Published online Oct 10, 2014. doi: 10.5306/wjco.v5.i4.705
Revised: April 28, 2014
Accepted: May 16, 2014
Published online: October 10, 2014
Phytoestrogens have multiple actions within target cells, including the epigenome, which could be beneficial to the development and progression of breast cancer. In this brief review the action of phytoestrogens on oestrogen receptors, cell signalling pathways, regulation of the cell cycle, apoptosis, steroid synthesis and epigenetic events in relation to breast cancer are discussed. Phytoestrogens can bind weakly to oestrogen receptors (ERs) and some have a preferential affinity for ERβ which can inhibit the transcriptional growth-promoting activity of ERα. However only saturating doses of phytoestrogens, stimulating both ERα and β, exert growth inhibitory effects. Such effects on growth may be through phytoestrogens inhibiting cell signalling pathways. Phytoestrogens have also been shown to inhibit cyclin D1 expression but increase the expression of cyclin-dependent kinase inhibitors (p21 and p27) and the tumour suppressor gene p53. Again these effects are only observed at high (> 10) µmol/L doses of phytoestrogens. Finally the effects of phytoestrogens on breast cancer may be mediated by their ability to inhibit local oestrogen synthesis and induce epigenetic changes. There are, though, difficulties in reconciling epidemiological and experimental data due to the fact experimental doses, both in vivo and in vitro, far exceed the circulating concentrations of “free” unbound phytoestrogens measured in women on a high phytoestrogen diet or those taking phytoestrogen supplements.
Core tip: Phytoestrogens have multiple actions within target cells, including the epigenome, which could be beneficial to the development and progression of breast cancer. In this brief review the action of phytoestrogens on oestrogen receptors, cell signalling pathways, regulation of the cell cycle, apoptosis, steroid synthesis and epigenetic events in relation to breast cancer are discussed. The difficulties in interpreting experimental evidence relating to the beneficial effects of phytoestrogens in light of dietary/supplementary intake and bioavailability of ingested phytoestrogens is also addressed.
- Citation: Bilal I, Chowdhury A, Davidson J, Whitehead S. Phytoestrogens and prevention of breast cancer: The contentious debate. World J Clin Oncol 2014; 5(4): 705-712
- URL: https://www.wjgnet.com/2218-4333/full/v5/i4/705.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i4.705
Phytoestrogens are naturally occurring plant compounds which are structurally similar to oestrogens and can have weak oestrogenic actions. There are six major classes of phytoestrogens, all of which have distinct common dietary sources (Table 1) but the most intensively investigated phytoestrogens are the isoflavones and the stilbene, resveratrol. The link between breast cancer and phytoestrogens arose from the early epidemiologal evidence showing that the incidence of breast cancer is lower in Asian populations who consume high dietary concentrations of soy products which have a high isoflavonone content[1,2]. This fuelled the widespread belief that consumption of soy foods reduces the risk of breast cancer and other hormone dependent cancers and led to further research on the protective effects of many other phytoestrogens[3,4].
| Flavanoids | Isoflavonoids | Lignans | Coumestans | Stilbenes |
| Apigenin | Genistein | Enterodiol | Coumesterol | Resveratrol |
| Quercetin | Biochanin A | Enterolactone | ||
| Narigenin | Diadzein → equol | |||
| Catechins | ||||
| Red/yellow fruits and vegetables, tea | Soy beans, soy foods, vegetables | Flaxseed, whole grains, fruit, vegetables | Peas, beans, alfafa, sunflower seeds | Red wine |
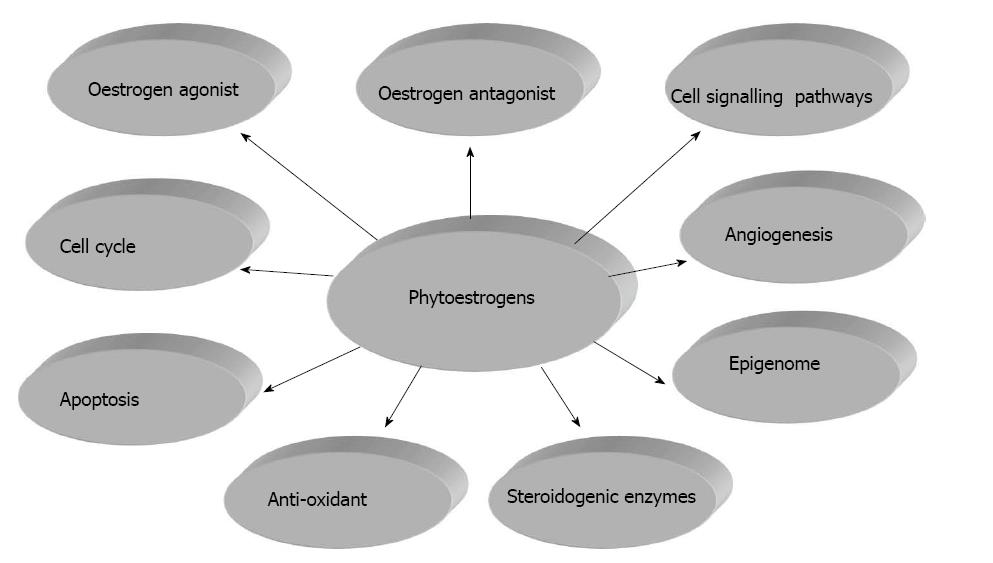
However reconciling experimental evidence, mainly based on high supraphysiological doses of single phytoestrogens, coupled with the limited bioavailability of orally consumed phytoestrogens has raised questions and concerns about the validity of promoting the health benefits of diets rich in phytoestrogens and/or taking dietary supplements[5-7]. Furthermore phytoestrogens exert a plethora of actions beyond weak oestrogenic effects (Figure 1) and these include antagonist effects oestrogen receptors (ERs), modulation of cell signalling pathways, regulation of the cell cycle, enzyme inhibition, anti-oxidant properties, angiogenesis and epigenetic alterations[8]. This review will focus on the action of phytoestrogens on oestrogen receptors and cell signalling pathways, their regulation of the cell cycle and apoptosis, inhibition of steroidogeneic enzymes and induced epigenetic changes.
In foods phytoestrogens are present as mixtures and are usually found as biologically inactive glycoside conjugates containing glucose or carbohydrate moieties. Blood levels can vary widely between individuals depending both on dietary preferences as well the phytoestrogen content of a particular food product resulting from local and/or seasonal variations[9]. For example Asian diets can result in isoflavone consumption as high as 50 mg/d compared with 1-3 mg/d for individuals eating a typical Western diet although a vegetarian diet or use of supplements can increase dietary intake to levels of an Asian diet[10-12] and references therein.
In the gut phytoestrogens are broken down by glucosidases to their respective aglycones allowing more efficient absorption, although intestinal bacteria may further metabolise these products. For example the phytoestrogens genistein and daidzein, can be further metabolised to p-ethyl phenol and to equol and/or O-desmethylangolensin (O-DMA) respectively though it should be noted that only 30%-50% of the population can produce equol and approximately 80%-90% 0-DMA[10,13]. Thus not only will dietary factors contribute to phytoestrogen intake but also individual variations in metabolism.
Once absorbed the aglycone phytoestrogens are rapidly conjugated to glucuronic acid and to a lesser extent sulphuric acid in the hepatic circulation. They are then de-conjugated prior to excretion with urinary concentrations increasing in parallel to consumption[14]. There is generally very low levels of biologically active ‘free’ unconjugated phytoestrogens in the circulation (< 3% of the total) and blood levels are in the ng/mL range or lower[10,15]. One may then argue what is the relevance of in vitro studies showing that only high micromolar doses of unconjugated phytoestrogens can inhibit the growth the breast cancer cells, inhibit oestrogen-dependent gene transcription or inhibit cell signalling pathways?[16,17]. Similarly in vivo studies have only shown that dietary supplements far in excess of those consumed with an Asian diet had any effect on inhibiting experimentally-induced tumour growth and even this data is conflicting[16,18,19] and references therein.
Two major oestrogen receptors (ERs) have been identified, ERα and ERβ, which are encoded by separate genes and have different tissue distributions and roles in gene regulation[20]. They also have differential effects in oestrogen-sensitive tissue and in breast tissue ERα activation can stimulate proliferation whilst ERβ activation can counteract this proliferative effect. This is thought to be mediated by dimerization of ERβ with ERα[20,21]. In breast tumours the ratio of ERα to ERβ is raised and tumour aggressiveness is increased in those that are ERβ negative[21].
The relative binding affinity (RBA) of phytoestrogens to ERs is weak and are in the order of 1000-10000 times less than that of oestradiol although some phytoestrogens such as genistein, coumestrol and apigenin have a higher affinity for ER’s and their RBAs are in the order of only 10-100 times that of oestradiol[22]. Interestingly several phytoestrogens such as genistein, daidzein and apigenin have a 9-10 fold increased affinity for ERβ than ERα[22] and a more recent study showed that after dietary supplementation total genistein and diadzein concentrations were 20-40 fold higher than oestradiol equivalents in breast adipose/glandular tissue[23]. Thus their ability to preferentially activate ERβ and their ability to accumulate in breast tissue may have some clinical significance. That said, the concentrations required to induce apoptosis or at least inhibit cell growth arrest are induced only by over-saturating doses (≥ 10 µmol/L) doses of phytoestrogens[16].
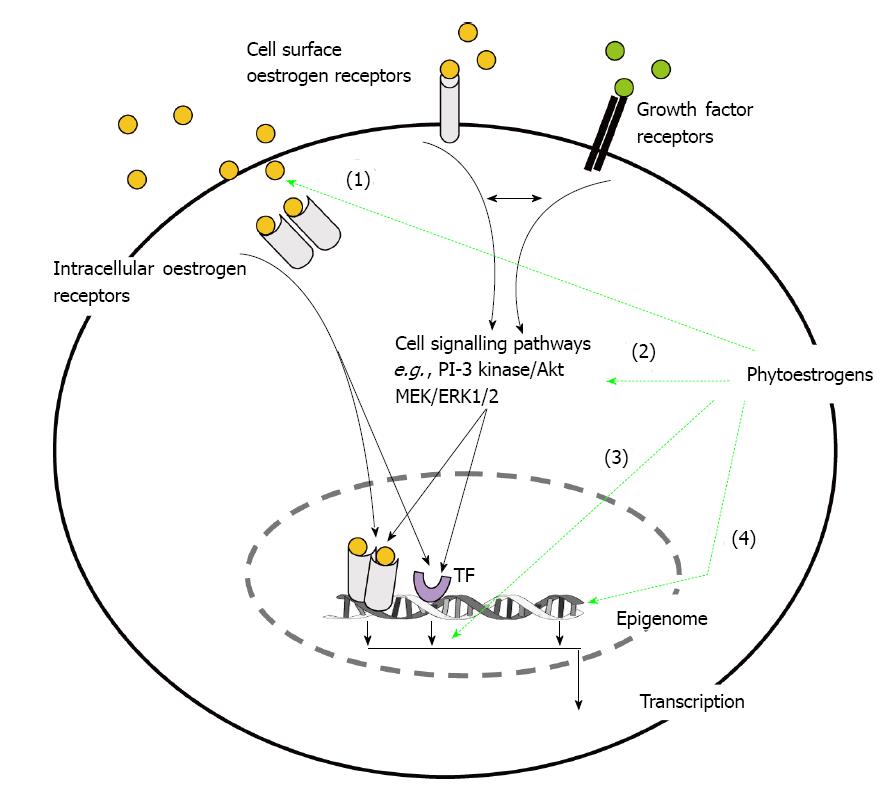
However, phytoestrogens can also act on cell surface oestrogen receptors or interact with growth factor and cytokine signalling pathways (Figure 2). Thus phytoetrogens can modulate the responses to growth factors or activate/inhibit kinases which may alter ligand-independent transcriptional activity of oestrogen receptors or other transcription factors such as AP-1 and NF-κB[24]. For example genistein is a tyrosine kinase inhibitor and has been shown to alter the activity/expression of both extracellular regulated kinase (ERK) and the PI-3/Akt pathway as have other phytoestrogens including resveratrol[25,26]. Long-term treatment of breast cancer cells with dietary levels of genistein (10-8 mol/L) have also been shown to down-regulate the expression of Akt[27]. In context of the growth-repressing effect of ERβ and cell signalling, a recent study showed that activation of the MEK 1/2 and PI-3K/Akt pathways inhibited the ERβ growth-mediated repression in breast cancer cells[28]. Thus down regulation of these pathways by long-term dietary phytoestrogens could promote the effectiveness of ERβ activation and inhibit proliferation.
Recently much attention has focussed on the action of phytoestrogens in regulating the expression of proteins regulating the cell cycle and apoptosis (Figure 2). Cyclins are a family of proteins which regulate transition of the cell cycle through the G1, S, G2 and metaphase (M) phases and, through coalescing with cyclin-dependent kinases(CDKs), they initiate gene transcription controlling regulation of the cell cycle. Cyclin D1, which regulates the G1 to S phase of the cell cycle, is the most widely investigated being established as an oncogene and over-expressed in more than 50% breast cancers[29]. The majority of studies have shown that high concentrations of phytoestrogens (≥ 10 µmol/L range) inhibit the expression of cyclin D1[30-32] although another study reported a transient increase mimicking the effects of oestradiol[33] and other studies reported no effects[34,35].
The activity of cyclin/CDK complexes is regulated by CDK inhibitors (CDIs) and thus these proteins can inhibit the cell cycle[36,37]. The two most widely investigated CDI’s are p21(CIP1/WAF1) and p27(Kip1) and the expression of p21 is controlled by the tumour suppressor gene p53[38] which has many other actions as a tumour suppressor including inducing apoptosis[39]. High doses of phytoestrogens have been shown to increase the expression/activity of p21, p27 and p53[33,35,40-43] which parallel changes in the reduction of cyclin D1. Such effects have been seen with high doses of phytoestrogens but a microarray analysis showed that low levels of genistein (1 and 5 µmol/L) that stimulated growth of MCF-7 cells had no effect on the expression of p53 target genes such as p21(CIP1/WAF1) and only at a higher pharmacological dose of genistein (25 µmol/L) cell growth was inhibited and increased the expression of p53 target genes. This would result in increased apoptosis and decreased proliferation[44].
The pro-apoptotic protein, Bax, is regulated positively by p53 whilst the anti-apoptotic protein, Bcl-2, is negatively regulated by p53[45]. Both in vivo[46-48] and in vitro studies[33,49-52] have shown that phytoestrogens can stimulate apoptosis and increase the Bax/Bcl-2 ratio but evidence as to whether this effect is due to increased or decreased activity of ERK1/2 is controversial[49-52].
Low doses of phytoestrogens are generally found to stimulate growth of breast cancer cells with only high supraphysiological doses inhibiting growth and with evidence that they regulate the expression of proteins involved in controlling the cell cycle and apoptosis as opposed to their action as weak oestrogen agonists/antagonists at ERα and ERβ. There is, however, evidence that phytoestrogens can also inhibit steroid synthesis and this may be particularly significant in relation to the local production of oestrogens in breast tissue[53] and references therein. Fatty tissues are a major storage site for phytoestrogens[13] and the most abundant cells surrounding breast cancer cells are mature adipocytes and progenitors which may be a key component of breast cancer progression by locally affecting breast cancer cell behaviour[54].
Approximately 60%-70% breast cancers express oestrogen receptors and these are considered to promote tumour growth. Hence initial treatments are directed towards reducing oestrogenic effects by inhibitors of oestrogen receptors and inhibitors of aromatase which converts androgens to active oestrogens[55]. The incidence of breast cancer increases with age despite the loss of ovarian hormones in post-menopausal women. Peripheral tissue can convert circulating androgens (dehydroepiandrosteneione/DHEA) and oestrone sulphate into 17β-oestradiol (E2) and in post-menopausal women with breast cancer concentrations of E2 in the tumour is at least 20-fold higher than in the circulation[56].
Flavones and isoflavones are the most potent phytoestrogens that inhibit aromatase and the IC50 values are in the order of 0.1-10 μM which is more than 100 times higher than the IC50 value for the steroidal inhibitor, 4-hydroxyandrostendione[53,57]. Isoflavones are generally weak inhibitors of aromatase but like other phytoestrogens can inhibit 17β-hydroxysteroid dehdrogenase (HSD) type 1 which reduces oestrone (E1) to E2 and 17β-HSD type 5 that converts androstenedione to testosterone, which can subsequently be converted to E2 by aromatase[53] and references therein. More recently certain phytoestrogens have been shown to alter the activation of breast cancer-associated aromatase promoters[58]. Overall, however, the inhibition of these enzymes by unconjugated phytoestrogens are in the order of 1-10 μmol/L whilst total circulating phytoestrogens are in the low nanomolar range except in vegetarians or those eating a high soy diet where concentrations of 100 mN to 1 μmol/L may be achieved[59]. Thus there is another discrepancy between experimental results and levels of phytoestrogens achieved by dietary means. However our study on human granulosa cells showed that low dose mixtures of three isoflavones in the nM range inhibited expression and activity of aromatase though a similar inhibition was only achieved with a single phytoestrogen at 100 times the dosage[60]. Further studies are required to investigate mixtures of phytoestrogens as occurs through dietary means.
Over the last decade there has been an explosion in the number of studies concerning epigenetic changes and the development and progression of breast cancer[61] and not surprisingly these have included studies on the ability of phytoestrogens to alter the epigenome which could be useful in the prevention of cancer[61-63]. In fact studies have indicated that early childhood exposure to phytoestrogens could protect against breast cancer in later life[62] and references therein and this could involve epigenetic events (Figure 2). Epigenetic changes are defined as heritable changes in gene expression which do not involve mutations of DNA nucleotide sequences. They include DNA methylation, histone acetylation and microRNA’s (miRNAs).
DNA methylation occurs on cytosine in the cytosine-phosphate-guanine (CpG) dinucleotide sequence of genomic DNA, a reaction catalysed by DNA methyl transferases (DNMTs). CpG dinucleotide rich regions (known as CpG islands) are found in the promoter region of approximately 60% of all human genes and, whilst most CpG islands are unmethylated in normal cells, they become hypermethylated in cancerous cells leading to gene suppression, including the tumour-suppressing genes[61]. Along with DNMTs are the methyl-CpG-binding domain family of proteins which bind to a methylated gene and can inhibit transcriptional activity by altering chromatin structure. Chromatin structure can also be modified by histone acetylation which is catalysed by histone acetylase (HAT) and results in a more open structure of chromatin allowing access for transcription factors to DNA. The reverse occurs when histone proteins become deacetylated and this reaction is catalysed by histone deacetylases (HDACs). Histones may also be methylated by histone methyl transferases (HMT’s) and generally methylation causes gene transcription to be switched off. The most recent participant of the epigenetic field are the miRNAs, small non-coding RNAs that inhibit protein expression of target genes by binding to the 3’-untranslated region of mRNA causing degradation or inhibition of mRNA of the target gene[61,62] and references therein.
The most widely studied dietary components in relation to epigenetic changes are the tea polyphenols, epicatechins and epigallocatechins (EGCCs), the isoflavones, genistein and diadzein, resveratrol and curcumin and all have been well reviewed recently[63-66]. Relatively few studies have been directed towards epigenetic changes in breast cancer models and results have been inconclusive[61,62].
Recent studies have shown that 20-40 µmol/L genistein stimulated expression of the tumour suppressing genes, p21WAF1 and p16INK4a, in breast cancer cells and that this was associated with a small reduction in the activity of HDACs but a large increase in the activity of HMTs[67]. The same group also showed that genistein can reactivate ERα expression in ER-ve breast cancer cells and that this effect was associated with increased markers of histone acetylation in the ERα promoter region and decreased activity of HDAC and DNMT[68]. Another study showed that µmol/L doses of genistein and diadzein “might reverse” DNA hypermethylation in breast cancer cells thus restoring expression of the oncosuppressor genes BRCA1 and BRCA2[69]. In biopsies of human breast tissue specific DNMT transcripts were increased in cells taken from the tumourous tissue compared to adjacent normal breast tissue and parallel studies showed that treatment of breast cancer cells lines with genistein, resveratrol, curcumin and EGCC also reduced the mRNA of the same DNMTs[70]. Whilst all these studies have been performed acutely with high doses of single phytoestrogens, we showed that long-term treatment with 10 nmol/L genistein down-regulated the expression of acetylated histone3, cyclin D1 and procaspase 9 and reduced the growth promoting effects of E2 and epidermal growth factor[71].
It is clear that both nutrition and exposure to phytoestrogens and other phytochemicals can have dramatic effects on epigenetic events and that these may become heritable through transgenerational mechanisms. Thus their impact on both disease and the health of future generations needs to be carefully considered.
Phytoestrogens have multiple targets within cells and whilst acute studies with supraphysiological doses of these compounds indicate that they may inhibit the development and progression of breast cancer, lower doses have been shown to promote the growth of breast cancer cells in vitro and experimentally induced tumours in vivo. More studies utilizing long-term exposure to lower doses and mixtures of phytoestrogens are required to demonstrate unequivocally that dietary supplements do have beneficial rather than detrimental effects on breast cancer. However their multiple targets in breast cancer cells and their ability to modulate epigenetic events associated with breast cancer and prevention may lead to new, non-toxic therapeutic approaches through development of highly specific and long-acting analogues of phytoestrogens.
P- Reviewer: Petmitr S, Shen J S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
| 1. | Mense SM, Hei TK, Ganju RK, Bhat HK. Phytoestrogens and breast cancer prevention: possible mechanisms of action. Environ Health Perspect. 2008;116:426-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Miller PE, Snyder DC. Phytochemicals and cancer risk: a review of the epidemiological evidence. Nutr Clin Pract. 2012;27:599-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Gikas PD, Mokbel K. Phytoestrogens and the risk of breast cancer: a review of the literature. Int J Fertil Womens Med. 2005;50:250-258. [PubMed] [Cited in This Article: ] |
| 4. | Pelekanou V, Leclercq G. Recent insights into the effect of natural and environmental estrogens on mammary development and carcinogenesis. Int J Dev Biol. 2011;55:869-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Velentzis LS, Woodside JV, Cantwell MM, Leathem AJ, Keshtgar MR. Do phytoestrogens reduce the risk of breast cancer and breast cancer recurrence? What clinicians need to know. Eur J Cancer. 2008;44:1799-1806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Helferich WG, Andrade JE, Hoagland MS. Phytoestrogens and breast cancer: a complex story. Immunopharmacol. 2008;16:291-216. [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Hilakivi-Clarke L, Andrade JE, Helferich W. Is soy consumption good or bad for the breast? J Nutr. 2010;140:2326S-2334S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Martin JH, Crotty S, Nelson PN. Phytoestrogens: perpetrators or protectors? Future Oncol. 2007;3:307-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Mortensen A, Kulling SE, Schwartz H, Rowland I, Ruefer CE, Rimbach G, Cassidy A, Magee P, Millar J, Hall WL. Analytical and compositional aspects of isoflavones in food and their biological effects. Mol Nutr Food Res. 2009;53 Suppl 2:S266-S309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Front Neuroendocrinol. 2010;31:400-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 407] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 11. | Vitale DC, Piazza C, Melilli B, Drago F, Salomone S. Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet. 2013;38:15-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 282] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 12. | Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood). 2005;230:155-170. [PubMed] [Cited in This Article: ] |
| 13. | de Cremoux P, This P, Leclercq G, Jacquot Y. Controversies concerning the use of phytoestrogens in menopause management: bioavailability and metabolism. Maturitas. 2010;65:334-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Karr SC, Lampe JW, Hutchins AM, Slavin JL. Urinary isoflavonoid excretion in humans is dose dependent at low to moderate levels of soy-protein consumption. Am J Clin Nutr. 1997;66:46-51. [PubMed] [Cited in This Article: ] |
| 15. | Verkasalo PK, Appleby PN, Allen NE, Davey G, Adlercreutz H, Key TJ. Soya intake and plasma concentrations of daidzein and genistein: validity of dietary assessment among eighty British women (Oxford arm of the European Prospective Investigation into Cancer and Nutrition). Br J Nutr. 2001;86:415-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Leclercq G, Jacquot Y. Interactions of isoflavones and other plant derived estrogens with estrogen receptors for prevention and treatment of breast cancer-considerations concerning related efficacy and safety. J Steroid Biochem Mol Biol. 2014;139:237-244. [PubMed] [Cited in This Article: ] |
| 17. | Rice S, Whitehead SA. Phytoestrogens and breast cancer – promoters or protectors? Endocr Rel Cancer. 2006;13:995-1015. [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Jenkins S, Betancourt AM, Wang J, Lamartiniere CA. Endocrine-active chemicals in mammary cancer causation and prevention. J Steroid Biochem Mol Biol. 2012;129:191-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Rietjens IM, Sotoca AM, Vervoort J, Louisse J. Mechanisms underlying the dualistic mode of action of major soy isoflavones in relation to cell proliferation and cancer risks. Mol Nutr Food Res. 2013;57:100-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Thomas C, Gustafsson JÅ. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011;11:597-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 481] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 21. | Saji S, Hirose M, Toi M. Clinical significance of estrogen receptor beta in breast cancer. Cancer Chemother Pharmacol. 2005;56 Suppl 1:21-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252-4263. [PubMed] [Cited in This Article: ] |
| 23. | Bolca S, Urpi-Sarda M, Blondeel P, Roche N, Vanhaecke L, Possemiers S, Al-Maharik N, Botting N, De Keukeleire D, Bracke M. Disposition of soy isoflavones in normal human breast tissue. Am J Clin Nutr. 2010;91:976-984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1186] [Cited by in F6Publishing: 1226] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 25. | Shimizu M, Weinstein IB. Modulation of signal transduction by tea catechins and related phytochemicals. Mutat Res. 2005;591:147-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Barnes S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat Res Biol. 2010;8:89-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Anastasius N, Boston S, Lacey M, Storing N, Whitehead SA. Evidence that low-dose, long-term genistein treatment inhibits oestradiol-stimulated growth in MCF-7 cells by down-regulation of the PI3-kinase/Akt signalling pathway. J Steroid Biochem Mol Biol. 2009;116:50-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Cotrim CZ, Fabris V, Doria ML, Lindberg K, Gustafsson JÅ, Amado F, Lanari C, Helguero LA. Estrogen receptor beta growth-inhibitory effects are repressed through activation of MAPK and PI3K signalling in mammary epithelial and breast cancer cells. Oncogene. 2013;32:2390-2402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Tobin NP, Bergh J. Analysis of Cyclin D1 in Breast Cancer: A Call to Arms. Curr Breast Cancer Rep. 2012;4:171-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Chen J, Sun L. Formononetin-induced apoptosis by activation of Ras/p38 mitogen-activated protein kinase in estrogen receptor-positive human breast cancer cells. Horm Metab Res. 2012;44:943-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Hsieh TC, Wu JM. Suppression of cell proliferation and gene expression by combinatorial synergy of EGCG, resveratrol and gamma-tocotrienol in estrogen receptor-positive MCF-7 breast cancer cells. Int J Oncol. 2008;33:851-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Rahal OM, Simmen RC. PTEN and p53 cross-regulation induced by soy isoflavone genistein promotes mammary epithelial cell cycle arrest and lobuloalveolar differentiation. Carcinogenesis. 2010;31:1491-1500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Sakamoto T, Horiguchi H, Oguma E, Kayama F. Effects of diverse dietary phytoestrogens on cell growth, cell cycle and apoptosis in estrogen-receptor-positive breast cancer cells. J Nutr Biochem. 2010;21:856-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Waite KA, Sinden MR, Eng C. Phytoestrogen exposure elevates PTEN levels. Hum Mol Genet. 2005;14:1457-1463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Seo HS, Ju JH, Jang K, Shin I. Induction of apoptotic cell death by phytoestrogens by up-regulating the levels of phospho-p53 and p21 in normal and malignant estrogen receptor α-negative breast cells. Nutr Res. 2011;31:139-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980-3985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 632] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 37. | Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 719] [Cited by in F6Publishing: 748] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 38. | May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene. 1999;18:7621-7636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 451] [Cited by in F6Publishing: 437] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 39. | Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis - the p53 network. J Cell Sci. 2003;116:4077-4085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 810] [Cited by in F6Publishing: 1011] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 40. | Choi EJ, Kim GH. Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomedicine. 2008;15:683-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 41. | Eto I. Nutritional and chemopreventive anti-cancer agents up-regulate expression of p27Kip1, a cyclin-dependent kinase inhibitor, in mouse JB6 epidermal and human MCF7, MDA-MB-321 and AU565 breast cancer cells. Cancer Cell Int. 2006;6:20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Privat M, Aubel C, Arnould S, Communal Y, Ferrara M, Bignon YJ. AKT and p21 WAF1/CIP1 as potential genistein targets in BRCA1-mutant human breast cancer cell lines. Anticancer Res. 2010;30:2049-2054. [PubMed] [Cited in This Article: ] |
| 43. | Hsieh TC, Wong C, John Bennett D, Wu JM. Regulation of p53 and cell proliferation by resveratrol and its derivatives in breast cancer cells: an in silico and biochemical approach targeting integrin αvβ3. Int J Cancer. 2011;129:2732-2743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Lavigne JA, Takahashi Y, Chandramouli GV, Liu H, Perkins SN, Hursting SD, Wang TT. Concentration-dependent effects of genistein on global gene expression in MCF-7 breast cancer cells: an oligo microarray study. Breast Cancer Res Treat. 2008;110:85-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1092] [Cited by in F6Publishing: 1326] [Article Influence: 120.5] [Reference Citation Analysis (0)] |
| 46. | Sahin K, Tuzcu M, Sahin N, Akdemir F, Ozercan I, Bayraktar S, Kucuk O. Inhibitory effects of combination of lycopene and genistein on 7,12- dimethyl benz(a)anthracene-induced breast cancer in rats. Nutr Cancer. 2011;63:1279-1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Liu X, Suzuki N, Santosh Laxmi YR, Okamoto Y, Shibutani S. Anti-breast cancer potential of daidzein in rodents. Life Sci. 2012;91:415-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Whitsett T, Carpenter M, Lamartiniere CA. Resveratrol, but not EGCG, in the diet suppresses DMBA-induced mammary cancer in rats. J Carcinog. 2006;5:15. [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Rajah TT, Peine KJ, Du N, Serret CA, Drews NR. Physiological concentrations of genistein and 17β-estradiol inhibit MDA-MB-231 breast cancer cell growth by increasing BAX/BCL-2 and reducing pERK1/2. Anticancer Res. 2012;32:1181-1191. [PubMed] [Cited in This Article: ] |
| 50. | Li Z, Li J, Mo B, Hu C, Liu H, Qi H, Wang X, Xu J. Genistein induces cell apoptosis in MDA-MB-231 breast cancer cells via the mitogen-activated protein kinase pathway. Toxicol In Vitro. 2008;22:1749-1753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Nguyen TH, Mustafa FB, Pervaiz S, Ng FS, Lim LH. ERK1/2 activation is required for resveratrol-induced apoptosis in MDA-MB-231 cells. Int J Oncol. 2008;33:81-92. [PubMed] [Cited in This Article: ] |
| 52. | Alkhalaf M. Resveratrol-induced apoptosis is associated with activation of p53 and inhibition of protein translation in T47D human breast cancer cells. Pharmacology. 2007;80:134-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | Rice S, Whitehead SA. Phytoestrogens oestrogen synthesis and breast cancer. J Steroid Biochem Mol Biol. 2008;108:186-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Wang YY, Lehuédé C, Laurent V, Dirat B, Dauvillier S, Bochet L, Le Gonidec S, Escourrou G, Valet P, Muller C. Adipose tissue and breast epithelial cells: a dangerous dynamic duo in breast cancer. Cancer Lett. 2012;324:142-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 55. | den Hollander P, Savage MI, Brown PH. Targeted therapy for breast cancer prevention. Front Oncol. 2013;3:250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 56. | Pasqualini JR, Chetrite GS. Recent insight on the control of enzymes involved in estrogen formation and transformation in human breast cancer. J Steroid Biochem Mol Biol. 2005;93:221-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 57. | Sanderson JT, Hordijk J, Denison MS, Springsteel MF, Nantz MH, van den Berg M. Induction and inhibition of aromatase (CYP19) activity by natural and synthetic flavonoid compounds in H295R human adrenocortical carcinoma cells. Toxicol Sci. 2004;82:70-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 58. | Khan SI, Zhao J, Khan IA, Walker LA, Dasmahapatra AK. Potential utility of natural products as regulators of breast cancer-associated aromatase promoters. Reprod Biol Endocrinol. 2011;9:91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Mazur W, Adlercreutz H. Overview of naturally occurring endocrine-active substances in the human diet in relation to human health. Nutrition. 2000;16:654-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Rice S, Mason HD, Whitehead SA. Phytoestrogens and their low dose combinations inhibit mRNA expression and activity of aromatase in human granulosa-luteal cells. J Steroid Biochem Mol Biol. 2006;101:216-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 61. | Huang Y, Nayak S, Jankowitz R, Davidson NE, Oesterreich S. Epigenetics in breast cancer: what’s new? Breast Cancer Res. 2011;13:225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 62. | Khan SI, Aumsuwan P, Khan IA, Walker LA, Dasmahapatra AK. Epigenetic events associated with breast cancer and their prevention by dietary components targeting the epigenome. Chem Res Toxicol. 2012;25:61-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 63. | Guerrero-Bosagna CM, Skinner MK. Environmental epigenetics and phytoestrogen/phytochemical exposures. J Steroid Biochem Mol Biol. 2014;139:270-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 64. | Hardy TM, Tollefsbol TO. Epigenetic diet: impact on the epigenome and cancer. Epigenomics. 2011;3:503-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 65. | Meeran SM, Ahmed A, Tollefsbol TO. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin Epigenetics. 2010;1:101-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 66. | Henning SM, Wang P, Carpenter CL, Heber D. Epigenetic effects of green tea polyphenols in cancer. Epigenomics. 2013;5:729-741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 67. | Li Y, Chen H, Hardy TM, Tollefsbol TO. Epigenetic regulation of multiple tumor-related genes leads to suppression of breast tumorigenesis by dietary genistein. PLoS One. 2013;8:e54369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 68. | Li Y, Meeran SM, Patel SN, Chen H, Hardy TM, Tollefsbol TO. Epigenetic reactivation of estrogen receptor-α (ERα) by genistein enhances hormonal therapy sensitivity in ERα-negative breast cancer. Mol Cancer. 2013;12:9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 69. | Bosviel R, Dumollard E, Déchelotte P, Bignon YJ, Bernard-Gallon D. Can soy phytoestrogens decrease DNA methylation in BRCA1 and BRCA2 oncosuppressor genes in breast cancer? OMICS. 2012;16:235-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 70. | Mirza S, Sharma G, Parshad R, Gupta SD, Pandya P, Ralhan R. Expression of DNA methyltransferases in breast cancer patients and to analyze the effect of natural compounds on DNA methyltransferases and associated proteins. J Breast Cancer. 2013;16:23-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 71. | Jawaid K, Crane SR, Nowers JL, Lacey M, Whitehead SA. Long-term genistein treatment of MCF-7 cells decreases acetylated histone 3 expression and alters growth responses to mitogens and histone deacetylase inhibitors. J Steroid Biochem Mol Biol. 2010;120:164-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |