INTRODUCTION
Osteoarthritis (OA) is a very complex and multifactorial disease of articular cartilage, which represents a leading cause of joint pain and disability worldwide[1]. The entire synovial joint is affected by the progression of this pathology, including the underlying bone, synovium, meniscus, ligaments/tendons, and cartilage[2,3]. OA is characterized by the degradation of the articular cartilage, which can be used as hallmark of pathological advancement beyond changes in subchondral bone, osteophyte formation, joint space narrowing and chronic synovial inflammation[4]. In normal joints, cartilage covers and cushions the ends of bones, reducing friction and absorbing shocks. Its destruction progress leads to stiffness, pain, mobility limitations and compromised overall quality of life[5,6]. Some of the most important risk factors include aging, inflammatory state, muscle atrophy, injury and metabolic disorders[7].
The management of OA focuses on alleviating its secondary effects since there is currently no resolutive cure. Nonsteroidal anti-inflammatory drugs and analgesics are generally prescribed to patients to reduce pain and improve joint function, but they fail in modifying disease progression in terms of prevention and chondroprotection[8]. The chronic nature of OA forces the use of pharmacological approaches that can be considered safe for long term use and, at the same time, might be able to slow its progression. The basis of articular damage relies on impaired balance between anabolic and catabolic mechanisms, which can be influenced by dietary compounds like nutraceuticals[9]. Due to their minimal side effects, especially in the long term, their easy extraction and low costs of production, they may represent a valid preventive management of OA.
Forty-seven percent of people who suffer from OA use complementary medications including nutraceuticals due to their anti-inflammatory and antioxidant activities[10]. Herbal and natural products have been used since ancient times. A 5000-year-old Sumerian clay tablet is the first proof of plants use as medicament, especially to treat pain and inflammation[11].
During the 19th century, improvement in chemical technologies allowed for the extraction of active substances from medicinal plants such as alkaloids, tannins, saponosides, etheric oils, vitamins and glycosides, isolated in pure form[12]. The term “nutraceutical”, resulting from the combination of the words “nutrition” and “pharmaceutical”, is used to define any natural or food-derived molecule able to exert a potential therapeutic effect that could be integrated into a daily diet[13].
Statutory law of these type of medicaments differ by country. For example, in the United States, they are considered dietary supplements by the Dietary Supplement Health and Education Act of 1994[14]. The Food and Drug Administration is in charge of reviewing and approving any health claims about these products. In some countries of the European Union, nutraceuticals may require registration whereas in others, they could be easily sold as food preparations[15].
This review will summarize natural-based approaches for chondroprotection, highlighting the peculiarity of some molecules whose positive effect in preserving cartilage health has recently been discovered. This approach may be useful both to prevent OA onset and to slow down its progression.
ANTI-INFLAMMATORY APPROACH
The involvement of an inflammatory component, marked by joint pain, swelling and stiffness, is now well recognized in the pathogenesis of OA. Indeed, chondrocytes undergo a loss of homeostatic balance, which includes expression of inflammatory cytokines, matrix proteins such as collagen and lubricin and proteolytic enzymes[16]. The most important pro-inflammatory cytokines involved are interleukin (IL)-1β and tumour necrosis factor (TNF)-α[17]. Some of the consequences of the development of an inflammatory scenario are as follows: downregulation of structural components, including type II collagen and proteoglycans[18-21], upregulation of proteolytic enzymes, such as matrix metalloproteinases (MMPs)-1, -3, -13, and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)[22-24] and stimulation of inflammatory mediators like prostaglandin E2 (PGE2), cyclooxygenase-2 (COX-2), and Reactive oxygen species (ROS)[25,26].
Recently, our lab carried out studies to determine the chondroprotective role of phytoactive molecules [e.g., polyphenols and monounsaturated fatty acids naturally present in olive tree-derived products, olive oil (-OO-) and olive leaf extract (-OLE-)] able to preserve the articular cartilage and skeletal muscle condition, in the context of early development of OA because of their antioxidant and anti-inflammatory properties[7]. In addition, the study examined differences between three types of oils in term of origin and polyphenol contents: Sicilian extra virgin olive oil (S-EVOO), Tunisian extra virgin olive oil (T-EVOO) and Tunisian extra virgin olive oil and leaves extract (T-enriched-EVOO), concluding that the first variety of oil (S-EVOO) is the best in exerting positive effects on the entire joint, remarkably reducing IL-6 release and increasing lubricin synthesis, compared to the other diet protocols (Figure 1). The effects of physical activity were also analysed in combination with the diet[27]. The studies demonstrated that an olive oil supplemented diet plus physical activity improved cartilage recovery after anterior cruciate ligament transection by lowering IL-6 and IL-1 expression and by increasing lubricin expression, suggestive of chondroprotective activity. Lubricin is a glycoprotein released by type B synoviocytes and chondrocytes from the superficial layer of articular cartilage, and its functions are to lubricate and nourish articular cartilage[28].
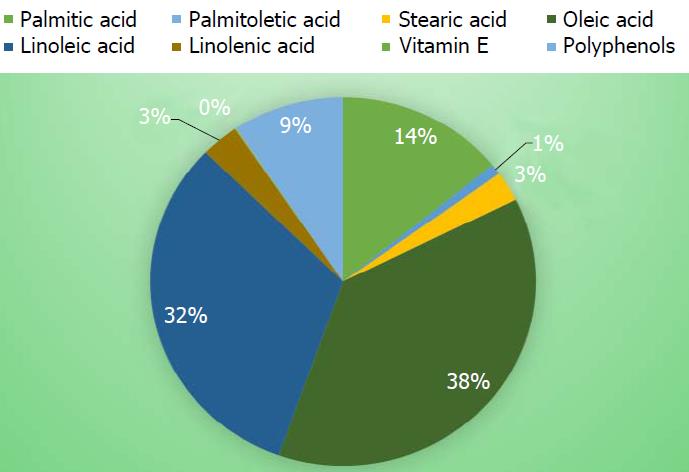
Figure 1 Composition of fatty acids, vitamin E and polyphenols in a Sicilian extra virgin olive oil-supplemented diet.
Palmitic acid (16:0) (mg/kg) 9002; palmitoleic acid (16:1) (mg/kg) 579; stearic acid (18:0) (mg/kg) 1689; oleic acid (18:1) (mg/kg) 24047; linoleic acid (18:2) (mg/kg) 20352; linolenic acid (18:3) (mg/kg) 2018; vitamin E (mg/kg) 72.167; polyphenols (mg/kg) 5.960.
Another recent study that confirms the healthy effect of OLE was presented by Maruyama et al[29], which addressed the main activity of hydroxytyrosol [4-(2-hydroxyethyl)-1, 2-benzenediol] (HT), an OLE polyphenol. STR/ort mice were used as a model for knee OA, and 100 mg/kg OLE was orally administered every day for 8 wk. The chondroprotective effect of the extract was proven by Mankin scores of the non-OA control group, OA control group and OLE-treated group, which were 3.50, 11.13 and 7.20, respectively. Moreover, the study suggests that these natural molecules were able to impair cartilage damage and, consequently, the pathology progression, since they stimulated the synthesis of high molecular weight hyaluronan in synovial cells in vitro. High molecular weight hyaluronan is involved in maintaining joint moisture and lubrication[30]. The authors suggested that OLE administration can effectively help suppress OA progression.
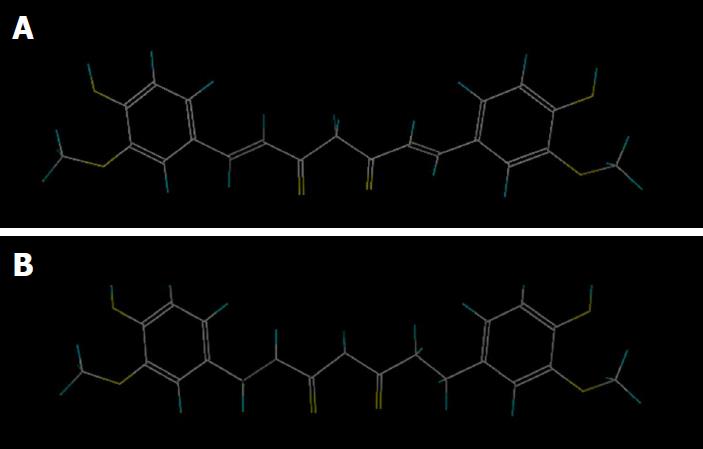
Traditionally used as an anti-inflammatory treatment in Chinese and Ayurvedic medicine, Curcuma longa is a plant rich in phytochemicals, which are responsible for its most impressive and wide-ranging health benefits. Some of its active components, curcumin and tetrahydrocurcumin (THC), a major metabolite of curcumin, have been studied because of their anti-inflammatory, anti-oxidant, chemopreventive, anti-aging and anti-bacterial activities[31,32]. Park et al[33] analysed the effects of long-term THC administration and curcumin in OA progression in rats with oestrogen deficiency. Ovariectomized obese rats underwent monoiodoacetate injections into the knee to simulate OA conditions, and then curcumin and THC were fed to prevent postmenopausal and OA symptoms. One of the most significant findings of the study was the differences between the two molecules. The chemical structures of curcumin involved in exerting the main activities are methoxy, hydroxyl, α,β-unsaturated carbonyl, and diketone groups, whereas its metabolite lacks the presence of the α,β-unsaturated carbonyl group, changing its functionality and efficacy (Figure 2). Park et al[33] found that both natural products showed similar abilities to decrease expression of TNF-α, IL-1β, IL-6 and MMP3 and MMP13, but only THC could enhance glucose tolerance, allowing it to decrease advanced glycation end products in articular cartilage, delaying the start of the pathological process of cartilage breakdown.
Figure 2 Chemical structure of curcumin and tetrahydrocurcumin.
A: Curcumin, C21H20O6; B: Tetrahydrocurcumin, C21H24O6.
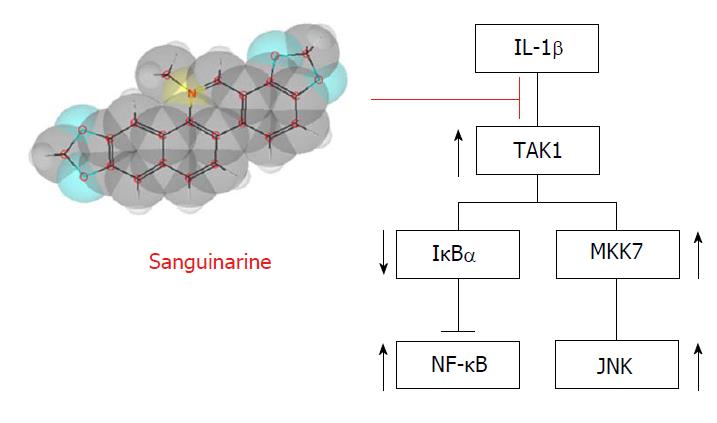
Furthermore, Ma et al[34] demonstrated for the first time the anti-inflammatory effect of sanguinarine (SA), a benzophenanthridine alkaloid isolated from the roots of Sanguinaria canadensis, on the pathogenesis of OA, in vitro, ex vivo and in vivo. Evaluation of the potential cytotoxicity of SA revealed that this compound does not affect cell viability at concentrations lower than 1.25 μmol/L. As stimulation of IL-1β increased the mRNA expression of MMP1a, MMP3, MMP13, and ADAMTS-5, SA downregulated these catabolic proteases through a dose-dependent manner indicative of IL-1β activity. More specifically, the anti-inflammatory molecule acts as suppressor of phosphorylation of the c-Jun N-terminal kinases (JNK) and nuclear factor kappa B (NF-κB) (Figure 3). These in vitro analyses were followed by ex vivo evaluation of SA’s effects on cartilage matrix degradation, which were consistent with the previous results. Intra-articular administration of different SA concentrations was used to determine whether the molecule could slow down the progression of ACLT-induced OA in mice. The hypothesis was finally and positively confirmed by immunochemistry results, evaluation of protease mRNA levels and Osteoarthritis Research Society International scoring.
Figure 3 Anti-inflammatory effect of sanguinarine.
Sanguinarine acts as suppressor of IL-1β, targeting the pathways involved in JNK activation and the degradation of IκBα, an inhibitory subunit of NF-κB. IL: Interleukin; IκBα: Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; NF-κB: Nuclear factor kappa B; JNK: c-Jun N-terminal kinases; MKK7: Dual specificity mitogen-activated protein kinase kinase 7.
ANTIOXIDANT APPROACH
It is well established that oxidative stress-induced ROS production (commonly experienced because of post-traumatic events or aging) is a crucial mediator of OA disease progression[35]. As a consequence, chondrocytes experience more significant senescence and cell death[36,37]. In addition, cartilage and joint fluid are not able to counteract this scenario because superoxide dismutase antioxidant levels are consistently decreased in OA[38]. This is the reason why an effective preventive approach to this pathology should consider boosting antioxidant shields to enhance the potency of constitutive defences such as the antioxidants catalase, superoxide dismutase, glutathione peroxidase and glutathione reductase.
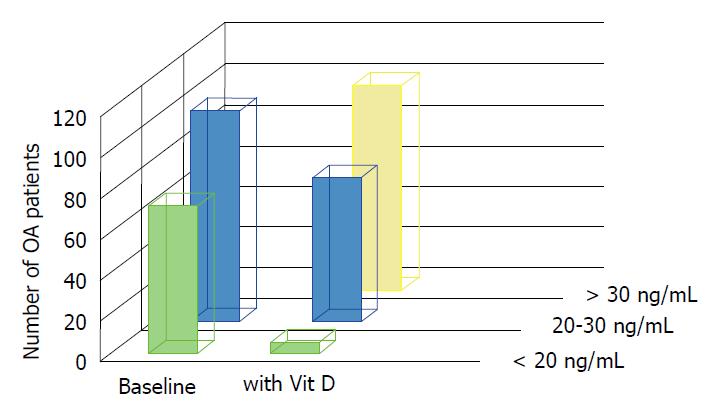
A study about dietary supplementation in OA by Manoy et al[39] highlighted the role of the commonly used antioxidant vitamin D. Even though the correlation between this vitamin and musculoskeletal diseases is still not clear, low levels of 25-hydroxyvitamin D [25(OH)D] have been observed in OA patient serum. In fact, evidence suggests that vitamin D deficiency is a co-factor for OA pathogenesis[40]. The study involved 175 primary knee OA patients who received 40000 IU vitamin D (ergocalciferol) per week. Six months after the first administration, the patients experienced ameliorated grip strength, physical performance and improved quality of life (Figure 4). Moreover, to confirm its anti-oxidant activity, analysis of protein carbonyl levels was performed to obtain information about oxidative damage. The results confirmed that vitamin D supplementation remarkably decreased carbonyl levels, and as a consequence, stress and the pro-inflammatory environment that can affect protein function and DNA. The underlying mechanism for this vitamin D activity may be explained by evidences for the downregulation of nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase), IL-6, TNF-α, NF-kB and p38[41,42].
Figure 4 Vitamin D levels in osteoarthritis patients at baseline and after vitamin D2 supplementation.
At baseline, 72 participants had vitamin D deficiency (< 20 ng/mL) and 103 patients had vitamin D insufficiency (20-30 ng/mL). After 40000 IU of vitamin D2 supplementation per week for 6 mo, 100 knee OA participants achieved concentration above 30 ng/mL, 70 knee OA participants had vitamin D insufficiency, and only 5 patients had vitamin D deficiency. Vit D: Vitamin D; OA: Osteoarthritis.
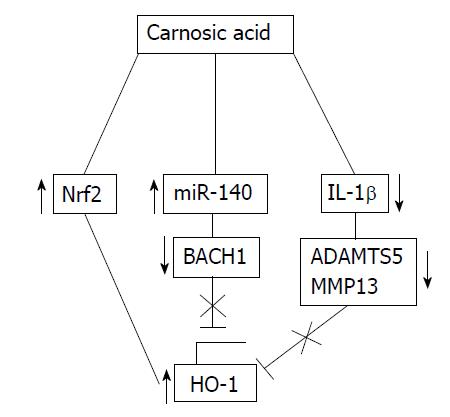
Heme oxygenase-1 (HO-1) is another potential target that can be used in an anti-oxidant strategy against OA. Constitutive expression of HO-1 in chondrocytes and the meniscus in mice has been linked to preserve cartilage degeneration[43]. For this reason, Hiroyuki et al[44] explored the effect of carnosic acid (CA) as an inducer of HO-1 upregulation in preventing OA progress. This molecule is a natural diterpene commonly found in rosemary and common sage, and it has demonstrated protective qualities in pathologies like cancer, diabetes and neurodegenerative disease[45]. Immunoblotting assays were used to test whether CA affected HO-1 expression in articular chondrocytes. The results showed that CA increased enzyme levels in a dose-dependent manner. More specifically, the best treatment seemed to require 10 to 50 μmol/L of CA. In addition, it was able to restore HO-1 levels under IL-1β treatment, which specifically inhibits the anti-oxidant effects of the enzyme. According to this study, the mechanisms by which this natural compound acts rely on downregulation of MMP-13 and ADAMTS-5, activation of nuclear factor erythroid 2-related factor 2 (Nrf2), regulation of the Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2 (KEAP1/NRF2) transcriptional pathway and an increase in microRNA 140 (miR-140) binding to the 3’UTR of Bach1 (an HO-1 repressor) in articular chondrocytes (Figure 5).
Figure 5 Mechanisms of heme oxygenase-1 upregulation by carnosic acid.
CA induces the expression of HO-1 by: Activation of the Nrf2 transcription factor, downregulation of Bach1 via miR-140 and downregulation of the IL-1β-induced expression of extracellular matrix degrading enzymes such as MMP-13 and ADAMTS-5. CA: Carnosic acid; HO-1: Heme oxygenase-1; Nrf2: Nuclear factor erythroid 2-related factor 2; IL: Interleukin; MMP: Matrix metalloproteinase; ADAMTS: A disintegrin and metalloproteinase with thrombospondin motifs; miR-140: MicroRNA 140.
Furthermore, our lab examined the relationship between oxidative stress and physical activity or a sedentary lifestyle, suggesting therapeutic solutions that involve natural dietary supplements. One study analysed the effects of oleic acid on ROS production induced by exhaustive physical activity in rat skeletal muscle[46]. The results highlight the importance of extra-virgin olive oil as a protective agent against oxidative stress following physical efforts. The group of rats subjected to exhaustive exercise but fed with a diet rich in oleic acid experienced a decrease in hydroperoxides and thiobarbituric acid reactive substances and an increase in antioxidant defences, rated as non-enzymatic antioxidant capacity and levels of 70 kDa heat shock proteins (Hsp70). OA cannot be completely prevented, but some precautions can help delay the progression of the pathology and manage the risk of its progression[47]. Since sarcopenia and sedentary life are possibly associated with knee OA[48], another study is worth citing because it evaluated whether different dietary profiles, containing or not containing vitamin D, could exert some effects on muscle fibres[49]. The study found that muscle fibres of rats fed with high-fat extra-virgin olive oil-based diets were hypertrophic compared to those of the regular diet group. These data confirmed that this natural supplement does not impair muscle fibre metabolism, unlike high-fat butter-based diets. In addition, Vitamin D exerted a trophic action on muscle fibres both in rats fed regular diets and in those fed a diet enriched with extra-virgin olive oil, suggesting that insulin-like growth factor-1 (IGF-1) and dickkopf-1 (DKK-1) may be involved in this mechanism.
CONCLUSION
When physical activity and a healthy lifestyle are not enough, anti-inflammatory drugs and painkillers are commonly used to alleviate pain, but sometimes rehabilitation and surgical intervention are unavoidable. For these reasons, trying to preserve the cartilage joint is imperative.
The use of natural approaches is a cutting-edge strategy. Nutraceuticals offer a wide range of molecules able to exert positive effects at different joint structures with several mechanisms of actions. In particular, this review focused on the anti-inflammatory and antioxidant properties of compounds that ameliorate cartilage conditions, suggesting that they should be integrated into a framework of prevention.
The presented studies offer thorough evaluations of olive oil, demonstrating that it reduces IL-6 release and increases lubricin synthesis, of olive leaf extract, as a stimulator of high molecular weight hyaluronan synthesis in synovial cells, of curcumin, addressing its ability to decrease TNF-α, IL-1β, IL-6 and MMP3 and MMP13 expression, of SA, as a downregulator of catabolic proteases through interaction with IL-1β, of vitamin D, since it influences the oxidative and pro-inflammatory environment and of CA, as an inducer of HO-1, preserving cartilage degeneration even under IL-1β treatment.
From a general analysis, it is worth noting that a common positive element of all these molecules is their availability in nature, which represents a huge advantage for food and pharmaceutical industries, and their low side effects, allowing for a broad range of safe uses for the derived products.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gheita TA, Yukata, K S- Editor: Ma RY L- Editor: Filipodia E- Editor: Wu YXJ