Published online Jul 27, 2015. doi: 10.5313/wja.v4.i2.44
Peer-review started: January 21, 2015
First decision: February 7, 2015
Revised: March 31, 2015
Accepted: April 27, 2015
Article in press: April 29, 2015
Published online: July 27, 2015
We present the case of a 13-year-old boy undergoing scoliosis repair utilizing skull-femoral traction who developed sudden, sustained bradycardia and hypotension during scoliosis repair, associated with loss of somatosensory evoked potentials and motor evoked potentials to all four limbs. A diagnosis of spinal shock and hypovolemia was made after ruling out primary cardiac causes, sepsis, anaphylaxis and intra-spinal pedicle screw placement. Acute complications of surgical scoliosis repair are reviewed along with anatomy of the sympathetic nervous system. In this case spinal shock may have been due to hypovolemia as well as spinal cord manipulation during T12 vertebral column resection that was needed to effect scoliosis correction. Treatment included volume expansion and inotropic support. Anesthesiologists caring for these patients should be mindful of the possibility of spinal shock during correction of severe scoliosis, particularly when vertebral column resection is undertaken.
Core tip: A child undergoing scoliosis repair developed sudden bradycardia and hypotension, associated with loss of somatosensory and motor evoked potentials to all four limbs. Spinal shock and hypovolemia were diagnosed after ruling out other causes. Acute complications of scoliosis repair are reviewed along with sympathetic nervous system anatomy. Spinal shock was likely due to hypovolemia and spinal cord manipulation during vertebral column resection that was needed to effect scoliosis correction. Treatment included volume expansion and inotropic support. Anesthesiologists should be mindful of the possibility of spinal shock during correction of severe scoliosis, particularly when vertebral column resection is undertaken.
- Citation: Karsli C, Strantzas S, Finnerty O, Holmes L, Lewis S. Bradycardia and hypotension during pediatric scoliosis surgery-hypovolemia or spinal shock? World J Anesthesiol 2015; 4(2): 44-48
- URL: https://www.wjgnet.com/2218-6182/full/v4/i2/44.htm
- DOI: https://dx.doi.org/10.5313/wja.v4.i2.44
Surgical correction of scoliosis may be associated with complications such as spinal cord or nerve root injuries[1], hypovolemic shock, superior mesenteric artery syndrome[2] and subtle sympathetic trunk/chain lesions[3]. We present a case of acute intraoperative spinal shock likely exacerbated by hypovolemia during thoracic vertebral column resection (VCR).
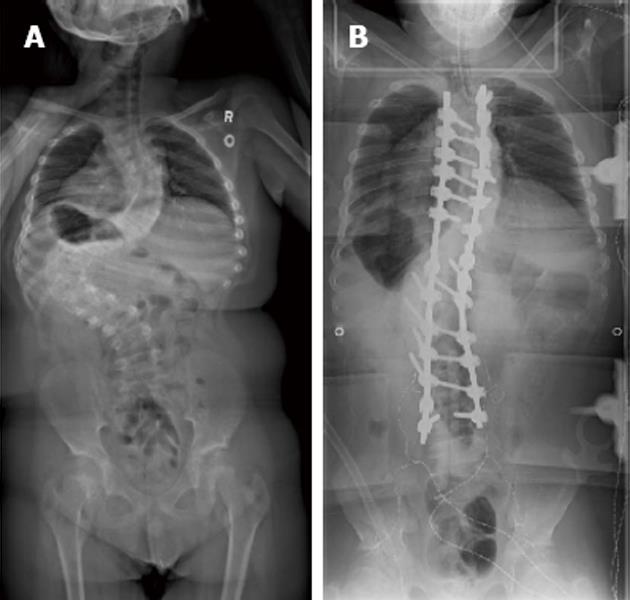
Our patient was a 13-year-old male with Prader-Willi syndrome and severe scoliosis, Cobb angle 128 degrees, (Figure 1A) who presented for posterior spinal instrumentation and fusion with the use of skull-femoral traction. Anesthesia was maintained with propofol and remifentanil to optimize somatosensory somatosensory evoked potentials (SEP) and motor evoked potentials (MEP) monitoring throughout the case. The use of skull-femoral traction to aid in deformity correction was abandoned after repeated alerts in MEP monitoring during the surgical exposure. With attempted rod insertion, the MEPs were completely abolished from the lower extremity muscle groups bilaterally, and responses from the abdominal recti were decreased in amplitude by more than 50% of baseline. The mean arterial blood pressure was elevated to aid the patient tolerate saggital plane correction of the spine. However, with the persistent absence of MEPs, the decision was made to eliminate the corrective forces by removing the rod. This resulted in an immediate return of all MEPs. With each subsequent attempt at rod insertion there was an associated loss of MEPs and the responses normalized only when attempts to correct the scoliosis were abandoned. It was decided to proceed with a vertebral column resection at the level of T12 in an effort to aid with the reduction of the severe curvature.
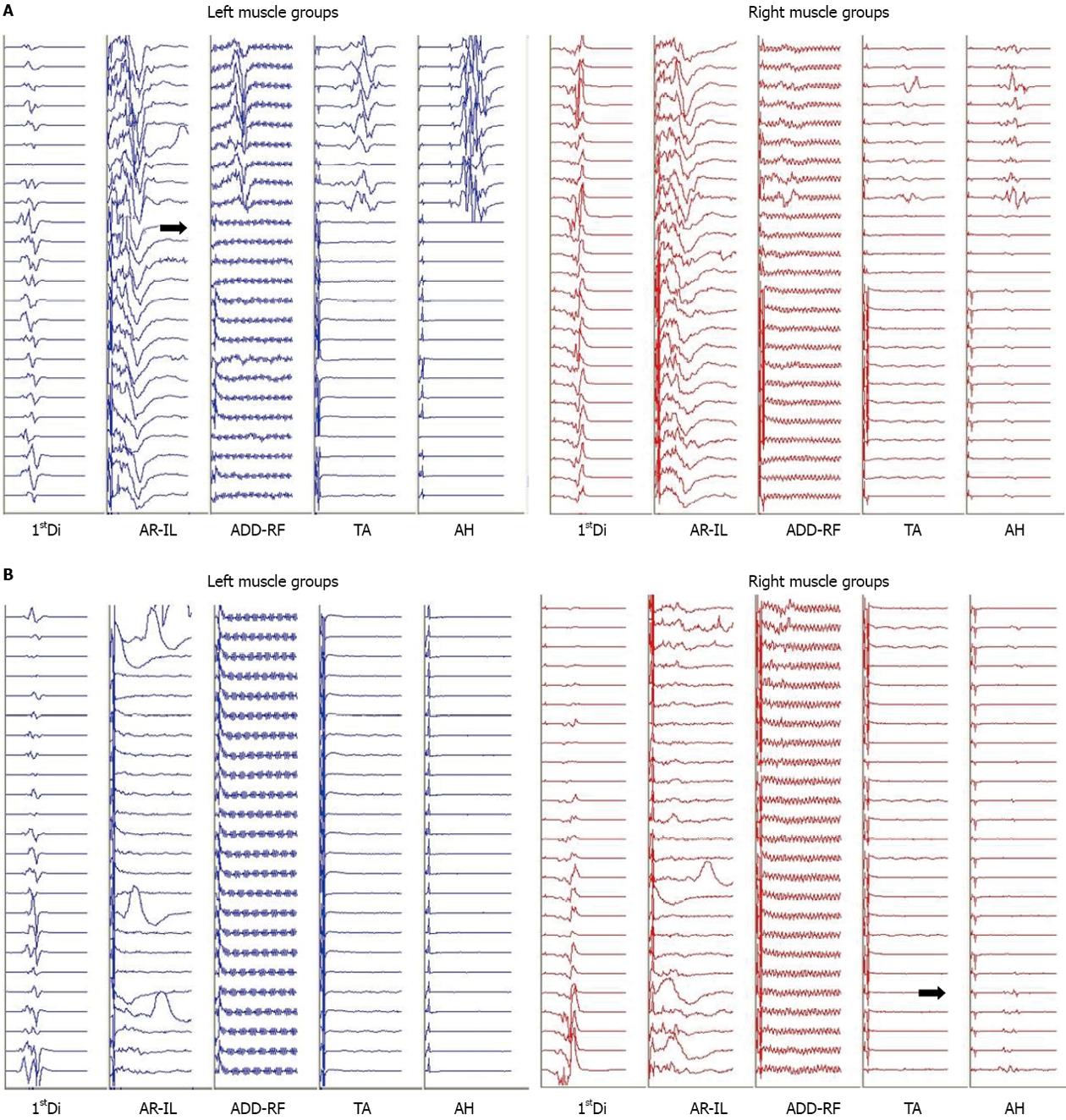
During the VCR there was an acute, complete loss of the left lower extremity MEPs and a significant reduction in amplitude of the right lower extremity MEPs to less than 10% of baseline (Figure 2A). Blood loss during the VCR was significant and over the next hour an estimated 60 mL/kg bleeding occurred. This was associated with a drop in mean arterial pressure from 70 to 35 mmHg and a slight increase in heart rate from 60 to 70 BPM. Coinciding with the drop in mean arterial pressure, the remaining small response from the right lower extremity MEPs disappeared. At this time, the upper extremity and abdominal rectus MEPs remained at baseline bilaterally. Aggressive fluid resuscitation included a total of one blood volume, comprising red blood cell concentrate: fresh frozen plasma at a ratio of 2:1. A dopamine 10 mcg/kg per minute infusion was initiated to address hypotension resistant to volume resuscitation and transfusion. Despite this, hypotension persisted and the heart rate remained between 60 and 70 BPM. With this the left and right upper extremity MEPs slowly declined in amplitude to less than 10% of baseline and the abdominal rectus responses were lost. This was followed by a complete loss of the upper extremity and greater than 50% amplitude decrease in the lower extremity SEP on both sides. The electroencephaography demonstrated burst suppression indicating global hypoperfusion.
With the instrumentation secured (Figure 1B) and continued fluid resuscitation with inotropic support, the mean arterial pressure improved over the next 20 min coinciding with partial return of MEPs, first in the right lower extremity followed by the left and right upper extremity. No improvement was seen from the left lower extremity MEPs (Figure 2B). Soon after, the left and right SEPs displayed recovery in both the upper and lower extremities. An intraoperative echocardiogram out-ruled hypovolemia or a primary cardiac cause for the decreased output and serum electrolytes and hemoglobin were in the normal range. With other causes ruled out, a diagnosis of acute intraoperative spinal shock was made. Upon emergence from anesthesia the patient was noted to be purposefully moving all but the left lower limb. This clinical presentation was consistent with the intraoperative findings of persistent complete loss of the left lower extremity motor evoked potentials.
Soon after intensive care unit admission dopamine was replaced with a norepinephrine infusion to maintain mean arterial pressure between 60 and 80 mmHg. Vasopressor support was discontinued on post-operative day 2 and the patient was transferred to the ward. Some motor function had returned to the left leg by that time and the heart rate and blood pressure returned to pre-operative values. Clinical neurologic examination revealed a left sided Brown-Séquard syndrome at the T12-L1 level.
To our knowledge, this is the first reported case of acute intra-operative spinal shock in a pediatric patient undergoing scoliosis repair. This was a diagnosis of exclusion, as many other confounding factors were present. There was significant blood loss during the VCR with hypovolemic shock and the absence of tachycardia in response to hypotension suggests a possible autonomic cause. The use of remifentanil may have clouded the overall clinical picture and delayed the diagnosis of spinal shock in this case as it often results in a relative bradycardia. However the authors feel the persistent bradycardia was not due to remifentanil as rises in heart rate were seen in response to incision and during deeper dissection earlier in the case. Trauma to the spinal cord as a result of a breached pedicle screw was out ruled using electrophysiological stimulation intraoperatively, and visual inspection of the X-rays post-operatively. It is possible during the VCR there was an insult to the left side of the spinal cord indicated by reductions in MEPs, and then confounded by ischemia during hemorrhagic shock. Despite aggressive resuscitation to normovolemia on ECHO, the need for inotropic support indicates a more sinister cause for the persistent shock. Given the acute and persistent loss of the left lower extremity motor evoked potentials during the VCR, and the clinical diagnosis of Brown-Séquard syndrome postoperatively, involvement of the spinal cord appears likely.
Spinal or neurogenic shock occurs usually after a serious injury to the spinal cord resulting in rapid loss of sympathetic output with persistent, relatively unopposed vagal tone causing the typical clinical picture of bradycardia with hypotension. The sympathetic nervous system includes preganglionic neurons in the lateral horn of the spinal cord from T1 to L2/3. Preganglionic axons exiting the spinal cord enter the white rami communicantes to join a network of the sympathetic chain, which run on either side of the vertebral bodies. Postganglionic axons follow the arterial tree to distal organs providing a constant balance between vasoconstriction and vasodilatation depending on clinical needs. Subtle sympathetic lesions have been reported after scoliosis repair and include altered sweating and sympathetic skin responses and an increase in temperature. We feel in this case two factors were at play: (1) a temporary but significant disruption in sympathetic activity due to spinal manipulation or vertebral column resection; and (2) hypovolemia from blood loss. The hypovolemia likely intensified the clinical picture of spinal shock. It is interesting that a single discrete injury at the level of T12 caused such rapid neurogenic shock. This scenario is more likely seen in high thoracic or cervical cord injuries. Hypovolemia leading to spinal cord ischemia likely aggravated the neurogenic shock. In caring for these patients the anesthesiologist should be mindful of this possibility and be prepared to treat spinal shock with fluids and vasopressor/inotropic support such as norepinephrine and dopamine.
Vertebral column resection is a challenging procedure that is reserved for patients with severe, rigid spinal deformity. Conventional methods such as posterior only instrumentation, or posterior instrumentation with anterior release, may not be adequate and a more aggressive method may be required[4]. It is associated with a higher degree of intraoperative blood loss and carries a risk of spinal or nerve root injuries, however, it has been performed safely and with excellent outcomes in several case series[5-7]. In our particular case, VCR was performed after it was determined that the patient could not tolerate the saggital corrective forces placed on the spinal cord by either skull-femoral traction or rod placement, as demonstrated by repeated MEP alerts. MEPs are highly sensitive in detecting ischemia to the anterior two-thirds of the spinal cord, and specifically the corticospinal tracts[7]. Vertebral column resection reduces both the coronal and saggital curves and aides in decompression of the spinal cord and reduces traction of the anterior spinal artery. Because of the high risk associated with VCR it is imperative that MEPs and SEPs be monitored to provide real time feedback and direct key surgical decisions[7].
A child undergoing scoliosis repair developed sudden bradycardia and hypotension.
The clinical findings coincided with loss of somatosensory and motor evoked potentials to all four limbs.
A diagnosis of spinal shock and hypovolemia was made after ruling out primary cardiac causes, sepsis, anaphylaxis and intra-spinal pedicle screw placement.
Somatosensory and motor evoked potentials identified the acute neurologic changes, and intraoperative echocardiography ruled out primary cardiac causes.
X-ray was used to rule out intraspinal pedical screw placement as a cause.
Fluid resuscitation as well as inotrope and vasoconstrictor therapy was required to treat the hypotension and spinal shock.
Spinal shock in pediatric scoliosis repair using vertebral column resection has not yet been reported.
Vertebral column resection is a surgical technique which involves resecting a or some segmental vertebral columns in their entirely in order to facilitate correction of severe scoliosis. It is associated with increased surgical blood loss and possible neurologic complications.
In caring for pediatric patients undergoing scoliosis surgery the anesthesiologist should be mindful of the possibility of spinal shock and be prepared to treat it with fluids and vasopressor/inotropic support such as norepinephrine and dopamine.
It is an interesting case.
P- Reviewer: Noll-Hussong M, Wong KL S- Editor: Gong XM L- Editor: A E- Editor: Yan JL
| 1. | Diab M, Smith AR, Kuklo TR. Neural complications in the surgical treatment of adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2007;32:2759-2763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Hod-Feins R, Copeliovitch L, Abu-Kishk I, Eshel G, Lotan G, Shalmon E, Anekstein Y, Mirovsky Y, Masharawi Y. Superior mesenteric artery syndrome after scoliosis repair surgery: a case study and reassessment of the syndrome’s pathogenesis. J Pediatr Orthop B. 2007;16:345-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Schulte TL, Adolphs B, Oberdiek D, Osada N, Liljenqvist U, Filler TJ, Marziniak M, Bullmann V. Approach-related lesions of the sympathetic chain in anterior correction and instrumentation of idiopathic scoliosis. Eur Spine J. 2010;19:1558-1568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Bradford DS, Tribus CB. Vertebral column resection for the treatment of rigid coronal decompensation. Spine (Phila Pa 1976). 1997;22:1590-1599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 200] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Papadopoulos EC, Boachie-Adjei O, Hess WF, Sanchez Perez-Grueso FJ, Pellisé F, Gupta M, Lonner B, Paonessa K, Faloon M, Cunningham ME. Early outcomes and complications of posterior vertebral column resection. Spine J. 2015;15:983-991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Xie J, Wang Y, Zhao Z, Zhang Y, Si Y, Li T, Yang Z, Liu L. Posterior vertebral column resection for correction of rigid spinal deformity curves greater than 100°. J Neurosurg Spine. 2012;17:540-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Jarvis JG, Strantzas S, Lipkus M, Holmes LM, Dear T, Magana S, Lebel DE, Lewis SJ. Responding to neuromonitoring changes in 3-column posterior spinal osteotomies for rigid pediatric spinal deformities. Spine (Phila Pa 1976). 2013;38:E493-E503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |