Published online Nov 18, 2019. doi: 10.5312/wjo.v10.i11.404
Peer-review started: January 28, 2019
First decision: March 18, 2019
Revised: May 17, 2019
Accepted: September 15, 2019
Article in press: September 15, 2019
Published online: November 18, 2019
Primary synovial chondromatosis (PSC) is a rare arthropathy of the synovial joints characterized by the formation of cartilaginous nodules, which may detach and become loose bodies within the joint and may undergo secondary proliferation. PSC of the foot and ankle is exceedingly rare, with only a few cases reported in the literature. The diagnosis may be difficult and delayed until operative treatment, when it is confirmed by histological assessment. PSC may degenerate into chondrosarcoma. Operative treatment is the gold standard aiming to minimize pain, improve function, prevent or limit progression of arthritis. Surgical treatment consists in debridement by arthrotomic or arthroscopic management, but there is no consensus in the literature about timing of surgery and surgical technique. Thus, the aim of this study is to report the outcomes of the surgical treatment of two cases, together with a literature review.
We report two cases of patients affected by PSC of the foot in stage III, according to the Milgram classification: the former PSC localized in the ankle that underwent open surgery consisted of loose bodies removal; the latter in the subtalar joint, and the choice of treatment was the arthrotomy and debridement from loose bodies, in addition to the subtalar arthrodesis. Both patients returned to complete daily and working life after surgery.
Synovial chondromatosis is a rare benign pathology, even rarer in the ankle joint and especially in the foot. Surgery should be minimal in patients with ankle PSC, choosing the correct timing, waiting if possible until stage III. More aggressive and early surgery should be performed in patients with PSC of the foot, particularly the subtalar joint, due to the high risk of arthritic evolution.
