Published online Jun 18, 2016. doi: 10.5312/wjo.v7.i6.383
Peer-review started: February 1, 2016
First decision: March 25, 2016
Revised: April 12, 2016
Accepted: May 7, 2016
Article in press: May 9, 2016
Published online: June 18, 2016
AIM: To develop a subset of simple outcome measures to quantify prosthetic gait deviation without needing three-dimensional gait analysis (3DGA).
METHODS: Eight unilateral, transfemoral amputees and 12 unilateral, transtibial amputees were recruited. Twenty-eight able-bodied controls were recruited. All participants underwent 3DGA, the timed-up-and-go test and the six-minute walk test (6MWT). The lower-limb amputees also completed the Prosthesis Evaluation Questionnaire. Results from 3DGA were summarised using the gait deviation index (GDI), which was subsequently regressed, using stepwise regression, against the other measures.
RESULTS: Step-length (SL), self-selected walking speed (SSWS) and the distance walked during the 6MWT (6MWD) were significantly correlated with GDI. The 6MWD was the strongest, single predictor of the GDI, followed by SL and SSWS. The predictive ability of the regression equations were improved following inclusion of self-report data related to mobility and prosthetic utility.
CONCLUSION: This study offers a practicable alternative to quantifying kinematic deviation without the need to conduct complete 3DGA.
Core tip: The number of available outcome measures and multi-dimensionality of functional status complicate appropriate selection. This study assists clinicians in choosing apposite measures by exploring the relationship between various measures and demonstrating that often expensive and unavailable measures can be estimated using a combination of readily available self-report and performance-based measures.
- Citation: Kark L, Odell R, McIntosh AS, Simmons A. Quantifying prosthetic gait deviation using simple outcome measures. World J Orthop 2016; 7(6): 383-391
- URL: https://www.wjgnet.com/2218-5836/full/v7/i6/383.htm
- DOI: https://dx.doi.org/10.5312/wjo.v7.i6.383
The multitude of available outcome measures and the multi-dimensional concept of functional status complicate the selection of appropriate outcome measures for use with lower-limb amputees (LLAs).
Numerous outcome measures are used with LLAs[1], and are generally classified as either self-report or performance-based. Self-report measures have been used in abundance with lower limb amputees and include the generic short-form 36[2] and amputee-specific prosthesis evaluation questionnaire (PEQ)[3]. Ease of administration make self-report measures attractive clinical tools, but answers are highly subjective and affected by a multitude of factors[4]. But, they provide a patient perspective, which in itself is an important part of functional status. Self-report measures also involve the patient in the decision-making process, which has been associated with improved patient outcome[5]. More objective than self-report measures are performance-based measures, which assess the ability to perform everyday tasks. These can be divided into clinical- or laboratory-based and real-world measures[6]. In contrast to self-report and real-world measures (for example, step-counters and accelerometers), which account for real-world experiences over a period of time, laboratory-based measures assess performance on defined tasks in an artificial environment and within a limited time period. Examples of laboratory-based measures used with LLAs include walking tests, such as the six-minute walk test and tests involving sit-to-stand and turning, such as the timed-up-and-go test (TUGT)[1]. Three-dimensional gait analysis (3DGA) is another such example. It is considered the assessment of choice for gait because it measures gait dynamics in detail with a high level of reliability[7,8]. Its low level of use with the LLA population has been attributed to its financial, personnel and time cost[9].
The correlation between outcome measures, including 3DGA, remains relatively unknown, particularly within the LLA population. Research into older people found that gait speed predicted self-perceived physical functioning[10], but the relationships between self-administered, interview-administered and performance-based measures was inconsistent and the strength of correlation ranged from weak to moderate[11,12]. Amongst diabetics and LLAs, research has shown that self-reported activity levels do not correlate with performance-based measures[6,13]. Relationships between 3DGA and other outcome measures have been investigated in the context of paediatric cerebral palsy, where gait analysis has demonstrated moderate to strong correlation with measures derived from observational gait analysis[14-17] and parent-report measures[18,19]. Gait velocity however was representative of functional capacity in children with cerebral palsy[20]. Archer et al[21] investigated the relationship between clinical factors, such as range of motion and strength, and observed gait deviation following lower extremity trauma, however excluded LLAs. Establishing the correlation between these outcome measures is important in order to: Assist in the development of appropriate research methods; assist in the interpretation of research results; advocate for resources to develop assessment facilities; and, identify the most appropriate assessments for individuals and populations. This paper will help establish the utility of selected measures in predicting gait deviation and contribute to the selection in research and clinical applications of cost-effective alternatives to 3DGA for the lower limb amputee population.
The aims of this study were to examine correlations between a selection of common outcome measures used to assess gait deviation and function in individuals with LLA, and to quantify kinematic deviation using a subset of these common outcome measures. This study was designed with the premise that 3DGA is the “gold standard” for measuring gait pathology, and it was hypothesised that simple outcome measures can be used to quantify overall kinematic deviation.
Ethics approval was obtained (University of New South Wales Human Research Ethics Committee, UNSW HREC 07247), and 20 unilateral LLAs and 28 able-bodied participants were recruited using direct mail to a number of support groups. Informed consent was obtained prior to participation in this study. Exclusion criteria for the LLA group included multiple amputations, upper limb amputations, amputations at a level other than transfemoral or transtibial, less than six months consistent prosthesis use, use of walking aids other than walking sticks, or cognitive disabilities. Able-bodied participants were included to create a normative database similar in age and body mass index to the LLA group. The exclusion criterion for the able-bodied participants was known gait pathology. Individuals aged less than 18 years were excluded.
Participants underwent 3DGA wearing their everyday prosthesis (with shoes) and using regular walking aids (if normally used) at UNSW’s Gait and Biomechanics Laboratory using an eight-camera Vicon 612 motion capture system (Oxford Metrics). Initial contact was detected by one of two embedded force plates (Kistler) located at the midpoint of the 15-m walkway. Markers were placed according to a modified Helen Hayes marker set[22] with additional markers placed over the anterior portion of the pelvis to address anterior pelvic marker dropout during the gait cycle[23]. Participants were recorded at a comfortable self-selected walking speed (SSWS), and at least six successful trials were collected for each limb. Success was defined by a complete foot strike of at least one of the in-ground force plates.
Following 3DGA, participants completed two performance-based tests - TUGT and the 6MWT as described in the literature[24,25]. Both measures have demonstrated validity for use with LLAs[26,27]. Practices were permitted for the TUGT, which was conducted three times. Participants completed a self-report measure, the PEQ, in rest periods throughout the test protocol. Able-bodied participants underwent the same protocol, but did not complete the PEQ.
Lower limb kinematics and temporospatial data were calculated using the Plug-In-Gait model (Vicon, Oxford Metrics). Step-length (SL) and SSWS were normalised against average leg-length for each participant. Leg-length was defined as the distance between the anterior superior iliac spine and the medial malleolus on the same side, and the arithmetic mean of the left and right leg-length formed the average leg-length value used for normalisation. The gait deviation index (GDI) was calculated using the template provided by its authors[28]. For the amputee group, the GDI was calculated for six trials per limb per participants and averaged to obtain the value used in subsequent analyses. A representative trial from the left and right limb was used from each able-bodied participant, and contributed to the normative database required for the calculation of the GDI. In doing so, the GDI distribution for the able-bodied participants has a mean value of 100, with every 10 points below equal to one standard deviation away from the mean. The average of the three TUGTs was used in further statistical analyses. The time taken to stand (tstand) was derived from the TUGT. Both the summary scales and individual questions from the PEQ were utilised.
Normalcy of data was assessed using the Anderson-Darling test. Summary statistics were calculated using measures appropriate to their distribution - mean and standard deviation for normal distributions, and median and interquartile range for non-normal distributions. Analysis of variance was used to compare results between the able-bodied group, transtibial amputee group and transfemoral amputee group for normally distributed data (Table 1). Kruskal-Wallis one-way analysis of variance was used for data that did not conform to a normal distribution. A P-value of less than 0.05 was considered significant.
| Participant characteristics | Summary statistic | Transtibial | Transfemoral | Able-bodied |
| Number | Count | 12 | 8 | 28 |
| Number of women | Count | 3 | 3 | 16 |
| Age (yr) | Mean (SD) | 61.7 (12.6) | 63.3 (12.0) | 60.6 (7.8) |
| BMI (kg/m2) | Mean (SD) | 27.3 (6.5) | 25.4 (4.4) | 25.6 (3.1) |
| Ageamp (yr) | Mean (SD) | 40.9 (19.2) | 38.9 (23.0) | NA |
| Time (yr) | Median (IQR) | 17.0 (27.3) | 22.5 (38.5) | NA |
| Use (h/d) | Median (IQR) | 15.5 (1.0) | 13.0 (10.0) | NA |
| Performance-based outcomes | ||||
| GDI (-)a | Mean (SD) | 81.2 (13.6) | 68.8 (8.8) | NA |
| nSL (-)ab | Mean (SD) | 0.76 (0.11) | 0.65 (0.10) | 0.87 (0.06) |
| nSSWS (/s)ab | Mean (SD) | 1.36 (0.27) | 1.01 (0.23) | 1.72 (0.19) |
| TUGT (s)ab | Median (IQR) | 10.0 (2.0) | 12.7 (7.5) | 7.9 (1.4) |
| 6MWD (m)ab | Mean (SD) | 412 (91) | 295 (85) | 520.3 (56.2) |
| Self reported outcomes | ||||
| AM (/100) | Mean (SD) | 78.4 (18.5) | 64.0 (19.9) | NA |
| AP (/100) | Mean (SD) | 72.2 (14.5) | 63.0 (14.2) | NA |
| FR (/100) | Median (IQR) | 76.0 (64.4) | 67.6 (59.6) | NA |
| PR (/100) | Median (IQR) | 94.7 (15.1) | 95.8 (20.9) | NA |
| RL (/100) | Mean (SD) | 63.4 (24.3) | 64.7 (25.4) | NA |
| SB (/100)a | Median (IQR) | 93.6 (11.3) | 80.13 (34.6) | NA |
| SO (/100) | Median (IQR) | 70.5 (46.0) | 85.3 (67.1) | NA |
| UT (/100) | Median (IQR) | 77.9 (18.0) | 68.3 (44.7) | NA |
| WB (/100) | Median (IQR) | 86.2 (23.4) | 53.8 (61.2) | NA |
Spearman’s rank correlation coefficient, ρ, was used to determine the relationships between the GDI and participant characteristics, performance-based measures and self-report measures. Strict significant criteria for the correlation coefficient were required to minimise the chance of coincidental findings, possible due to the large number of relationships investigated in this study[29]. Significance was set at P ≤ 0.001, or |ρ| ≥ 0.70.
Stepwise regression analyses were used to determine the major predictors of the GDI (dependent variable), with participant characteristics (as listed in Table 1 and including aetiology), performance-based measures and responses from the PEQ used as independent variables in the regression models. The alpha-to-enter and alpha-to-exclude were set to 0.2 to accommodate the small sample size[30]. Predicted R2 values were calculated using a leave one out cross-validation protocol. Three types of regression analyses were performed for various reasons. The GDI was the dependent variable in all models.
All independent variables: A purely explorative model, including all independent variables to determine the best possible predictors of the GDI.
Omission of SL relationships: Clinical utility requires that reliance on instrumentation be minimised. Of the outcome measures adopted in this study, with the exception of the GDI, instrumentation was required only for the calculation of SL. Other measures needed little more than a stopwatch to obtain. SL relationships were omitted from the second regression analysis to minimise the need for instrumentation and consider applicability.
Forced inclusion of walking speed relationships, omission of SL relationships: Walking speed is often considered a robust measure of functional ability[1] in population groups with movement disorders. This was investigated in the final regression analysis by forcing the inclusion of walking speed relationships as independent variables.
Since frustration is known to affect self-efficacy[31], responses to self-report measures will differ between participants reporting frustration and participants reporting an absence of frustration. The PEQ contains within it questions relating to frustration. To account for differences in self-efficacy, participants were separated based upon the presence (n = 16) and absence of frustration (n = 4) as measured by the frustration questions in the PEQ [Larger studies (n = 135) by our group have shown that approximately 75% of LLAs experience some form of frustration as measured by the PEQ]. Regression analyses were performed using only participants reporting frustration. The small sample size prohibited separate analysis of the participants who were not frustrated.
The utility of a regression equation in diagnosing presence of a gait pathology was assessed by constructing receiver operating characteristic (ROC) curves as follows. Participants were classified as either pathological or non-pathological according to their measured GDI and a chosen cut-off, GDImeas,cut. In this study, a range of cut-off values for GDImeas,cut were investigated (65-95 in increments of five) because a definitive threshold for amputee gait is not yet available. They were then classified as pathological or non-pathological according to the GDI predicted by the regression equation and a range of cut-off values, GDIpred,cut (55-105, as determined by the GDIpred of each amputee participant). Finally, sensitivity and specificity were calculated for each value of GDIpred,cut and plotted as sensitivity against 1 - specificity. The area under the curve (AUC) was used as an overall measure of performance (AUC = 1 is perfect, AUC = 0.5 is no better than random[32]). The significance of the two-by-two classification table for a specific value of GDIpred,cut was assessed using Fisher’s exact test.
ROC curve analyses were performed using MedCalc for Windows, version 11.4.2.0 (MedCalc Software, Mariakerke, Belgium). All other analyses, unless otherwise stated, were performed at the 0.05 significance level using Minitab Statistical Software (Version 15).
The participant characteristics are summarised in Table 1. The sample was predominantly male (70%) with trauma being the most common reason for amputation (65%). Other reasons for amputation included cancer (10%), infection (10%) and vascular insufficiencies (15%). Two participants with transfemoral amputation used a walking stick during testing; all other participants completed testing unaided. The participant characteristics were similar for the able-bodied group, transtibial amputee group and transfemoral amputee group (Table 1).
Results for the self-report and performance-based measures are summarised in Table 1. Significant differences were present between the transfemoral and transtibial amputee groups for all performance variables. The transtibial amputee group reported values closer to able-bodied than the transfemoral amputee groups, but significant differences existed between the amputee groups and able-bodied participants. The transfemoral and transtibial amputees were similar for all scales of the PEQ, except Social Burden, where the transfemoral amputee group reported greater feelings of burden on friends and family as a result of their amputation.
The relationship between the GDI and participant characteristics, performance variables and scales of the PEQ are summarised in Table 2. The GDI demonstrated significant relationships with normalised average step-length, normalised self-selected walking speed (nSSWS) and the 6MWD. Significant correlations were not observed between the GDI, participant characteristics and scales from the PEQ.
| Parameter | Correlation coefficient, ρ |
| Participant characteristics | |
| Age | -0.13 |
| BMI | -0.27 |
| Age at amputation | -0.16 |
| Time since amputation | 0.14 |
| Performance-based outcomes | |
| nSLpro | 0.73b |
| nSLint | 0.83b |
| nSLave | 0.78b |
| nSSWS | 0.7b |
| TUGT | -0.60 |
| 6MWD | 0.74b |
| Self-report measures | |
| Ambulation | 0.44 |
| Appearance | 0.22 |
| Frustration | -0.14 |
| Perceived response | 0.02 |
| Residual limb health | -0.22 |
| Social burden | 0.37 |
| Sounds | -0.01 |
| Utility | 0.33 |
| Well-being | 0.20 |
Table 3 presents the correlation coefficients for the relationships between the performance-based measures used in this study. The strongest correlation was between nSSWS and 6MWD (ρ = 0.96), and all correlations were significant.
| nSLpro | nSLint | nSLave | nSSWS | TUGT | |
| nSLint | 0.87 | ||||
| nSLave | 0.98 | 0.95 | |||
| nSSWS | 0.84 | 0.91 | 0.88 | ||
| TUGT | -0.70 | -0.71 | -0.71 | -0.82 | |
| 6MWD | 0.86 | 0.89 | 0.89 | 0.96 | -0.83 |
The results of multivariate analysis are summarised in Table 4. The 6MWD was the strongest individual predictor of GDI [adjusted R2 (R2adj) = 68.6, predictive R2 (R2pred) = 60.4], despite intact limb step length producing the greatest adjusted R2 value (R2adj = 70.9, R2pred = 56.8). The forced inclusion of SSWS produced regression equations with the lowest adjusted and predicted R2 values. The time taken to stand from a chair with arms (tstand; derived from the TUGT) and mobility-related questions (particularly AM_C “Over the past four weeks, rate your ability to walk up stair when using your prosthesis”, see Table 4) contributed significantly to all regression equations with at least two independent variables.
| Independent variables | ||||||
| No. | R2adj | R2pred | 1 | 2 | 3 | 4 |
| All variables | ||||||
| 1 | 70.9 | 56.8 | nSLint | |||
| 2 | 76.1 | 59.6 | nSLint | AM_C | ||
| 3 | 82.6 | 66.5 | nSLint | AM_C | tstand | |
| 4 | 89.3 | 81.4 | nSLint | AM_C | tstand | 6MWD |
| No step-length parameters | ||||||
| 1 | 68.6 | 60.4 | 6MWD | |||
| 2 | 79.8 | 72.0 | 6MWD | AM_C | ||
| 3 | 86.1 | 82.7 | 6MWD | AM_C | tstand | |
| 4 | 90.2 | 86.2 | 6MWD | AM_C | tstand | Age |
| Forced inclusion of walking speed | ||||||
| 1 | 57.4 | 46.4 | nSSWS | |||
| 2 | 71.1 | 53.7 | nSSWS | AM_C | ||
| 3 | 79.6 | 65.2 | nSSWS | AM_C | tstand | |
| 4 | 82.1 | 70.7 | nSSWS | AM_C | tstand | UT_D1 |
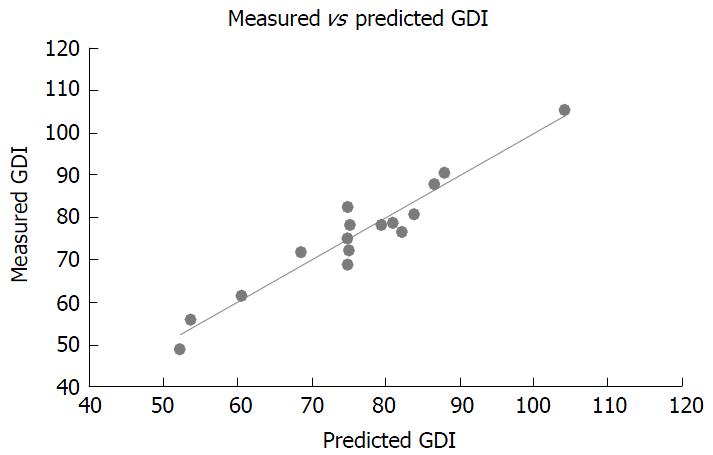
The equation selected for further analysis predicted GDI using 6MWD, AM_C, tstand and age (R2adj = 90.2; R2pred = 86.2; Table 4). It was chosen because of its superior predictive strength and clinical applicability when compared to other regression equations (Table 4). The plot of measured GDI against predicted GDI shown in Figure 1 illustrates the concordance between measured and predicted values for participants in this study.
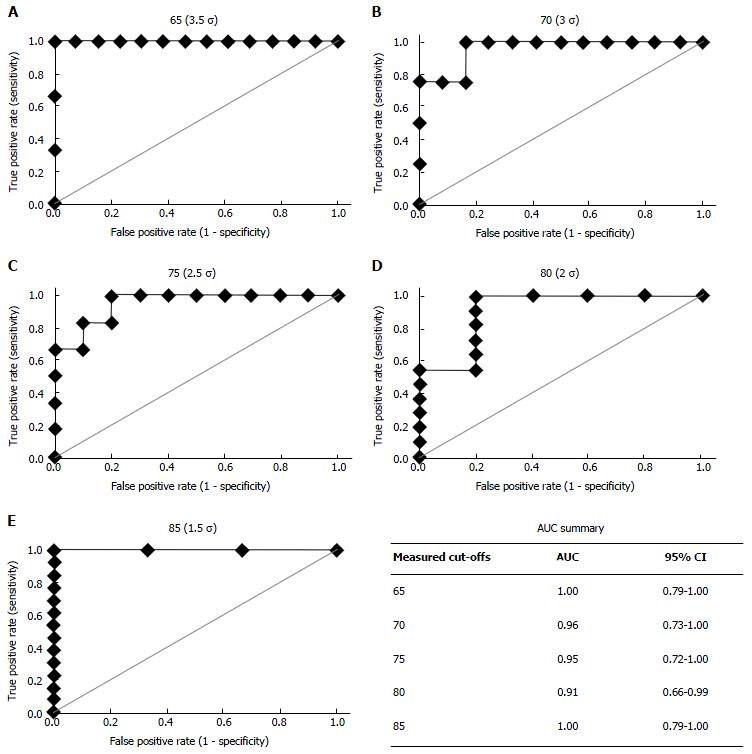
The resulting ROC curves for a range of measured cut-offs (65-85) are shown in Figure 2. Also in this figure are mean values and 95%CI for AUC for each of the measured cut-offs. The curves and AUC values showed that the diagnostic capability of the regression equation was not sensitive to choice of measured cut-off. Fisher’s exact test of the 2 × 2 classification table gave P < 0.05 for all ROC curves.
This study has shown that it is possible to predict overall gait deviation, as measured by the GDI, using combinations of simple performance-based and self-report outcome measures in a sample of persons with lower-limb amputation. Of the outcome measures investigated in this study, temporospatial data were the strongest correlates of the GDI (Table 2).
The strongest correlation was observed between the intact limb SL and the GDI (Table 2). This parameter provides insight into the extent of gait asymmetry, which is considered an indication of gait pathology[33], and explains its strong correlation with the GDI. Asymmetries in prosthetic gait have been attributed to a number of factors, including lack of plantarflexion and decreased range of motion of the prosthetic ankle joint, absence of proprioception and sensory feedback, pain, and prosthetic alignment[34]. Despite good predictive abilities, these equations are not clinically practical, requiring non-standard technology for the measurement of SL.
Unlike SL, 6MWD and SSWS can be measured with relative ease in clinical contexts. SL and SSWS were mutually exclusive in regression models because of their strong correlation with one another. The 6MWD was more strongly associated with the GDI than walking speed (Table 2) most likely because it is strongly correlated with energy expenditure[35], and energy expenditure is correlated with gait deviation[36]. In this study, energy expenditure would have had only minimal impact on SSWS, because the latter was calculated over a distance of only 15 m. The inclusion of 6MWD (Table 4) in the first regression model provides further evidence that energy expenditure is better correlated than walking speed over short distances with gait deviation.
One goal of this study was to develop a set of simple tests that can be used to identify patients whose gait is sufficiently impaired to warrant intervention. The ability of a regression equation to predict measured GDI is encouraging, but an R2pred is a measure of agreement, not a measure of the performance of the equation when used as a diagnostic tool. On the other hand, the ROC curve does provide an overall measure of performance. The areas under the curve (all greater than 0.7; Figure 2), implies that this can be an effective diagnostic tool. Fisher’s exact test applied to a range of measured cut-off values confirmed that that the selected regression equation was better than chance, P < 0.05.
The results from this study indicate that using a battery of outcome measures (excluding 3DGA) in combination provides a better perspective on functional status than using single or only a few measures. This is encouraging, because it implies that low cost and more readily available outcome measures can be highly informative. For example, the effects on function and gait deviation that may arise with changes to componentry would be best assessed using a standardised battery of outcome measures rather than an ad hoc selection of single measures. The statistical analyses demonstrate that characteristics observed across a number of outcome measures may contribute collectively to the quantification of kinematic deviation. In this study, a combination of performance-based and self-report measures provided the best indication of kinematic deviation. The results from this study have shown the ideal outcome measures to assess gait deviation to be: 6MWD, tstand, self-report questions addressing stair climbing and chronological age. Walking speed over longer distances provides a better indication of gait deviation than SSWS over short distances (< 15 m).
The sample size was small and comprised mainly traumatic amputees and experienced prosthetic users. This study did not assess test responsiveness, a necessity for clinical utility. Future work should investigate the responsiveness of the regression models either in response to rehabilitation or componentry modifications, and extend the sample to include more participants with various aetiologies, levels of amputation and prosthetic experience.
The GDI is an overall summary measure of kinematic patterns. It does not, and cannot, substitute for clinical experience and 3DGA. Rather, it provides an efficient method to communicate overall gait pathology. The GDI has demonstrated applicability for use with children with cerebral palsy[28] and adults with unilateral lower limb amputation[37], making it an appropriate outcome measure for use in this study. In addition, kinetic characteristics were excluded, as were data on muscle activation patterns. Both are important measures of gait biomechanics.
Time to stand was derived from the TUGT. In lower functioning individuals there was a clear demarcation between the time to stand and the initiation of walking gait, making the measurement of the time taken to stand relatively straightforward. In contrast, the transition between the standing phased and initiation of walking was sometimes difficult to discern in high functioning individuals. Some of these participants tucked the intact limb under the chair prior to the start of the test to facilitate forward progression during the standing phase of the TUGT. Where this occurred, the time to stand was recorded as the point at which the trailing leg aligned with the stance limb. Future studies should consider using a designated sit-to-stand test, and contemplate using multiple sit-to-stand assessment such as the five-times sit-to-stand test due to their demonstrated correlation with functional status in older people[38].
This study offers a practical alternative to quantifying kinematic deviation without conducting complete 3DGA. Performance-based measures were strong correlates of the GDI, thus rejecting the null hypothesis. It was possible to predict the GDI using a combination of performance-based measures and self-report items related to mobility and prosthetic utility. Accuracy was reasonably high for a range of designated cut-off points for the GDI. Further work is required to determine appropriate GDI cut-off points for each level of amputation.
The authors would like to thank: The Amputee Association of NSW, Inc., and its affiliated branches, for their assistance with recruitment; and Mrs Deborah Vickers for her role in data collection.
Successful prosthetic fitting is reliant on the appropriate matching of functional ability to prosthetic componentry. But, functional status is not a straightforward concept and its measurement even less so. There are numerous outcome measures currently available, and selection of appropriate measures for the assessment of functional status following lower-limb amputation is complicated. Identifying the smallest subset of appropriate measures required to encapsulate functional status could encourage the systemic and standardised use of outcomes measures throughout the rehabilitation system.
In comparing the benefits of using self-report vs performance-based measures with older persons, it was shown that neither type of measure is superior, nor are these measures interchangeable. Instead, each assess distinct, although related constructs. Further, self-report and performance-based measures may be complementary. Studies have shown that by complementing performance-based measures with self-report measures it was possible to improve prognostic information in a sample of older person. The challenge remains to determine a suitable combination of self-report and performance-based measures to adequately assess functional status in individuals with lower-limb amputation.
This study has provided a set of simple self-report and performance-based measures to facilitate evaluation of functional status in the physical domain. In this study, functional status was best assessed using a timed walking test, a sit-to-stand assessment and an evaluation of advanced levels of mobility such as stair ambulation. These measures, in combination, enable calculation of overall kinematic deviation in prosthetic users.
The subset of outcome measures developed in this study that are able to assess kinematic deviation and functional status (in the physical domain) in individuals with lower-limb amputation require no more than a stopwatch and a self-administered questionnaire. All are relatively straightforward to implement in clinical practice. It is hoped that this research will contribute to the development of a standardised set of outcome measures for use within the lower-limb amputation population group, which in turn, will facilitate comparison between rehabilitation facilities and ultimately result in improved outcomes for individuals with lower-limb amputation.
GDI: Gait deviation index; a one-dimensional index that summarises kinematic deviation; ROC curve: Receiver operator characteristic curve; illustrates the sensitivity and specificity of a diagnostic test.
This study provides a great foundation for clinicians and researchers looking for simple, low cost objective and subjective measures to approximate more complex gait analysis. Study design and statistical analysis were appropriate.
P- Reviewer: Guerado E, Metzger PD S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Condie E, Scott H, Treweek S. Lower limb prosthetic outcome measures: A review of the literature 1995 to 2005. JPO. 2006;18:P13-P45. [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23352] [Cited by in F6Publishing: 22717] [Article Influence: 709.9] [Reference Citation Analysis (0)] |
| 3. | Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79:931-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 4. | Kempen GI, van Heuvelen MJ, van den Brink RH, Kooijman AC, Klein M, Houx PJ, Ormel J. Factors affecting contrasting results between self-reported and performance-based levels of physical limitation. Age Ageing. 1996;25:458-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Carr AJ, Donovan JL. Why doctors and patients disagree. Br J Rheumatol. 1998;37:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Smith DG, Domholdt E, Coleman KL, Del Aguila MA, Boone DA. Ambulatory activity in men with diabetes: relationship between self-reported and real-world performance-based measures. J Rehabil Res Dev. 2004;41:571-580. [PubMed] [Cited in This Article: ] |
| 7. | DeLuzio KJ, Wyss UP, Li J, Costigan PA. A procedure to validate three-dimensional motion assessment systems. J Biomech. 1993;26:753-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | McGinley JL, Baker R, Wolfe R, Morris ME. The reliability of three-dimensional kinematic gait measurements: a systematic review. Gait Posture. 2009;29:360-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 665] [Cited by in F6Publishing: 608] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 9. | Czerniecki JM, Gitter AJ. Gait analysis in the amputee: Has it helped the amputee or contributed to the development of improved prosthetic components? Gait Posture. 1996;4:258-268. [DOI] [Cited in This Article: ] |
| 10. | Cress ME, Schechtman KB, Mulrow CD, Fiatarone MA, Gerety MB, Buchner DM. Relationship between physical performance and self-perceived physical function. J Am Geriatr Soc. 1995;43:93-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 183] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Reuben DB, Valle LA, Hays RD, Siu AL. Measuring physical function in community-dwelling older persons: a comparison of self-administered, interviewer-administered, and performance-based measures. J Am Geriatr Soc. 1995;43:17-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 175] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Sherman SE, Reuben D. Measures of functional status in community-dwelling elders. J Gen Intern Med. 1998;13:817-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Stepien JM, Cavenett S, Taylor L, Crotty M. Activity levels among lower-limb amputees: self-report versus step activity monitor. Arch Phys Med Rehabil. 2007;88:896-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Damiano DL, Abel MF. Relation of gait analysis to gross motor function in cerebral palsy. Dev Med Child Neurol. 1996;38:389-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Hillman SJ, Hazlewood ME, Schwartz MH, van der Linden ML, Robb JE. Correlation of the Edinburgh Gait Score with the Gillette Gait Index, the Gillette Functional Assessment Questionnaire, and dimensionless speed. J Pediatr Orthop. 2007;27:7-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 16. | Romei M, Galli M, Fazzi E, Maraucci I, Schwartz M, Uggetti C, Crivellini M. Analysis of the correlation between three methods used in the assessment of children with cerebral palsy. Funct Neurol. 2007;22:17-21. [PubMed] [Cited in This Article: ] |
| 17. | Wren TA, Do KP, Hara R, Dorey FJ, Kay RM, Otsuka NY. Gillette Gait Index as a gait analysis summary measure: comparison with qualitative visual assessments of overall gait. J Pediatr Orthop. 2007;27:765-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 18. | Novacheck TF, Stout JL, Tervo R. Reliability and validity of the Gillette Functional Assessment Questionnaire as an outcome measure in children with walking disabilities. J Pediatr Orthop. 2000;20:75-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Tervo RC, Azuma S, Stout J, Novacheck T. Correlation between physical functioning and gait measures in children with cerebral palsy. Dev Med Child Neurol. 2002;44:185-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Drouin LM, Malouin F, Richards CL, Marcoux S. Correlation between the gross motor function measure scores and gait spatiotemporal measures in children with neurological impairments. Dev Med Child Neurol. 1996;38:1007-1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Archer KR, Castillo RC, Mackenzie EJ, Bosse MJ. Gait symmetry and walking speed analysis following lower-extremity trauma. Phys Ther. 2006;86:1630-1640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Kadaba MP, Ramakrishnan HK, Wootten ME, Gainey J, Gorton G, Cochran GV. Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. J Orthop Res. 1989;7:849-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | McClelland JA, Webster KE, Grant C, Feller J. Alternative modelling procedures for pelvic marker occlusion during motion analysis. Gait Posture. 2010;31:415-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6981] [Cited by in F6Publishing: 7597] [Article Influence: 345.3] [Reference Citation Analysis (0)] |
| 25. | Podsiadlo D, Richardson S. The timed “Up & amp; Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 26. | Lin SJ, Bose NH. Six-minute walk test in persons with transtibial amputation. Arch Phys Med Rehabil. 2008;89:2354-2359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Schoppen T, Boonstra A, Groothoff JW, de Vries J, Göeken LN, Eisma WH. The Timed “up and go” test: reliability and validity in persons with unilateral lower limb amputation. Arch Phys Med Rehabil. 1999;80:825-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 28. | Schwartz MH, Rozumalski A. The Gait Deviation Index: a new comprehensive index of gait pathology. Gait Posture. 2008;28:351-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 474] [Cited by in F6Publishing: 405] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 29. | Zar JH. Significance Testing of the Spearman Rank Correlation Coefficient. J Am Stat Assoc. 1972;67:578-580. [DOI] [Cited in This Article: ] |
| 30. | Steyerberg EW, Eijkemans MJ, Harrell FE, Habbema JD. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19:1059-1079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 31. | Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, NJ: Prentice Hall 1986; . [Cited in This Article: ] |
| 32. | Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561-577. [PubMed] [Cited in This Article: ] |
| 33. | Sadeghi H, Allard P, Prince F, Labelle H. Symmetry and limb dominance in able-bodied gait: a review. Gait Posture. 2000;12:34-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 646] [Cited by in F6Publishing: 584] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 34. | Nadollek H, Brauer S, Isles R. Outcomes after trans-tibial amputation: the relationship between quiet stance ability, strength of hip abductor muscles and gait. Physiother Res Int. 2002;7:203-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Ross RM, Murthy JN, Wollak ID, Jackson AS. The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm Med. 2010;10:31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 36. | Waters RL, Mulroy S. The energy expenditure of normal and pathologic gait. Gait Posture. 1999;9:207-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 716] [Cited by in F6Publishing: 598] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 37. | Kark L, Vickers D, McIntosh A, Simmons A. Use of gait summary measures with lower limb amputees. Gait Posture. 2012;35:238-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Walston JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52:625-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 640] [Cited by in F6Publishing: 632] [Article Influence: 31.6] [Reference Citation Analysis (0)] |