Published online Dec 10, 2015. doi: 10.5306/wjco.v6.i6.281
Peer-review started: May 30, 2015
First decision: August 14, 2015
Revised: September 3, 2015
Accepted: October 20, 2015
Article in press: October 27, 2015
Published online: December 10, 2015
Cervical cancer (CC) represents the fourth most common malignancy affecting women all over the world and is the second most common in developing areas. In these areas, the burden from disease remains important because of the difficulty in implementing cytology-based screening programmes. The main obstacles inherent to these countries are poverty and a lack of healthcare infrastructures and trained practitioners. With the availability of new technologies, researchers have attempted to find new strategies that are adapted to low- and middle-income countries (LMIC) to promote early diagnosis of cervical pathology. Current evidence suggests that human papillomavirus (HPV) testing is more effective than cytology for CC screening. Therefore, highly sensitive tests have now been developed for primary screening. Rapid molecular methods for detecting HPV DNA have only recently been commercially available. This constitutes a milestone in CC screening in low-resource settings because it may help overcome the great majority of obstacles inherent to previous screening programmes. Despite several advantages, HPV-based screening has a low positive predictive value for CC, so that HPV-positive women need to be triaged with further testing to determine optimal management. Visual inspection tests, cytology and novel biomarkers are some options. In this review, we provide an overview of current and emerging screening approaches for CC. In particular, we discuss the challenge of implementing an efficient cervical screening adapted to LMIC and the opportunity to introduce primary HPV-based screening with the availability of point-of-care (POC) HPV testing. The most adapted screening strategy to LMIC is still a work in progress, but we have reasons to believe that POC HPV testing makes part of the future strategies in association with a triage test that still needs to be defined.
Core tip: Cervical cancer (CC) burden in developing countries remains important because of the difficulty in implementing cytology-based screening programmes. With the introduction of new technologies, researchers have attempted to find new strategies for CC screening adapted to these countries. Rapid human papillomavirus (HPV) tests are one of these advantageous methods. However, HPV testing has a low positive predictive value for CC, so a triage test is needed. Visual inspection tests, cytology and novel biomarkers are some options. We provide an overview of current and emerging screening approaches for CC. We discuss the challenge of implementing an efficient CC screening adapted to developing countries and the opportunity to introduce primary HPV-based screening with the availability of point-of-care tests.
- Citation: Catarino R, Petignat P, Dongui G, Vassilakos P. Cervical cancer screening in developing countries at a crossroad: Emerging technologies and policy choices. World J Clin Oncol 2015; 6(6): 281-290
- URL: https://www.wjgnet.com/2218-4333/full/v6/i6/281.htm
- DOI: https://dx.doi.org/10.5306/wjco.v6.i6.281
The incidence of cervical cancer (CC) varies greatly worldwide. There is a large difference between developing and developed countries, where CC cases have been significantly reduced since the implementation of effective screening programmes. However, in developing countries, the burden from CC remains because of the difficulty in implementing cytology-based screening programmes. According to the latest world cancer statistics[1], CC is the fourth most common cancer in women globally (528000 new cases each year) and the second most common in developing areas (445000 new cases each year). CC is also the fourth most lethal cancer in women worldwide (266000 deaths) and the third cause of cancer-related death in developing countries (230158 deaths)[1], which means that more than 80% of the global burden occurs in developing areas.
In addition, the incidence and mortality of CC is variable within low- and middle-income countries (LMIC). In India, there are 20.2 per 100000 new cases of CC diagnosed and 11.1 per 100000 deaths annually, accounting for more than one fifth of the global CC deaths[2]. In sub-Saharan Africa, 34.8 per 100000 women are diagnosed with CC annually and 22.5 per 100000 women die from this disease[1]. In contrast, in western Asian countries, only 3.8 per 100000 new cases are diagnosed per year and 1.6 per 100000 die from CC[1]. Therefore, if the chances to survive CC are considered, a woman in Thailand will have an approximately 58% chance of survival, while in India she will only have a 42% chance. This survival is even more critical in Sub-Saharan Africa, where women only have a 21% chance to survive CC[3]. Overall, the mortality to incidence ratio of CC is 52%[4].
Human papillomavirus (HPV) is a major co-factor of CC. Development of vaccines against HPV has been a major advance for prevention of this cancer. Nevertheless, large-scale implementation of HPV vaccination is still lacking in developing countries and will not replace the need for CC screening.
In LMIC, there are several issues and challenges associated with CC screening. The main failure to implement an effective screening programme is related to the complexity of the screening process and the obstacles inherent in these countries. Poverty, limited access of the population to information, lack of knowledge of CC, the absence of sustained prevention programmes, lack of healthcare infrastructure required and lack of trained practitioners are the main obstacles to implementation of CC screening programmes[5]. Socio-religious and cultural barriers may also play an important role, as shown in an attempt to screen for CC in Peru[6]. Finally, government resources may be allocated to competing public health programmes with higher visibility and international attention than CC screening.
In this review, we discuss the challenge of implementing an efficient cervical screening adapted to LMIC and the opportunity to introduce a primary HPV-based screening with availability of a rapid HPV test.
At the present, very few developing countries have been able to implement CC screening programmes. To screen successfully in LMIC different requirements are important. The programme shall ensure wide coverage of the target population; it must guarantee screening, management and adequate follow-up of patients; it shall be provided on-site and be low-cost, with minimum infrastructure requirement that can lead to immediate treatment if abnormal. CC screening should be planned in line with other national programmes for cancer control. Moreover, in order to implement CC screening policies in these countries, a support and funding from the Ministry of Health is indispensable.
In the Middle East and North Africa, the first steps to implement national screening programmes based on visual inspection tests are being currently completed[7]. In contrast, in Sub-Saharan Africa, it is estimated that less than 5% of women at risk have ever been screened[8]. In India’s case, guidelines for population-based screening programmes for cervical cancer are established for about 10 years[9] and are based on visual inspection tests. However, despite the introduction of these national guidelines, screening coverage is still very low[10]. Several obstacles are responsible for the failure to implement an effective screening program in LMIC. A summary of these obstacles is respresented in Table 1.
| Practical/logistical reasons |
| Widespread poverty |
| Lack of healthcare infrastructure |
| Absence of sustained prevention programmes |
| Lack of trained practitioners |
| Lack of laboratory supplies |
| Lack of patient management guidelines |
| Limited physical access of the population |
| Knowledge, religion and beliefs |
| Lack of knowledge of cervical cancer |
| Limited access of the population to information |
| Women disempowerment |
| Socio-religious and cultural barriers to routine pelvic screening |
| Political |
| Lack of support from the Ministery of Health |
| Competing healthcare priorities |
| War and civil strife |
| Others |
| High temperatures in tropical countries with lack of proper climatisation |
| Particularities about the screening test |
| VIA |
| Significant number of unnecessary and unsustainable treatment |
| Cytology |
| Need important health-care resource and infrastructure |
| Need important laboratory supplies |
| Screening requires more than one visit (important drop out) |
| Further testing with colposcopy wouldn't be possible, leading to unnecessary agressive and unsustainable treatment |
| HPV |
| Need important healthcare infrastructure |
| Need important laboratory supplies |
Cytology screening (Pap test) for CC, especially as part of organised screening programmes, is the oldest and most widespread cancer screening technique. This technique has lead to effective reduction in the incidence and mortality from CC in many developed countries[11-13]. CC screening is one of the most successful disease-prevention programmes. However, this approach has failed to attain the same results in developing areas. A cytology-based screening programme requires repeat testing and visits to identify women who need treatment. Besides a cytopathologist, a colposcopy specialist and a pathologist should also be involved. To guarantee the success of a screening programme, training and continuing education are essential[14]. Previous experience has shown no decline in the incidence and/or mortality of CC, and this is probably because of low-quality cytology smears[8]. Consequently, implementation and execution of the whole process is too complex and expensive.
Moreover, even if implementing a high-quality cytology programme in these countries is possible, it would only be moderately effective. This is because the currently used Pap test misses approximately 50% of high-grade precursor lesions and cancers with a single screening[15]. Additionally, in low-resource settings, women would probably only be screened once or twice in their lifetime.
Visual inspection tests with 3%-5% acetic acid (VIA) and/or Lugol’s iodine (VILI) appear to be a satisfactory alternative screening approach to cytology. These tests have been used since the 1990s, mainly in poor resource settings. They are simple, cost-effective with relative ease of use[16-19], and may be performed by different healthcare workers (physicians, nurse, midwives and technicians). Moreover, this approach does not require high technology or infrastructure and has been shown to reduce mortality in developing countries[20,21]. The visible changes that occur in the cervix after application of acetic acid are immediate, and can be categorised as negative or positive for cervical neoplasia. These immediate results facilitate a same-day screen and management strategy. Therefore, this allows most of the eligible women to participate in the programme by minimising repeat visits. Evidence shows that this single-visit approach leads to the most significant decrease in high-grade cervical intraepithelial neoplasia (CIN)[22] and it is regarded safe, acceptable and fairly effective in India and Sub-Saharan Africa[17,23]. Despite the limitations of the concept of “screen and treat”, it helps to overcome barriers of time, distance and loss to follow-up. This is relevant, because in a low-resource context, recalling patients for additional testing or treatment can be a critical component to a programme’s success (Figure 1).
The performance of VIA has been evaluated in numerous studies[18,19,24-26]. An extensive meta-analysis by Sauvaget et al[19] pooled data from 26 studies that were conducted in different high- and low-income countries. They found an overall sensitivity of 80% and a specificity of 92% for the VIA method, although sensitivities greatly varied between studies. Close values were found in a meta-analysis where pooled data from 11 studies that were performed in Africa and India showed a sensitivity for VIA of 79% (range: 73%-85%) and a specificity of 85% (range: 81%-89%) for CIN2 lesions or worse (CIN2+)[18]. With regard to VILI, its use appears to increase VIA’s sensitivity by 10%, without affecting the specificity[18,24,26].
VIA and VILI also have some drawbacks that need to be addressed. Interpretation of a visual test of the cervix has limited value in older women because of degenerating cervical epithelium and partial or lack of visibility of the transition zone with ageing. Indeed, studies have shown that VIA sensitivity declines substantially in women aged 40 years or older[27,28]. VIA-based screening is also healthcare provider dependent and lacks reliable quality assurance control. As a consequence and to maintain high quality, implementation of VIA screening at primary and secondary facilities would require close supervision, which is difficult to attain at a national level.
More importantly, reported sensitivity for detecting CIN2+ widely varies in different studies (37%-96%), as does specificity (49%-98%)[27,29], which makes it dependent on the skill of the provider. Finally, studies that were conducted under screening conditions to assess the sensitivity and specificity of VIA used the gold standard of colposcopy, and this technique has been proven to yield error in the recognition of disease[30]. Because of these drawbacks, alternative methods need to be developed to improve, complement, or even replace VIA.
In recent years, there has been overwhelming evidence that HPV testing is more effective than cytology for CC screening, providing increased reassurance and allowing longer screening intervals to be adopted[31]. Highly sensitive tests have been developed and are currently used to replace cervical cytology for primary screening[32].
Currently, in the worldwide market, there are at least 150 different HPV tests available for the detection of alpha-HPVs and over 95 variants of the original tests. However, only some commercial HPV tests have documented clinical performance compared with the standard HPV test. According to guidelines, a candidate test should present a clinical sensitivity for CIN2+ of at least 90%, and a clinical specificity of at least 98% of that of the reference assays[33,34]. Regardless, the number of assays for HPV that have been approved by the Food and Drug Administration is increasing over time[33,35,36].
Moreover, among HPV tests, there is an important difference concerning the choices of primers to be used. Because of this overwhelming amount of choice available, choosing which HPV test is more suitable given a certain context can be difficult. Furthermore, and paradoxically, clinicians are generally not involved in choosing the HPV test.
Evidence shows that HPV tests should not only be type specific but also virial region specific (specific regions in the HPV genome are L1, E1/E2 and E6/E7). Indeed, during integration of HPV in the human genome, L1 expression is sometimes lost, but E6/E7 expression always remains present, which explains why there are not E6- or E7-negative cancers[37]. A test designed only for L1 will miss approximately 10% of all invasive cancers. This is why an HPV test is not recommended by some authors as a stand-alone test in CC screening programmes[37].
Current HPV tests are able to detect the presence of viral markers by signal amplification techniques, such as the Digene Hybrid Capture® II assay or by amplification of nucleic acid with polymerase chain reaction. When combined with Pap smears, HPV tests can achieve nearly 100% sensitivity and a specificity of 93% in women aged 30 years and older, with a negative predictive value of almost 100%[38].
Several studies support that HPV testing is feasible in low-resource settings and appears to be the best strategy for CC in this context[17,24,39]. A large-cluster randomised trial from rural India showed that a single round of HPV screening could reduce the incidence and mortality from CC of approximately 50%, whereas approaches based on VIA and cytology had little effect on these outcomes[40].
Until recently, the greatest limitations of HPV testing were the need for expensive laboratory infrastructure and the 4-7 h time to process the test. The development of rapid molecular methods for detecting HPV DNA (e.g., care HPV® - Qiagen, GeneXpert® - Cepheid) for screening or other POC type of tests is a milestone in CC screening in low-resource settings. This is because these new options may make screening more feasible in the future and reduce the infrastructural requirements of previous screening programmes.
In a cohort of unscreened women aged 30 and over from South Africa, HPV testing followed by the treatment of HPV-positive women at the second visit was the most effective option (27% reduction in the incidence of CC) at a cost of 39 USD/years of life saved (YLS)[41]. VIA combined with the immediate treatment of women who tested positive at the first visit was cost saving and was the next most effective strategy, with a 26% decrease in the incidence of CC[41]. In another cost-effectiveness analysis in a rural Chinese population, where the careHPV® test (Qiagen, Gaithersburg, MD, United States) was directly compared with VIA, a once-per-lifetime screening at the age of 35 years would reduce CC mortality by 8% combined with VIA (cost of 557 USD/YLS), compared with 12% with the careHPV test (cost of 959 USD/YLS)[42].
HPV‐based screening requires that a sample be taken using a swab or brush by a healthcare provider or by the patient herself. The greatest advantage of HPV-based testing is obvious in that it allows sample collection to be performed by the patient herself, not requiring trained personnel and infrastructure to perform a pelvic examination. The criteria for a good quality sample are less rigorous with HPV testing compared with cytology. Many studies have shown that offering self-sampling for HPV testing (Self-HPV) can improve attendance to a CC screening programme and it is well accepted among women[39,43-45]. This strategy can not only be more appealing to non-attendees in developed countries, but also makes CC screening accessible to women in LMIC[46,47]. Evidence from multiple prospective studies has shown that the accuracy of Self-HPV versus clinician-collected specimens to detect precancerous lesion is comparable for the detection of precancerous and cancerous lesions[39,48,49]. Because of the numerous advantages of self-HPV, it will become a major focus of CC screening programmes worldwide in the near future.
The most relevant approach to identify women at risk for CC or pre-cancer is by age restriction. The World Health Organization (WHO) recommends targeting HPV screening to women who are 30 years of age and older because of their higher risk of CC, and that priority should be given to screening women aged 30-49 years (WHO screening recommendation update 2014). In addition, VIA is less effective in women aged older than 50 years because the squamocolumnar junction is less visible in menopausal women. If HPV is used as primary screening, recent evidence supports its use in women aged 30 years and over[50,51]. Most HPV infections are transient at an age younger than 30 years Therefore, the screening of young women leads to unrequired assessment and potentially to treatment of cervical lesions that might have regressed spontaneously[52,53]. However, even in women aged ≥ 30 years, most of HPV infections are transient, and only a small fraction of cases with persistent infection are at risk of CC[54]. Therefore, selecting HPV-positive women aged older than 30 years who are most likely to have or to develop a CC precursor in the future and require treatment is necessary for further evaluation (triage).
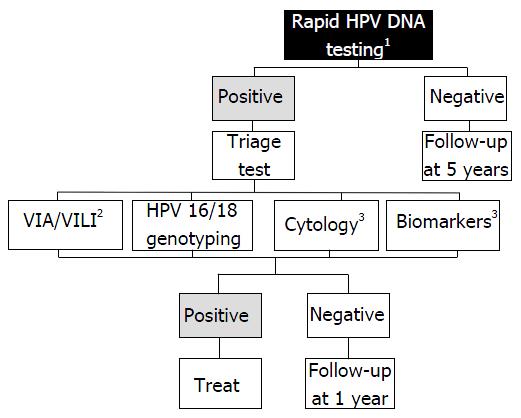
HPV-based screening has a low positive predictive value for CC because it does not directly test for cancer, but for HPV infection instead. A negative HPV test only indicates a low probability for the patient to develop CC within 5-10 years, and a positive result is only an indication of the presence of an essential risk factor. Therefore, women who test positive for HPV must be further evaluated to determine the optimal management. At the present time, three candidates can potentially be used as triage test: (1) visual methods (VIA/VILLI; (2) cytology; and (3) molecular testing. To date, there is no clear evidence to determine which strategy should be prioritised. Therefore, the choice of test essentially depends on the available health resource (Figure 2).
Triage with VIA/VILI offers the dual benefit of HPV screening to maximise detection of the disease and VIA/VILI for triage. In low-resource areas where the necessary equipment is lacking, VIA/VILI following an HPV-positive test is probably a good option, offering the possibility to adopt a “see and treat” approach. VIA/VILI will identify women with a precancerous change requiring immediate treatment by cryotherapy or cold coagulation, and those women in which cancer is suspected who should be referred to a specialised centre to receive aggressive multimodal treatment. Women with a negative VIA/VILI will be followed without treatment.
Triage with cytology is proposed in developing (middle income) countries where infrastructure exists with experience of screening[55]. However, healthcare providers should be aware that cytology is associated with multiple clinic visits and delays between screening, laboratory results, colposcopy and ultimately treatment, which are major barriers to the success of this method.
Cervical carcinogenesis is characterized by the integration of HPV DNA into the host cell genome resulting in abnormal proliferation of basal and parabasal cells due to the deregulated expression of viral oncoproteins, leading ultimately to the development of CC[56]. Therefore, the detection of HPV DNA is used by many assays and is the only molecular marker fully developed and approved for primary CC screening. These tests can be based on the detection of specific types of oncogenic HPV that identify women at a higher cancer risk (e.g., HPV genotypes 16 and 18)[36]. However, many other molecular mechanisms associated with HPV infection are necessary for CC development, such as chromosomal abnormalities, expression of oncogenes[57], epigenetic regulation (hypermethylation)[58] and apoptotic markers, which covers a large number of potential biomarkers. Molecular tests have been lately under intensive study as a potential alternative and triage tests for CC screening[59].
Expression of oncogenes: Oncoproteins expressing viral oncogenic activity could potentially be used as biomarkers in the triage of HPV-positive women or directly as a primary screening method. When HPV-infected cervical cells undergo precancerous or cancerous changes, oncoprotein E6 is expressed in cervical cells at elevated levels. Only E6 protein from high-risk HPV types promotes carcinogenesis by binding to a human PDZ domain. This allows E6 protein to bind to cellular molecules and deregulate cellular proliferation and differentiation, which may lead to the development of cancer[60]. An HPV E6 test using lateral flow (OncoE6™, Arbor Vita Corporation) has been developed to detect E6 protein of HPVs 16, 18 and 45[61]. Weaknesses of the OncoE6™ Cervical Test are low sensitivity (approximately 45%)[62] because it only detects HPV 16, 18 or 45. Additionally, specimens stored in buffers/transport medium used for HPV DNA testing cannot be used, and thus new cervical collection is always required. The oncogenic activity of E7 protein may also be tested indirectly by the host cyclin-dependent kinase inhibitor p16Ink4a. This kinase inhibitor decelerates the cell cycle by inactivating the cyclin-dependent kinases (CDK4/CDK6) involved in retinoblastoma protein phosphorylation[63]. Overexpression of p16INK4a in almost all cervical precancer (High-grade lesions) and invasive CC[64,65] has been shown to be directly linked to the transforming activity of E7 oncoprotein, which is produced by HPV[66]. Cellular accumulation of p16INK4a can be measured by cytochemistry using ELISA assays, which are commercially available (CINtec® p16, Roche mtm laboratories, Mannheim, Germany).
Modulation of host microRNAs and methylation status of protein-coding genes: HPVs modulate expression of host microRNAs (miRNAs)[67] via deletion, amplification, or genomic rearrangement. Recent studies have explored the role of the miRNAs in the development of CC. They found that several miRNAs are dysregulated in CC, such as miR-21, miR-127, miR-143, miR-145, miR-155, miR-203, miR-218 and miR-214, among others[68-72]. The miRNA-203 is downregulated in HPV-positive cells and its repression leads to maintenance of increased levels of p63 in infected suprabasal cells, maintaining cells in an active state in the cell cycle[73]. Other well studied miRNA is the miRNA-21, whose upregulation has been associated with aggressive progression and poor prognosis in CC[74]. Also miRNA-143 and -145 were found to be less expressed in CC[67,70]. Despite being a hot-spot topic, some discordance exists between studies concerning miRNAs, therefore further studies need to be conducted before these molecular biomarkers can be safely introduced in CC screening routine.
Epigenetic silencing of tumor suppressor genes is also responsible for cervical carcinogenesis[58]. Quantification of DNA methylation can be easily done and has been drawing attention in the recent years, making it a promising biomarker in CC[75]. L1 genes from HPV16 and 18 L1 are always highly methylated in CC[76,77]. A recent study using a rapid and sensitive technique[77], methylation-sensitive high-resolution melting analysis, has shown that L1 HPV16 methylation was highly associated with cervical pre-cancer and cancer and can be used as a triage test for women positive for HPV16 who are at greater risk to develop invasive cancer. Another study on HPV DNA methylation[78] tested 14 methylated candidate genes (ADRA1D, AJAP1, COL6A2, EDN3, EPO, HS3ST2, MAGI2, POU4F3, PTGDR, SOX8, SOX17, ST6GAL2, SYT9, and ZNF614) and found that POU4F3 gene methylation had the highest area under the ROC curve (0.86; 95%CI: 0.78-0.95) in detecting CIN3+, which makes it a potential molecular tool for triage in HPV-positive women.
Other protein biomarkers: Promising additional molecular markers for triage of HPV-positive women are molecular markers expressing aberrant S-phase induction (BD ProEx™ C reagent), including two proteins: Topoisomerase IIA and minichromosome maintenance protein. Both proteins are overexpressed in HPV-infected cells as a result of the uncontrolled activation of the gene transcription and are linked to severity of cervical lesions[79,80]. Moreover, carcinoma embryonic antigen has found to be a good biomarker for CC prognosis and disease management[81], though it is elevated in different non-cancerous and cancerous conditions. Many other biomarkers, such as integral membrane protein CD44, enzyme cyclooxygenase-2, cytokine vascular endothelial growth factor and membrane protein caveolin-1 might be useful in CC screening, by being more or less associated with cervical lesions severity, disease progression and prognosis[82-85].
Emerging technology places CC screening in developing countries at a crossroad and a choice of new policies is warranted. Primary HPV testing is widely accepted as being more effective than cytology for CC screening. Primary HPV testing increases sensitivity for the detection of CIN2+ compared with cytology and its high negative predictive value allows screening intervals to be extended. However, HPV testing has a mediocre specificity and positive predictive value. Additionally, HPV testing could be impractical in developing countries without a triage strategy to further characterise and evaluate the risk of an HPV-positive woman. Therefore, follow-up and management should be carried out. The emergence of rapid POC HPV tests that are performed in self-obtained vaginal samples will permit not only first-line screening, but also a triage of HPV-positive women during the same visit. As a result, a new concept can be achieved in a single visit, consisting of self-HPV testing, triage and treatment. This could allow most of the eligible women living in low-resource settings to participate in a CC screening programme by minimising repeated visits.
P- Reviewer: Dirier A, Reinhold WC, Tran CD
S- Editor: Tian YL L- Editor: A E- Editor: Li D
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20108] [Cited by in F6Publishing: 19774] [Article Influence: 2197.1] [Reference Citation Analysis (17)] |
| 2. | Bruni L, Barrionuevo-Rosas L, Albero G, Aldea M, Serrano B, Valencia S, Brotons M, Mena M, Cosano R, Mu-oz J. ICO Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases Reports. Accessed. 2015;March 20 Available from: http://www.hpvcentre.net/statistics/reports/XWX.pdf. [Cited in This Article: ] |
| 3. | LaMontagne DS, Barge S, Le NT, Mugisha E, Penny ME, Gandhi S, Janmohamed A, Kumakech E, Mosqueira NR, Nguyen NQ. Human papillomavirus vaccine delivery strategies that achieved high coverage in low- and middle-income countries. Bull World Health Organ. 2011;89:821-830B. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 4. | World Health Organization. Burden of cervical cancer globally. WHO/ICO Information Center on HPV and Cervical Cancer. Accessed. 2011; Available from: http: /www.hpvcentre.net/dataquery.php. [Cited in This Article: ] |
| 5. | Ngugi CW, Boga H, Muigai AW, Wanzala P, Mbithi JN. Factors affecting uptake of cervical cancer early detection measures among women in Thika, Kenya. Health Care Women Int. 2012;33:595-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Winkler J, Bingham A, Coffey P, Handwerker WP. Women’s participation in a cervical cancer screening program in northern Peru. Health Educ Res. 2008;23:10-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Sancho-Garnier H, Khazraji YC, Cherif MH, Mahnane A, Hsairi M, El Shalakamy A, Osgul N, Tuncer M, Jumaan AO, Seoud M. Overview of cervical cancer screening practices in the extended Middle East and North Africa countries. Vaccine. 2013;31 Suppl 6:G51-G57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Sankaranarayanan R, Budukh AM, Rajkumar R. Effective screening programmes for cervical cancer in low- and middle-income developing countries. Bull World Health Organ. 2001;79:954-962. [PubMed] [Cited in This Article: ] |
| 9. | Department of Cytology and Gynaecological Pathology, Postgraduate Institute of Medical Education, and Research, Chandigarh, India. Guidelines for Cervical Cancer Screening Programme. [accessed. 2006;Jun] Available from: http://screening.iarc.fr/doc/WHO_India_CCSP_guidelines_2005.pdf. [Cited in This Article: ] |
| 10. | Aswathy S, Quereshi MA, Kurian B, Leelamoni K. Cervical cancer screening: Current knowledge & amp; practice among women in a rural population of Kerala, India. Indian J Med Res. 2012;136:205-210. [PubMed] [Cited in This Article: ] |
| 11. | Nygård M. Screening for cervical cancer: when theory meets reality. BMC Cancer. 2011;11:240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Arbyn M, Rebolj M, De Kok IM, Fender M, Becker N, O’Reilly M, Andrae B. The challenges of organising cervical screening programmes in the 15 old member states of the European Union. Eur J Cancer. 2009;45:2671-2678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | Austoker J. Cancer prevention in primary care. Screening for cervical cancer. BMJ. 1994;309:241-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Denny L, Quinn M, Sankaranarayanan R. Chapter 8: Screening for cervical cancer in developing countries. Vaccine. 2006;24 Suppl 3:S3/71-S3/77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 15. | Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, Szarewski A, Birembaut P, Kulasingam S, Sasieni P. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 738] [Cited by in F6Publishing: 733] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 16. | Visual inspection with acetic acid for cervical-cancer screening: test qualities in a primary-care setting. University of Zimbabwe/JHPIEGO Cervical Cancer Project. Lancet. 1999;353:869-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 213] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Denny L, Kuhn L, Pollack A, Wainwright H, Wright TC. Evaluation of alternative methods of cervical cancer screening for resource-poor settings. Cancer. 2000;89:826-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 18. | Arbyn M, Sankaranarayanan R, Muwonge R, Keita N, Dolo A, Mbalawa CG, Nouhou H, Sakande B, Wesley R, Somanathan T. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer. 2008;123:153-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Sauvaget C, Fayette JM, Muwonge R, Wesley R, Sankaranarayanan R. Accuracy of visual inspection with acetic acid for cervical cancer screening. Int J Gynaecol Obstet. 2011;113:14-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Sankaranarayanan R, Anorlu R, Sangwa-Lugoma G, Denny LA. Infrastructure requirements for human papillomavirus vaccination and cervical cancer screening in sub-Saharan Africa. Vaccine. 2013;31 Suppl 5:F47-F52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Wright TC, Kuhn L. Alternative approaches to cervical cancer screening for developing countries. Best Pract Res Clin Obstet Gynaecol. 2012;26:197-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Gaffikin L, Blumenthal PD, Emerson M, Limpaphayom K. Safety, acceptability, and feasibility of a single-visit approach to cervical-cancer prevention in rural Thailand: a demonstration project. Lancet. 2003;361:814-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Blumenthal PD, Gaffikin L, Deganus S, Lewis R, Emerson M, Adadevoh S. Cervical cancer prevention: safety, acceptability, and feasibility of a single-visit approach in Accra, Ghana. Am J Obstet Gynecol. 2007;196:407.e1-e8; discussion 407.e8-e9. [PubMed] [Cited in This Article: ] |
| 24. | Sankaranarayanan R, Basu P, Wesley RS, Mahe C, Keita N, Mbalawa CC, Sharma R, Dolo A, Shastri SS, Nacoulma M. Accuracy of visual screening for cervical neoplasia: Results from an IARC multicentre study in India and Africa. Int J Cancer. 2004;110:907-913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 196] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Bigoni J, Gundar M, Tebeu PM, Bongoe A, Schäfer S, Fokom-Domgue J, Catarino R, Tincho EF, Bougel S, Vassilakos P. Cervical cancer screening in sub-Saharan Africa: a randomized trial of VIA versus cytology for triage of HPV-positive women. Int J Cancer. 2015;137:127-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Murillo R, Luna J, Gamboa O, Osorio E, Bonilla J, Cendales R. Cervical cancer screening with naked-eye visual inspection in Colombia. Int J Gynaecol Obstet. 2010;109:230-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Li N, Shi JF, Franceschi S, Zhang WH, Dai M, Liu B, Zhang YZ, Li LK, Wu RF, De Vuyst H. Different cervical cancer screening approaches in a Chinese multicentre study. Br J Cancer. 2009;100:532-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S, Fayette JM, Cherian J. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370:398-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 327] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 29. | Sankaranarayanan R, Gaffikin L, Jacob M, Sellors J, Robles S. A critical assessment of screening methods for cervical neoplasia. Int J Gynaecol Obstet. 2005;89 Suppl 2:S4-S12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 30. | Massad LS, Jeronimo J, Katki HA, Schiffman M. The accuracy of colposcopic grading for detection of high-grade cervical intraepithelial neoplasia. J Low Genit Tract Dis. 2009;13:137-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, Kitchener H, Segnan N, Gilham C, Giorgi-Rossi P. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1041] [Cited by in F6Publishing: 1078] [Article Influence: 107.8] [Reference Citation Analysis (0)] |
| 32. | Vassilakos P, Catarino R, Frey Tirri B, Petignat P. Cervical cancer screening in Switzerland: time to rethink the guidelines. Swiss Med Wkly. 2015;145:w14112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Phillips S, Garland SM, Tan JH, Quinn MA, Tabrizi SN. Comparison of the Roche Cobas(®) 4800 HPV assay to Digene Hybrid Capture 2, Roche Linear Array and Roche Amplicor for Detection of High-Risk Human Papillomavirus Genotypes in Women undergoing treatment for cervical dysplasia. J Clin Virol. 2015;62:63-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Meijer CJ, Berkhof J, Castle PE, Hesselink AT, Franco EL, Ronco G, Arbyn M, Bosch FX, Cuzick J, Dillner J. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124:516-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 500] [Cited by in F6Publishing: 465] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 35. | Monsonego J, Hudgens MG, Zerat L, Zerat JC, Syrjänen K, Halfon P, Ruiz F, Smith JS. Evaluation of oncogenic human papillomavirus RNA and DNA tests with liquid-based cytology in primary cervical cancer screening: the FASE study. Int J Cancer. 2011;129:691-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 36. | Castle PE, Stoler MH, Wright TC, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol. 2011;12:880-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 371] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 37. | Tjalma WA, Depuydt CE. Cervical cancer screening: which HPV test should be used--L1 or E6/E7? Eur J Obstet Gynecol Reprod Biol. 2013;170:45-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, Ratnam S, Coutlée F, Franco EL. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579-1588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 767] [Cited by in F6Publishing: 704] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 39. | Ogilvie GS, Patrick DM, Schulzer M, Sellors JW, Petric M, Chambers K, White R, FitzGerald JM. Diagnostic accuracy of self collected vaginal specimens for human papillomavirus compared to clinician collected human papillomavirus specimens: a meta-analysis. Sex Transm Infect. 2005;81:207-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 40. | Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, Hingmire S, Malvi SG, Thorat R, Kothari A. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385-1394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 812] [Cited by in F6Publishing: 811] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 41. | Goldie SJ, Kuhn L, Denny L, Pollack A, Wright TC. Policy analysis of cervical cancer screening strategies in low-resource settings: clinical benefits and cost-effectiveness. JAMA. 2001;285:3107-3115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 236] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 42. | Shi JF, Canfell K, Lew JB, Zhao FH, Legood R, Ning Y, Simonella L, Ma L, Kang YJ, Zhang YZ. Evaluation of primary HPV-DNA testing in relation to visual inspection methods for cervical cancer screening in rural China: an epidemiologic and cost-effectiveness modelling study. BMC Cancer. 2011;11:239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Catarino R, Vassilakos P, Stadali-Ullrich H, Royannez-Drevard I, Guillot C, Petignat P. Feasibility of at-home self-sampling for HPV testing as an appropriate screening strategy for nonparticipants in Switzerland: preliminary results of the DEPIST study. J Low Genit Tract Dis. 2015;19:27-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Dzuba IG, Díaz EY, Allen B, Leonard YF, Lazcano Ponce EC, Shah KV, Bishai D, Lorincz A, Ferris D, Turnbull B. The acceptability of self-collected samples for HPV testing vs. the pap test as alternatives in cervical cancer screening. J Womens Health Gend Based Med. 2002;11:265-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 45. | Snijders PJ, Verhoef VM, Arbyn M, Ogilvie G, Minozzi S, Banzi R, van Kemenade FJ, Heideman DA, Meijer CJ. High-risk HPV testing on self-sampled versus clinician-collected specimens: a review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int J Cancer. 2013;132:2223-2236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 46. | Untiet S, Vassilakos P, McCarey C, Tebeu PM, Kengne-Fosso G, Menoud PA, BoulvainZ M, Navarria I, Petignat P. HPV self-sampling as primary screening test in sub-Saharan Africa: implication for a triaging strategy. Int J Cancer. 2014;135:1911-1917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Lazcano-Ponce E, Lorincz AT, Cruz-Valdez A, Salmerón J, Uribe P, Velasco-Mondragón E, Nevarez PH, Acosta RD, Hernández-Avila M. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011;378:1868-1873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 48. | Petignat P, Faltin DL, Bruchim I, Tramèr MR, Franco EL, Coutlée F. Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis. Gynecol Oncol. 2007;105:530-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 49. | Gravitt PE, Belinson JL, Salmeron J, Shah KV. Looking ahead: a case for human papillomavirus testing of self-sampled vaginal specimens as a cervical cancer screening strategy. Int J Cancer. 2011;129:517-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, Gillio-Tos A, Minucci D, Naldoni C, Rizzolo R. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008;100:492-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 51. | Leinonen M, Nieminen P, Kotaniemi-Talonen L, Malila N, Tarkkanen J, Laurila P, Anttila A. Age-specific evaluation of primary human papillomavirus screening vs conventional cytology in a randomized setting. J Natl Cancer Inst. 2009;101:1612-1623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 52. | Tjalma WA, Arbyn M, Paavonen J, van Waes TR, Bogers JJ. Prophylactic human papillomavirus vaccines: the beginning of the end of cervical cancer. Int J Gynecol Cancer. 2004;14:751-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur DC. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 854] [Cited by in F6Publishing: 781] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 54. | Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890-907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1829] [Cited by in F6Publishing: 1758] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 55. | Muwonge R, Wesley RS, Nene BM, Shastri SS, Jayant K, Malvi SG, Thara S, Sankaranarayanan R. Evaluation of cytology and visual triage of human papillomavirus-positive women in cervical cancer prevention in India. Int J Cancer. 2014;134:2902-2909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Snijders PJ, Steenbergen RD, Heideman DA, Meijer CJ. HPV-mediated cervical carcinogenesis: concepts and clinical implications. J Pathol. 2006;208:152-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 57. | McLaughlin-Drubin ME, Münger K. Oncogenic activities of human papillomaviruses. Virus Res. 2009;143:195-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 58. | Steenbergen RD, Snijders PJ, Heideman DA, Meijer CJ. Clinical implications of (epi)genetic changes in HPV-induced cervical precancerous lesions. Nat Rev Cancer. 2014;14:395-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 249] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 59. | Dasari S, Wudayagiri R, Valluru L. Cervical cancer: Biomarkers for diagnosis and treatment. Clin Chim Acta. 2015;445:7-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 60. | Ibeanu OA. Molecular pathogenesis of cervical cancer. Cancer Biol Ther. 2011;11:295-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 61. | Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C. Clinical performance of the APTIMA HPV Assay for the detection of high-risk HPV and high-grade cervical lesions. J Clin Virol. 2009;45 Suppl 1:S55-S61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 62. | Qiao YL, Jeronimo J, Zhao FH, Schweizer J, Chen W, Valdez M, Lu P, Zhang X, Kang LN, Bansil P. Lower cost strategies for triage of human papillomavirus DNA-positive women. Int J Cancer. 2014;134:2891-2901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 63. | Benevolo M, Vocaturo A, Mottolese M, Mariani L, Vocaturo G, Marandino F, Sperduti I, Rollo F, Antoniani B, Donnorso RP. Clinical role of p16INK4a expression in liquid-based cervical cytology: correlation with HPV testing and histologic diagnosis. Am J Clin Pathol. 2008;129:606-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2008;17:2536-2545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 65. | Tsoumpou I, Arbyn M, Kyrgiou M, Wentzensen N, Koliopoulos G, Martin-Hirsch P, Malamou-Mitsi V, Paraskevaidis E. p16(INK4a) immunostaining in cytological and histological specimens from the uterine cervix: a systematic review and meta-analysis. Cancer Treat Rev. 2009;35:210-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 66. | McLaughlin-Drubin ME, Crum CP, Münger K. Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proc Natl Acad Sci USA. 2011;108:2130-2135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 67. | Lajer CB, Garnæs E, Friis-Hansen L, Norrild B, Therkildsen MH, Glud M, Rossing M, Lajer H, Svane D, Skotte L. The role of miRNAs in human papilloma virus (HPV)-associated cancers: bridging between HPV-related head and neck cancer and cervical cancer. Br J Cancer. 2012;106:1526-1534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 68. | Pereira PM, Marques JP, Soares AR, Carreto L, Santos MA. MicroRNA expression variability in human cervical tissues. PLoS One. 2010;5:e11780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 69. | Lee JW, Choi CH, Choi JJ, Park YA, Kim SJ, Hwang SY, Kim WY, Kim TJ, Lee JH, Kim BG. Altered MicroRNA expression in cervical carcinomas. Clin Cancer Res. 2008;14:2535-2542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 238] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 70. | Deftereos G, Corrie SR, Feng Q, Morihara J, Stern J, Hawes SE, Kiviat NB. Expression of mir-21 and mir-143 in cervical specimens ranging from histologically normal through to invasive cervical cancer. PLoS One. 2011;6:e28423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 71. | Martinez I, Gardiner AS, Board KF, Monzon FA, Edwards RP, Khan SA. Human papillomavirus type 16 reduces the expression of microRNA-218 in cervical carcinoma cells. Oncogene. 2008;27:2575-2582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 270] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 72. | Hu X, Schwarz JK, Lewis JS, Huettner PC, Rader JS, Deasy JO, Grigsby PW, Wang X. A microRNA expression signature for cervical cancer prognosis. Cancer Res. 2010;70:1441-1448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 73. | Melar-New M, Laimins LA. Human papillomaviruses modulate expression of microRNA 203 upon epithelial differentiation to control levels of p63 proteins. J Virol. 2010;84:5212-5221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 74. | Han Y, Xu GX, Lu H, Yu DH, Ren Y, Wang L, Huang XH, Hou WJ, Wei ZH, Chen YP. Dysregulation of miRNA-21 and their potential as biomarkers for the diagnosis of cervical cancer. Int J Clin Exp Pathol. 2015;8:7131-7139. [PubMed] [Cited in This Article: ] |
| 75. | Fernandez AF, Rosales C, Lopez-Nieva P, Graña O, Ballestar E, Ropero S, Espada J, Melo SA, Lujambio A, Fraga MF. The dynamic DNA methylomes of double-stranded DNA viruses associated with human cancer. Genome Res. 2009;19:438-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 76. | Bryant D, Tristram A, Liloglou T, Hibbitts S, Fiander A, Powell N. Quantitative measurement of Human Papillomavirus type 16 L1/L2 DNA methylation correlates with cervical disease grade. J Clin Virol. 2014;59:24-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 77. | Qiu C, Zhi Y, Shen Y, Gong J, Li Y, Li X. High-resolution melting analysis of HPV-16L1 gene methylation: A promising method for prognosing cervical cancer. Clin Biochem. 2015;48:855-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Pun PB, Liao YP, Su PH, Wang HC, Chen YC, Hsu YW, Huang RL, Chang CC, Lai HC. Triage of high-risk human papillomavirus-positive women by methylated POU4F3. Clin Epigenetics. 2015;7:85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Pinto AP, Degen M, Villa LL, Cibas ES. Immunomarkers in gynecologic cytology: the search for the ideal ‘biomolecular Papanicolaou test’. Acta Cytol. 2012;56:109-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 80. | Conesa-Zamora P, Doménech-Peris A, Orantes-Casado FJ, Ortiz-Reina S, Sahuquillo-Frías L, Acosta-Ortega J, García-Solano J, Pérez-Guillermo M. Effect of human papillomavirus on cell cycle-related proteins p16, Ki-67, Cyclin D1, p53, and ProEx C in precursor lesions of cervical carcinoma: a tissue microarray study. Am J Clin Pathol. 2009;132:378-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 81. | Borras G, Molina R, Xercavins J, Ballesta A, Iglesias J. Tumor antigens CA 19.9, CA 125, and CEA in carcinoma of the uterine cervix. Gynecol Oncol. 1995;57:205-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Dasari S, Rajendra W, Valluru L. Evaluation of soluble CD44 protein marker to distinguish the premalignant and malignant carcinoma cases in cervical cancer patients. Med Oncol. 2014;31:139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Kulkarni S, Rader JS, Zhang F, Liapis H, Koki AT, Masferrer JL, Subbaramaiah K, Dannenberg AJ. Cyclooxygenase-2 is overexpressed in human cervical cancer. Clin Cancer Res. 2001;7:429-434. [PubMed] [Cited in This Article: ] |
| 84. | Van Trappen PO, Steele D, Lowe DG, Baithun S, Beasley N, Thiele W, Weich H, Krishnan J, Shepherd JH, Pepper MS. Expression of vascular endothelial growth factor (VEGF)-C and VEGF-D, and their receptor VEGFR-3, during different stages of cervical carcinogenesis. J Pathol. 2003;201:544-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 85. | Suprynowicz FA, Disbrow GL, Krawczyk E, Simic V, Lantzky K, Schlegel R. HPV-16 E5 oncoprotein upregulates lipid raft components caveolin-1 and ganglioside GM1 at the plasma membrane of cervical cells. Oncogene. 2008;27:1071-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |