Published online Apr 18, 2014. doi: 10.5312/wjo.v5.i2.124
Revised: January 8, 2014
Accepted: January 15, 2014
Published online: April 18, 2014
Neuromuscular disorders are a group of diseases affecting the neuro-musculo-skeletal system. Children with neuromuscular disorders frequently develop progressive spinal deformities with cardio-respiratory compromise in the most severe cases. The incidence of neuromuscular scoliosis is variable, inversely correlated with ambulatory abilities and with a reported risk ranging from 80% to 100% in non-ambulatory patients. As surgical and peri-operative techniques have improved, more severely affected children with complex neuromuscular deformities and considerable co-morbidities are now believed to be candidates for extensive surgery for spinal deformity. This article aimed to provide a comprehensive review of how neuromuscular spinal deformities can affect normal spine balance and how these deformities can be treated with segmental instrumentation and sub-laminar devices. Older concepts have been integrated with newer scientific data to provide the reader with a basis for better understanding of how treatment of neuromuscular scoliosis has evolved over the past few decades. Recent advances, as well as challenges that remain to be overcome, in the surgical treatment of neuromuscular curves with sub-laminar devices and in the management of post-operative infections are outlined.
Core tip: In patients with neuromuscular disease, the likelihood and severity of the scoliosis increase with the degree of neuromuscular involvement. There is little doubt that segmental instrumentation techniques have revolutionized the care of patients with neuromuscular scoliosis by providing lasting correction and significant relief of pain and by restoring quality of life and sitting position. The state of knowledge regarding neuromuscular scoliosis is a dynamic process, and a current literature review is mandatory. The somewhat large bibliography for this subject reflects the many opinions and findings currently available.
- Citation: Canavese F, Rousset M, Le Gledic B, Samba A, Dimeglio A. Surgical advances in the treatment of neuromuscular scoliosis. World J Orthop 2014; 5(2): 124-133
- URL: https://www.wjgnet.com/2218-5836/full/v5/i2/124.htm
- DOI: https://dx.doi.org/10.5312/wjo.v5.i2.124
Scoliosis is a three-dimensional deformity of the spine with lateral, antero-posterior and rotational components. In most cases, the disease is idiopathic. Non-idiopathic cases are often secondary to neuromuscular diseases affecting the neuro-musculo-skeletal system.
Children with neuromuscular disorders frequently develop progressive spinal deformities, with cardio-respiratory compromise in the most severe cases. Among patients with neuromuscular disorders, the probability of developing scoliosis is inversely correlated with ambulatory ability, with a reported risk ranging from 80% to 100% in non-ambulatory patients (Table 1). Patients with neuromuscular disorders have many similarities in curve patterns, despite different etiologies of the main disease; therefore similar strategies are implemented for treatment.
| Diagnosis | Incidence of scoliosis |
| Cerebral palsy | 25% (GMFCS I and II) to 100% (GMFCS IV and V) |
| Charcot-Marie-Tooth disease | 30% |
| Myelodysplasia | 60% (lumbar level) to 100% (thoracic level) |
| Spinal muscular atrophy | 70% |
| Friedreich ataxia | 80% |
| Duchenne muscular dystrophy | 90%1 |
| Paralysis from spinal cord injury | 100% |
Neuromuscular scoliosis is characterized by a long collapsing spine, pelvic obliquity and changes in sagittal plane alignment that can affect sitting balance and cardio-respiratory function. Long C-shaped thoraco-lumbar and lumbar curves are very often diagnosed in patients with underlying neuromuscular pathologies. Associated negative predictors include osteopenia and concomitant congenital malformations, which are responsible for rapid progression (collapsing spine)[1-3].
Patients with neuromuscular disorders tend to develop scoliosis at younger ages than patients with idiopathic scoliosis, and a large proportion of neuromuscular curves are progressive and often non-responsive to orthotic management. Unlike idiopathic scoliosis, neuromuscular spine deformities can progress beyond skeletal maturity, particularly in wheelchair-bound patients[1,3-6].
Surgical treatment is more complex in neuromuscular scoliosis than in idiopathic scoliosis. Complex reconstruction can be necessary to obtain satisfactory results. However, a bone stock of poor quality, longer fusions, the frequent need for fusion to the pelvis and increased bleeding can significantly affect operative time and make such surgery difficult.
As surgical and peri-operative techniques have improved, more severely affected children with complex neuromuscular deformities and considerable co-morbidities are now believed to be candidates for extensive surgery for spinal deformity[1,4-6].
Pulmonary, neurologic, genitourinary, nutritional and gastroenterological comorbidities are common in patients with neuromuscular scoliosis and must be managed (pre- and post-operatively) by a multidisciplinary team of care providers. A multidisciplinary approach is the key to a successful outcome. Comorbidities often make corrective operations high-risk procedures. Orthopedic surgeons experienced in undertaking major spine reconstruction, anesthesiologists, pulmonologists, cardiologists, nutritionists and pediatricians must work together to evaluate and handle these complex surgical patients to obtain the best possible outcomes[1,3,7-9].
Furthermore, the post-operative complication rate is much higher (approximately 30%) in patients with neuromuscular deformities, compared to patients with idiopathic scoliosis. Therefore, the risk-to-benefit ratio is an important parameter that must be considered before surgery as the results can be gratifying if patients are properly selected[2,6,10].
Luque rods, or variations on the Luque technique, often remain the preferred instrumentation for neuromuscular curves. The success of treatment depends on the maintenance of a balanced spine on the coronal and sagittal planes over a level pelvis[11-13].
This article aims to provide a comprehensive review of how neuromuscular spinal deformities can affect normal spine balance and how such deformities can be treated with segmental instrumentation and sub-laminar devices.
