Published online Jul 24, 2022. doi: 10.5306/wjco.v13.i7.577
Peer-review started: January 29, 2022
First decision: May 12, 2022
Revised: June 5, 2022
Accepted: June 21, 2022
Article in press: June 21, 2022
Published online: July 24, 2022
Adjuvant chemotherapy is recommended in high-risk breast cancer. However, no universally accepted guidelines exist on pre-chemotherapy assessment. In particular, the number and frequency of medical visits vary according to each institution’s policy. We hypothesised that the Edmonton Symptom Assessment Scale (ESAS) may have a favourable impact on the pre-treatment assessment in candidates for adjuvant chemotherapy.
To investigate whether the ESAS can be used to safely reduce the number of medical visits in women with breast cancer undergoing adjuvant chemotherapy.
In a retrospectively prospective matched-pair analysis, 100 patients who completed the ESAS questionnaire before administration of adjuvant chemotherapy (ESAS Group) were compared with 100 patients who underwent chemotherapy according to the traditional modality, without ESAS (no-ESAS Group). Patients of the ESAS Group received additional visits before treatment if their ESAS score was > 3. The primary endpoint was the total number of medical visits during the entire duration of the chemotherapy period. The secondary endpoints were the occurrence of severe complications (grade 3-4) and the number of unplanned visits during the chemotherapy period.
The study variables did not statistically differ between patients of the ESAS Group and no-ESAS Group (age P = 0.880; breast cancer stage P = 0.56; cancer histology P = 0.415; tumour size P = 0.258; lymph node status P = 0.883; immunohistochemical classification P = 0.754; type of surgery P = 0.157), except for premenopausal status (P = 0.015). The study variables did not statistically differ between patients of the ESAS Group and no-ESAS Group regarding age, cancer stage, histology, tumour size, lymph node status, immunohistochemical classification, and type of surgery. Unplanned visits during the entire duration of chemotherapy were 8 in the ESAS Group and 18 in the no-ESAS Group visits (P = 0.035). Grade 3-4 toxicity did not differ between the study groups (P = 0.652). Forty-eight patients of the ESAS Group received additional visits due to an ESAS score > 3. The mean number of medical visits was 4.38 ± 0.51 in the ESAS Group and 16.18 ± 1.82 in the no-ESAS group (P < 0.001). With multivariate analysis, women of the ESAS group were more likely to undergo additional visits for an ESAS score > 3 if they were aged 60 or older, received a mastectomy, or had tumour stage II/III.
The ESAS score may safely reduce the number of medical visits in candidates for adjuvant chemotherapy for early breast cancer. Our results suggest that the ESAS score may be used for selecting a group of breast cancer patients for whom it is safe to reduce the number of medical visits in the setting of adjuvant chemotherapy. This may translate into several advantages, such as a more rational utilization of human resources and a possible reduction of coronavirus pandemic infection risk in oncologic patients.
Core Tip: Adjuvant chemotherapy is recommended in high-risk breast cancer. We hypothesized that the Edmonton Symptom Assessment Scale (ESAS) can be used to safely reduce the number of medical visits in women with breast cancer undergoing adjuvant chemotherapy. The main result of this case-matched analysis is that ESAS screening may safely reduce the frequency of medical visits in the setting of AC in patients with breast cancer. This finding may have some advantageous implications in oncological practice, especially in the current scenario, where an increase in coronavirus pandemic cases throughout the world has imposed measures for minimising the risk of infection among patients and health care providers.
- Citation: Sanna V, Fedele P, Deiana G, Alicicco MG, Ninniri C, Santoro AN, Pazzola A, Fancellu A. Edmonton Symptom Assessment Scale may reduce medical visits in patients undergoing chemotherapy for breast cancer. World J Clin Oncol 2022; 13(7): 577-586
- URL: https://www.wjgnet.com/2218-4333/full/v13/i7/577.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i7.577
The multidisciplinary treatment of breast cancer has permitted achieving high survival rates over the last 20 years[1-4]. According to current accepted worldwide guidelines, many patients with breast cancer receive recommendation for adjuvant chemotherapy (AC), which continues to be a cornerstone of treatment for high-risk patients. In fact, AC has been linked to a reduced risk of developing locoregional and systemic recurrences, as well as to increased overall survival in some subgroups of patients who have undergone surgery for breast cancer[5-7]. However, it is known that toxicity of chemotherapy regimens can expose patients to adverse effects, unplanned medical visits, or hospitalisation[4,7,8].
There are no globally standardised guidelines that regulate the pre-treatment assessment of candidates for AC. While it is established that administration of chemotherapy drugs should be done by oncology nurses under the supervision of a medical oncologist, some aspects of the treatment vary according to each institution’s policy. In common practice, prior to every session of chemotherapy patients are evaluated during a medical visit. A pre-chemotherapy medical visit before every cycle of AC represents a time- and resource-demanding practice, especially in high-volume centres. The Edmonton Symptom Assessment Scale (ESAS) is a useful and simple tool for evaluating patients undergoing therapy for cancer. The ESAS consists of a questionnaire developed to rate the intensity of nine common symptoms experienced by patients with cancer[9-11]. We hypothesised that the ESAS can be used to safely reduce the number of medical visits in women undergoing AC for breast cancer. Therefore, we conducted a prospective matched-pair analysis to evaluate the impact of the ESAS in this subgroup of patients.
Patients receiving treatment for breast cancer were prospectively registered in an institutional board-registered database at the Breast Unit of the University Hospital of Sassari (Italy). According to the institutional policy, all patient cases were presented in a weekly multidisciplinary meeting, in which preoperative and postoperative management was discussed. After metastatic work up, each patient received neoadjuvant chemotherapy or upfront surgery (mastectomy or breast-conserving surgery [BCS] and sentinel node biopsy with or without axillary lymphadenectomy, according to the status of the sentinel node). Radiotherapy was given after BCS and in selected high-risk patients after mastectomy, in accordance with current guidelines. Adjuvant endocrine therapy was administered for 5 years to all women with oestrogen receptor-positive breast cancer after the completion of chemotherapy. Trastuzumab was recommended for women with HER2-positive tumours (immunohistochemistry 3+) for a total duration of 1 year. For the purpose of this study, we asked our database for patients who had undergone AC for Stages I-III breast cancer from January 2018 to November 2021. To be eligible for the present study, patients had to fulfil the following criteria: female gender, age ranging from 18 to 75 years old, diagnosis of unilateral or bilateral operable primary breast carcinoma without distant metastases, and sequential chemotherapy comprising epirubicin and cyclophosphamide followed by taxane. Exclusion criteria were neoadjuvant chemotherapy, metastatic disease, recurrent breast cancer, pregnancy, or lactation.
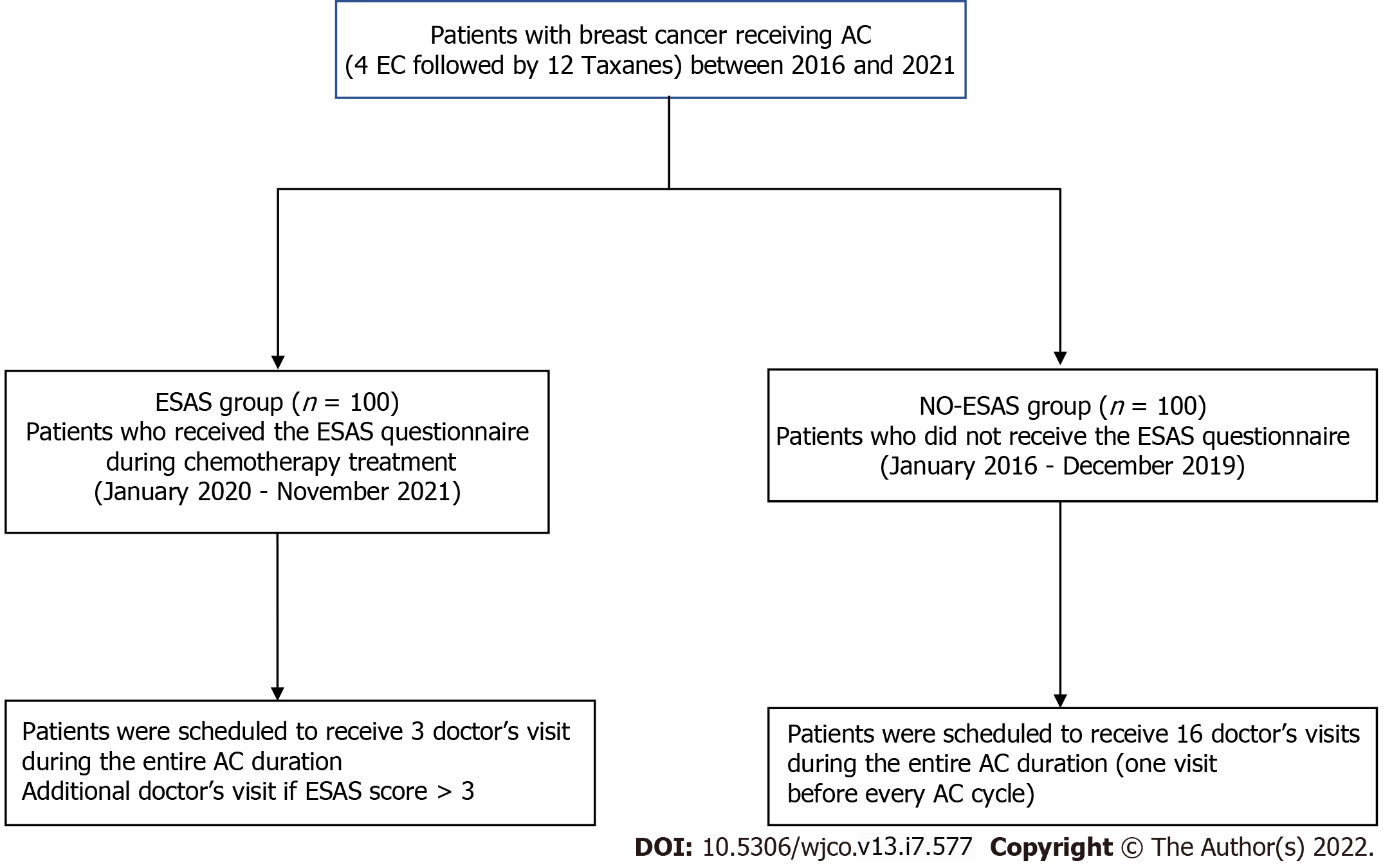
The study was approved by the Institutional Board of the AOU of Sassari. From January 2020, patients scheduled for AC were offered to participate in a programme where the ESAS was provided during the chemotherapy treatment period. All patients signed a written consent form before entering the ESAS programme. In a case-matched analysis, data from 100 patients taking the ESAS (the ESAS Group) in the period January 2020 to November 2021 were compared with data of 100 patients who underwent AC according to the traditional modality, without the ESAS (the no-ESAS Group) during the previous period (January 2016-December 2019). All patients of the study were scheduled to receive the following sequential regimen: Four cycles of epirubicin and