Core tip: Synovial chondromatosis of the ankle and foot is a particularly rare, benign pathology. Despite the etiology remaining unknown, it can severely impair common daily activities of affected patients because of severe pain and limitation of joint motion. Surgical treatment should be performed in patients with Milgram’s stage III primary ankle synovial chondromatosis. Earlier surgery should be limited to primary synovial chondromatosis of the foot only, because of the higher frequency of subsequent degenerative pathologies.
- Citation: Monestier L, Riva G, Stissi P, Latiff M, Surace MF. Synovial chondromatosis of the foot: Two case reports and literature review. World J Orthop 2019; 10(11): 404-415
- URL: https://www.wjgnet.com/2218-5836/full/v10/i11/404.htm
- DOI: https://dx.doi.org/10.5312/wjo.v10.i11.404
Synovial chondromatosis (SC) is a rare arthropathy of the synovial joints characterized by the formation of cartilaginous nodules in the synovium[1-3]. These nodules may detach and become loose bodies within the joint, and may undergo secondary calcification and proliferation[4] .
Some authors suggested metaplastic or neoplastic origin. Nevertheless, the exact initial stimulus resulting in synovial transformation is still unknown[2,5,6].
Males in their third to fifth decades are typically affected. SC can be classified as primary (benign neoplastic process) or secondary, and associated with joint abnormalities, such as mechanical or arthritic conditions[7]; the pathogenesis of the latter being related to certain synoviocyte dysfunction. Particularly, cells like synovial macrophages, other synoviocytes and chondrocytes may produce different enzymes and cytokines, inducing inflammation and damage to articular tissues[8].
Knee (up to 65%) and hip are mostly involved, followed by elbow and shoulder; uncommon cases have been reported in wrist, interphalangeal and temporo-mandibular joints, as well as in extra-articular locations[5,7,9,10].
Primary (P) SC of the foot and ankle is exceedingly rare, with only a few cases reported in the literature worldwide: Reports include tibiotalar, calcaneocuboid, talonavicular, subtalar, navicular-cuneiform, tarsometatarsal and meta-tarsophalangeal joints[11-15].
We report two cases of patients affected by PSC of the foot: The former in the ankle, the latter in the subtalar joint. The aim of the study is to report the outcomes of the surgical treatment of those two rare cases, together with a review of the literature.
Male, Caucasian, 50-years-old. The patient complained of pain, stiffness, crepitation and catching sensation at his right ankle for more than 1 year. No traumatic events were reported. Personal and family history were silent at the time of onset.
At admission, clinical assessment revealed swelling of the ankle and good range of motion (ROM) (dorsiflexion 10°; plantarflexion 10°). Anterior impingement with moderate pain was detected. No vascular or neurological abnormalities were referred; laboratory test, blood and urine were normal.
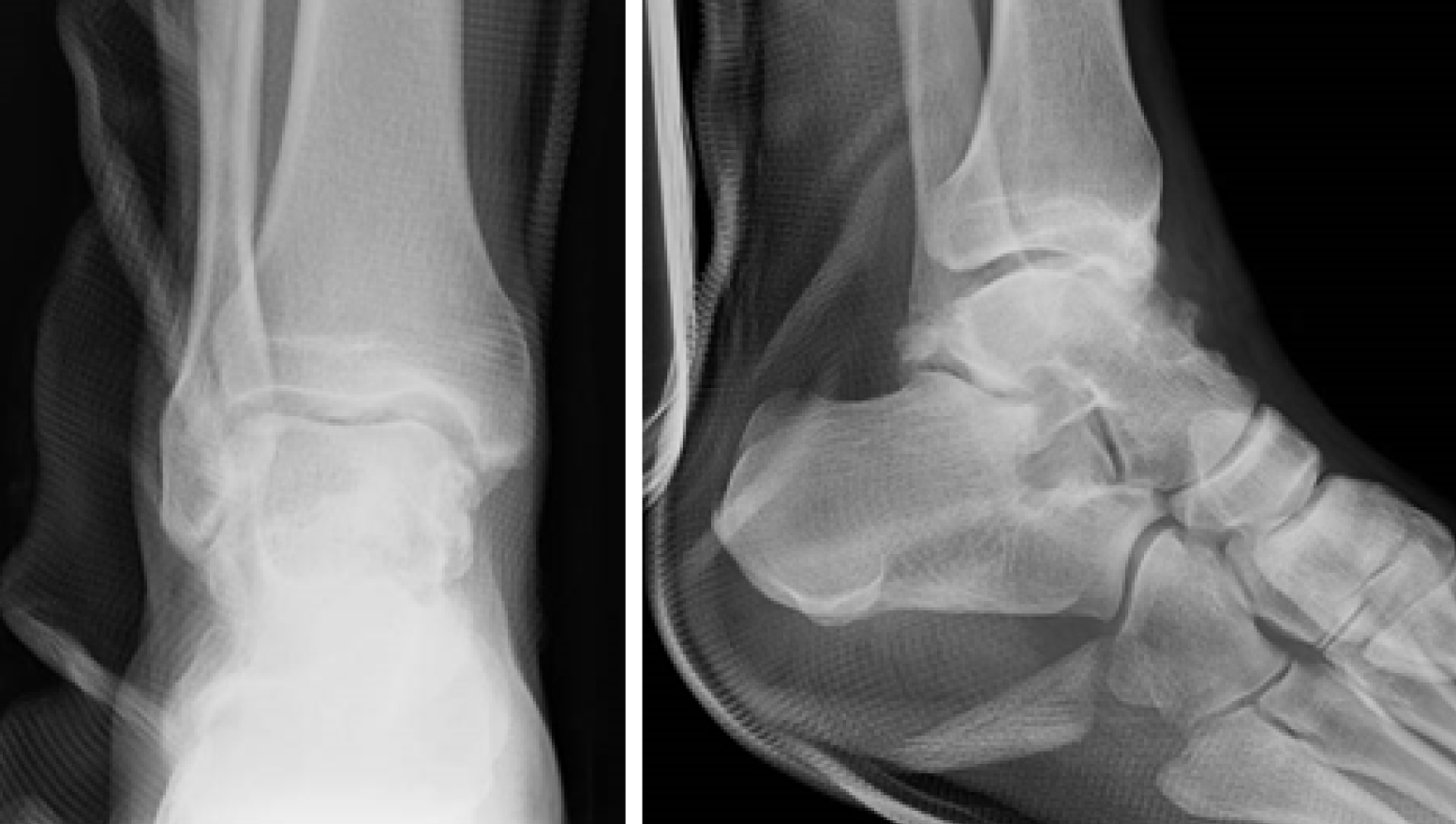
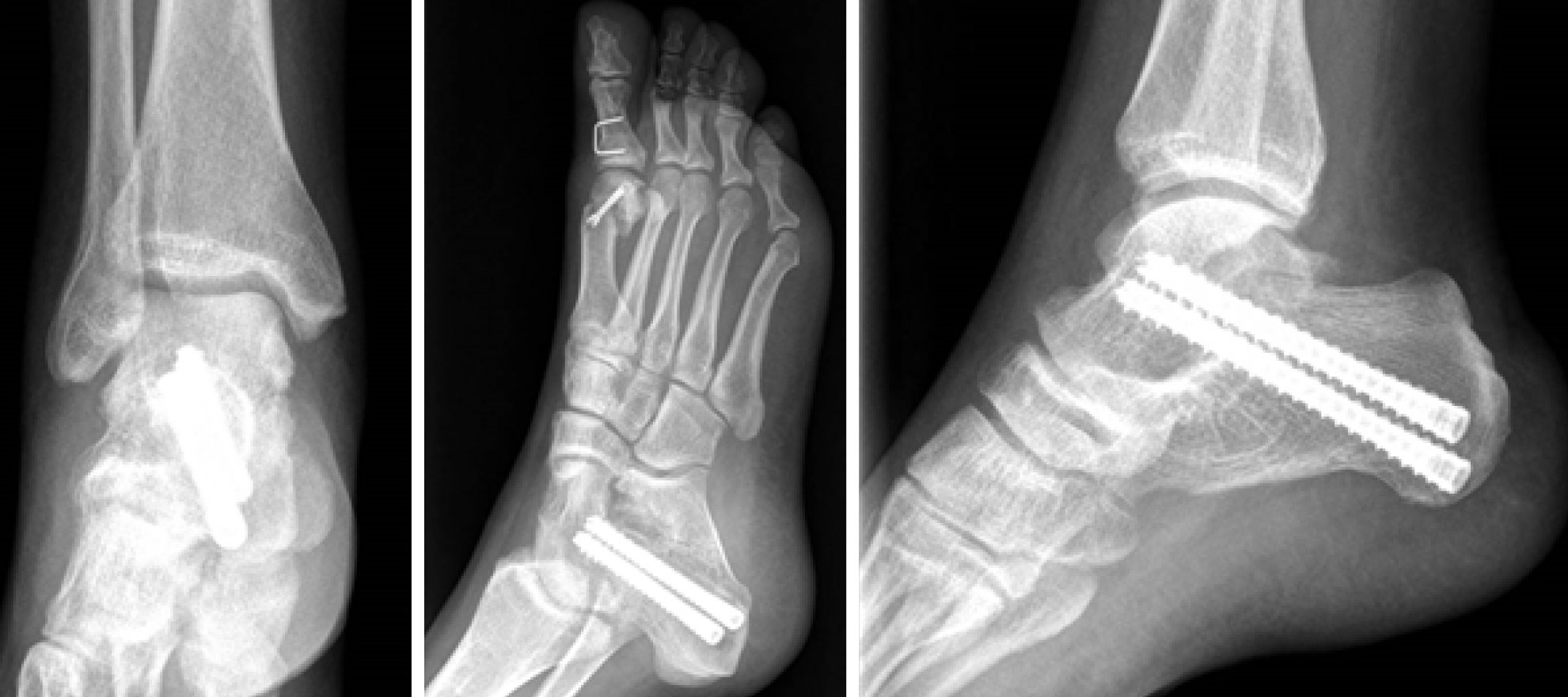
Radiographs are shown in Figure 1. Multiple intra-articular loose bodies were seen around the ankle joint, suggesting possible PSC.
Female, Caucasian, 43-years-old. The patient complained of pain at the right rearfoot, and two crutches were necessary for walking. These symptoms have been present and worsening over the last 2 years, and a previous surgery for tarsal tunnel syndrome was reported several years before. No other significant information on personal or family history was collected.
At admission, the right ankle was swollen and aching; ROM was good (dorsiflexion 10°; plantarflexion 30°). Palpation of sinus tarsi was painful. as was its passive motion. Ipsilateral hallux valgus was present. No vascular or neurological abnormalities were referred nor seen, and all laboratory tests were normal.
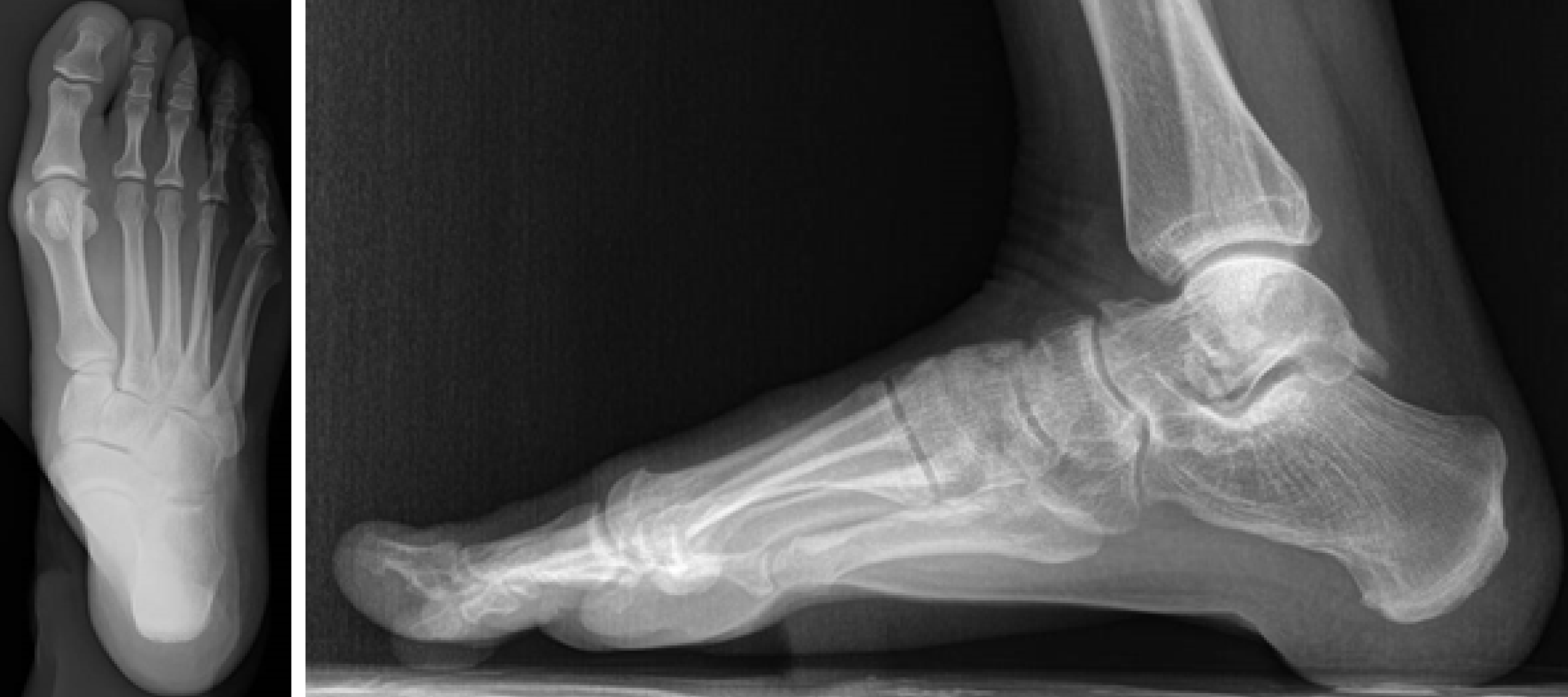
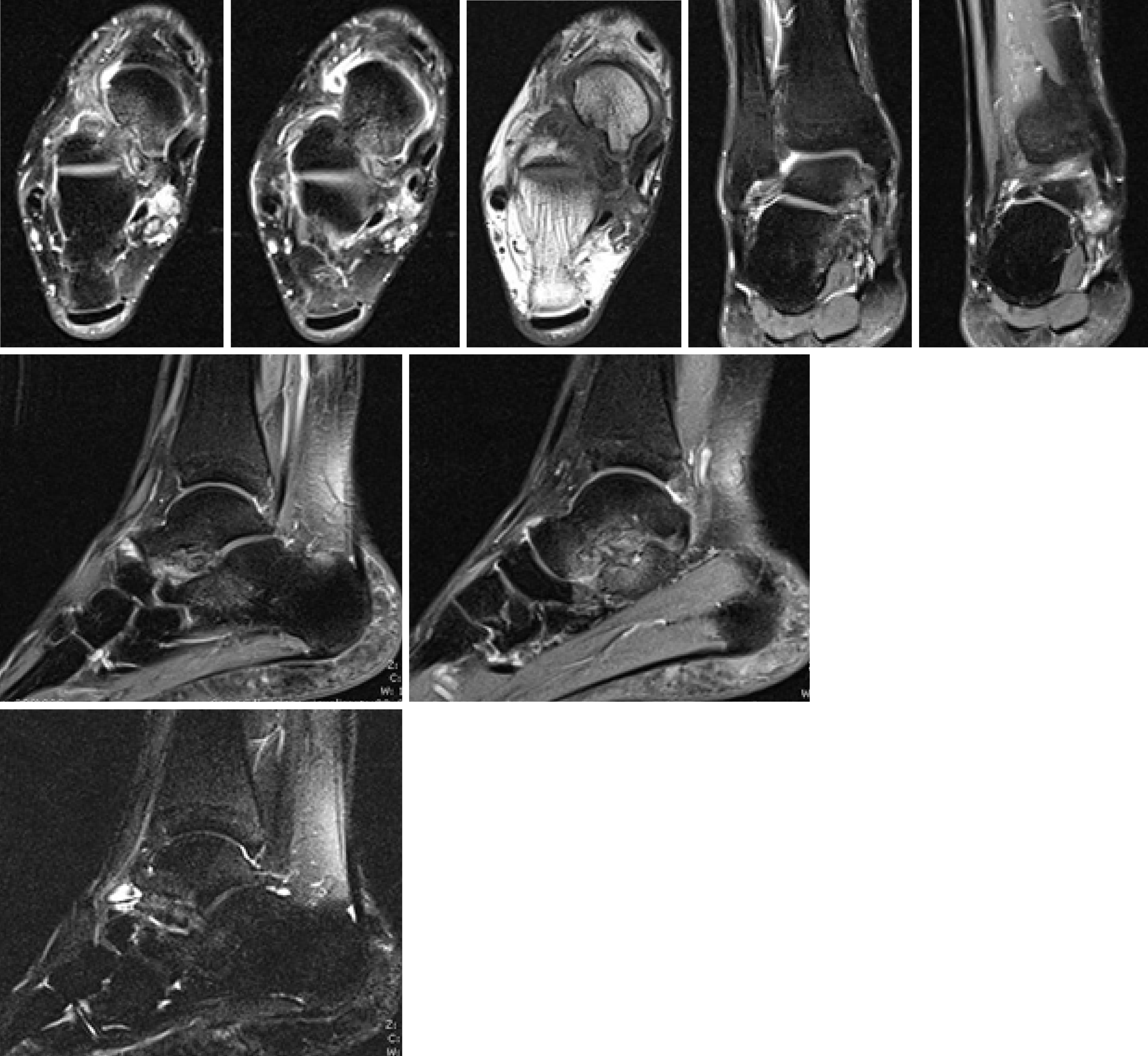
Plain radiographs of the rearfoot revealed no osseous abnormality: a cavus right foot with hallux valgus, and an arthritic degeneration of the talo-navicular joint. No loose bodies or osteochondral defects were seen (Figure 2). Magnetic resonance imaging (MRI) identified a mass extending from the subtalar joint to the soft tissue posteriorly to the medial malleolus, as an arthritic formation at the subtalar joint (Figure 3).
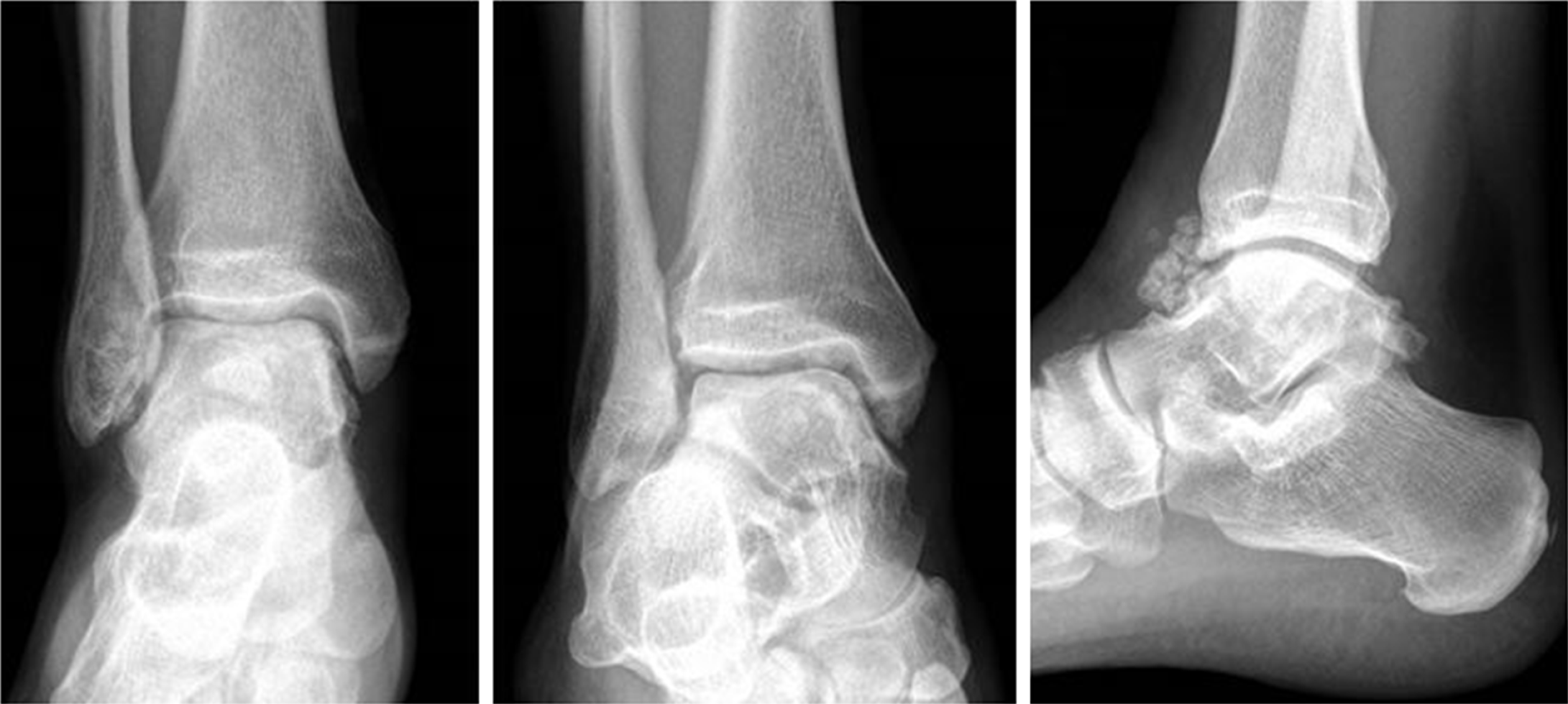
Patient 1 underwent surgery, with removal of all the intra-articular bodies; the joint was irrigated by copious amounts of normal saline. The ankle was immobilized with a cast, and weight-bearing was avoided for 1 mo (Figure 4).
Also, patient 2 did undergo operative treatment. Direct approach to the subtalar joint was performed; fibrous, scar tissue was found, probably due to the previous surgery; multiple loose bodies and arthritic degeneration were found in the subtalar joint. A medial approach was also necessary to release the tibialis posterior nerve trapped within the synovial degenerative tissue. After removal of all loose bodies, an arthrodesis of subtalar joint was performed. At the same time, Youngswick and Akin osteotomies were performed for the hallux valgus (Figure 5).
Postoperatively, the patient was immobilized in a non-weightbearing cast for a month.
Histological examination of surgically harvested samples from “patient 1” revealed SC, with no evidence of malignant transformation.
Histology of patient 2’s loose bodies showed multiple osseous-cartilaginous fragments from the synovium.
At 1-year follow up, “patient 1” was pain-free with a full ROM (dorsiflexion 20°; plantarflexion 30°), and full return to his previous daily activity level. No recurrence of pathology was observed.
At the same interval follow up, patient 2 was also pain-free with a full ROM of the ankle (dorsiflexion 20°; plantarflexion 40°), and went back to her previous daily activities. Similarly, no recurrence of pathology was seen.
SC shows a 1:100,000 incidence rate[16]. The disease affects males twice as often as females, with peak incidence in the third to fifth decades[17]. Secondary forms mainly affect older subjects, between the fourth and seventh decades of life[6].
SC has rarely been reported in the prepubescent age group, and only a handful of cases have been reported extra-articularly in children[18]. Mishra finally described a PSC case in a post-partum woman[19].
SC is usually a monoarticular disease[2,11]. The knee joint is the predilection site (up to 50%-65%), followed by the hip, shoulder and elbow[7,20]. In some conditions, authors referred to “snow storm knee”; larger loose bodies fragments under the force of joint movements, giving rise to smaller bodies that may grow again in the synovium through real mitoses and matrix synthesis. This vicious circle leads to a myriad of loose bodies (from 200 to more than 1,500 bodies, often with a diameter of 1–2mm)[21]. Uncommon cases have been reported in wrist, metacarpophalangeal or interphalangeal joints, acromioclavicular, temporo-mandibular and tibiofibular articulations, as well as extra-articular locations[5,9,10]. Littrel et al[22] described 28 cases of PSC in the spine from 1984-2011.
PSC of the foot and ankle is exceedingly rare, with only a few cases reported in the literature worldwide. The subtalar joint is mostly affected[11,13-15]. Other reports include tibiotalar, calcaneocuboid, talonavicular, navicular-cuneiform, tarsometatarsal and metatarsophalangeal joints[1,13,15]. Recently, Isbell described a PSC case of the ankle with associated talar syndrome[23].
SC can be differentiated into primary (PSC) and secondary forms (SSC).
PSC occurs in an otherwise normal joint[10,15,18]. This form is generally thought to be progressive, more likely to recur, and may lead to severe degenerative arthritis with long term presence[15]. Primary cases are thought to be more aggressive, and a relationship with osteoarthritic processes was mentioned[24,25]. Stensby et al[15] reported a history of trauma in 24% of PSC cases involving the feet.
Its pathogenesis is unknown; it is considered that undifferentiated mesenchymal stem cells proliferate in the synovium, forming nodular foci of hyaline cartilage[2,5,6,21]. It is assumed that chondroid metaplasia (with high numbers of MIB-1-positive chondrocytes) occurs as a precursor of cartilaginous nodules, under the influence of bone morphogenetic protein (BMP). Cartilage develops in the synovium, leading to sessile nodules that often detach and remain as loose bodies within the synovial folds or articular cavity, being nourished by the synovial fluids, as they may calcify and even ossify over time[5,9,10,16,21,23,26]. Silva hypothesized that PSC is a secondary disorder following cartilage shedding into a joint[16].
There are rare reports of familial association (2% of cases) related to type 2 collagen abnormalities, such as those described for Wagner-Stickler syndrome. The hedgehog signaling pathway, measured by its target genes PTC1 and GLI1, may play a role in the development of PSC[20].
Several studies suggested cytogenetic implications: Mertens described complex structural chromosomal aberrations in three PSC lesions[27]. Robinson reported diploid chromosomes and expression of C-ERB B-2 in about half of the cells, indicating that the disorder is probably metaplasia of the synovium[28]. The same proto-oncogene was found in a familial case of two brothers with PSC at the ankle[20]. Apte and Athanasou[29] claimed that synovial cells in PSC express CD68 and HLA-DR. Sciot et al[17] reported a case of SC with clonal chromosomal changes.
Mertens reported the rearrangement of band lpl3, as loss of band IOq26 and translocations involving 12ql13-15 were as frequent as in other benign and malignant chondroid tumors[27].
Dysplasia of fibroblasts is also reported in PSC[27,30]. Pau discussed the metaplasia due to the presence of fibroblast growth factor FGF-9 and FGF receptor-3, which activate bone morphogenetic proteins 2 and 3[25]. Robinson showed changed levels of FGF-9 and FGF receptor-3, which can increase the proliferation of mesenchymal cells. The presence of FGF-9 and FGF receptor-3 creates a feedback loop that results in the continued proliferation of loose bodies[25].
Although PSC is considered a benign condition (metaplasia), some authors suggested a possible neoplastic origin; indirect evidence for a neoplastic origin could be derived from the existence of well-documented cases of chondrosarcoma originating in SC[1,25,27,30,31]. Ozyurek et al[33] suggested neoplastic origin with chromosome 6 abnormalities[32]. In his review of 155 cases of PSC, McCarthy identified four cases of aggressive chondrosarcoma-like masses.
Secondary SC is associated with joint abnormalities, such as mechanical or arthritic conditions: Degenerative arthritis, trauma, inflammatory and non-inflammatory arthropathies, neuropathic arthropathy and avascular necrosis[20,24,34,35]. More frequent than primary chondromatosis, SSC occurs when cartilage fragments detach from articular surfaces or become embedded in the synovium. These loose bodies are nourished by the synovium, and consequently produce chondroid nodules[16,35]. Cytogenetic aberrations are absent in secondary SC[20]. SSC is not likely to recur after operative removal[10].
Diagnosis may be difficult and delayed until operative treatment, as symptoms are vague and physical assessment non-specific[6].
Although a history of ankle sprains is often described, patient may not experience any trauma, inflammatory or other joint disease[13,19,21,34].
Clinical symptoms typically include pain, swelling, stiffness and restriction of ROM[2,20,35]. Aching is described as chronic, subtle, a “catching sensation”, or dull; in some cases, patients refer to “walking on pebbles”[3,10,32]. Discomfort is aggravated by physical activity, running, shoe-wearing, weight bearing and climbing stairs, which could last all-day long[10,13,23,32]. Patients may also report tingling and burning along the plantar aspect of the foot[13,23].
Physical examination demonstrated normal symmetrical alignment of the hindfoot, midfoot, and forefoot[12]. Christensen and Poulsen proposed that a clinical diagnosis may be made in the presence of any one of three diagnostic criteria: changes within the synovial membrane, metaplasia or more than three intra-articular nodules[24].
Assessment reveals focal swelling or diffuse joint enlargement, articular tenderness, articular crepitus, locking, and palpable bony nodules or a mass[18-20]. The overlying skin is usually normal, without erythema[11,18,19,36].
Movement is painful and limited[37]. Instability is normally absent as drawer ankle test negative[14,34,38].
In case of known PSC with rapid deterioration of clinical symptoms, transformation to synovial chondrosarcoma should be suspected[2].
Laboratory studies including C-reactive protein and erythrocyte sedimentation rate were normal[3,5,11,14,15].
Radiographs: Plain radiographs are mandatory for the diagnosis of SC.
Features of PSC include multiple intraarticular chondral bodies of uniform size distributed within the joint, with "ring-and-arc" chondroid mineralization[8,16,20]; calcified bodies are usually smooth, round and finely stippled[1,14]. Edema and calcifications of surrounding soft tissues are characteristic[36,37].
Calcifications or mineralization are common (67%-95% of cases)[1,15,20,38]. Only 29% of PSC cases presenting in the subtalar joint demonstrated mineralization, compared to 91% of cases of PSC presenting in other joints of the feet[11,12,15,24,26,39,40]. This discrepancy may in part be attributed to the average time of diagnosis from the onset of symptoms; 12 mo in the subtalar joint and 27 mo in other joints[15].
Osseous erosion is also typical (20%-80% of all cases)[1,12,20,41]. Osseous erosions are not correlated with symptoms, while relationships with the presence of tightly adherent synovium have been reported[42].
In long-stage PSC disorders, mineralization of the nodules may evolve to enchondral ossification with peripheral calcified rim cortex and inner trabecular bone[5,41]. Although juxta-articular osteopenia may develop, bone density is usually preserved[16]. Secondary osteoarthritis may arise in more advanced untreated disease, as well as displacement or even dislocation of the joint[16,43].
SSC shows fewer osteochondral bodies with a greater variability in size, suggesting various times of origin; they exhibit several rings of calcification, in contradistinction to the single ring seen in primary disease. Osteoarthritis degeneration is usually evident as an extrinsic erosion of bone[16].
Computed tomography (CT): CT is for identifying the calcified intra-articular nodules; calcified nodules may present a “ring and arc” pattern of mineralization or a target appearance[16]. CT can also help differentiate PSC loose bodies from loose bodies that are secondary to degenerative osteoarthritis or joint destruction[1].
CT depicts document joint destruction and extrinsic bone erosion[1,20]. After intravenous administration of contrast material, peripheral and septal enhancement may be seen[16].
MRI: MRI is mandatory to assess alterations of synovium, define the stage of the disorder (Milgram’s classification) and depict bone erosion. It represents the best modality to evaluate lesion extension, extrinsic bone erosion and marrow invasion[16].
Nodules of low or isointense signal on T1-weighted images and high signal on T2-weighted images, with thin peripheral and septal enhancement, are typical described; nidus of low signal intensity could be referred, depending on minerali-zation/ossification and joint effusion[1,16,20,36,39].
Villonodular hypertrophic synovitis is often revealed with hemosiderin deposits or mineralization, as well as calcified lesions of ankle ligaments[5,38,44].
Kramer et al[45] described three patterns of PSC, based on the signal intensity of the nodules. Pattern A (16% of cases) consists of lobulated homogeneous intra-articular isointense/hypointense T1-weighted and hyperintense T2-weighted nodules without mineralization. They are difficult to distinguish from the synovial fluid. If vascular supply from the synovium is present, nodules may only show peripheric gadolinium enhancement. Pattern B (75% of cases) had a similar appearance; nonetheless, foci of low signal intensity due to calcification are depicted. Pattern C (9% of cases) reveals both A and B features, but nodules are characterized by an external hypointense signal ring and fat signal core, corresponding to ossification. Among SC of the foot, pattern A is described in 44% of cases, pattern B in 33% and pattern C in 22%[15,16]. MR is also crucial to evaluate enlarging erosions, cortical destruction of bones and early marrow infiltration[33].
Other investigations: Ultrasonography may show heterogeneous avascular masses surrounded by fluid, with hyperechoic foci (cartilaginous nodules or osseous loose bodies). Posterior acoustic shadowing may be present, depending on mineralization[16]. A bone scan may be useful in determining the level of activity of the disease, and the assessment of possible recurrence after operative excision[24].
The diagnosis is confirmed by histopathological examination, which reveals a «cobblestone appearance» of lobulated hyaline cartilage, surrounded by synovium and some degree of nuclear atypia without the presence of mitosis[9,19,20,22]. The cobblestone appearance may extend into adjacent soft tissues and bursae, as it may erode bone. Fusion or coalescence of multiple chondral bodies may occur, creating a giant conglomerate appearance; lobules of hyaline cartilage surrounded by synovial lining (a two-cell layer of cuboidal epithelium) that is usually attenuated[20].
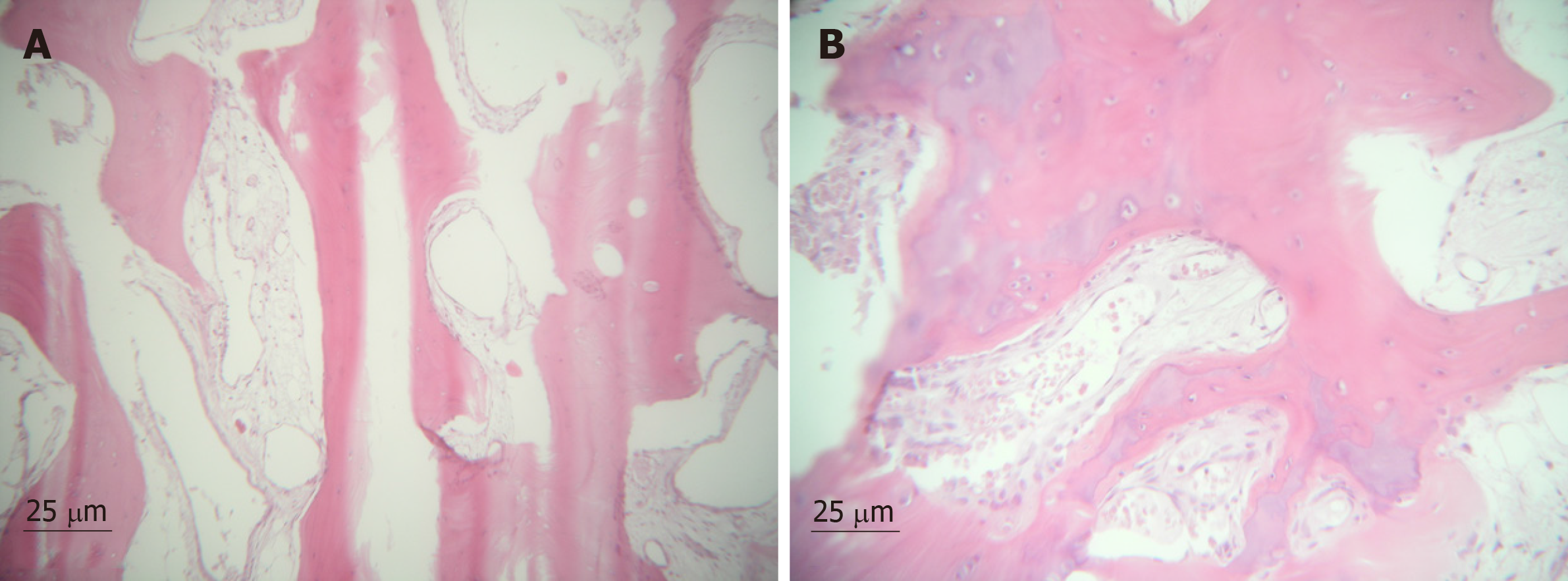
The degree of cellularity is generally striking. Double or multiple as enlarged pleomorphic nuclei may be seen within individual chondrocytes[6]. There was no necrosis or myxoid change in the stroma. The primary and recurrent lesions showed an identical morphology[17]. Cells at the periphery of the cartilage nodules express FGFR3 and PCNA (proliferating cell nuclear antigen)[28]. DNA image cytometry suggests that chondrocytes in primary SC are active[1]. Figure 6 shows the histological pattern.
The differential diagnosis includes[5,10,18,24,31,34,38,46,47]: (1) Joint disorders: degenerative arthropathy, osteochondritis dissecans, neurotrophic, tuberculous, rheumatoid or septic arthritis, injury-related soft tissue calcification, osteochondral fractures and avascular necrosis; (2) Benign disorders: Synovial hemangioma, pigmented Villonodular synovitis, chondroma, tenosynovial giant cell tumor, calcifying aponeurotic fibroma, periosteal chondroma; and (3) Malignant disorders: Chondrosarcoma, synovial chondrosarcoma, synovial sarcoma; in case of proximity of nerves, neurofibroma, schwannoma and peripheral nerve sheath tumor.
Although PSC is considered completely benign, recent interest in diagnosis has occurred due to about 2.5%-5% relative risk for malignant degeneration[30,33,48]. Bojanic et al[49] reported malignant transformation to chondrosarcoma in 17%-25% of all cases, while Galat et al[1] reported two cases of transformation in PSC of the foot. The high rate of malignant progression could be referred to difficulty in distinguishing these two entities, and some authors argue it is simply a case of misdiagnosis[1,11]. An accurate diagnosis is based on clinical, radiographic or advanced imaging and histological evidence[10]. They appear histologically indistinguishable from low-grade chondrosarcomas; although mitotic figures were identified in the chondrosarcomas but not in cases of SC or in the enchondromas, binucleate chondrocytes were present in all cases of SC. Chondrosarcomas usually show loss of the micro-nodularity of the chondrocytes and myxoid transformation of the matrix, as chondrocyte necrosis. The most reliable histological sign consists of osseous permeation by cartilage, with the extension of cartilage matrix into the intertrabecular space[2].
Davis suggests assessing proliferative activity by Ki-67 immunohistochemical staining, since there is no detectable staining for Ki-67 protein in SC or enchondromas. Moreover, C-erb B2 staining is positive in SC, while negative in normal articular cartilage, enchondromas, or grade I chondrosarcomas. Thus, the authors concluded that detection of C-erb B-2 protein may be a more sensitive indicator of cell proliferation than nuclear expression of Ki-67 protein[48]. A DNA index of >1 has also been suggested to carry prognostic value[50].
Bertoni et al[31] described five features to distinguish PSC from chondrosarcoma: First, there is a typical clustering pattern with abundant matrix juxtaposed to areas in PSC cartilage, while the tumor cells were arranged in sheets. Second, there is a myxoid change in the matrix; while it appears solid in PSC, any tendency to «run» when cut into should be viewed with suspicion for malignancy. Third, hypercellularity with spindling of the nuclei at the periphery is present in sarcoma not in PSC. Fourth, necrosis cannot be considered crucial, as it is absent in PSC and very rare in chondrosarcoma. Finally, SC may erode bone and soft tissues, but when it does, the tumor usually has pushing margins. Permeation of trabecular bone with filling-up of marrow spaces should be considered a sign of malignancy[31].
In 1977, Milgram described a three-stage classification of SC, based on the position of the loose bodies and pathologic findings[41].
Phase I (early or florid stage) is characterized by metaplasia of the synovial intima, active synovitis and formation of nodules, with no calcification. Phase II (transitional stage) shows both active intra-synovial proliferation or calcification of the nodules and free loose bodies. Phase III (late or quiescent stage) demonstrates only the loose bodies, without any evidence of synovial metaplasia but occasional, slight inflammation of the membrane. Calcification or ossification of loose bodies is present only in the third phases.
The target of treatment is to minimize pain, improve function, and prevent or limit the progression of arthritis and chondral damage[5].
SC is a benign condition with a tendency to progressive resolution[2,7]. Since SC tends to be progressive but self-limiting, indications for surgery depend on symptomatic presentation in addition to the functional demands of the patients[7,10]. In asymptomatic patients, the nodules may resorb over time and invasive procedures should be avoided; treatment can be planned conservatively with frequent follow-up assessments. Patient age is also important for indications[51]; children should initially be treated conservatively with NSAIDs, cryotherapy and ultrasound because they are skeletally immature[18].
Indeed, surgery is considered the treatment of choice for PSC. The operative approach can vary based on the stage of disease: complete synovectomy for stage I, complete synovectomy and removal of intra articular bodies for stage II, removal of loose bodies for stage III[15,39,40]. Milgram recommended simple excision of the foreign bodies in the late inactive stages, although adding synovectomy and foreign body removal if the disease is active or transitional[40]. Some authors reported the same results by the removal of loose bodies alone, as well as synovectomy added to the removal of loose bodies[15,52].
Maurice distinguished two groups of patients, with extra- and intra-articular SC, and recommended treatment in each case: Synovectomy is recommended for both groups with localized disease; in intra-articular cases, synovectomy can be associated with removal of the loose bodies[53]. Nevertheless, for the extra-articular group, Shearer stated that surgery to extract the calcifications from the tendons would be too invasive, and that the surgeon should avoid excessively weakening tendon structure[10].
The traditional technique for the treatment of PSC of the ankle is arthrotomy and debridement. However, arthroscopy gained more and more indications in the last two decades.
Advantages of the arthroscopic approaches include decreased morbidity, wide visualization and treatment feature for intra- and extra-articular pathologies[5,34]. The excision of the loose bodies is the standard arthroscopic treatment with synovectomy[23]. Minimally invasive surgery offers a multitude of advantages in comparison with open techniques: Less local pain, swelling, infection, shorter recovery times, increased patient satisfaction, no need of immobilization post-operatively, and the patient can walk without pain[5,23,34,54]. Finally, Iossifidis[7] stated that such extensive surgery predisposes to tissue scarring and compromises articular function.
The ankle joint is entered via anteromedial and anterolateral arthroscopic portals during spinal anesthesia and tourniquet application, under manual traction and manipulation[5,13,34]. Bojanic also performed arthroscopy by posteromedial and posterolateral portals with patients in the prone position and by using a 4.5-mm/30-degree arthroscope[49].
Before and after closure of the wound in layers, an anterior drawer stress test should be performed to demonstrate joint stability[12]. The subtalar joint is entered by both medial and lateral approach, at the base of the position of loose bodies.
For the Lisfranc joint, Fujita performed a synovectomy and removal of the loose bodies in the dorsal, lateral, and plantar aspect of the articulation[39]. By a dorsal approach with dislocation of the fourth and fifth metatarsal bases, adequate visualization of both the dorsal and plantar aspects of the Lisfranc joint is allowed. Repair of dorsal ligamentous structures and percutaneous pinning of the Lisfranc joint is necessary for anatomic reduction at the end of surgery. The disadvantages of this approach include the possibility of postoperative instability and degenerative arthritis of the fourth and fifth Lisfranc joints. In severe cases, arthrodesis may be considered[15].
The use of intraoperative C-arm fluoroscopy is mandatory to ensure that all calcified loose bodies are removed; they may not be found in the joint cavity because they can be encysted in bursae or in the synovium, or because they have not yet been liberated from the synovial membrane[5,9].
Before closing the articular capsule, abundant washing with normal saline is required[3]. However, Saxena suggested irrigation with 3% hydrogen peroxide for PSC and synovial tumors in the foot and ankle[30]. Hydrogen peroxide serves as a chemo-cautery by removing microscopic chemical elements; this appears to decrease recurrence[54].
Portals or wounds should be primarily closed after drain insertion. Post-operative immobilization in a cast for a varying period is usually necessary after open operative treatment. For arthroscopy, after the compressive dressing that is removed in the first post-operative day as it drains, early active motion of the joint can start. Bearing weight as tolerated is recommended for 2 wk, and is then allowed to gradually progress to full weightbearing[5,34].
In untreated patients or those without the appropriate therapy, degenerative arthritis or joint dislocation could occur in later stages of the disease[34,39].
Another usual complication of PSC is recurrence. It is thought to be related to the presence of the stimulus that caused the metaplasia, or to incomplete synovectomy[3,13,18,23,30,34,40,52]. Recurrence after loose body removal and synovectomy varies from 3%-23% of cases[1,3,16,36]. Saxena suggested irrigation with hydrogen peroxide, as used by other authors for synovial tumors in the foot and ankle, which appears to decrease recurrence[30,55].
When recurrence is associated with particularly aggressive features, such as rapid growth or destruction of joints, the surgeon should consider the potential for malignant transformation[1,56]. Surgeons ought to also consider malignancy in case of longstanding PSC after several surgeries[36]. In these patients, wide operative margin is mandatory, as the post-operative administration of radiation therapy is suggested[31,50].
SC is a rare benign monoarticular disease characterized by the presence of cartilaginous nodules in the synovium of joints, tendon sheaths and bursae, which often occur without trauma or inflammation[1,2,3,44].
Described firstly by Leannac in 1813, SC is also named chondromatosis, synoviochondrometaplasia, synovial chondrosis, synovial osteochondromatosis and articular chondrosis[9,10]. The earliest description in the literature of synovial osteo-chondromatosis was from Henderson and Jones in 1923[24].
SC is a rare benign disease with extremely rare presentation in foot and ankle joints. Clinical relevance to clinicians and surgeons may be summarized in the need for open or arthroscopic surgery, and subsequent to clinical assessment and imaging. Postoperative histopathological characterization should lead to a definitive diagnosis. Also, as malignant transformation may occur, surgeons should perform a complete synovectomy with wide margins, and remove all loose bodies.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Emara KM, Guerado E, Musumeci G S-Editor: Gong ZM L-Editor: Filipodia E-Editor: Liu MY
| 1. | Galat DD, Ackerman DB, Spoon D, Turner NS, Shives TC. Synovial chondromatosis of the foot and ankle. Foot Ankle Int. 2008;29:312-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Jeffreys TE. Synovial chondromatosis. J Bone Joint Surg Br. 1967;49:530-534. [PubMed] [Cited in This Article: ] |
| 3. | Sedeek SM, Choudry Q, Garg S. Synovial Chondromatosis of the Ankle Joint: Clinical, Radiological, and Intraoperative Findings. Case Rep Orthop. 2015;2015:359024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Adelani MA, Wupperman RM, Holt GE. Benign synovial disorders. J Am Acad Orthop Surg. 2008;16:268-275. [PubMed] [Cited in This Article: ] |
| 5. | Doral MN, Uzumcugil A, Bozkurt M, Atay OA, Cil A, Leblebicioglu G, Tetik O. Arthroscopic treatment of synovial chondromatosis of the ankle. J Foot Ankle Surg. 2007;46:192-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Hermann G, Klein MJ, Abdelwahab IF, Kenan S. Synovial chondrosarcoma arising in synovial chondromatosis of the right hip. Skeletal Radiol. 1997;26:366-369. [PubMed] [Cited in This Article: ] |
| 7. | Iossifidis A. Synovial chondromatosis of the ankle. The Foot. 1995;5:44-46. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Zhang M, Wang J. Epigenetics and Osteoarthritis. Genes Dis. 2015;2:69-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Scholl DM, Taddie KL. Asymptomatic synovial chondromatosis of the ankle: an incidental finding. J Foot Ankle Surg. 2010;49:565.e13-565.e17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Shearer H, Stern P, Brubacher A, Pringle T. A case report of bilateral synovial chondromatosis of the ankle. Chiropr Osteopat. 2007;15:18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Bahari S, McKenna J. Subtalar and Extra Articular Synovial Chondromatosis. J Case Rep. 2012;2:120-124. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Brodsky JW, Jung KS, Tenenbaum S. Primary synovial chondromatosis of the subtalar joint presenting as ankle instability. Foot Ankle Int. 2013;34:1447-1450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Kim JB, Song IS, Shin SY, Yoon JY. Primary synovial chondromatosis of the talonavicular joint: A case report. Foot Ankle Surg. 2016;22:e25-e28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Kim SR, Shin SJ, Seo KB, Teong CT, Hyun CL. Giant extra-articular synovial osteochondromatosis of the sinus tarsi: a case report. J Foot Ankle Surg. 2013;52:227-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Derek Stensby J, Fox MG, Kwon MS, Caycedo FJ, Rahimi A. Primary synovial chondromatosis of the subtalar joint: case report and review of the literature. Skeletal Radiol. 2018;47:391-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Silva L, Castro MOE, Santos BMQ, Bilreiro C, Aleixo F. Synovial Chondromatosis: a pictorial review. ECR. 2014;C-1307. [DOI] [Cited in This Article: ] |
| 17. | Sciot R, Dal Cin P, Bellemans J, Samson I, Van den Berghe H, Van Damme B. Synovial chondromatosis: clonal chromosome changes provide further evidence for a neoplastic disorder. Virchows Arch. 1998;433:189-191. [PubMed] [Cited in This Article: ] |
| 18. | Damodaran P, Talawadekar GD, Cornell M. Bilateral, symptomatic synovial chondromatosis of ankle in a prepubescent 7-year-old boy. Eur J Rad Extra. 2009;72:e137-e139. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Mishra P, Mishra PK, Pandey D, Tripathi BN. Primary ankle synovial chondromatosis in a young post-partum female. J Orthop Allied Sci. 2014;2:60-62. [DOI] [Cited in This Article: ] |
| 20. | Murphey MD, Vidal JA, Fanburg-Smith JC, Gajewski DA. From the Archives of the AFIP: Imaging of Synovial Chondromatosis with Radiologic-Pathologic Correlation. Radio Graphics. 2007;27:1465-1488. [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 21. | Mohr W. Is synovial osteo-chondromatosis a proliferative disease? Pathol Res Pract. 2002;198:585-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Littrell LA, Inwards CY, Sim FH, Wenger DE. Imaging features of synovial chondromatosis of the spine: a review of 28 cases. Skeletal Radiol. 2016;45:63-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Isbell JA, Morris AC, Araoye I, Naranje S, Shah AB. Recurrent Extra- and Intra-articular Synovial Chondromatosis of the Ankle with Tarsal Tunnel Syndrome: A Rare Case Report. J Orthop Case Rep. 2017;7:62-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 24. | Dawson-Bowling S, Hamilton P, Bismil Q, Gaddipati M, Bendall S. Synovial osteochrondomatosis of the talonavicular joint: a case report. Foot Ankle Int. 2008;29:241-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Pau M, Bicsák A, Reinbacher KE, Feichtinger M, Kärcher H. Surgical treatment of synovial chondromatosis of the temporomandibular joint with erosion of the skull base: a case report and review of the literature. Int J Oral Maxillofac Surg. 2014;43:600-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Young-in Lee F, Hornicek FJ, Dick HM, Mankin HJ. Synovial chondromatosis of the foot. Clin Orthop Relat Res. 2004;Jun; 423:186-190. [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Mertens F, Jonsson K, Willén H, Rydholm A, Kreicbergs A, Eriksson L, Olsson-Sandin G, Mitelman F, Mandahl N. Chromosome rearrangements in synovial chondromatous lesions. Br J Cancer. 1996;74:251-254. [PubMed] [Cited in This Article: ] |
| 28. | Robinson D, Hasharoni A, Evron Z, Segal M, Nevo Z. Synovial chondromatosis: the possible role of FGF 9 and FGF receptor 3 in its pathology. Int J Exp Pathol. 2000;81:183-189. [PubMed] [Cited in This Article: ] |
| 29. | Apte SS, Athanasou NA. An immunohistological study of cartilage and synovium in primary synovial chondromatosis. J Pathol. 1992;166:277-281. [PubMed] [Cited in This Article: ] |
| 30. | Saxena A, St Louis M. Synovial Chondromatosis of the Ankle: Report of Two Cases With 23 and 126 Loose Bodies. J Foot Ankle Surg. 2017;56:182-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Bertoni F, Unni KK, Beabout JW, Sim FH. Chondrosarcomas of the synovium. Cancer. 1991;67:155-162. [PubMed] [Cited in This Article: ] |
| 32. | Ozyurek S, Atik A, Sivrioglu AK, Ege T. Primary synovial osteochondromatosis of the ankle. BMJ Case Rep. 2013;2013:pii: bcr2013009046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | McCarthy C, Anderson WJ, Vlychou M, Inagaki Y, Whitwell D, Gibbons CL, Athanasou NA. Primary synovial chondromatosis: a reassessment of malignant potential in 155 cases. Skeletal Radiol. 2016;45:755-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Ozmeric A, Aydogan NH, Kocadal O, Kara T, Pepe M, Gozel S. Arthroscopic treatment of synovial chondromatosis in the ankle joint. Int J Surg Case Rep. 2014;5:1010-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Peh WC, Shek TW, Davies AM, Wong JW, Chien EP. Osteochondroma and secondary synovial osteochondromatosis. Skeletal Radiol. 1999;28:169-174. [PubMed] [Cited in This Article: ] |
| 36. | Brucher N, Faruch-Bilfeld M, Molinier F, Brouchet-Gomez A, Lapegue F, Sans N. Primary synovial osteochondromatosis of the first interphalangeal joint of the foot: A case report. Diagn Interv Imaging. 2014;95:451-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Santiago T, Mariano C. Primary synovial chondromatosis of the ankle joint presenting as monoarthritis. BMJ Case Rep. 2013;2013:pii: bcr2013202186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Kyriakopoulos CK, Mavrogenis AF, Benetos IS, Koulalis D, Mitsou A, Papagelopoulos PJ. Arthroscopic treatment of synovial chondromatosis of the ankle. Eur J Orthop Surg Traumatol. 2006;16:75-79. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Fujita I, Matsumoto K, Maeda M, Kizaki T, Okada Y, Yamamoto T. Synovial osteochondromatosis of the Lisfranc joint: a case report. J Foot Ankle Surg. 2006;45:47-51. [PubMed] [Cited in This Article: ] |
| 40. | Milgram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59:792-801. [PubMed] [Cited in This Article: ] |
| 41. | Wittkop B, Davies AM, Mangham DC. Primary synovial chondromatosis and synovial chondrosarcoma: a pictorial review. Eur Radiol. 2002;12:2112-2119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Norman A, Steiner GC. Bone erosion in synovial chondromatosis. Radiology. 1986;161:749-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Harish S, Saifuddin A, Cannon SR, Flanagan AM. Synovial chondromatosis of the foot presenting with Lisfranc dislocation. Skeletal Radiol. 2005;34:736-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Hocking R, Negrine J. Primary synovial chondromatosis of the subtalar joint affecting two brothers. Foot Ankle Int. 2003;24:865-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Kramer J, Recht M, Deely DM, Schweitzer M, Pathria MN, Gentili A, Greenway G, Resnick D. MR appearance of idiopathic synovial osteochondromatosis. J Comput Assist Tomogr. 1993;17:772-776. [PubMed] [Cited in This Article: ] |
| 46. | Duymus TM, Yucel B, Mutlu S, Tuna S, Mutlu H, Komur B. Arthroscopic treatment of synovial chondromatosis of the shoulder: A case report. Ann Med Surg (Lond). 2015;4:179-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Taglialavoro G, Moro S, Stecco C, Pennelli N. [Bilateral synovial chondromatosis of the first metatarsophalangeal joint: a case report]. Reumatismo. 2003;55:263-266. [PubMed] [Cited in This Article: ] |
| 48. | Davis RI, Hamilton A, Biggart JD. Primary synovial chondromatosis: a clinicopathologic review and assessment of malignant potential. Hum Pathol. 1998;29:683-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 194] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 49. | Bojanic I, Bergovec M, Smoljanovic T. Combined anterior and posterior arthroscopic portals for loose body removal and synovectomy for synovial chondromatosis. Foot Ankle Int. 2009;30:1120-1123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Sah AP, Geller DS, Mankin HJ, Rosenberg AE, Delaney TF, Wright CD, Hornicek FJ. Malignant transformation of synovial chondromatosis of the shoulder to chondrosarcoma. A case report. J Bone Joint Surg Am. 2007;89:1321-1328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Yu GV, Zema RL, Johnson RW. Synovial osteochondromatosis. A case report and review of the literature. J Am Podiatr Med Assoc. 2002;92:247-254. [PubMed] [Cited in This Article: ] |
| 52. | Shpitzer T, Ganel A, Engelberg S. Surgery for synovial chondromatosis. 26 cases followed up for 6 years. Acta Orthop Scand. 1990;61:567-569. [PubMed] [Cited in This Article: ] |
| 53. | Maurice H, Crone M, Watt I. Synovial chondromatosis. J Bone Joint Surg Br. 1988;70:807-811. [PubMed] [Cited in This Article: ] |
| 54. | Kunzler DR, Shazadeh Safavi P, Warren BJ, Janney CF, Panchbhavi V. Arthroscopic Treatment of Synovial Chondromatosis in the Ankle Joint. Cureus. 2017;9:e1983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 55. | Goni V, Gopinathan NR, Radotra BD, Viswanathan VK, Logithasan RK, S B. Giant cell tumour of peroneus brevis tendon sheath--a case report and review of literature. BMJ Case Rep. 2012;2012:pii: bcr0120125703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |