999;340:1406-1410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 155] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 130. | Anhalt GJ. Paraneoplastic pemphigus. Adv Dermatol. 1997;12:77-96; discussion 97. [PubMed] [Cited in This Article: ] |
| 131. | Kim SC, Chang SN, Lee IJ, Park SD, Jeong ET, Lee CW, Ahn CM, Anhalt GJ. Localized mucosal involvement and severe pulmonary involvement in a young patient with paraneoplastic pemphigus associated with Castleman’s tumour. Br J Dermatol. 1998;138:667-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 132. | Chorzelski T, Hashimoto T, Maciejewska B, Amagai M, Anhalt GJ, Jablonska S. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J Am Acad Dermatol. 1999;41:393-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 133. | Nikolskaia OV, Nousari CH, Anhalt GJ. Paraneoplastic pemphigus in association with Castleman’s disease. Br J Dermatol. 2003;149:1143-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 134. | Mondino BJ, Brown SI. Ocular cicatricial pemphigoid. Ophthalmology. 1981;88:95-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 148] [Article Influence: 3.4] [Reference Citation Analysis (0)] |