Older concepts have been integrated with newer scientific data to provide the reader with a basis for better understanding of how treatment of neuromuscular scoliosis has evolved over the past few decades. Recent advances, as well as challenges that remain to be overcome, are outlined in the surgical treatment of neuromuscular curves with sub-laminar devices and in the management of post-operative infections.
Surgical management of scoliotic curves in patients with neuromuscular conditions has evolved over the past five decades. Segmental fixation, sub-laminar wires, L-rods, unit-rods and sub-laminar bands have been progressively developed for the treatment of neuromuscular curves and they now form part of the armamentarium of the spinal surgeon in addressing such deformities. The surgical treatment must be adapted to the severity of the deformity and the neuromuscular disease. The treating surgeon should not be a prisoner of a single strategy; rather, the strategy should depend on the health of the patient (Table 2).
| Surgical risk | Walking abilities | Weight | Cardiac Function | Respiratory function (VC) | Sleep | Comorbidities |
| Average | Ambulatory | > 40 kg | Normal | Normal | Normal | No |
| Increased | Ambulates with aid | 20-40 kg | Reduced | Reduced, but > 50% | Hypersomnia | |
| High | Non ambulatory | < 20 kg or obese | Significantly impaired | < 50% | Nocturnal hypercapnic Hypoventilation, Obstructive sleep apnea | Yes |
The concept of segmental fixation was pioneered in 1963 by Resina and Alves from Portugal, by fixation with segmental wiring for the treatment of scoliotic curves[11].
Stainless steel wires are passed through a hole at the base of the spinous processes (one wire per vertebra) and are twisted around to two straight rods placed on either side of the spine.
This technique allows for translation of the spine and correction of the deformity (mostly on the frontal plane) using an even distribution of corrective forces.
Eduardo Luque from Mexico popularized sub-laminar wires to attach to L-shaped rods during the late 1970s (Table 3)[12].
| Decade-Year of publication | Authors | Patients (n) | Neuromuscular condition | Instrumentation | Complications (number of patients) |
| 1980-1989 | |||||
| 1982 | Allen et al[13] | 10 | Cerebral palsy | L-rod | |
| 1986 | Sponseller et al[14] | 34 | Cerebral palsy | Interspinous process instrumentation | |
| 1988 | Gersoff et al[16] | 33 | Cerebral palsy | L-rod | 5 deep wound infections |
| Complications rate: 15% | |||||
| 1989 | Broom et al[18] | 74 | Various | L-rod | 1 death; |
| 3 deep wound infections; | |||||
| 2 pressure sores; | |||||
| 6 sets of broken rods; | |||||
| 1 distal rotation and migration of the rod | |||||
| Complications rate: 18% | |||||
| 1989 | Boachie-Adjei et al[17] | 46 | Various | L-rod | 3 cases of pseudarthrosis; |
| 3 deaths | |||||
| Complications rate: 13% |
In Luque’s system, two L-shaped rods are placed on either side of the spine, and they are wired to each of the vertebrae. The L-rods are contoured or bent to conform to the curve and to provide proper sagittal alignment. The wires are threaded through the spinal canal at each vertebral level and are then twisted around the rods on each side of the spine. The wires are usually doubled (to reduce the risk of fracturing the lamina), with one end joined by a bead and the other by a loop. The beaded end is contoured by creating a small bend at the tip that will emerge on the cephalad side of the lamina. A flatter contour minimizes intrusion into the canal. However, it is important to bear in mind that once metal wires are passed under the lamina, great care must be taken to ensure that none of the operating team inadvertently pushes one of the wires into the canal. To minimize this danger, each wire should be temporarily bent over the lamina.
Segmental instrumentation with sub-laminar wires results in even distribution of corrective forces with two lateral fixation points on each segment, which provide good rotatory control. The rods apply pressure on the spine to correct the deformity[12-16].
The L-rods and wire constructs aim at translation and coronal and sagittal balancing, rather than derotation, as their principle, so the extent of derotation is not the purpose that is intended to be achieved with this technique. Because there are multiple points of fixation with the Luque technique, the patient does not have to wear a brace after surgery. Therefore, segmental instrumentation with sub-laminar wires has been widely adopted in the treatment of neuromuscular curves because it provides rigid fixation and allows for early mobilization without external support[12,16-18].
Overall, the technique has proved to be safe and relatively easy to perform with a relatively low complications rate, providing rigid fixation and predictable correction with minimal post-operative external support required, and it is applicable for a wide variety of spinal deformities, offers a high rate of fusion with a low incidence of failure of the instrumentation and provides sagittal plane correction comparable to more recent implants.
The unit-rod (U-rod) technique was developed in the late 1980s by Bell, Moseley and Koreska from Canada[19].
This technique uses a U-shaped, double, prebent rod, and it is a modification of Luque’s segmental instrumentation technique, which, in contrast, must link together two single L-shaped rods[20-22].
The distal portion of the U-rod is inserted into both iliac wings, while the middle and proximal portions are wired to sub-laminar wires threaded through the spinal canal at each vertebral level, from the upper thoracic (T1-T2) to the lower lumbar (L4-L5) spine. Sub-laminar wires are progressively twisted around the U-rod from caudal to cephalad to provide gradual deformity correction.
