Published online May 20, 2022. doi: 10.5317/wjog.v11.i2.8
Peer-review started: February 13, 2021
First decision: March 31, 2021
Revised: May 12, 2021
Accepted: March 27, 2022
Article in press: March 27, 2022
Published online: May 20, 2022
Processing time: 458 Days and 17.4 Hours
Microglandular hyperplasia (MGH) is a proliferation of endocervical glands, related to estrogen stimulation, mainly occurring in the reproductive age group. The differential diagnosis includes endometrial adenocarcinoma with MGH-like pattern (MGA), a distinction that may be particularly problematic in curettage specimen.
A 57-year-old, postmenopausal woman was admitted in our hospital for surgical treatment. She had been diagnosed with a uterine leiomyoma, after complaints of irregular vaginal bleeding. She underwent dilatation and curettage (D&C) and subsequent total abdominal hysterectomy with bilateral salpingo-oophorectomy. D&C were compatible with MGA. Histologically, a proliferation of small glands, without intervening stroma, with mucin production, accumulation of neutrophils in the gland lumen and stroma, mild nuclear atypia and rare mitoses, were seen. In the hysterectomy specimen, the endometrium was thickened, but without apparent tumor formation. On microscopic examination, a residual similar adenocarcinoma was seen in the isthmus and more conventional-of endometrioid and mucinous type, in the rest of the endometrium.
MGH-like proliferation with mild cytologic atypia, detected in the endometrial curettage specimen of a postmenopausal woman, should alert pathologists for MGA of the endometrium. VIM, p16, PAX-2, CD10 and CD34 may help in the differential diagnosis.
Core Tip: When microglandular hyperplasia (MGH)-like proliferation is detected in the endometrial curettage of a postmenopausal woman, the pathologists must be vigilant for endometrial MGH-like endometrial adenocarcinoma type of carcinoma, as it may be misdiagnosed. The examination of scant biopsy specimens remains a challenge. Its recognition can avoid underdiagnosis and mistreatment of the patient.
- Citation: Trihia HJ, Souka E, Galanopoulos G, Pavlakis K, Karelis L, Fotiou A, Provatas I. Microglandular hyperplasia-like mucinous adenocarcinoma of the endometrium: A rare case report. World J Obstet Gynecol 2022; 11(2): 8-16
- URL: https://www.wjgnet.com/2218-6220/full/v11/i2/8.htm
- DOI: https://dx.doi.org/10.5317/wjog.v11.i2.8
Microglandular hyperplasia (MGH) is a characteristic proliferation of endocervical glands, often associated with estrogen and progesterone stimulation (oral contraceptives and pregnancy). It occurs in the reproductive age group and occasionally in postmenopausal women. The differential diagnosis is usually with endometrial adenocarcinomas resembling MGH, a distinction that can be very difficult in a biopsy or curettage (D&C). We report a case of a 57-year-old woman, with vaginal bleeding and a known uterine leiomyoma, diagnosed with MGH-like endometrial adenocarcinoma (MGA) in dilatation and curettage specimen. We address the difficulties of differential diagnosis and the hysterectomy findings.
The patient suffered from uterine bleeding.
A 57-year-old, postmenopausal woman, was referred to our hospital for surgical treatment of a radiographically detected uterine leiomyoma, after irregular vaginal bleeding of seven days’ duration.
None.
The patient had undergone four in vitro fertilization attempts in the past, one of which resulted in a failed pregnancy. Last attempt took place eleven years before her clinical presentation with vaginal bleeding.
No clinical findings.
No increase of tumour markers.
No mass forming findings.
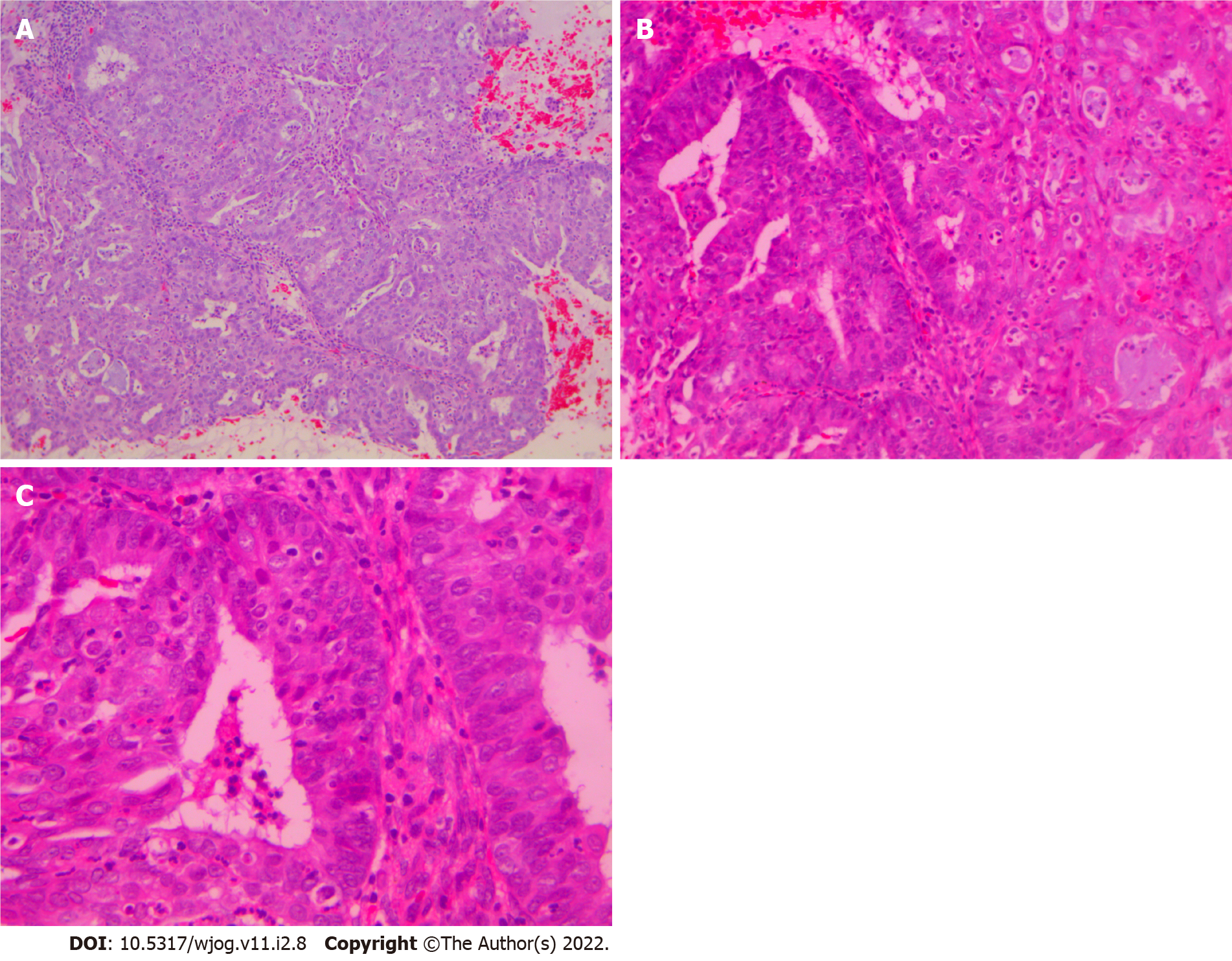
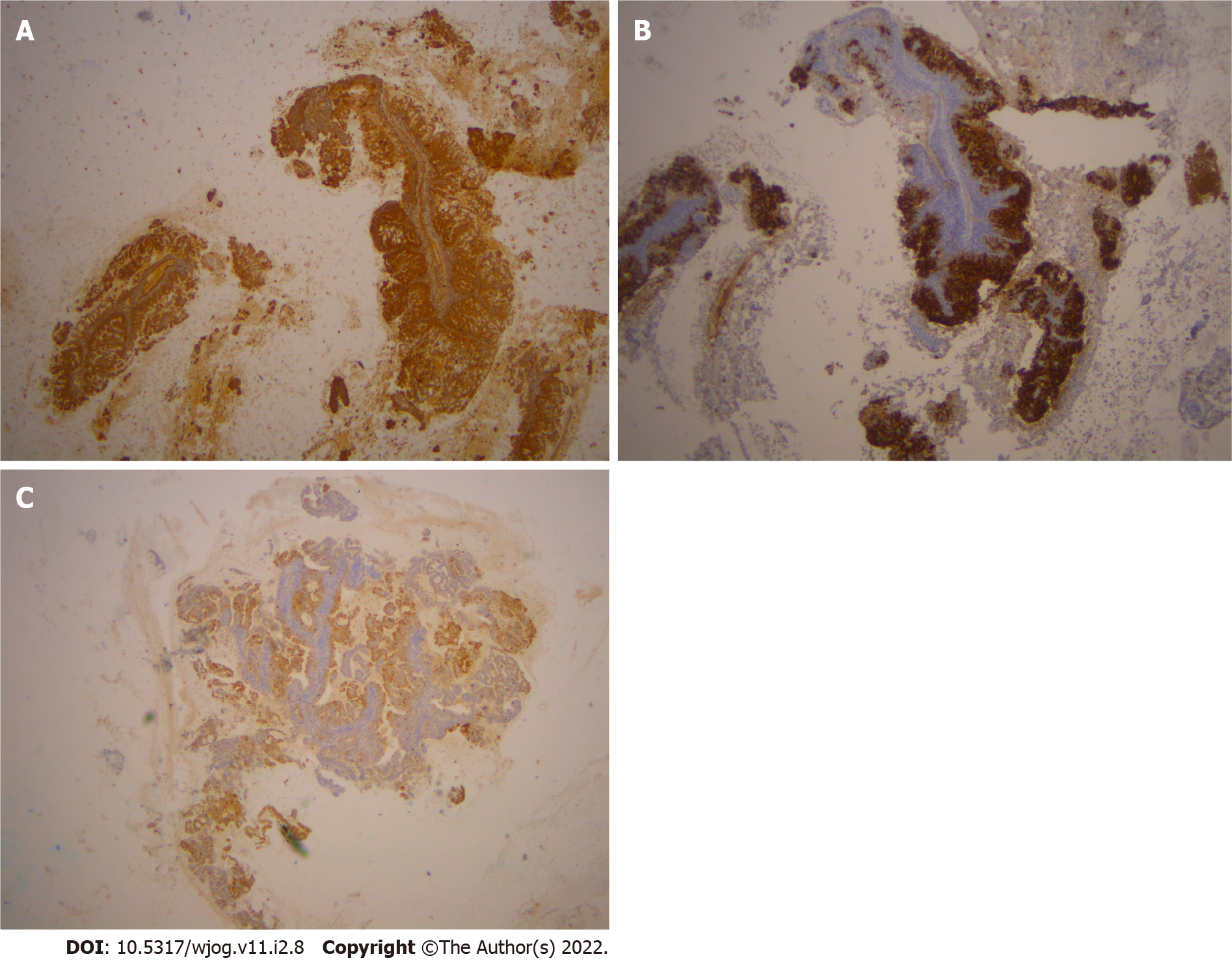
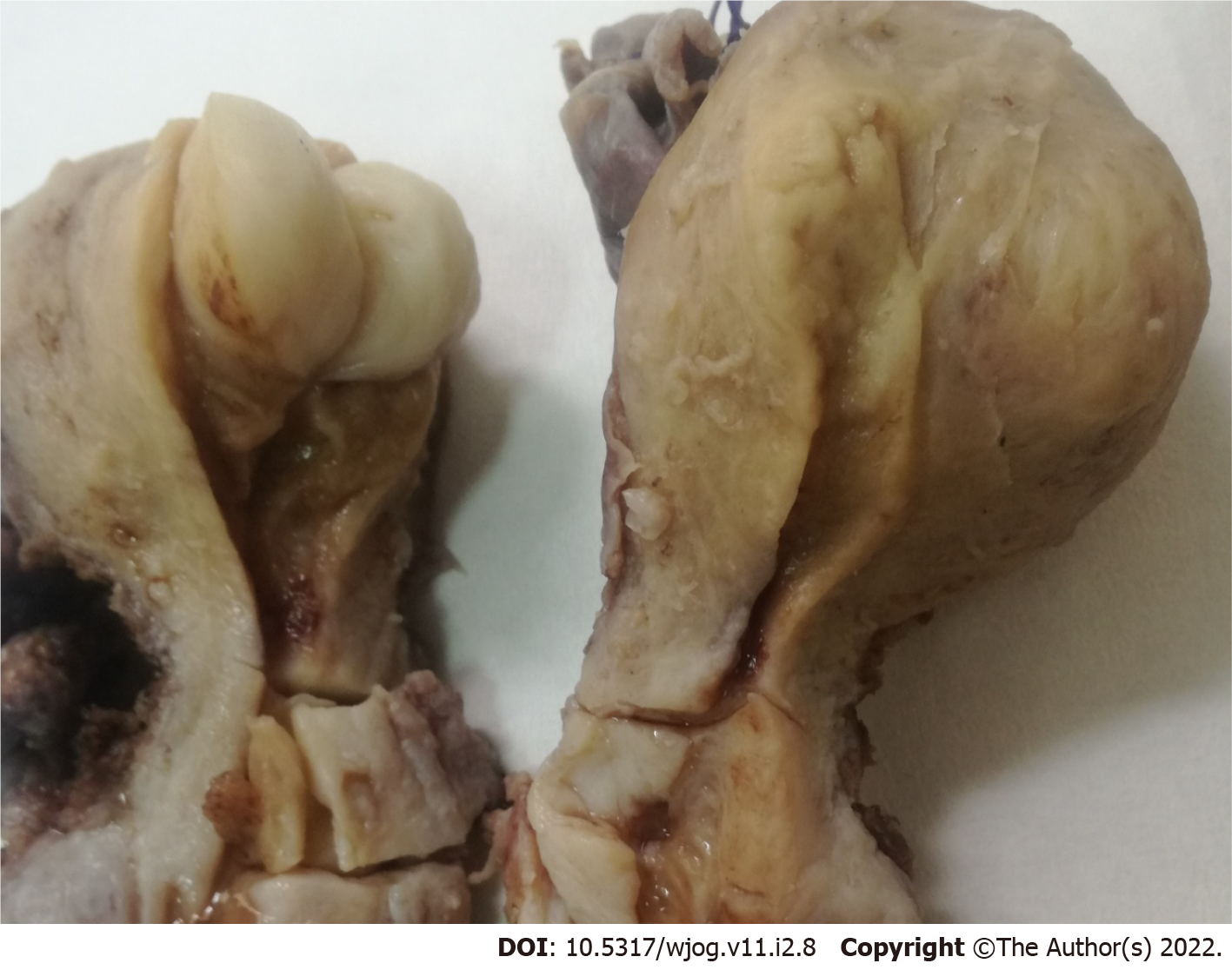
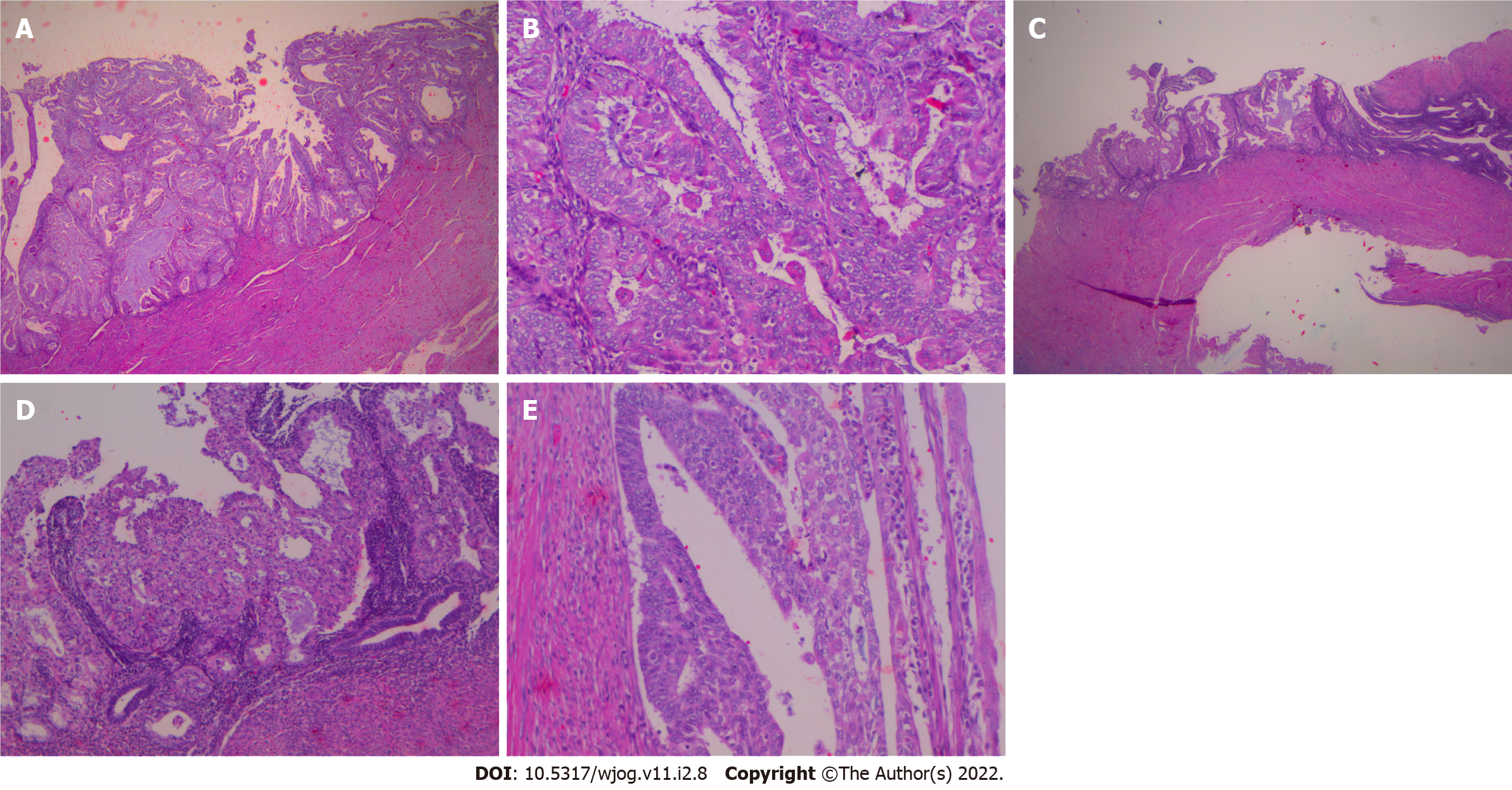
Histological examination of D&C revealed a complex-microglandular proliferation of small back-to-back glands, without intervening stroma, with mucin production, accumulation of neutrophils in the gland lumen and stroma, mild nuclear atypia and rare mitotic figures (Figure 1). Alcian blue and PAS-D stains showed abundant luminal and occasional intracytoplasmic mucin. Invasion could not be assessed in the insufficient and fragmented curettage specimen. Our diagnosis was compatible with adenocarcinoma of the endometrium with extensive features mimicking microglandular hyperplasia of the cervix (microglandular hyperplasia-like mucinous adenocarcinoma of the endometrium- EAMGHP). The diagnosis was based mainly on the extent of the lesions, the finding of a very limited element of glands with complex back-to-back, cribriform and tubule-papillary architectural patterns, with focal pseudo-stratification and the presence of rare mitoses (Figure 1). Immunohistochemically, there was positivity for VIM (Figure 2), mCEA (Figure 1), ER, PR and p16 (Figure 2). The slides were reviewed by an eminent Gynecologic Pathologist (K.P), who agreed with our diagnosis of a low grade endometrial endometrioid carcinoma with features mimicking microglandular hyperplasia of the cervix. The patient underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH & BOOP). In the hysterectomy specimen, a pink polypoid lesion of 0.5cm was seen at the isthmus area. There were several Nabothian cysts seen in the cervix. Macroscopically, there was no obvious mass formation. The endometrial area was determined in a flat appearance macroscopically (Figure 3). On microscopic examination, a residual adenocarcinoma with partly similar features with those observed in the D&C specimen was seen in the isthmus area and more conventional carcinoma of mucinous (Figure 4) and endometrioid types with areas of ciliated cells and small non-villous papillae (Figure 4), in the rest of the endometrium. MGH-like areas were mostly replacing the surface areas of the more conventional carcinoma of the endometrium (Figure 4). The carcinoma was superficially infiltrative and was extending in adenomyosis areas. The final diagnosis was of a FIGO I, grade I-II, MGH-like endometrial adenocarcinoma.
In the cervix, apart from Naboth cysts, there was also a cystic structure, underneath the polyp, compatible with mesonephric remnant of ductal type, as well as a focus of reserve cell metaplasia in an endocervical crypt.
The postoperative recovery course was uneventful.
The patient was diagnosed with microglandular-like adenocarcinoma of the endometrium.
Then, this patient underwent TAH & BOOP (total abdominal hysterectomy and bilateral salpingo-oophorectomy).
The postoperative recovery course was uneventful. The patient is well 15 mo after surgical treatment.
MGH is a lesion, mostly seen in women of reproductive age, although it can be found in up to 6% of postmenopausal women[1]. It is a benign proliferation of endocervical glands and is often an incidental finding. It usually occurs in women who are either pregnant or are taking progesterone[2]. It was first described in a study of changes in the cervix of pregnant women[3]. The term MGH, was used for the first time, by Kyriakos et al[4] in 1968, for a group of patients on oral contraceptives. One year earlier, the resemblance of this lesion with endocervical adenocarcinoma, was thus far aknowledged[5]. Although, this is commonly associated with pregnancy and oral contraceptive use, it can occur in patients without this clinical history. Most cases are found incidentally, but gross abnormalities such as an erosion, polyp formation, or friable raised areas in the cervix can be seen. MGH can be focal or multifocal and can involve the surface epithelium and/or the endocervical glands. It is composed of closely packed glands of variable size and shape, with acute and chronic inflammatory cells and little intervening stroma. The epithelium lining the glands is columnar or cuboidal and mucin producing and contains supra-or subnuclear vacuoles. The nuclei are usually uniform, but focal atypia can be encountered. Reserve cell hyperplasia and squamous metaplasia may be present. Mitotic figures are rare. Immunohistochemically, there is positivity of MGH for p63 in the reserve/immature squamous cells. It is therefore usually negative for p16; however, in rare cases can be strongly, but usually patchy-positive. Cases of MGH with p16 expression do not co-localize MIB-1 or cyclin E expression and are not associated with human papilloma virus infection. The differential diagnosis of the lesion includes endometrial adenocarcinoma with a microglandular pattern (EAMGP). Otherwise, typical endometrioid (or mixed endometrioid-mucinous or pure mucinous) carcinomas may have prominent microglandular pattern with eosinophilic secretions and acute inflammatory cells in the lumens and stroma. The differential diagnosis is with MGH, although this is invariably a purely endocervical lesion. The distinction rests on the merging of the microglandular pattern with that of a typical endometrioid carcinoma, with nuclear atypia and mitotic activity exceeding those in MGH. Positivity for p16 and vimentin and > 10% MIB-1 index, also favors microglandular adenocarcinoma. There is a great diagnostic challenge of differentiating between endocervical MGH and well differentiated endometrial adenocarcinoma with microglandular pattern, in biopsy and D&C. Therefore the above consist one of the most common reasons for consultation in gynecologic pathology. Both entities can be quite similar. Although, the presence of nuclear atypia and mitotic figures can be of help in the differential diagnosis-favoring endometrial adenocarcinoma- yet the latter may be desceptively underestimated.
The WHO Classification of endometrial carcinomas (2014) is mostly based on morphologic features[6] and according to that they are classified in two broad categories, endometrioid non-serous carcinomas, or Bokham type 1 tumors and type 2, non-endometrioid, serous carcinomas. Type 1 includes endometrioid and mucinous carcinoma. Type 2 includes serous, clear cell, undifferentiated carcinoma and carcinosarcoma. Mucinous carcinomas, are classified as non-endometrioid carcinomas, with more than 50% of tumor cells containing intracytoplasmic mucin. A subset of mucinous carcinomas, designated MGA, due to its similarity to MGH of the endocervical glands (MGH), is acknowledged by the WHO 2014. According to the revised 5th edition of WHO Classification of Female Genital Tumours, mucinous carcinoma is not included as a separate type 1 carcinoma type, but has been incorporated in the endometrioid type, as endometrioid carcinoma with mucinous differentiation[7]. Unusual histological patterns that may be seen in endometrioid carcinoma which are not associated with different prognosis, include among others the microglandular pattern. Tumors with notable microglandular pattern are characterized by small-to medium-sized, closely packed glands with eosinophilic secretions and numerous acute inflammatory cells. There is mild cytologic atypia and low mitotic rate. Mucinous pattern may be present in varying degrees and may predominate.
Mucinous differentiation of the endometrium can occur in a spectrum of lesions ranging from benign, like metaplasia to malignant, like adenocarcinomas with mucinous differentiation. It is very difficult to make a diagnosis of carcinoma in endometrial biopsies and curretings that show proliferative mucinous lesions, because the desceptively bland appearance of invasive mucinous adenocarcinoma at this site. Only limited information is available regarding criteria for distinguishing mucinous carcinoma from atypical mucinous proliferations and mucinous metaplasia of the endometrium. The threshold for diagnosing mucinous carcinoma in endometrial biopsies/curretings may be possibly lower than that of endometrioid carcinoma. There are three categories of mucinous proliferations of the endometrium (A, B or C), based on increasing degree of architectural complexity and cytologic atypia[8]. Type A, is characterized only by mucin-containing epithelial cells, single or in small tufts, within architecturally benign glands or in the endometrial surface. Type B, lesions are by definition more complex, and are characterized by mucin-containing epithelial cells forming pseudoglands with rigid punched out spaces with no supporting stroma. Type C alterations, are characterized by conspicuous cytologic atypia and architectural complexity, such as filliform growth. A high percentage of type B lesions are known to be associated with well differentiated endometrial adenocarcinoma, with no or minimal invasion. Mucinous lesions with complex (cribriform or prominent villous) architecture and absence of cytologic atypia are also characterized by low concurrent risk for deeply invasive cancer. The fact that type B microglandular lesions, are presented predominantly on the endometrial surface, without co-existing atypical hyperplasia, implies that a subset of well-differentiated adenocarcinomas arise via neoplastic alterations in the surface epithelium.
MGA is by definition a rare type of endometrial mucinous carcinoma with microglandular architecture and mucinous and squamous features, which can mimic lesions of the endometrium and the cervix, both benign and malignant. MGA, was described for the first time by Young and Scully, in 1992[9]. The D&C in six cases was reported as “suspicious for malignancy that might be compatible with MGA of the endometrium or MGH of the cervix”. The patients were 37-84 years old and all women were postmenopausal, except of one case of cervical adenocarcinoma, which was premenopausal[8-13]. The clinical complaints were of vaginal spotting, discharge or bleeding. Six women were on exogenous hormones. From the clinical point of view, MGH is mostly presented in young women under hormone therapy. On the contrary, MGA mostly occurs in women of postmenopausal age. It is therefore known that the age of the patient and whether pre- or postmenopausal can be major clues for the correct diagnosis. Histologically, MGH and MGA share similar histological features. In MGH there is mild nuclear atypia and scarce mitotic activity. On the contrary, when nuclear atypia and mitotic figures are more pronounced, are in favor of MGA. In addition, subnuclear vacuolization can be present in MGH, which is not a feature in MGA. Staining for vimentin can help in the differential diagnosis, as it is positive in MGA and negative in MGH[14]. Both MGA and MGH have variable expression of estrogen and progesterone receptors[14]. Carcinoembryonic antigen (CEA) is often expressed in endometrial and endocervical adenocarcinomas, but is negative in endometrial mucinous adenocarcinoma and cervical MGH[11,12]. Qiu et al[14], describes absence of CEA staining in all cases of MGA and MGH. Immunostaining for CEA, ER, PR or p53 does not aid the differential diagnosis. Chekmareva et al[15] suggested that p16, CD10 and CD34 immunostaining could help in the differential diagnosis between MUC-AD and MGA of the endometrium on the one hand and benign endocervical lesions on the other. As reported, MUC-AD and MGA cases were positive for p16, whereas none of the cases with benign endocervical epithelial lesions and MGH showed p16 positivity, except from the reserve cells, typically located on the outer aspect of the endocervical glands. Also Baroetta et al[16] showed that ‘CD34-dominant phenotype’ of stromal cells was in favor of the cervical origin of the lesion and ‘CD10-dominant phenotype’ of stromal cells was compatible with the endometrial origin of the lesion. Overall, the immunohistochemical profile of endometrioid carcinomas, including mucinous carcinoma, overlaps with that of MGH (ER positive, p16 negative or patchy, variable Ki-67).
Loss of PAX2 expression in the epithelium, would favor the diagnosis of MGA[17]. Although, there are no antibodies completely sensitive and specific, a p16-positive/PAX2-negative phenotype, favors MGA (Table 1). Additionally, pathologists should be aware that MGA, are commonly p16-positive, as primary endocervical neoplasms.
| MGH | MGA | |
| Subnuclear Vacuoles | + | - |
| Foamy stromal cells | - | + |
| VIM | - | + |
| p16 | - (positivity in reserve cells) | + |
| PAX2 | + | - |
| CD10-dominant phenotype of stromal cells | - | + (endometrial origin) |
| CD34-dominant phenotype of stromal cells | + (cervical origin) | - |
| Menopausal age | Reproductive (mostly) | Peri-or postmenopausal |
Apart from MGH, MGH-like carcinoma should be differentiated from benign mucinous endometrial proliferations. Benign mucinous proliferation is supported by simple glandular architecture with mucin containing cells, absence of nuclear atypia and epithelial stratification[8].
In cases with no clues, a descriptive diagnosis is advised, such as ‘atypical mucinous glandular proliferation’ with a discussion of the differential diagnosis of under-sampled adenocarcinoma vs endocervical MGH. These patients should undergo further clinical and radiologic evaluation, including thorough endometrial curettage. The likelihood of finding adenocarcinoma on subsequent hysterectomy is partly related to the degree of architectural complexity. Nevertheless, this can be challenging in actual practice[18]. The presence of MGH-like glands in an endometrial sampling in peri- or postmenopausal women, regardless of the degree of complexity, should be mentioned and discussed.
Mutational analysis for KRAS has been suggested to be of aid in cases of small and fragmented biopsies[19], as complex mucinous proliferations largely harbor KRAS mutations.
Features that mimic endocervical MGH may be seen on the surface or at the periphery of some endometrioid adenocarcinomas. Often, these are grade I tumors[10] with mucinous differentiation, with predilection for post-menopausal women.
In all similar with ours presented cases in the literature, residual carcinomas were seen in the hysterectomy specimen, consisting of conventional carcinomas of mucinous or endometrioid type, in association with MGH-like carcinoma. These findings support the idea that the microglandular pattern represents ‘a line of differentiation that is more mature and less aggressive in comparison with conventional carcinoma and this microglandular pattern usually occurs on the tumor surface, where an area permits a proliferation of non-invasive cells’[7]. Plaque-like microglandular differentiation is found on the surface of conventional adenocarcinoma[10]. The studies of Jacques et al[20] and Fukunaga[12] supported this argument but Zaloudek et al[11] and McCluggage and Perenyei[21] found MGH-like patterns in invasive areas of the tumor. In our case we found MGH-like carcinoma on the surface of the conventional endometrioid carcinoma in the hysterectomy specimen (ph 10). The conventional carcinoma was of mucinous or endometrioid type. There was no atypical hyperplasia present.
There is no clinical significance to MGH-like features in an endometrioid adenocarcinoma. The significance is purely to pathologists concerning the differential diagnosis in biopsy or curettage specimens, because under-sampling of this type of tumor may lead to hypo-diagnosis of MGA. The latter can present a true diagnostic challenge that many times may not be solved upon review of a limited sampling. The biopsy may only contain fragments of mucinous glandular proliferation with no nuclear atypia or mitotic activity and with no features of either hyperplasia or carcinoma. The only clue may be the patient’s age and in some cases the clinical history of an endometrial tumor, endometrial thickening or uterine bleeding. If no other clues present, there is an important diagnostic rule of thumb, to consider the patient’s age when considering a diagnosis of endocervical MGH in an endometrial sampling: if peri- or postmenopausal age, then the possibility of under-sampled adenocarcinoma with MGH-like features should be considered. The diagnosis of MGH in endometrial samples of postmenopausal women should not be made unless thorough examined[8]. Furthermore, features that favor EAMGP are a large amount of tissue in a biopsy, a lack of subnuclear vacuoles, transition to other patterns of endometrial adenocarcinoma, connection with endometrial stroma, an association with foamy stromal cells and the presence of complex endometrial hyperplasia or mucinous metaplasia in the background of endometrium. A descriptive diagnosis should be reserved for cases where the distinction is not possible, such as ‘glandular proliferation with a microglandular-like pattern’. Additionally, the report should include a comment, suggesting either acquirement of additional tissue (i.e., fractional curettage) or clinical correlation , to reach a definitive diagnosis.
When MGH-like proliferation is detected in the D&C of a postmenopausal woman, endometrial MGA type of carcinoma, should be excluded. The examination of scant biopsy specimens remains a challenge. Look for areas of typical endometrioid adenocarcinoma. Look for subnuclear vacuolation. Staining for vimentin, p16, PAX2, CD10 and CD34 can be of help in the differential diagnosis with MGH.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aldera AP, South Africa; Bains L, India; Chiu KW, Taiwan S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Witkiewicz AK, Hecht JL, Cviko A, McKeon FD, Ince TA, Crum CP. Microglandular hyperplasia: a model for the de novo emergence and evolution of endocervical reserve cells. Hum Pathol. 2005;36:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Greeley C, Schroeder S, Silverberg SG. Microglandular hyperplasia of the cervix: a true "pill" lesion? Int J Gynecol Pathol. 1995;14:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Arnold MP, Hirschmann MT. Letter to the editor concerning the editorial ‘‘Innovation in orthopaedic surgery as it relates to evidence-based practice’’ by M. Hofbauer, B. Muller, C. D. Murawski, J. Karlsson and Freddie H. Fu. Knee Surg Sports Traumatol Arthrosc. 2014;22:710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Kyriakos M, Kempson RL, Konikov NF. A clinical and pathologic study of endocervical lesions associated with oral contraceptives. Cancer. 1968;22:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Taylor HB, Irey NS, Norris HJ. Atypical endocervical hyperplasia in women taking oral contraceptives. JAMA. 1967;202:637-639. [PubMed] |
| 6. | Kurman RJ, Carcanglu ML, Herrington CS, Young RH. WHO Classification of Tumours of Female Reproductive Organs. Lyon: IARC 2014: 6. |
| 7. | Höhn AK, Brambs CE, Hiller GGR, May D, Schmoeckel E, Horn L-C. 2020 WHO Classification of Female Genital Tumors. Geburtshilfe und Frauenheilkunde. 2021;81:1145-1153. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 8. | Nucci MR, Prasad CJ, Crum CP, Mutter GL. Mucinous endometrial epithelial proliferations: a morphologic spectrum of changes with diverse clinical significance. Mod Pathol. 1999;12:1137-1142. [PubMed] |
| 9. | Young RH, Scully RE. Uterine carcinomas simulating microglandular hyperplasia. A report of six cases. Am J Surg Pathol. 1992;16:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 47] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Zamecnik M, Skalova A, Opatrny V. Microglandular adenocarcinoma of the uterus mimicking microglandular cervical hyperplasia. Ann Diagn Pathol. 2003;7:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Zaloudek C, Hayashi GM, Ryan IP, Powell CB, Miller TR. Microglandular adenocarcinoma of the endometrium: a form of mucinous adenocarcinoma that may be confused with microglandular hyperplasia of the cervix. Int J Gynecol Pathol. 1997;16:52-59. [PubMed] |
| 12. | Fukunaga M. Mucinous endometrial adenocarcinoma simulating microglandular hyperplasia of the cervix. Pathol Int. 2000;50:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Da Forno PD, McGregor AH, Brown LJ. Microglandular hyperplasia: a pitfall in the diagnosis of microglandular type endometrioid adenocarcinoma. Histopathology. 2005;46:346-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Qiu W, Mittal K. Comparison of morphologic and immunohistochemical features of cervical microglandular hyperplasia with low-grade mucinous adenocarcinoma of the endometrium. Int J Gynecol Pathol. 2003;22:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Chekmareva M, Ellenson LH, Pirog EC. Immunohistochemical differences between mucinous and microglandular adenocarcinomas of the endometrium and benign endocervical epithelium. Int J Gynecol Pathol. 2008;27:547-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Barroeta JE, Pasha TL, Acs G, Zhang PJ. Immunoprofile of endocervical and endometrial stromal cells and its potential application in localization of tumor involvement. Int J Gynecol Pathol. 2007;26:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Stewart CJ, Crook ML. PAX2 and cyclin D1 expression in the distinction between cervical microglandular hyperplasia and endometrial microglandular-like carcinoma: a comparison with p16, vimentin, and Ki67. Int J Gynecol Pathol. 2015;34:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Vang R, Tavassoli FA. Proliferative mucinous lesions of the endometrium: analysis of existing criteria for diagnosing carcinoma in biopsies and curettings. Int J Surg Pathol. 2003;11:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Alomari A, Abi-Raad R, Buza N, Hui P. Frequent KRAS mutation in complex mucinous epithelial lesions of the endometrium. Mod Pathol. 2014;27:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Jacques SM, Qureshi F, Lawrence WD. Surface epithelial changes in endometrial adenocarcinoma: diagnostic pitfalls in curettage specimens. Int J Gynecol Pathol. 1995;14:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | McCluggage WG, Perenyei M. Microglandular adenocarcinoma of the endometrium. Histopathology. 2000;37:285-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |