Published online Jun 18, 2021. doi: 10.5312/wjo.v12.i6.403
Peer-review started: January 27, 2021
First decision: March 8, 2021
Revised: March 15, 2021
Accepted: May 22, 2021
Article in press: May 22, 2021
Published online: June 18, 2021
Glenohumeral osteoarthritis (OA) is a common cause of pain and disability affecting nearly a third of the world’s population over 60 years of age. As in other joints, shoulder arthroplasty appears to be the most effective treatment. The implant design has evolved during time transitioning to shorter humeral stem lengths or even stemless components.
To evaluate the medium-term outcome and survival of a cementless humeral head resurfacing (HHR) in a group of patients affected with OA or avascular necrosis.
This is a retrospective study of prospectively collected data using HHR in 23 patients (15 female and 8 male) after a 7.4 year follow-up. The collected data included clinical and radiographical evaluation. The Constant score, the visual analogue scale, and a clinical evaluation of range of motion were registered pre- and postoperatively. Fifteen patients affected with OA (2 cases of mild, 6 moderate, and 7 severe) and 10 with avascular necrosis (stage III according to Cruess classification) were enrolled. X-rays were evaluated to detect loosening signs, degenerative changes, and superior humeral head migration. Magnetic resonance preoperatively was also performed to assess the rotator cuff status. Tendon integrity was mandatory to implant the HHR.
In total, 19 patients (21 shoulders) completed the follow-up. Data on 4 shoulders, in 4 patients, were lost because of prosthesis failure. The global revision rate was 16%. A statistically significant improvement in the mean Constant score, visual analogue scale, and range of motion have been reported. No signs of loosening were registered, while in 12 cases a glenoid erosion was found. The osteophytes appeared 7 times on the humeral side and 12 on the glenoid. Superior humeral migration was recorded in only 1 case.
HHR remains a reasonable option in patients with an intact rotator cuff for the treatment of OA and avascular necrosis.
Core Tip: Shoulder arthroplasty is the most effective treatment of glenohumeral osteoarthritis. The medium-term outcome and survival of a cementless humeral head resurfacing was retrospectively evaluated in 23 patients affected with osteoarthritis or avascular necrosis after a 7.4 year follow-up. The global revision rate was 16%. A statistically significant improvement in the mean Constant score, visual analogue scale, and range of motion have been reported. No signs of loosening were registered, while in 12 cases a glenoid erosion was found. Humeral head resurfacing remains a reasonable option in patients with an intact rotator cuff for the treatment of osteoarthritis and avascular necrosis.
- Citation: Chillemi C, Paglialunga C, De Giorgi G, Proietti R, Carli S, Damo M. Outcome and revision rate of uncemented humeral head resurfacing: Mid-term follow-up study. World J Orthop 2021; 12(6): 403-411
- URL: https://www.wjgnet.com/2218-5836/full/v12/i6/403.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i6.403
Glenohumeral osteoarthritis (OA) is a common cause of pain and physical limitation, with an estimated prevalence between 4% and 26%[1], affecting nearly a third of the world’s population over 60 years of age[2]. The choice of treatment of shoulder OA is often controversial, and it is related to the surgeon and based on the patient’s age, severity of symptoms, level of activity, radiographic findings, and medical comorbidities[3]. Activity modification, physical therapy, and anti-inflammatory drugs are the major nonoperative treatment options. In case of failure (nonresponding) of this therapeutic approach and when the patients may not wish to progress directly to surgery, intra-articular injection with either corticosteroid or hyaluronic acid or platelet-reach plasma[4] represents a valid alternative. Finally, surgery may be considered. As in other joints affected by severe OA, different surgical procedures are available, being joint arthroplasty the most effective treatment[3].
Shoulder arthroplasty was introduced by Neer et al[5]; from that date prosthesis design has evolved during time to better reproduce and accommodate the individual shoulder anatomy and variability[3,6]. During this evolution, implants have transitioned to shorter humeral stem lengths or even stemless components. The latter has been introduced in 2004 by Copeland, who designed a cementless humeral head resurfacing (HHR) prosthesis for the treatment of glenohumeral OA[7], providing good clinical results[8]. Over time, the indications to implant a cementless HHR changed and extended to other shoulder pathologies, such as humeral head avascular necrosis (AVN), instability arthropathy, rheumatoid arthritis, post-traumatic arthropathy, and cuff tear arthropathy, with good functional results[8,9]. The rationale to implant a cementless HHR is to restore the patient’s individual humeral head anatomy, characterized by articular retroversion, neck shaft angle, lateral offset, and center of rotation, and it is easier to remove, preserving the bone stock for a possible future revision[8,10].
The main criticism referred to HHR is usually related to the incorrect sizing and orientation of the prosthesis, resulting in an oversizing of the joint. A deviation of the center of rotation higher than 5 mm has been shown to correlate to clinical failure of the implant[10]. HHR failure rate is among 6% and 37%[9,11,12]. A direct correlation between overstuffing and glenoid wear has been demonstrated, leading to prosthesis failure[13]. Moreover, an operative change of the lateral glenohumeral offset as a predictive factor for implant failure has been reported[14]. Among other causes for prosthesis failure, rotator cuff tear, painful stiffness without glenoid erosion, or infections were reported[14].
The aim of the present study was to evaluate the medium-term outcomes and survival of a specific cementless HHR in a group of patients operated by a single surgeon in a single center.
This is a report of prospectively collected data of 23 patients who have undergone cementless Aequalis HHR (Tornier, Warsaw, IN, United States) with a mean follow-up of 89 mo (range 44-131 mo).
All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with 1964 Helsinki Declaration and its later amendments. This study was reviewed and approved by the Ethics Committee/Scientific Council of the Istituto Chirurgico Ortopedico Traumatologico of Latina, Italy.
All participants gave informed written consent.
Between February 2009 and May 2014, 25 shoulders in 23 patients (15 females and 8 males; mean age: 67.2 years, min/max: 39-83 years) were surgically treated with the same type of cementless HHR performed by the same surgeon (CC) in a single center. The indications for surface replacement were primary OA (15 shoulders) and humeral head AVN (10 shoulders). The exclusion criteria were the presence of rotator cuff tears (partial or complete) and a damaged glenoid (evaluated with magnetic resonance).
All patients were operated under general anesthesia in the beach-chair position, by using the delto-pectoral approach. An L-shaped tenotomy of the subscapularis tendon was performed, followed by a capsular release for soft tissue balancing. The glenoid surface status was then assessed by retracting the humeral head. Once the humeral head was exposed, all osteophytes were carefully circumferentially removed. This step is critical because head sizing and head orientation are based off the anatomic neck. If the actual humeral head appeared to be in between sizes, the smaller size was selected. The humeral head was then reamed down to restore the original head height. The uncemented press-fit technique was the method of choice for fixing the resurfacing implant (Aequalis RH Tornier, Warsaw, IN, United States). Primary stability was achieved in all cases thanks to the presence of a tapered press-fit, tri-fin antirotational stem and a diamond-shaped macrotexture. In addition, all bone contacting surfaces were hydroxyapatite-coated.
In all cases, the long head biceps tenotomy was performed close to its glenoid attachment, followed by tenodesis in the bicipital groove. After implant reduction, the subscapularis was repaired in a tendon-to-tendon fashion using three to five nonabsorbable sutures. In no cases drains were placed in the shoulder.
The patient’s arm was placed in a shoulder abduction pillow for 4 wk. Passive rehabilitation was started from day 2 with external rotation restricted to 0°. Patients were asked to support these movements actively. Free range of motion was allowed 6 wk after surgery.
Clinical evaluation included pre- and postoperative administration of the Constant scale and the visual analogue scale. The active range of motion was evaluated in pre- and postoperative time, with particular attention to the forward elevation, abduction, and external rotation with the arm at the side.
Pre- and postoperative radiographs were performed in two projections: A true anteroposterior view in neutral rotation and an axillary view. All radiographs were digitalized and scaled on the same size thanks to the software of Picture Archiving and Communication System monitor (General Electric, Chicago, IL, United States), available in the radiology department of our institute.
In the preoperative, the glenohumeral OA and humeral head AVN were radiologically classified according to Samilson and Prieto (mild: inferior humeral and/or glenoid exostosis < 3 mm in height; moderate: inferior humeral and/or glenoid exostosis measuring 3 mm to 7 mm, slight glenohumeral irregularity; severe: inferior humeral and/or glenoid exostosis measuring > 7 mm, glenohumeral joint narrowing and sclerosis) and Cruess (stage I: normal X-ray, changes on magnetic resonance; stage II: sclerosis, osteopenia; stage III: crescent sign indicating a subchondral fracture; stage IV: flattening and collapse; stage V: degenerative changes extend to glenoid).
Preoperative X-ray images revealed OA in 15 shoulders (2 cases of mild, 6 moderate, and 7 severe) and 10 shoulders affected by humeral head AVN (10 cases stage III).
During follow-up, the radiographic evaluation was useful to check signs of: (1) Loosening around the peg and in the eighth humeral zone. Probably loosening is intended as a radiolucent 2 mm wide line or greater around the implant without any change in position of the implant. Definite loosening was defined as a change in position of the implant over time; (2) Degenerative changes (i.e. glenoid erosion, humeral head or glenoidal osteophytes); and (3) Vertical humeral migration of the head intended as a superior migration of the prosthesis outside the center of the glenoid.
The paired t-test was used to determine whether there was a significant difference between preoperative and postoperative Constant score, visual analogue scale, and range of motion obtained at the latest check-up at 7.4 years. A P value of < 0.05 was considered to be statistically significant.
In total, 19 patients (21 shoulders) completed the follow-up.
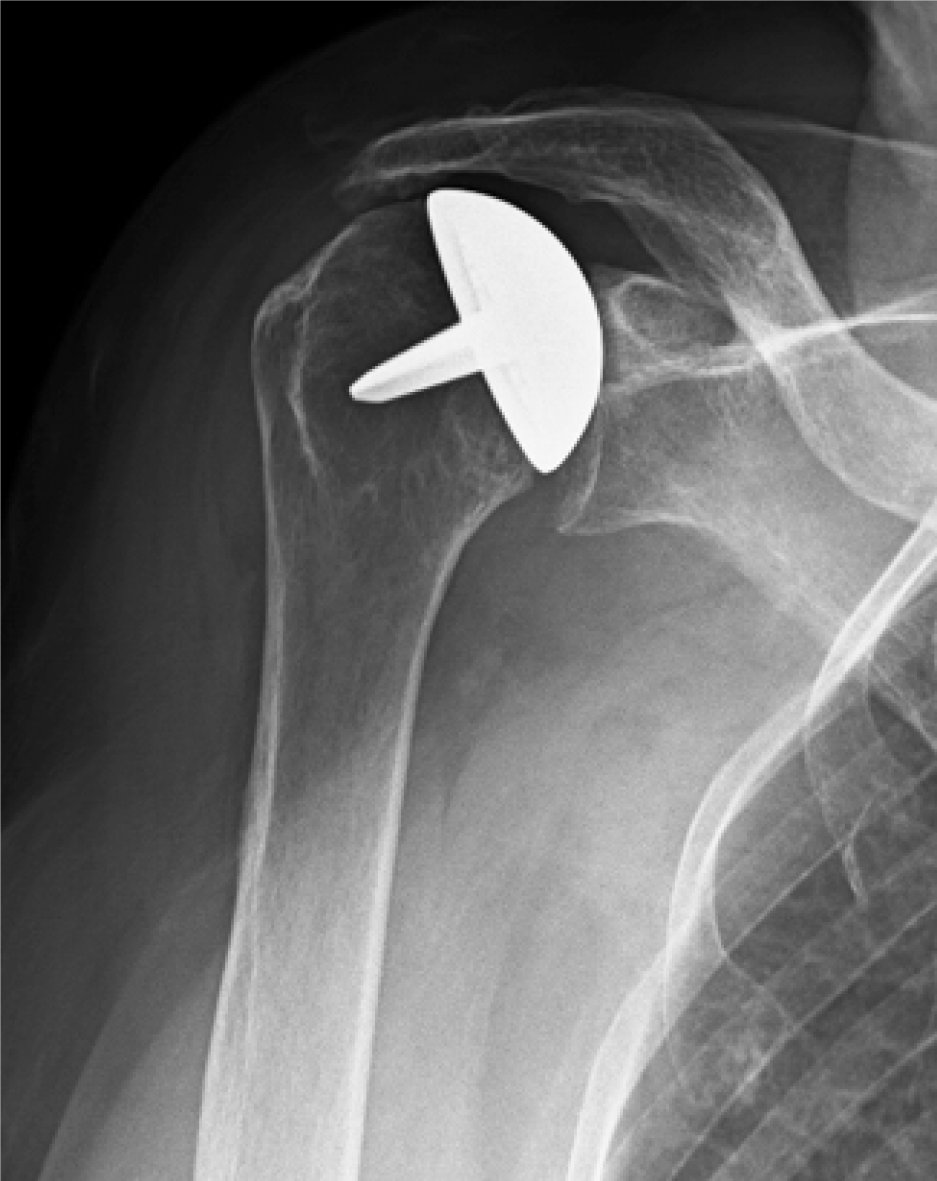
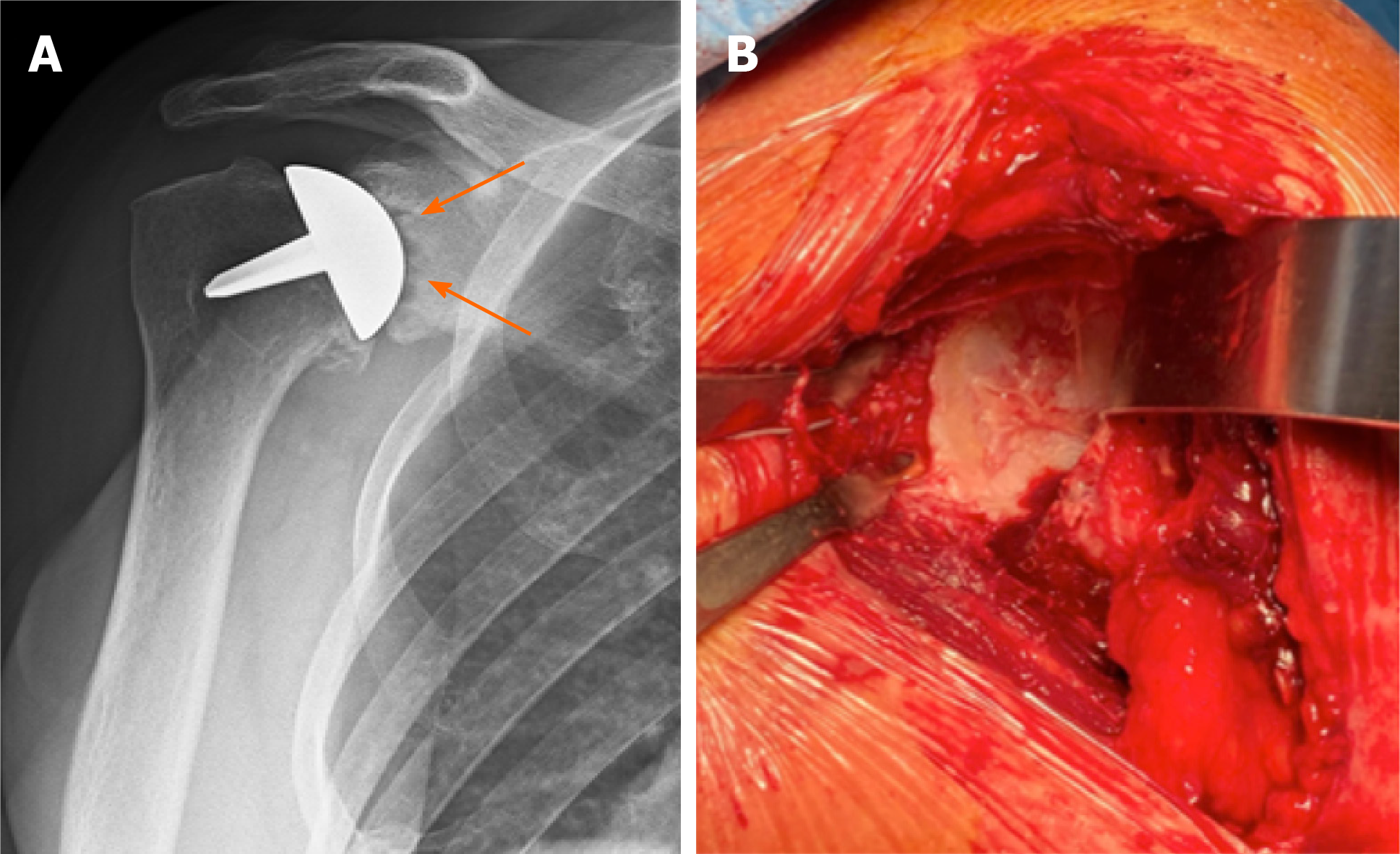
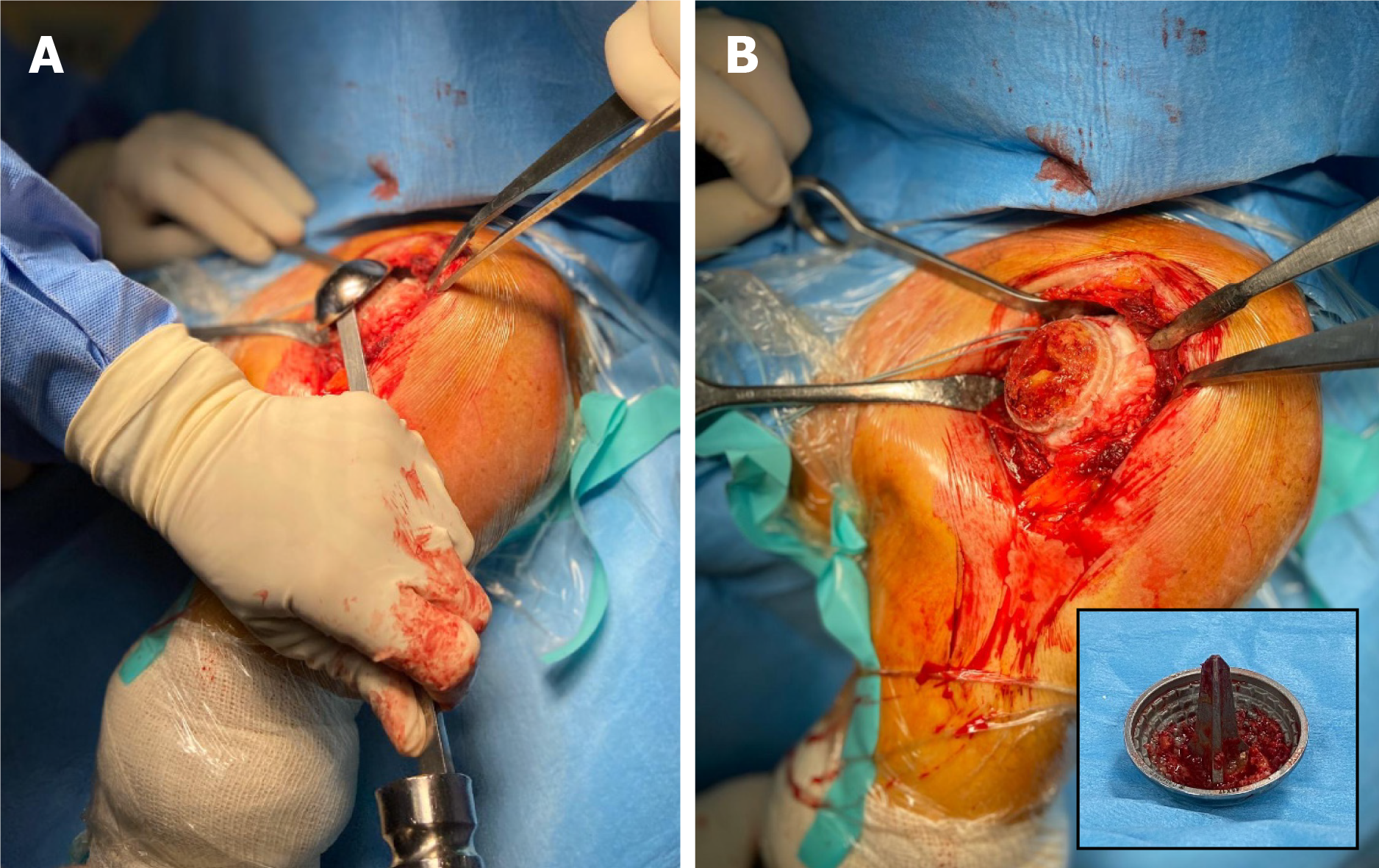
Data on 4 shoulders in 4 patients were lost because of prosthesis failure due to a rotator cuff tear after a traumatic accident (1 case) (Figure 1) and glenoid erosion (3 cases) (Figure 2) that required a revision surgery (Table 1). In all the revised cases the implant was removed without complications, leaving a sufficient bone stock for reimplantation (Figure 3)
| Patient | Sex | Age | Indication | Lifespan of the prosthesis | Failure cause | Revision |
| 1 | Male | 66 | Right OA | 65 mo (5.41 yr) | Glenoiditis | rTSA |
| 2 | Female | 78 | Right AVN | 44 mo (3.66 yr) | Traumatic RCT | rTSA |
| 3 | Female | 75 | Right AVN | 50 mo (4.16 yr) | Glenoiditis | rTSA |
| 4 | Male | 48 | Left AVN | 66 mo (5.50 yr) | Glenoiditis | TSA |
The Constant score improved from the preoperative mean value of 31.1 (range 16-57) to the postoperative mean value of 74.9 (range 57-100) (P < 0.001). In detail, an improvement of all the sections of the Constant score was registered from the preoperative to the postoperative latest evaluation and was statistically significant (P < 0.001) except for the recovery of the strength (P = 0.3).
The pretreatment visual analogue scale grading scale was 7.8 (range 5-10), while at the final follow-up decreased to 2.1 (range 0-5) (P < 0.001).
An improvement in the clinical evaluation of the active range of motion was also registered. The mean flexion forward changed from 75° (range 30°-120°) in the preoperative to 111° (range 60°-160°) in the postoperative (P = 0.06). The pre- and postoperative mean value of abduction were respectively 68° (range 30°-120°) and 104° (range 60°-160°) (P < 0.001), while the mean external rotation value pre- and postoperative were respectively 30° (range 10°-50°) and 54° (range 30°-75°) (P < 0.001) (Table 2).
| Preoperative value (min-max) | Postoperative value (min-max) | P value | |
| Pain | 3.20 (0-5) | 12.38 (10-15) | < 0.001 |
| Daily activity level | 7.96 (6-11) | 16.66 (12-20) | < 0.001 |
| ROM | 10.48 (2-17) | 35.47 (28-40) | < 0.001 |
| Strength | 9.44 (3-25) | 10.42 (4-25) | 0.3 |
| Constant score | 31.08 (16-57) | 74.95 (57-100) | < 0.001 |
| VAS | 2.88 (1-4) | 7.76 (5-10) | < 0.001 |
| Flexion forward | 74.80° (30°-120°) | 111.42° (60°-160°) | 0.06 |
| Abduction | 68.00° (30°-120°) | 104.28° (60°-160°) | < 0.001 |
| External rotation | 30.40° (10°-50°) | 54.04° (30°-75°) | < 0.001 |
No signs of loosening around the peg and in the eighth humeral zone[15] were detected at the latest follow-up. In 11 cases, a glenoidal erosion has been reported. In 3 patients, this condition was symptomatic, while in the remaining 8 it was not. In 12 cases the presence of inferior glenoidal osteophytes was registered, and in 7 cases humeral head osteophytes were registered. Only 1 case of superior humeral migration had been reported due to the traumatic rotator cuff tear (Figure 1).
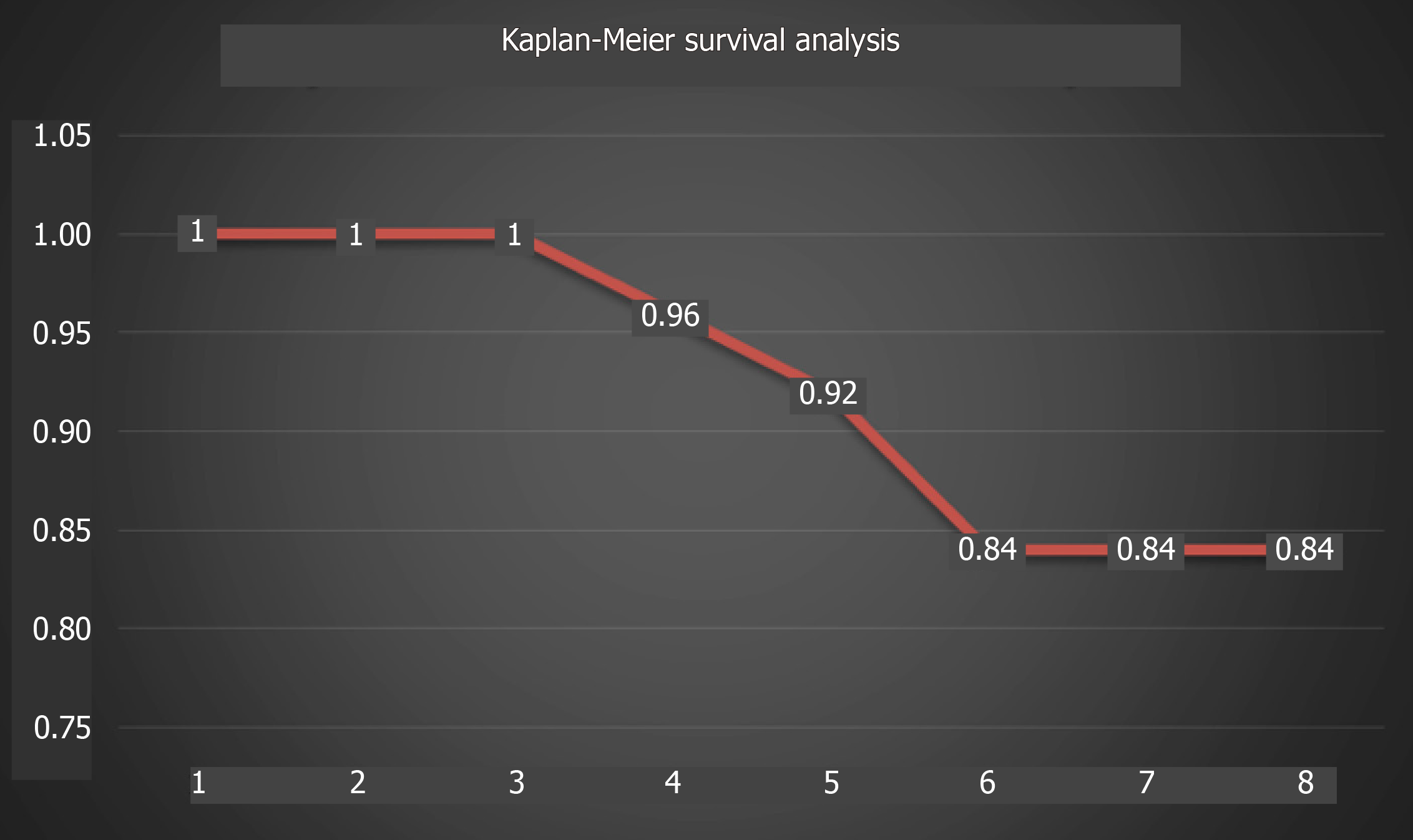
The global revision rate was 16% at a mean follow-up of 7.4 years, and the survival rate was 84% (Figure 4).
Even if during the years the indications to implant a cementless HHR changed and extended from OA to numerous shoulder pathologies[8,9], the authors’ rationale to implant a cementless HHR was to restore the patient’s individual humeral head anatomy, and two major aspects were considered crucial: (1) The integrity of the rotator cuff; and (2) A good bone stock to fix the implant.
The latter aspect may be a problem in AVN. This condition in fact is characterized by the death of cellular components of bone secondary to an interruption of the subchondral blood supply determining a deep bone distortion with abnormal architecture and for this reason may be considered not an absolute indication to implant a cementless HHR, especially in its late stages (IV and V according to Cruess).
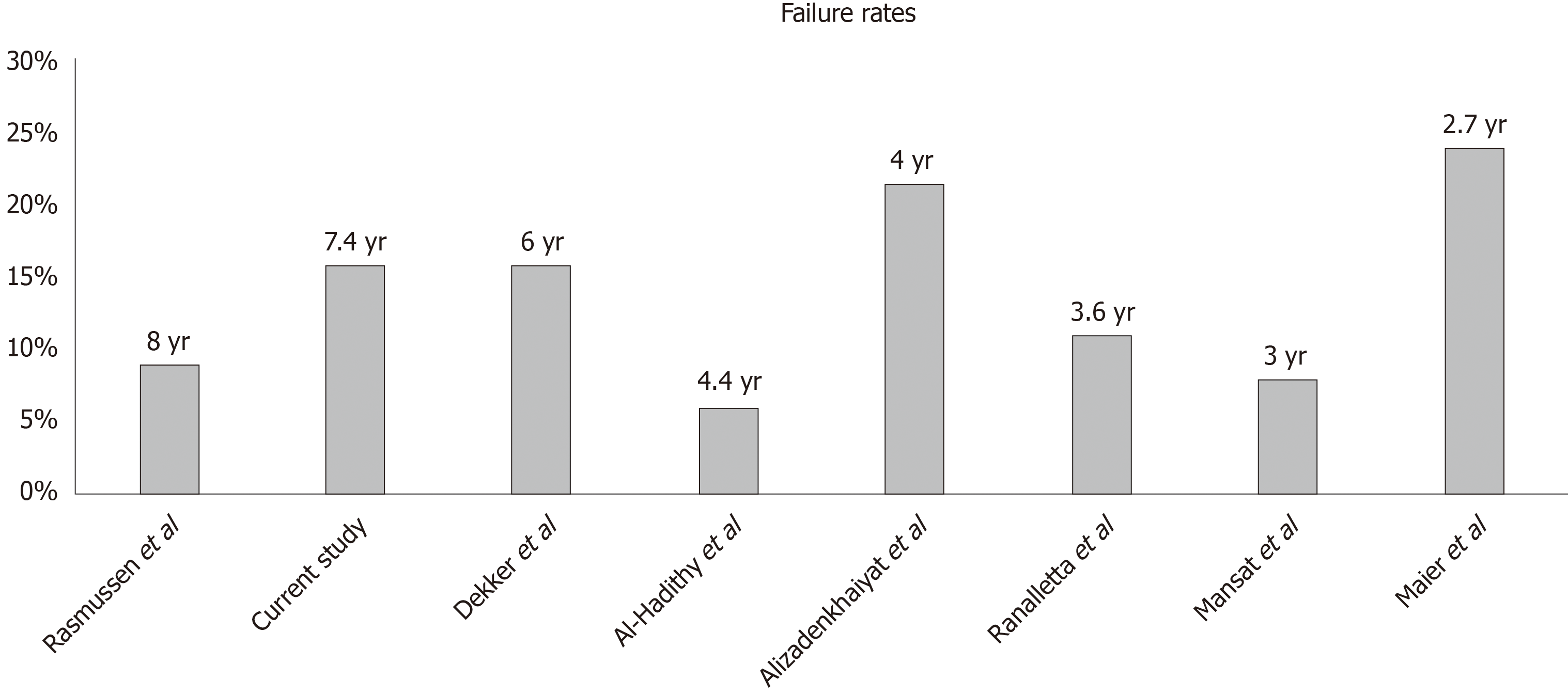
According to the findings of the present study, the clinical and radiological results were good in a mean term follow-up of more than 7 years using the Aequalis HHR in the treatment of glenohumeral OA and humeral head AVN compared to similar data reported in the literature[9,11,14,16-19] (Figure 5).
In contrast, to our knowledge there exists only one paper reporting no failure at a long-term follow-up[20]. In that paper, the study population consisted of 14 young patients (aged 19-49 years) affected by juvenile idiopathic arthritis and treated with HHR with a 10.4 year follow-up period. The authors reported that only two shoulders required early arthroscopic subacromial decompression. This interesting study may have a limitation/bias in the age of its population because it was limited to young patients.
As underlined above, the use of HHR as the primary implant presents numerous advantages in all ages, but especially in younger patients. The cementless implant may preserve the bone stock, and this aspect could be useful during a future revision[7]. Moreover, HHR may be considered a valid alternative in the post-traumatic arthropathy to restore shoulder anatomy[7,14].
As already underlined in the literature and confirmed by this study, glenoid erosion is considered as the most important reported cause of prosthesis failure[14,16,17]. This complication could be caused by an incorrect assessment of the humeral head size and orientation, producing an overstuffing of the joint[10,21].
In the past, different attempts were suggested to limit glenoid erosion, and particular interest was given to the procedure employing a biological glenoid resurfacing[22-24]. After promising early results, all these attempts have failed. The reported long-term failure rate of the biological glenoid resurfacing was 56% using the anterior shoulder capsule even though the authors registered good clinical results after 5 years[22].
Also, the use of autograft Achilles tendon or allograft fascia lata for biological glenoid resurfacing was inconsistent. Elhassan et al[23] registered 10 cases of erosion in 13 patients (failure rate > 70%), so they do not recommend this kind of treatment. The same conclusion was drawn by Lollino et al[24] who proposed to resurface glenoid with the lateral meniscus. After a 2 year follow-up period, the authors reported a narrowing of the articular space, probably related to the meniscal reabsorption.
Taking into consideration the overall results of HHR, the weak part is to date the glenoid. This conclusion is apparently similar to the results of the traditional anatomical shoulder arthroplasty[25]. In fact, many studies have reported that the glenoid component loosening, and failure represented the most common long-term complication of total shoulder arthroplasty. This accounted for approximately 24% of all total shoulder arthroplasty complications[26], so that we have to conclude that the result of a shoulder replacement highly depends on the status of the glenoid, be it native or implanted. Unfortunately, this remains still true although glenoid component design, material, and surgical technique of implant including cement use have been rapidly changed and evolved during the last decades[25].
HHR can be considered a good treatment option in OA and humeral head AVN in patients with an intact rotator cuff. This paper, even though is based on a small cohort of patients, shows a good outcome with a failure rate of 16% in a 7.4 year follow-up. No loosening or infection issues were encountered. The main problem of this prosthesis is the higher revision rate due to glenoid erosion, which is comparable to reported rates on total shoulder arthroplasty. Its advantage is bone preservation in the proximal humerus.
Glenohumeral osteoarthritis and avascular necrosis are causes of shoulder pain and disability. Shoulder arthroplasty is the most effective treatment. The implant design has evolved during time transitioning to shorter humeral stem lengths or even stemless components.
The rationale to implant a cementless humeral head resurfacing (HHR) is to restore the patient’s individual humeral head anatomy, characterized by articular retroversion, neck shaft angle, lateral offset, and center of rotation, and it is easier to remove, preserving the bone stock for a possible future revision. The reported revision rate at a mid-term follow-up is not so high, so this could be an alternative to a total shoulder arthroplasty.
Our aim is to evaluate the medium-term outcome and survival of a cementless HHR in a group of patients affected with osteoarthritis or avascular necrosis.
This is a report of prospectively collected data using HHR in 23 patients (15 female and 8 male) after a 7.4 year follow-up.
The global revision rate was 16%. Data on 4 shoulders in 4 patients were lost because of prosthesis failure. Nineteen patients (21 shoulders) completed the follow-up. No signs of loosening were registered, while in 12 cases a glenoid erosion was found. The osteophytes appeared 7 times on the humeral side and 12 on the glenoid. Superior humeral migration was recorded in only one case.
The use of a cementless HHR in the treatment of osteoarthritis and early stage avascular necrosis could nowadays be consider a valid therapeutic option.
Further research based on well-designed studies with longer follow-up examination and with a bigger patient population need to be performed in order to elucidate the efficacy of cementless HHR.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: European Society for Surgery of the Shoulder and the Elbow; Italian Orthopaedic Society; and Italian Society of Shoulder and Elbow Surgery.
Specialty type: Orthopedics
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Silvestre-Muñoz A, Widmer KH S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | Thomas M, Bidwai A, Rangan A, Rees JL, Brownson P, Tennent D, Connor C, Kulkarni R. Glenohumeral osteoarthritis. Shoulder Elbow. 2016;8:203-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Fourman MS, Beck A, Gasbarro G, Irrgang JJ, Rodosky MW, Lin A. Humeral head resurfacing is associated with less pain and clinically equivalent functional outcomes compared with stemmed hemiarthroplasty at mid-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2019;27:3203-3211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Chillemi C, Franceschini V. Shoulder osteoarthritis. Arthritis. 2013;2013:370231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Werner BC, Cancienne JM, Browning R, Verma NN, Cole BJ. An Analysis of Current Treatment Trends in Platelet-Rich Plasma Therapy in the Medicare Database. Orthop J Sports Med. 2020;8:2325967119900811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64:319-337. [PubMed] [Cited in This Article: ] |
| 6. | Liu EY, Kord D, Horner NS, Leroux T, Alolabi B, Khan M. Stemless anatomic total shoulder arthroplasty: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2020;29:1928-1937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Copeland S. The continuing development of shoulder replacement: "reaching the surface". J Bone Joint Surg Am. 2006;88:900-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Levy O, Copeland SA. Cementless surface replacement arthroplasty (Copeland CSRA) for osteoarthritis of the shoulder. J Shoulder Elbow Surg. 2004;13:266-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Ranalletta M, Bertona A, Tanoira I, Rossi LA, Bongiovanni S, Maignón GD. Results of partial resurfacing of humeral head in patients with avascular necrosis. Rev Esp Cir Ortop Traumatol. 2019;63:29-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Geervliet PC, Willems JH, Sierevelt IN, Visser CPJ, van Noort A. Overstuffing in resurfacing hemiarthroplasty is a potential risk for failure. J Orthop Surg Res. 2019;14:474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Al-Hadithy N, Domos P, Sewell MD, Naleem A, Papanna MC, Pandit R. Cementless surface replacement arthroplasty of the shoulder for osteoarthritis: results of fifty Mark III Copeland prosthesis from an independent center with four-year mean follow-up. J Shoulder Elbow Surg. 2012;21:1776-1781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Werner BS, Stehle J, Abdelkawi A, Plumhoff P, Hudek R, Gohlke F. Progressive glenoid bone loss caused by erosion in humeral head resurfacing. Orthopade. 2017;46:1028-1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Soudy K, Szymanski C, Lalanne C, Bourgault C, Thiounn A, Cotten A, Maynou C. Results and limitations of humeral head resurfacing: 105 cases at a mean follow-up of 5 years. Orthop Traumatol Surg Res. 2017;103:415-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Maier MW, Hetto P, Raiss P, Klotz M, Bülhoff M, Spranz D, Zeifang F. Cementless humeral head resurfacing for degenerative glenohumeral osteoarthritis fails at a high rate. J Orthop. 2018;15:349-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Sperling JW, Cofield RH, O'Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 246] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Rasmussen JV, Olsen BS, Al-Hamdani A, Brorson S. Outcome of Revision Shoulder Arthroplasty After Resurfacing Hemiarthroplasty in Patients with Glenohumeral Osteoarthritis. J Bone Joint Surg Am. 2016;98:1631-1637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Mansat P, Coutié AS, Bonnevialle N, Rongières M, Mansat M, Bonnevialle P. Resurfacing humeral prosthesis: do we really reconstruct the anatomy? J Shoulder Elbow Surg. 2013;22:612-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Alizadehkhaiyat O, Kyriakos A, Singer MS, Frostick SP. Outcome of Copeland shoulder resurfacing arthroplasty with a 4-year mean follow-up. J Shoulder Elbow Surg. 2013;22:1352-1358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Dekker AP, Joshi N, Morgan M, Espag M, A Tambe A, Clark DI. 6-Year clinical results and survival of Copeland Resurfacing hemiarthroplasty of the shoulder in a consecutive series of 279 cases. J Clin Orthop Trauma. 2020;11:S265-S269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Ibrahim EF, Rashid A, Thomas M. Resurfacing hemiarthroplasty of the shoulder for patients with juvenile idiopathic arthritis. J Shoulder Elbow Surg. 2018;27:1468-1474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Mechlenburg I, Amstrup A, Klebe T, Jacobsen SS, Teichert G, Stilling M. The Copeland resurfacing humeral head implant does not restore humeral head anatomy. A retrospective study. Arch Orthop Trauma Surg. 2013;133:615-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Lee KT, Bell S, Salmon J. Cementless surface replacement arthroplasty of the shoulder with biologic resurfacing of the glenoid. J Shoulder Elbow Surg. 2009;18:915-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Elhassan B, Ozbaydar M, Diller D, Higgins LD, Warner JJ. Soft-tissue resurfacing of the glenoid in the treatment of glenohumeral arthritis in active patients less than fifty years old. J Bone Joint Surg Am. 2009;91:419-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Lollino N, Pellegrini A, Paladini P, Campi F, Porcellini G. Gleno-Humeral arthritis in young patients: clinical and radiographic analysis of humerus resurfacing prosthesis and meniscus interposition. Musculoskelet Surg. 2011;95 Suppl 1:S59-S63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Castagna A, Garofalo R. Journey of the glenoid in anatomic total shoulder replacement. Shoulder Elbow. 2019;11:140-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Gonzalez JF, Alami GB, Baque F, Walch G, Boileau P. Complications of unconstrained shoulder prostheses. J Shoulder Elbow Surg. 2011;20:666-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |