Published online Jun 18, 2022. doi: 10.5312/wjo.v13.i6.578
Peer-review started: December 25, 2021
First decision: March 7, 2022
Revised: March 20, 2022
Accepted: May 22, 2022
Article in press: May 22, 2022
Published online: June 18, 2022
Orthopedic implant-related infection remains one of the most serious complications after orthopedic surgery. In recent years, there has been an increased scientific interest to improve prevention and treatment strategies. However, many of these strategies have focused on chemical measures.
To analyze the effect of alternating current electrical fields on bacterial adherence to titanium surfaces.
Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) were exposed to 6.5 V electrical currents at different frequencies: 0.5 Hz, 0.1 Hz, and 0.05 Hz. After exposure, a bacterial count was then performed and compared to the control model. Other variables registered included the presence of electrocoagulation of the medium, electrode oxidation and/or corrosion, and changes in pH of the medium.
The most effective electrical model for reducing S. aureus adhesion was 6.5 V alternating current at 0.05 Hz achieving a 90% adhesion reduction rate. For E. coli, the 0.05 Hz frequency model also showed the most effective results with a 53% adhesion reduction rate, although these were significantly lower than S. aureus. Notable adhesion reduction rates were observed for S. aureus and E.coli in the studied conditions. However, the presence of electrode oxidation makes us presume these conditions are not optimal for in vivo use.
Although our findings suggest electrical currents may be useful in preventing bacterial adhesion to metal surfaces, further research using other electrical conditions must be examined to consider their use for in vivo trials.
Core Tip: Current strategies to prevent orthopedic implant infections have focused on chemical measures. Our data suggest electrical currents may be useful in preventing Staphylococcus aureus and Escherichia coli adhesion to titanium surfaces. Reduction in adhesion rates of up to 90% were observed when applying low intensity alternating currents on titanium surfaces. Further research is needed to consider the use of electrical currents for infection prevention in an in vivo scenario.
- Citation: Bernaus M, Guillem-Marti J, Bermúdez-Castel A, Calero JA, Torres D, Veloso M, Font-Vizcarra L. Reducing bacterial adhesion to titanium surfaces using low intensity alternating electrical pulses. World J Orthop 2022; 13(6): 578-586
- URL: https://www.wjgnet.com/2218-5836/full/v13/i6/578.htm
- DOI: https://dx.doi.org/10.5312/wjo.v13.i6.578
Orthopedic implant-related infection remains one of the most serious complications after orthopedic surgery. The incidence of infection after fracture surgery varies depending on the mechanism of injury from 1% in low energy closed fractures and up to 30% in high energy open fractures[1]. The incidence of infection after primary hip and knee arthroplasty is 1%-3% and increases to up to 15% in revision surgery[2]. The management of orthopedic implant-related infection usually requires a combination of surgical and prolonged courses of antibiotic treatment. In recent years, there has been an increased scientific interest to improve prevention and treatment strategies. Many of these strategies have focused on chemical measures to reduce and treat infections.
Although the exact physiopathology of implant-related infections is unknown, infections are preceded by contamination of the surgical site (during surgery, postoperatively, or by hematogenous seeding) followed by bacterial adhesion and biofilm formation on the metal surface. Depending on several variables such as bacterial load, host immunity, and microorganism virulence, early or delayed implant infections can occur.
The antibacterial properties of electric currents have been used since the past century. In 1919, Anderson et al[3] published a method to sterilize milk using low frequency alternating current (AC). In non-medical related fields such as industrial water or food processing, high voltage electrical currents are commonly used to eliminate microorganisms and debris.
Focusing on the medical field, Costerton et al[4] were the first to postulate the potential uses of electrical currents for the treatment of artificial implant infections. From that point on, an increasing number of in vitro studies have demonstrated the capacity of low-intensity electrical currents to inhibit bacterial growth of different Staphylococcus aureus (S. aureus), Pseudomonas aeruginosa, and Staphylococcus epidermidis strains. Moreover, they described the “bioelectric” effect caused by the antibacterial synergies between antibiotics and electric fields against biofilm bacteria[5].
However, many of these studies are focused on the use of direct current (DC). It is important to note that DC has potential drawbacks for its in vivo use. DC generates water electrolysis and can cause changes in pH and free radicals that could be cytotoxic. Furthermore, DC generates higher amounts of energy that can cause electrocorrosion and/or oxidation of the metal electrodes making its applicability limited in clinical scenarios with metallic implants.
Orthopedic surgical procedures commonly involve the use of biocompatible metals such as titanium. The reduction of bacterial adherence to metal surfaces using electrical fields has been described for stainless steel[6]. To the best of our knowledge, there is no evidence of the effect of electrical currents on reducing bacterial adherence to titanium surfaces.
The objective of our study was to analyze the effect of AC electrical fields on bacterial adherence to a titanium surface for gram-positive and gram-negative bacteria. The secondary objective was to analyze the effect of electrical fields to titanium and the medium.
The medical titanium (Ti-6Al-4V) components used in our study were manufactured and supplied by AMES S.A. (Sant Feliu de Llobregat, Barcelona, Spain) Circular disks were obtained from cutting titanium bars. Each disk was mechanically polished to remove irregularities and to promote identical physical conditions at the surface for all disks.
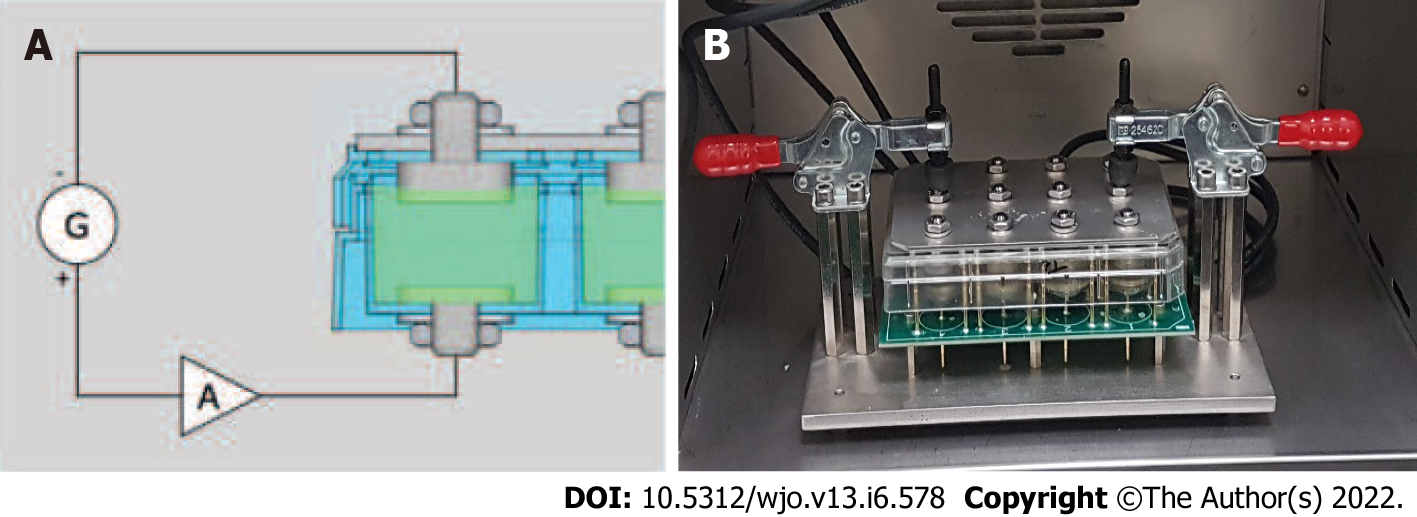
The design of a culture plate that would allow a simultaneous number of tests with different electrical conditions was important to standardize the study process and optimize resources. A self-designed 12-well culture plate was designed and manufactured by Innovative Minds S.L. to achieve this goal. Using a standard plastic 12-well culture plate with 3 rows and 4 columns (Nunclon-Thermo Scientific), holes were drilled at the bottom of each of the wells to fit titanium electrodes at the base. The top or lid of the wells was also drilled to place a disk at the top to serve as an electrode. This setup allowed the 12 wells to have cylindrical titanium electrodes at the top and bottom of each well. All electrodes were connected to a generator and an amplifier as seen in Figure 1A. The function generator had four separate connections to allow four different trials to be performed simultaneously and in a triplicated manner. To generate the desired waveforms, an AFG1022 Dual Channel Arbitrary Function Generator (Tektronix Inc., Beaverton, OR, United States) was used. The signal was amplified by an OPA548EVM Power Operational Amplifier (Texas Instruments Incorporated, Dallas, TX, United States). One amplifier per channel was used. The power op-amps were powered by an off-the-shelf 15 V symmetric power supply. (Figure 1A and B).
Before conducting the study with bacteria, we performed a series of analyses to evaluate possible interactions between electrical fields and the titanium plates and brain heart infusion (BHI) medium.
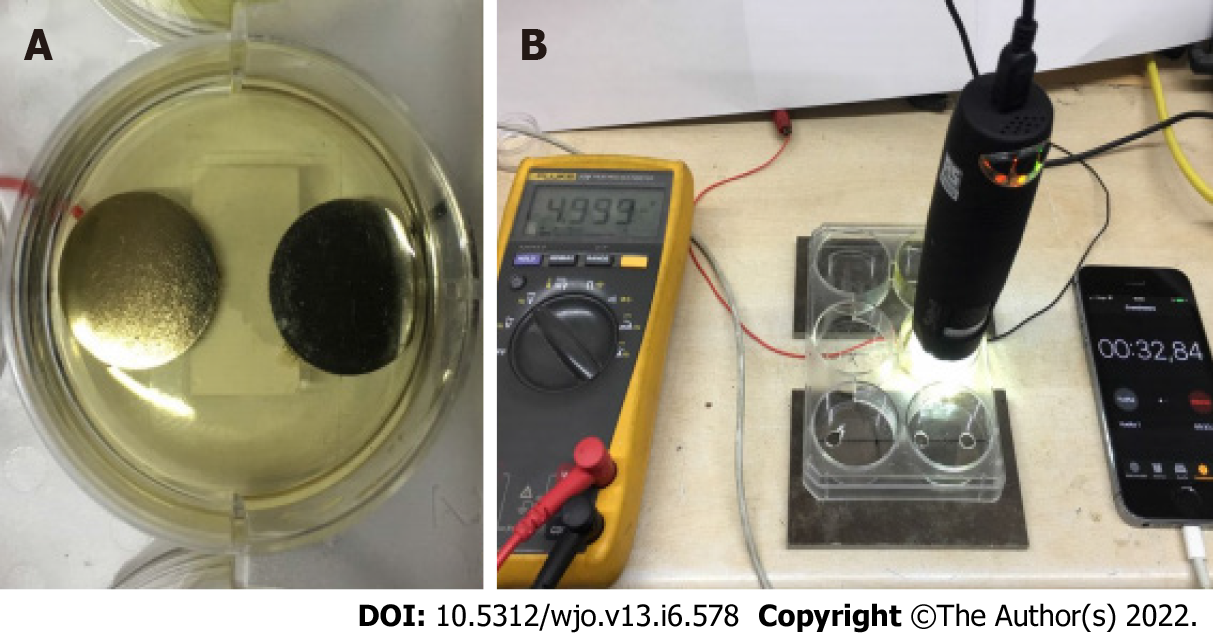
The main objectives of this test were: (1) To obtain the threshold voltage at which electrocoagulation and electrolysis were seen in the culture medium; and (2) To evaluate if at this point of voltage there was macroscopic signs of oxidation of titanium plates. For these reasons, two 15 mm diameter and 2 mm thick titanium disks were suspended at the base of a well in a 6-well culture plate (Nuclon Delta Surface, Thermo Fisher Scientific, Waltham, MA, United States) petri dish covered in 4 mm of BHI. The two disks were separated by a 5 mm distance as shown in Figure 2A. The left electrode was connected to the negative (catode) pole of a DC power supply, while the right electrode was connected to the positive (anode) electrode of the same supply. A USB microscope [913-2478, Electrocomponents PLC (RS Pro), London, United Kingdom] was set up and shown in Figure 2B to visualize and count bubble formation. A constant potential difference was applied between the two electrodes during a 10-min period. We then evaluated: (1) The presence of titanium oxidation by controlling the change of color of the surface; (2) The presence of bubbles and the quantity during one minute to estimate the number of bubbles formed during exposure; and (3) The formation of lumps as a consequence of medium electrocoagulation.
The presence of lumps was evaluated using a Zeiss Neon scanning electron microscope operated at 15 keV, equipped with energy dispersive X-ray spectroscopy to examine their composition. Energy dispersive X-ray spectroscopy line profile and energy dispersive X-ray spectroscopy mapping analyses were performed to detect the presence of calcium, phosphate, sodium, and potassium.
Using the self-designed 12-well culture plate mentioned above, we analyzed the potential effect of electrical currents in reducing bacterial adhesion to a titanium metal surface. Our self-designed culture plate allowed four different conditions to be tested by triplicate at the same time.
The experiments were performed using a gram-positive strain, S. aureus (CCUG 15915), and a gram-negative strain, Escherichia coli (E. coli) (CECT 101). Both bacteria were individually streaked in BHI (Scharlab SL, Spain) agar plates. Colonies were grown in BHI broth overnight at 37 ºC and diluted to an OD600 of 0.2. Three milliliters of this medium were then inoculated to the self-designed plates containing titanium discs. Bacteria were incubated at 37 ºC for 2 h, applying 6.5 V pulsed electrical currents at different frequencies, i.e. 0.5 Hz, 0.1 Hz, and 0.05 Hz, to prevent attachment. A control without exposure to electrical fields was used as maximum bacterial adhesion. Each condition was tested with titanium electrodes provided at each end of the wells.
After exposure, non-adhered bacteria were removed by aspiration of the medium followed by three rinses with PBS. Then, 1 mL PBS was added, and the plate was sonicated in a Xuba1 Ultrasonic bath (Grant Instruments, United Kingdom) to detach the bacteria adhered to the bottom titanium electrode. Subsequently, serial dilutions of detached bacteria were streaked onto BHI agar plates and incubated overnight at 37 ºC. Colony-forming units per milliliter were counted. All tests were repeated three times, and mean values were reported.
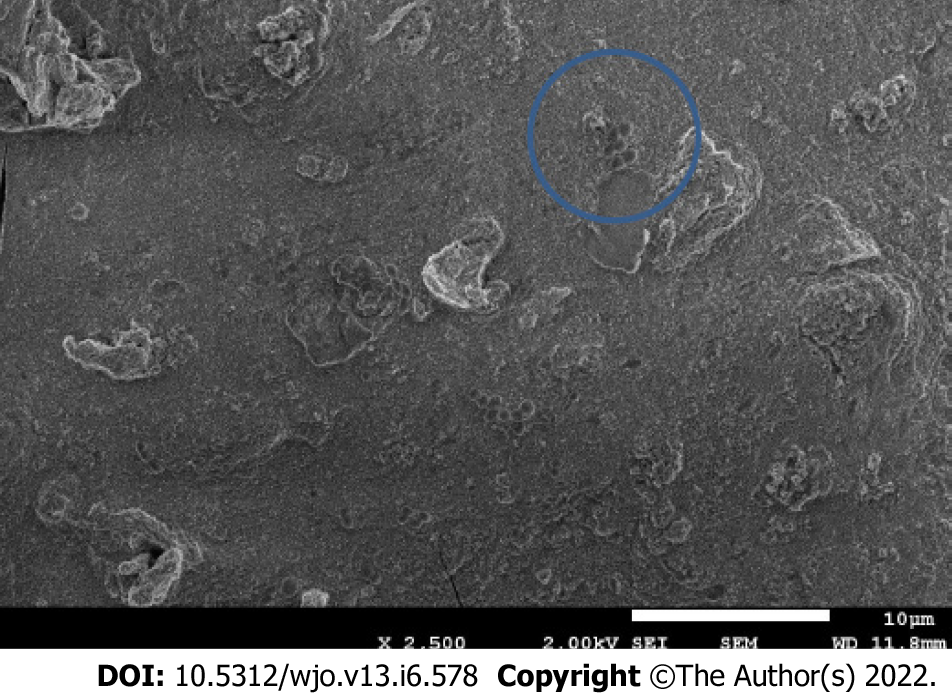
Presence of bacteria at the titanium disc surface was evaluated by scanning electron microscope. After incubating the bacteria with different electrical currents as described above, discs were rinsed thrice with PBS, and bacteria were fixed with a 2.5% glutaraldehyde solution. Discs were then washed and dehydrated in a series of increasing ethanol solutions for 20 min each. Complete dehydration was performed after incubation in hexamethyldisilazane. Dried samples were covered with a thin carbon layer and mounted on aluminum stubs. Samples were observed using a Zeiss Neon at an operating voltage of 5 kV.
Other variables registered included presence of electrocoagulation of the medium (lumps), electrode oxidation, and changes in pH of the medium. The pH was measured in the collected supernatants using a selective MultiMeter MM41 (Crison Instruments) electrode.
The results shown in this manuscript are part of a thesis project titled “Utility of electrical currents for the prevention and treatment of orthopedic implant-related infections” (Universitat de Barcelona).
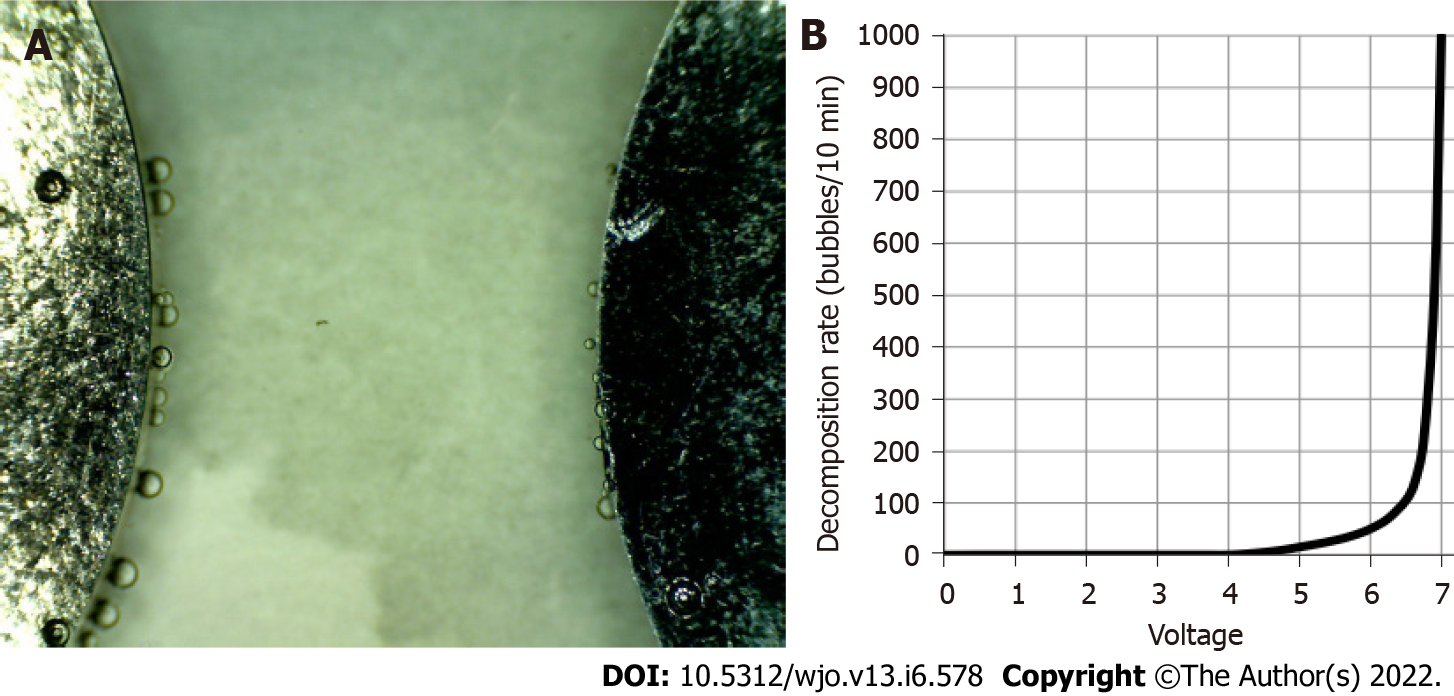
During our preliminary tests, we had observed the formation of hydrogen microbubbles from the negative electrode and a fewer amount of oxygen bubbles from the positive electrode when high voltages were used. This phenomenon was attributed to electrolysis. We therefore had to establish the threshold potential at which electrolysis did not occur for our setup. Our results showed the decomposition potential for our setup started at 5 V. An exponential increase in bubble formation was reported at 6.5 V (Figure 3).
We also noted the formation of lumps in the culture medium due to electrocoagulation. For this reason, we tested two different voltages, 7.5 V and 6.5 V, to evaluate the effect of electrocoagulation in our medium. We applied these potential differences and observed there was an increased agglomeration when using 7.5 V. We did not observe agglomeration using 6.5 V. Taking into consideration these results, we decided to establish 6.5 V as a safe potential difference in our setup to minimize the effect of electrolysis and electrocoagulation on our results.
After establishing a reference voltage at which electrocoagulation and electrolysis were minimized, we proceeded to carry out the experiments on bacteria following the previously described methodology.
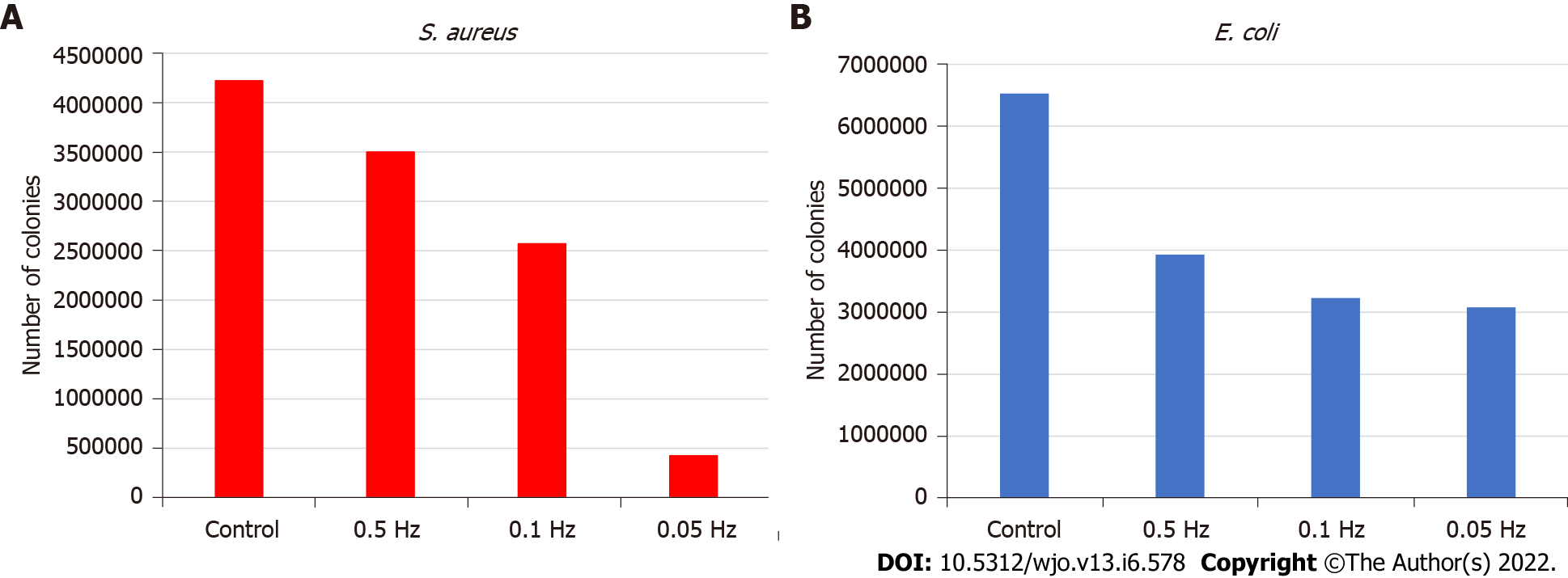
The most effective electrical model for reducing S. aureus adhesion was 6.5 V AC at 0.05 Hz achieving a 90% adhesion reduction rate. Bacterial counts in the control were 4.22e6 colony-forming units (Figure 4A).
For E. coli, the 0.05 Hz frequency model also showed the most effective results with a 53% adhesion reduction rate, although these were lower than S. aureus. Bacterial counts in the control were 6.53e6 colony-forming units (Figure 4B).
Although we performed tests to establish a high threshold voltage to minimize the effects of electrocoagulation and electrolysis of the culture medium, these phenomena were observed in all tests when bacteria were present in the medium. The lumps were analyzed using energy dispersion X-ray spectroscopy to determine their composition. We concluded these were formed by titanium ions, due to oxidation of the electrode, and other ions (P, K, Na) from the salts in the medium. Furthermore, when the lumps were visualized under an electron microscope, bacteria were seen in the surface and inside the lumps as shown in Figure 5.
No changes in pH were found in any of the tests performed. In all studied conditions, anodizing at the titanium electrodes was observed creating an ochre and purple coloring (Figure 6).
The results of our study suggest it is possible to achieve a reduction in the adhesion of S. aureus and E. coli to titanium surfaces using low intensity alternating pulsed electrical currents. Most current research regarding electrical currents and bacterial adhesion to metal surfaces have been performed in a variety of custom-designed electrical devices to serve the purpose. Previous results using parallel plate flow chambers as described by Poortinga et al[7] and performed by van der Borden et al[8,9] showed that a variety of staphylococcal strains could be detached from surgical stainless steel by the application of a direct electrical current. The authors of this study attributed this detachment mechanism to an ionic-strength dependent transfer of electrons. This explained the increased detachment rate with higher currents. They also described the detachment force to be proportional to the voltage applied and that this force needs to overcome the attractive Lifshitz-Van der Waals and the acid-base forces between the adhering bacteria and the surface.
To reduce the number of bacteria adhered to a metal surface; these forces also need to be neutralized. The mechanism of bacterial adhesion to metal surfaces is complicated. Bacterial adhesion to a metal surface can be described as a two-phase process including an initial and reversible phase (phase one) and a time-dependent irreversible phase (phase two), which was first proposed by Marshall et al[10,11]. Planktonic bacteria interact with metal surfaces following a series of physical forces such as gravity, electrostatic charges, hydrophobic interactions, Brownian motion, and van der Waals attraction forces. In phase one, when the cell and the surface come into close contact (< 3 nm), short-range interactions such as hydrogen bonding and ionic and dipole interactions become effective for bacterial adhesion. At this point the electrical currents play an important role for preventing bacterial adhesion to metal surfaces by interacting with these forces. In the second phase of adhesion, molecular reactions between bacterial surface structures become predominant[12]. Other factors affecting bacterial adhesion include characteristics of bacteria themselves, the target material surface, and environmental factors such as the presence of serum proteins or bactericidal substances[12].
Regarding the electrical model, the most successful studies have used DC currents. Theoretically, under the influence of ACs, fewer electrons and therefore less energy is applied to the system. This could explain the lower rate of adhesion reduction achieved by ACs compared to DCs described by van der Borden et al[8]. However, the use of AC could prove to be feasible for the treatment in clinical in vivo scenarios.
Our study results show adhesion reduction rates of up to 90% for S. aureus using AC low intensity alternating pulses. However, we only achieved 53% adhesion reduction rates for E. coli. As previously pointed out, many factors influence bacterial adhesion. We believe this difference could be explained by the different cell structure of the studied bacteria. Most prokaryotic cells are known to have a rigid cell wall. This cell wall is essential for viability, protection, and structure. There are significant differences in the composition of S. aureus and E. coli cell walls. The Gram stain test is a quick way to classify bacteria into two broad categories (gram-positive or gram-negative) according to their cell wall characteristics. The most significant difference between these two groups of bacteria is that gram-positive bacteria are marked by the absence of an outer lipid cell membrane. In general, gram-positive bacteria are considered monoderms and have a single lipid layer as opposed to gram negative bacteria that are diderms and have two lipid layers. We have hypothesized that our results improve when increasing the amount of energy applied to S. aureus but remain constant with E. coli due to these structural differences, making gram negative bacteria more resistant to forces caused by electrical currents.
Changes in pH have also been described to influence bacterial growth. This principle is commonly used in detergents and disinfectants to kill bacteria[13]. Large variations in external pH have biocidal effects on bacteria[14]. In our study, we did not observe changes in pH. For this reason, we can establish that the effect on bacterial adhesion is directly related to the electrical current and not caused by a change of pH in the medium.
Additionally, the effects of electrolysis and electrocoagulation in the presence of bacteria and BHI have shown several a priori unexpected reactions. The formation of lumps formed due to electrocoagulation of the medium showed the presence of bacteria when examined under an electron microscope. Additionally, we cultured these lumps to establish if there was living bacteria. Although we cannot be sure if the samples were contaminated by the suspended bacteria in the culture medium, we can almost be sure electrical fields, at the voltages used, do not directly kill bacteria, and prevent them from adhering to the metal surface. To the best of our knowledge there are no current studies in the literature that evaluated this phenomenon. Therefore, we have not found any references regarding this topic.
Notable adhesion reduction rates were observed for S. aureus and E. coli in the studied conditions. However, the presence of electrolysis and electrode oxidation lead us to presume these conditions are not optimal for in vivo use. Although our findings suggest alternating electrical currents may be useful in preventing bacterial adhesion to titanium, further research using other electrical conditions must be examined to consider their use for in vivo trials.
Orthopedic implant-related infection remains one of the most serious complications after orthopedic surgery. In recent years, there has been an increased scientific interest to improve prevention and treatment strategies. Many of these strategies have focused on chemical measures to reduce and treat infections.
Our study group has been developing novel strategies for the treatment and prevention of orthopedic implant-related infections. These have been focused on the application of electrical currents to surface metals in the presence of bacteria to prevent adhesion and disrupt biofilm formation.
The objective of our study was to analyze the effect of alternating current electrical fields on bacterial adherence to a titanium surface for gram-positive and gram-negative bacteria.
Using a self-designed 12-well culture plate, we analyzed the potential effect of electrical currents in reducing bacterial adhesion to a titanium metal surface. Gram-positive bacteria, represented by Staphylococcus aureus (S. aureus), and gram-negative bacteria, represented by Escherichia coli (E. coli), were exposed to 6.5 V electrical currents at different frequencies: 0.5 Hz, 0.1 Hz, and 0.05 Hz. After exposure, colony-forming units per milliliter were counted and compared to a control without exposure to electrical currents.
The most effective electrical model for reducing S. aureus adhesion was 6.5 V alternating current at 0.05 Hz achieving a 90% adhesion reduction rate. For E. coli, the 0.05 Hz frequency model also showed the most effective results with a 53% adhesion reduction rate, although these were significantly lower than S. aureus.
Our results demonstrate electrical fields may have promising applications in preventing bacterial adhesion to titanium metal surfaces. However, the presence of electrolysis and electrode oxidation lead us to presume these conditions may not be optimal for in vivo use.
Further research using other electrical conditions must be examined to consider their use for in vivo trials.
This study was developed through the DIRECT project (Desarrollo de Nuevos Dispositivos Biomiméticos Mejorados Superficialmente con Nuevos Recubrimientos y Tratamientos Físicos), which has been funded with a grant from the Centre for Development of Industrial Technology (CDTI). The CDTI is a Public Business Entity, answering to the Ministry of Science and Innovation of the Spanish Government.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hooper GJ, New Zealand; Liu P, China A-Editor: Yao QG, China S-Editor: Zhang H L-Editor: Filipodia CL P-Editor: Zhang H
| 1. | Papakostidis C, Kanakaris NK, Pretel J, Faour O, Morell DJ, Giannoudis PV. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo-Anderson classification. Injury. 2011;42:1408-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 2. | Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468:52-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 464] [Cited by in F6Publishing: 496] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 3. | Anderson AK, Finkelstein R. A Study of the Electro-Pure Process of Treating Milk. J Dairy Sci. 1919;2:374-406. [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 72] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Costerton JW, Ellis B, Lam K, Johnson F, Khoury AE. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob Agents Chemother. 1994;38:2803-2809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 201] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Blenkinsopp SA, Khoury AE, Costerton JW. Electrical enhancement of biocide efficacy against Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 1992;58:3770-3773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 128] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Muñoz-Mahamud E, González-Cuevas A, Sierra JM, Diaz-Brito V, Bermúdes A, Soriano A, Castellanos J, Font-Vizcarra L. Pulsed electric fields reduce bacterial attachment to stainless steel plates. Acta Orthop Belg. 2018;84:11-16. [PubMed] [Cited in This Article: ] |
| 7. | Poortinga AT, Bos R, Busscher HJ. Controlled electrophoretic deposition of bacteria to surfaces for the design of biofilms. Biotechnol Bioeng. 2000;67:117-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 8. | van der Borden AJ, van der Mei HC, Busscher HJ. Electric-current-induced detachment of Staphylococcus epidermidis strains from surgical stainless steel. J Biomed Mater Res B Appl Biomater. 2004;68:160-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | van der Borden AJ, van der Mei HC, Busscher HJ. Electric block current induced detachment from surgical stainless steel and decreased viability of Staphylococcus epidermidis. Biomaterials. 2005;26:6731-6735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Marshall KC. Mechanisms of Bacterial Adhesion at Solid-Water Interfaces. In: Savage DC, Fletcher M. Bacterial Adhesion. New York: Plenum Press, 1985: 133-161. [Cited in This Article: ] |
| 11. | Marshall KC, Stout R, Mitchell R. Mechanism of the Initial Events in the Sorption of Marine Bacteria to Surfaces. J Gen Microbiol. 1971;68:337-348. [DOI] [Cited in This Article: ] |
| 12. | Katsikogianni M, Missirlis YF. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cell Mater. 2004;8:37-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 816] [Cited by in F6Publishing: 650] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 13. | Garrett TR, Bhakoo M, Zhang Z. Bacterial adhesion and biofilms on surfaces. Prog Nat Sci. 2008;18:1049-1056. [DOI] [Cited in This Article: ] |
| 14. | Booth IR. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985;49:359-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 441] [Article Influence: 11.3] [Reference Citation Analysis (0)] |