U-rod instrumentation has become a common, standard technique and the primary instrumentation system for the treatment of pediatric patients with neuromuscular spine deformities and pelvic obliquity (Tables 4 and 5). The technique is simple to apply, it is less expensive than most other systems, and it can achieve good deformity correction and a low reoperation rate[19].
| Decade-Year of publication | Authors | Patients(n) | Neuromuscular condition | Instrumentation | Complications(number of patients) | Outcome/Conclusions |
| 1990-1999 | ||||||
| 1991 | Gau et al[34] | 68 | Various | Luque-Galveston instrumentation | 14 hardware problems; | |
| 7 cases of pseudarthrosis; | ||||||
| 3 neurologic deficits | ||||||
| Complications rate: 35% | ||||||
| 1992 | Hopf et al[35] | 44 | Various | |||
| 1992 | Neustadt et al[36] | 18 | Various | CDI of the pelvis | 1 hardware failure; | Posterior spinal fusion with CDI of the pelvis is an effective treatment for patients with neuromuscular scoliosis. |
| 1 deep wound infection | ||||||
| Complications rate: 11% | ||||||
| 1992 | Onimus et al[37] | 32 | Cerebral palsy | 3 deaths; | Pain disappeared in 2/3 of cases; | |
| 10 other | sitting position was acquired in all the cases at follow-up; | |||||
| motor possibilities improved in 25% of cases; | ||||||
| Complications rate: 41% | associated medical pathologies were reduced in 67% of cases. | |||||
| 1996 | Sussman et al[38] | 25 | Cerebral palsy | L-rod | Posterior fusion and instrumentation from the upper thoracic spine to L5 without anterior fusion provides adequate correction and control of spinal deformity for many patients with cerebral palsy | |
| 1997 | Frischhut et al[39] | 41 | Various | 29 L-rod, Luque-Galveston, CDI and ISOLA; 12 Harrington instrumentation | 3 deep wound infections Complications rate: 7% | |
| 1997 | Marchesi et al[40] | 25 | Duchenne muscular dystrophy | L-rod with sacral screws | In every patient, a good sitting balance could be restored after surgery |
| Decade-Year of publication | Authors | Patients(n) | Neuromuscular condition | Instrumentation | Complications (number of patients) | Outcome/Complications |
| 2000-2011 | ||||||
| 2000 | Yazici et al[25] | 47 | Various | ISOLA-Galveston | 2 deep wound infections; | ISOLA-Galveston instrumentation is as safe and effective as other types of instrumentation |
| 2 hardware removals; | ||||||
| 4 cases of pseudarthrosis; | ||||||
| 1 pseudarthrosis repair | ||||||
| Complications rate: 19% | ||||||
| 2009 | Modi et al[20] | 52 | Cerebral palsy | U-rod and pedicle screws | 2 deaths; | U-rod with pedicle screws provides good frontal and sagittal plane correction, as well as pelvic obliquity improvement (56% correction) |
| 1 neurologic deficit; | ||||||
| 17 respiratory complications (atelectasia, pneumonia, hemothorax) | ||||||
| Complications rate: 38% | ||||||
| 2010 | Nectoux et al[21] | 28 | Cerebral palsy | Luque-Galveston, U-rod | 1 case of blindness; | |
| 1 death; | ||||||
| 16 respiratory complications (atelectasia, pneumonia, pneumothorax) | ||||||
| Complications rate: 64% | ||||||
| 2010 | Modi et al[22] | 27 | Spinal muscular atrophy and Duchenne muscular dystrophy | U-rod and pedicle screws | 1 death; | Although flaccid neuromuscular scoliosis can be corrected well with U-rod and posterior-only pedicle screws, there is a high rate of associated complications |
| 4 respiratory failure; | ||||||
| 2 neurological deficits; | ||||||
| 1 ileus; | ||||||
| 2 cases of atelectasia; | ||||||
| 3 UTIs; | ||||||
| 7 cases of coccydynia; | ||||||
| 1 rod dislodgement | ||||||
| Complications rate: 77% | ||||||
| 2011 | La Rosa et al[24] | 84 | Cerebral palsy | Universal clamps, hooks and L-rod | 5 respiratory complications | |
| Complications rate: 6% |
The sub-laminar band devices and technique were first described in 2009 by Mazda et al[23] from France. The technique is also known as the universal clamp technique (Table 5).
The technique of placing sub-laminar bands is similar to Luque’s wire technique. However, while Luque’s wires are made of steel or titanium alloy, the bands with this technique are made of acrylic or polyester material. The bands are supple and, once inserted, cannot be inadvertently pushed into the canal.
The sub-laminar system is composed of a connector, a band-locking set screw, a polyester or acrylic band and, depending on the manufacturer, a rod-locking set screw.
Compared to Luque’s metal wires, the technique described by Mazda et al[23] allows the surgeon to perform progressive tensioning and deformity correction because of the simplicity of the implant and the tensioning of the strips[24].
Sub-laminar bands have the same stress resistance as steel or titanium alloy sub-laminar wires. Moreover, the increased contact area between the bands and bone improves corrective forces and reduces laminar fracture risk[24].
Today, band-only and hybrid constructs, with lumbar transpedicular screws, thoracic sub-laminar bands and pedicle-transverse hooks at the upper end of the curve, have become widely used and have been shown to provide good correction of spinal deformities, as well as reduced operating time, radiation exposure, and blood loss, compared to all-screw constructs.
Patients can be treated either with band-only or hybrid instrumentation.
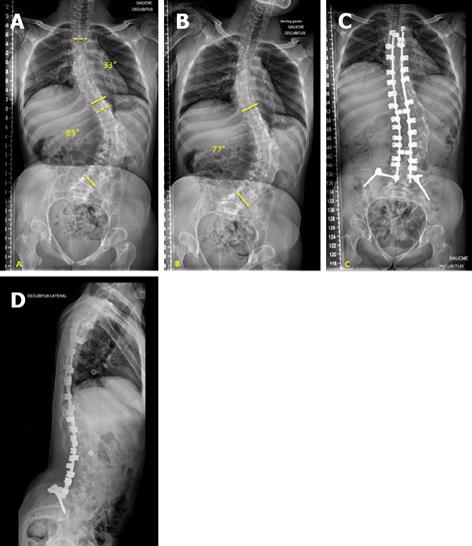
Band-only instrumentation (Figure 1) is a construct characterized by two bilateral claws at the upper instrumented vertebra (one per side) and sub-laminar bands as thoracic and lumbar anchorages. Band-only instrumentation should be preferred in non-ambulatory patients.
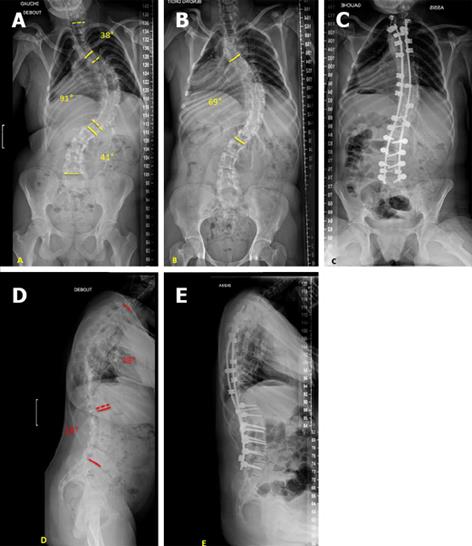
The hybrid construct (Figure 2) consists of two bilateral claws at the upper instrumented vertebra (one per side), multiple transpedicular screws as distal anchorages and sub-laminar bands between the upper claws and distal screws. Hybrid instrumentation can be used in both ambulatory and non-ambulatory patients.
In cases of severe pelvic obliquity, iliac screws can be added to both constructs. Moreover, Ponte’s posterior osteotomies can be performed at and around the apex for rigid curves (less than 30% reduction on bending films).
Instrumentation is performed with the PASS® LP side connection segmental system (MEDICREA, Neyron, France), using 5.5 mm titanium (Ti) or cobalt-chrome (Co-Cr) rods, pedicle screws, auto-stable claws, cross-links and various rod-anchorage connectors locked by nuts.
Transpedicular screws can be inserted with the free-hand technique, and they are mostly placed at the lumbar level.
Auto-stable claws consist of a main pedicular hook and a counter-hook, which can be placed under the lamina or above the transverse process of the upper instrumented vertebra (thoracic region). Upper claws and lumbar screws should be placed before band insertion.
Sub-laminar fixation is performed with the LigaPASS® system (MEDICREA, Neyron, France), which consists of a titanium alloy connector, a rod-locking set screw, a polyester band and a band-locking set screw.
A portion of the ligamentum flavum must be removed from each intervertebral space. Once the canal is opened, the bands can be placed from caudal to cephalad.
Each band ends with malleable Ti leads. The malleable Ti end is contoured by creating a small bend at the tip that will emerge on the cephalad side of the lamina. Contouring the malleable Ti leads helps to slide the band under the lamina from caudal to cephalad, with a very low risk of damaging the thecal sac.
If two bands must be slid under the same lamina, the second band placement can be facilitated using the first band as a guide. The second band should be inserted between the first band and the lamina. By doing so, the thecal sac is protected throughout the whole insertion maneuver of the second band. Moreover, insertion is easier as the first band can be glided from caudal to cephalad, thus bringing the second band into its movement.
The polyester band can be simply passed under the lamina or inserted in a figure-8 belt (under the lamina and around the transverse process). In any case, attention should be paid to ensuring that the band is not twisted during the passing maneuver.
Once band placement is completed, all the connectors can be slid on the previously contoured rod. Subsequently, the two extremities of each band are introduced into the opening situated below the band-locking set screw, ensuring that each end of the band is paired correctly and inserted through the corresponding connector.
The rods are then placed and properly oriented on both the sagittal and coronal planes without any attempt to reduce the deformity. In hybrid constructs, rods can be locked to the lowest lumbar transpedicular screws and pelvic screws. If band-only constructs are used, the rods are locked to pelvic screws. Locking of the rods stabilizes the construct and avoids undesired rotation during band tensioning. The rods should not be locked to the upper claws to allow for rod movements during band tensioning and subsequent deformity correction.
The LigaPASS connectors must be locked perpendicularly to the rod on the coronal plane by setting the rod-locking set screw. This procedure prevents the connector from rotating around the rod and creates a platform for self-stable band tensioning.
The reduction is performed sequentially by progressively tensioning all the bands with the tension pulleys. This process gradually translates the spine toward the rods and reduces the deformity by sharing forces among all the implants. The tension applied to each band can be assessed by observing the strain gauge on the pulley.
Maximum reduction is achieved when the band connector is seated on the vertebra. Once the desired reduction is achieved, the band is locked within the connector by tightening the band-locking set screw. At this point, the remaining screws and upper claws can also be locked.
In neuromuscular scoliosis, bracing is usually not effective, and surgery becomes the primary treatment option[8].
The type of spinal stabilization is influenced by the age of the patient, the severity of the deformity, the ambulatory status, and the underlying neuromuscular condition.
Posterior instrumentation for neuromuscular deformity treatment should be segmental and low-profile, with sound pelvic purchase if needed[5,11,18,25]. In all cases, fitness for surgery and psychological status should be assessed prior to surgery, as the results can be gratifying if patients are properly selected.
Surgical treatment is indicated in large curves (> 50°) and in curves progressing beyond skeletal maturity. However, puberty can begin earlier or, more frequently, later in patients with neuromuscular disease (than the puberty of children with idiopathic curves)[1]. Depending on the neuromuscular disease, the rate of progression of the scoliotic deformity during pubertal growth spurt can increase by 2° to 4° per month, especially in patients who are wheelchair-bound. Scoliosis continues to progress beyond skeletal maturity at a rate of approximately 1° to 4° per year if the curvature is greater than 50° at the end of growth, compared to approximately 0.5° to 1° per year for curves of less than 50°[1,8,26].
In addition, neuromuscular curves are responsible overall for a greater decrease in lung volumes compared to idiopathic curves, which, in contrast, are characterized by normal muscle function[27-29].
Sleep-disordered breathing, with or without nocturnal hypercapnic hypoventilation, is a common complication of respiratory muscle weakness in children and adolescents with neuromuscular disorders (Table 2). Nocturnal hypercapnic hypoventilation is a sign of respiratory muscle fatigue and a poor prognosis. It is recommended to perform a polysomnographic evaluation, searching for sleep-disordered breathing in patients with neuromuscular compromise and spinal deformities. Children with neuromuscular scoliosis are at risk for sleep-disordered breathing when the inspiratory vital capacity is less than 60% during daytime hours. Moreover, they are at risk for hypercapnic hypoventilation during the nighttime if their inspiratory vital capacity is less than 40%, and PaCO2 is greater than 40 mmHg[30].
Overall, the indications for surgery are: (1) A significant curve resulting in functional disturbance and/or cardio-respiratory compromise; (2) A progressive spinal deformity not controllable with orthosis; (3) A small curve with inevitable progression; and (4) Painful deformities.
The goals of surgical treatment are: (1) To prevent curve progression; (2) To maintain the spine balanced on the coronal and sagittal planes, with a level and upright trunk position; (3) To provide a balanced and comfortable sitting position to reduce repositioning; (4) To reduce pain; (5) To reduce the discomfort caused by the impingement of the ribs against the iliac crest on the concave side of the curve; (6) To maximize patients’ health and function; and (7) To maintain walking ability in ambulatory patients.
Although spinal surgery can restore proper spinal alignment, it has some potential disadvantages. In particular, spinal fusion and instrumentation can adversely affect those patients with neuromuscular disorders who have developed functional compensation techniques requiring a short and mobile trunk. Moreover, surgery stops any further growth over the fused segments, and it can accentuate hip deformity[1,2,6,31].
A large number of patients with neuromuscular scoliosis have involvement of the sacrum and subsequent pelvic obliquity. However, patients with neuromuscular scoliosis can develop pelvic obliquity from other sources, such as hip joint and other lower extremity contractures, which will eventually affect the lumbar spine. Furthermore, deformity progression can interfere with trunk stability[32,33].
It is important to assess hip motion and contracture carefully in any patient with neuromuscular spinal deformity. Hip contracture and dislocation can secondarily deform the spine dynamically when the patient attempts to accommodate the hip deformity while sitting.
In cases of unilateral dislocation, pelvic obliquity increases spine deformities and can cause ischiatic pressure sores and loss of sitting position. In such situations, hip surgery is recommended. The choice of surgical procedure depends on the morphology of the femoral head and the presence of necrosis and degenerative cartilage changes. The current recommendations are to reconstruct the hip whenever it is possible. Otherwise, a total hip prosthesis or a femoral head resection can be considered. For patients who present with scoliosis and hip dislocation, hip surgery is usually performed before spinal fusion unless pelvic obliquity is caused by the spine deformity. The goal of orthopedic surgery in non-ambulatory patients is to achieve a sitting position with a level pelvis and an upright trunk position[19,22,24,32].
Instrumentation and fusion should be extended to the pelvis in non-ambulatory patients with pelvic obliquity. In contrast, instrumented fusion can stop at L5 or above when the patient is still ambulatory and shows minimal or no signs of pelvic obliquity. Small amounts of pelvic obliquity (less than 10° to 15°) are compatible with comfortable sitting. in contrast, larger fixed obliquities are not compatible with comfortable sitting and must be corrected surgically or, if not fixed, with wheelchair modifications[34-36].
Surgical treatment of neuromuscular spine deformities is more complex than the treatment of idiopathic scoliosis[37-40]. Complex reconstruction can be necessary to obtain satisfactory outcomes. However, the post-surgical complication rate is higher in neuromuscular scoliosis patients, compared to patients with idiopathic scoliosis. The Scoliosis Research Society Morbidity and Mortality Committee reported an infection rate of 5.5% for neuromuscular cases compared to 1.4% in idiopathic patients and a new neurological deficit rate of 1.03% vs 0.73%, respectively[41,42]. These rates are often due to the presence of multiple comorbidities. Chronic cardiovascular disease as a consequence of a severe scoliotic deformity can lead to complications such as hypoxemia, hypercapnia, cor pulmonale, and pulmonary hypertension. A preoperative forced vital capacity less than 30% is strongly predictive of pulmonary complications, and a significant association between restrictive lung disease and increased pulmonary complications has been reported[16-18,22,39].
The nutritional status of patients with neuromuscular disorders is extremely important, as nutritional depletion has been associated with increased complication rates[7,34,36,37].
Complications can be divided into early and late. Early complications are those diagnosed immediately after or within 4 to 6 wk from the index surgery. In contrast, late complications are diagnosed more than 6 wk after the index surgery.
Early post-operative complications include infections, cardio-respiratory, neurologic and nutritional issues, prolonged ileus, constipation, fluid overload, skin breakdown, bleeding and death. Late post-operative complications include chronic infections, non-union, coccigodinia, crankshaft phenomena, implant-related issues, loss of correction and inadequate correction[20,21,25,36,37].
Deep infections after instrumented fusion for the management of scoliosis are uncommon. However, when they do occur, they can result in considerable morbidity, costs and compromise of correction. As surgical and peri-operative techniques have improved, more severely affected children with complex neuromuscular deformities and considerable co-morbidities are now believed to be candidates for extensive surgery for spinal deformity. In the literature, the rate of spinal infections has been reported to increase with the complexity of the procedure, ranging between 1.9% and 20.0%[5,7,21,33,43].
In acute deep spinal infection, the goals are to eradicate the infection by proper debridement of infected and devitalized tissues and to maintain the hardware to avoid losing correction. Most common organisms are Staphylococcus aureus and Enterococcus spp. However, rises in methicillin-resistant S. aureus (MRSA) and S. aureus has been observed.
Various treatment protocols for debridement, soft-tissue management and antibiotic therapy have been recommended with mixed results. The use of the wound vacuum-assisted closure (VAC) system (KCI Inc., San Antonio, Texas, USA) has gained increasing popularity in the management of acute, sub-acute and chronic wounds. Vacuum-assisted closure is a relatively new technique for promoting the healing of infected wounds that are resistant to treatment by established methods[33,43].
The controlled application of sub-atmospheric pressure facilitates the formation of granulation tissue, assists debridement of necrotic tissue and acts as a sterile barrier. Increasing use of the VAC system for complex soft-tissue injuries has generally resulted in accelerated wound healing, compared with traditional methods.
The VAC system consists of a polyurethane ether foam sponge with open pores, 400 µm to 600 µm in size, a connecting tube and a plastic sealant. After thorough lavage and removal of all macroscopic contamination, devitalized tissue and loose bone grafts, the VAC sponge is cut and fitted into the wound. The plastic sealant is used to cover the sponge and is applied several centimeters beyond the margins of the wound to create an airtight seal. A cruciate incision is made in the plastic sealant covering the sponge, through which a suction tube is inserted and fixed. The tubing is connected to a negative pressure device. The sponge is compressed at sub-atmospheric pressure (-125 mmHg), continuously or intermittently. “Controlled negative pressure” is used to evacuate edema from the wound, increase blood flow, decrease bacterial load and increase the formation of granulation tissue. The system also assists the debridement of necrotic tissue and acts as a sterile barrier[33,43].
Intra-operative debridement should involve thorough lavage and removal of all macroscopic contamination, devitalized tissue and loose bone grafts. No attempt should be made to remove grafts that are partially or fully fused. Intra-operative specimens for bacteriological culture must be obtained before application of the VAC system[33,43].
Canavese et al[43] treated 14 patients with early post-operative infections in which removal of the implant was undesirable because fusion had not been achieved. These authors had no patients who required removal of hardware and no loss of correction at an average of 44 mo of follow-up.
In patients with neuromuscular disease, the likelihood and severity of scoliosis increase with the degree of neuromuscular involvement. There is little doubt that segmental instrumentation techniques have revolutionized the care of patients with neuromuscular scoliosis by providing lasting correction, significant relief of pain, and restoration of quality of life and sitting positions. Moreover, continuous evolution in segmental instrumentation has increased the percentage of successful surgical corrections.
However, it must be stressed that although neuromuscular scoliosis can be well corrected with different constructs (hooks, screws, sub-laminar bands, U-rods, L-rods), there is a high rate of associated complications[5,33,44,45]. The complication rates have increased with time, and an increasing number of complications have been reported in the recent literature. This can be explained by the improvement in complication recording but also that more severe patients are now operated. The evidence of such complications should never be underestimated by the treating surgeon, and the rate of such complications is particularly high in patients with flaccid neuromuscular scoliosis, i.e., spinal muscular atrophy and Duchenne muscular dystrophy[22].
The literature regarding the surgical management of spinal deformities in neuromuscular disorders has suggested that bilateral instrumentation and fusion to either L5 or the sacrum are the most effective, and multiple fixation points, such as sub-laminar wires or bands, are preferred[23,24,36,38,45]. In our opinion, instrumentation of the pelvis is indicated in non-ambulatory patients with pelvic obliquity. Fixation of the pelvis can be obtained with iliac screws, while hybrid instrumentation (screws, hooks, sub-laminar bands) or band-only instrumentation can be used for deformity correction. Fusion to the sacrum should be avoided in patients with residual walking ability.
In contrast, ambulatory patients should be fused to L5 at most with hybrid constructs (screws as distal anchorages, hooks and sub-laminar bands as proximal anchorages).
Restoring the sagittal balance of the spine is one of the most challenging goals in scoliosis surgery. Sub-laminar bands have been demonstrated to provide good deformity correction on both the coronal and sagittal planes[46]. Moreover, the operative time, bleeding and radiation exposure are reduced, with a low rate of early or late surgical complications.
The state of knowledge regarding neuromuscular scoliosis is a dynamic process, so a current literature review was mandatory. The somewhat large bibliography for this subject reflects the many opinions and findings presented here[1,10,43,46,47].
P- Reviewers: Aota Y, Lykissas MG S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
| 1. | Madigan RR, Wallace SL. Scoliosis in the institutionalized cerebral palsy population. Spine (Phila Pa 1976). 1981;6:583-590. [Cited in This Article: ] |
| 2. | Comstock CP, Leach J, Wenger DR. Scoliosis in total-body-involvement cerebral palsy. Analysis of surgical treatment and patient and caregiver satisfaction. Spine (Phila Pa 1976). 1998;23:1412-1424; discussion 1412-1424. [Cited in This Article: ] |
| 3. | Saito N, Ebara S, Ohotsuka K, Kumeta H, Takaoka K. Natural history of scoliosis in spastic cerebral palsy. Lancet. 1998;351:1687-1692. [Cited in This Article: ] |
| 4. | Bowen RE, Abel MF, Arlet V, Brown D, Burton DC, D’Ambra P, Gill L, Hoekstra DV, Karlin LI, Raso J. Outcome assessment in neuromuscular spinal deformity. J Pediatr Orthop. 2012;32:792-798. [Cited in This Article: ] |
| 5. | Lonstein JE, Koop SE, Novachek TF, Perra JH. Results and complications after spinal fusion for neuromuscular scoliosis in cerebral palsy and static encephalopathy using luque galveston instrumentation: experience in 93 patients. Spine (Phila Pa 1976). 2012;37:583-591. [Cited in This Article: ] |
| 6. | Cady RB, Bobechko WP. Incidence, natural history, and treatment of scoliosis in Friedreich’s ataxia. J Pediatr Orthop. 1984;4:673-676. [Cited in This Article: ] |
| 7. | Jevsevar DS, Karlin LI. The relationship between preoperative nutritional status and complications after an operation for scoliosis in patients who have cerebral palsy. J Bone Joint Surg Am. 1993;75:880-884. [Cited in This Article: ] |
| 8. | Miller A, Temple T, Miller F. Impact of orthoses on the rate of scoliosis progression in children with cerebral palsy. J Pediatr Orthop. 1996;16:332-335. [Cited in This Article: ] |
| 9. | Sponseller PD, LaPorte DM, Hungerford MW, Eck K, Bridwell KH, Lenke LG. Deep wound infections after neuromuscular scoliosis surgery: a multicenter study of risk factors and treatment outcomes. Spine (Phila Pa 1976). 2000;25:2461-2466. [Cited in This Article: ] |
| 10. | Thomson JD, Banta JV. Scoliosis in cerebral palsy: an overview and recent results. J Pediatr Orthop B. 2001;10:6-9. [Cited in This Article: ] |
| 11. | Resina J, Alves AF. A technique of correction and internal fixation for scoliosis. J Bone Joint Surg Br. 1977;59:159-165. [Cited in This Article: ] |
| 12. | Luque ER. Segmental spinal instrumentation for correction of scoliosis. Clin Orthop Relat Res. 1982;192-198. [Cited in This Article: ] |
| 13. | Allen BL, Ferguson RL. L-rod instrumentation for scoliosis in cerebral palsy. J Pediatr Orthop. 1982;2:87-96. [Cited in This Article: ] |
| 14. | Sponseller PD, Whiffen JR, Drummond DS. Interspinous process segmental spinal instrumentation for scoliosis in cerebral palsy. J Pediatr Orthop. 2001;6:559-563. [Cited in This Article: ] |
| 15. | Herring JA, Wenger DR. Segmental spinal instrumentation: a preliminary report of 40 consecutive cases. Spine (Phila Pa 1976). 1982;7:285-298. [Cited in This Article: ] |
| 16. | Gersoff WK, Renshaw TS. The treatment of scoliosis in cerebral palsy by posterior spinal fusion with Luque-rod segmental instrumentation. J Bone Joint Surg Am. 1988;70:41-44. [Cited in This Article: ] |
| 17. | Boachie-Adjei O, Lonstein JE, Winter RB, Koop S, vanden Brink K, Denis F. Management of neuromuscular spinal deformities with Luque segmental instrumentation. J Bone Joint Surg Am. 1989;71:548-562. [Cited in This Article: ] |
| 18. | Broom MJ, Banta JV, Renshaw TS. Spinal fusion augmented by luque-rod segmental instrumentation for neuromuscular scoliosis. J Bone Joint Surg Am. 1989;71:32-44. [Cited in This Article: ] |
| 19. | Bell DF, Moseley CF, Koreska J. Unit rod segmental spinal instrumentation in the management of patients with progressive neuromuscular spinal deformity. Spine (Phila Pa 1976). 1989;14:1301-1307. [Cited in This Article: ] |
| 20. | Modi HN, Hong JY, Mehta SS, Srinivasalu S, Suh SW, Yi JW, Yang JH, Song HR. Surgical correction and fusion using posterior-only pedicle screw construct for neuropathic scoliosis in patients with cerebral palsy: a three-year follow-up study. Spine (Phila Pa 1976). 2009;34:1167-1175. [Cited in This Article: ] |
| 21. | Nectoux E, Giacomelli MC, Karger C, Herbaux B, Clavert JM. Complications of the Luque-Galveston scoliosis correction technique in paediatric cerebral palsy. Orthop Traumatol Surg Res. 2010;96:354-361. [Cited in This Article: ] |
| 22. | Modi HN, Suh SW, Hong JY, Cho JW, Park JH, Yang JH. Treatment and complications in flaccid neuromuscular scoliosis (Duchenne muscular dystrophy and spinal muscular atrophy) with posterior-only pedicle screw instrumentation. Eur Spine J. 2010;19:384-393. [Cited in This Article: ] |
| 23. | Mazda K, Ilharreborde B, Even J, Lefevre Y, Fitoussi F, Penneçot GF. Efficacy and safety of posteromedial translation for correction of thoracic curves in adolescent idiopathic scoliosis using a new connection to the spine: the Universal Clamp. Eur Spine J. 2009;18:158-169. [Cited in This Article: ] |
| 24. | La Rosa G, Giglio G, Oggiano L. Surgical treatment of neurological scoliosis using hybrid construct (lumbar transpedicular screws plus thoracic sublaminar acrylic loops). Eur Spine J. 2011;20 Suppl 1:S90-S94. [Cited in This Article: ] |
| 25. | Yazici M, Asher MA, Hardacker JW. The safety and efficacy of Isola-Galveston instrumentation and arthrodesis in the treatment of neuromuscular spinal deformities. J Bone Joint Surg Am. 2000;82:524-543. [Cited in This Article: ] |
| 26. | Thometz JG, Simon SR. Progression of scoliosis after skeletal maturity in institutionalized adults who have cerebral palsy. J Bone Joint Surg Am. 1988;70:1290-1296. [Cited in This Article: ] |
| 27. | Bergofsky EH, Turino GM, Fishman AP. Cardiorespiratory failure in kyphoscoliosis. Medicine (Baltimore). 1959;38:263-317. [Cited in This Article: ] |
| 28. | Bjure J, Grimby G, Kasalický J, Lindh M, Nachemson A. Respiratory impairment and airway closure in patients with untreated idiopathic scoliosis. Thorax. 1970;25:451-456. [Cited in This Article: ] |
| 29. | Kafer ER. Respiratory function in paralytic scoliosis. Am Rev Respir Dis. 1974;110:450-457. [Cited in This Article: ] |
| 30. | Mellies U, Ragette R, Schwake C, Boehm H, Voit T, Teschler H. Daytime predictors of sleep disordered breathing in children and adolescents with neuromuscular disorders. Neuromuscul Disord. 2003;13:123-128. [Cited in This Article: ] |
| 31. | Cotton LA. Unit rod segmental spinal instrumentation for the treatment of neuromuscular scoliosis. Orthop Nurs. 1991;10:17-23. [Cited in This Article: ] |
| 32. | Senaran H, Shah SA, Glutting JJ, Dabney KW, Miller F. The associated effects of untreated unilateral hip dislocation in cerebral palsy scoliosis. J Pediatr Orthop. 2006;26:769-772. [Cited in This Article: ] |
| 33. | Canavese F, Emara K, Sembrano JN, Bialik V, Aiona MD, Sussman MD. Varus derotation osteotomy for the treatment of hip subluxation and dislocation in GMFCS level III to V patients with unilateral hip involvement. Follow-up at skeletal maturity. J Pediatr Orthop. 2010;30:357-364. [Cited in This Article: ] |
| 34. | Gau YL, Lonstein JE, Winter RB, Koop S, Denis F. Luque-Galveston procedure for correction and stabilization of neuromuscular scoliosis and pelvic obliquity: a review of 68 patients. J Spinal Disord. 1991;4:399-410. [Cited in This Article: ] |
| 35. | Hopf C, Rompe JD, Heine J. [Indications and results of surgical treatment of neuromuscular scoliosis]. Z Orthop Ihre Grenzgeb. 1992;130:146-151. [Cited in This Article: ] |
| 36. | Neustadt JB, Shufflebarger HL, Cammisa FP. Spinal fusions to the pelvis augmented by Cotrel-Dubousset instrumentation for neuromuscular scoliosis. J Pediatr Orthop. 1992;12:465-469. [Cited in This Article: ] |
| 37. | Onimus M, Manzone P, Lornet JM, Laurain JM. [Surgical treatment of scoliosis in bed-ridden patients with cerebral palsy]. Rev Chir Orthop Reparatrice Appar Mot. 1992;78:312-318. [Cited in This Article: ] |
| 38. | Sussman MD, Little D, Alley RM, McCoig JA. Posterior instrumentation and fusion of the thoracolumbar spine for treatment of neuromuscular scoliosis. J Pediatr Orthop. 1996;16:304-313. [Cited in This Article: ] |
| 39. | Frischhut B, Sterzinger W, Rachbauer F, Klestil T, Krismer M, Bauer R. Surgical treatment of neuropathic scoliosis: morphologic and functional outcome. Arch Orthop Trauma Surg. 1997;116:367-372. [Cited in This Article: ] |
| 40. | Marchesi D, Arlet V, Stricker U, Aebi M. Modification of the original Luque technique in the treatment of Duchenne’s neuromuscular scoliosis. J Pediatr Orthop. 1997;17:743-749. [Cited in This Article: ] |
| 41. | Smith JS, Shaffrey CI, Sansur CA, Berven SH, Fu KM, Broadstone PA, Choma TJ, Goytan MJ, Noordeen HH, Knapp DR. Rates of infection after spine surgery based on 108,419 procedures: a report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976). 2011;36:556-563. [Cited in This Article: ] |
| 42. | Hamilton DK, Smith JS, Sansur CA, Glassman SD, Ames CP, Berven SH, Polly DW, Perra JH, Knapp DR, Boachie-Adjei O. Rates of new neurological deficit associated with spine surgery based on 108,419 procedures: a report of the scoliosis research society morbidity and mortality committee. Spine (Phila Pa 1976). 2011;36:1218-1228. [Cited in This Article: ] |
| 43. | Canavese F, Gupta S, Krajbich JI, Emara KM. Vacuum-assisted closure for deep infection after spinal instrumentation for scoliosis. J Bone Joint Surg Br. 2008;90:377-381. [Cited in This Article: ] |
| 44. | Sharma S, Wu C, Andersen T, Wang Y, Hansen ES, Bünger CE. Prevalence of complications in neuromuscular scoliosis surgery: a literature meta-analysis from the past 15 years. Eur Spine J. 2013;22:1230-1249. [Cited in This Article: ] |
| 45. | Mattila M, Jalanko T, Puisto V, Pajulo O, Helenius IJ. Hybrid versus total pedicle screw instrumentation in patients undergoing surgery for neuromuscular scoliosis: a comparative study with matched cohorts. J Bone Joint Surg Br. 2012;94:1393-1398. [Cited in This Article: ] |
| 46. | La Rosa G, Giglio G, Oggiano L. Sagittal profile control in patients affected by neurological scoliosis using Universal Clamps: a 4-year follow-up study. Eur Spine J. 2012;21 Suppl 1:S32-S36. [Cited in This Article: ] |
| 47. | Lebel DE, Corston JA, McAdam LC, Biggar WD, Alman BA. Glucocorticoid treatment for the prevention of scoliosis in children with Duchenne muscular dystrophy: long-term follow-up. J Bone Joint Surg Am. 2013;95:1057-1061. [Cited in This Article: ] |