cyclophosphamide followed by 12 cycles of paclitaxel (4EC-12T). Patients of the ESAS Group received the ESAS questionnaire translated into Italian before every cycle of AC; a medical visit was scheduled before the first cycle of epirubicin and cyclophosphamide, before the first cycle of taxane, and before the last cycle of taxane[12,13]. Therefore, each patient of the ESAS Group was scheduled to receive a total of three medical visits for the entire AC duration; an additional medical visit before each chemotherapy session was carried out according to the ESAS score (specifically in the all cases where the ESAS score was > 3). Patients of the no-ESAS Group received a medical visit before every cycle of AC. Therefore, each patient of the no-ESAS Group was scheduled to receive a total of 16 medical visits for the entire AC duration (Figure 1).
The matching variables included age and breast cancer stage. We decided to perform a case-matched analysis to obtain a more homogenous control group, and to minimise differences between groups due to the extent of disease. For all patients, the following data were extracted: Age, year of diagnosis, menopausal status, tumour size, histological type, axillary lymph nodes status, immunohistochemical classification, type of upfront surgery, and breast cancer stage. In the ESAS Group, patients who needed additional medical visits based on the ESAS score > 3, were identified. In both study groups (ESAS and no-ESAS) percentage of patients requiring unplanned medical visits (defined as visits for problems related to the surgical procedure or chemotherapy-related side effects), the number of unplanned medical visits, and grade 3-4 adverse effects during chemotherapy treatment, were calculated.
The primary endpoint was the total number of medical visits per patient during the entire duration of AC. The secondary endpoints were the occurrence of severe complications (grade 3-4) during the administration of AC and the number of unplanned visits during the cycles of chemotherapy. In addition, independent factors associated with the likelihood of receiving additional visits due to an ESAS score > 3 were analysed. Quantitative variables are presented as a mean; qualitative variables are presented as absolute numbers and percentages. Categorical variables were compared by the chi-square test or Fisher’s exact test where appropriate. Continuous variables were assessed by Student’s t-test or the Mann-Whitney U test. A P value < 0.05 was used as the threshold for statistical significance. In the ESAS Group, the likelihood of receiving additional visits on the basis of an ESAS score > 3 was analysed with a multivariable logistic regression model. Each factor was dichotomised to a binary variable: Age (≤ 60 years vs > 60 years), type of surgery (BCS vs mastectomy), immunohistochemical classification (luminal vs non-luminal), and tumour stage (stage I vs stage II/III). Covariates were chosen on the basis of clinical significance. For each dichotomous variable, a reference category was chosen, generally the majority category, and compared with the other category. The odds ratio (OR) in each category vs the reference category was estimated. The goodness of fit of the model was assessed by the Hosmer-Lemeshow test, and P > 0.05 indicated a good fit. Statistical analyses were conducted by using SPSS Statistics 20 (IBM Corp., Armond, NY, United States).
Demographic and tumour characteristics are presented in Table 1. The mean age at diagnosis was 57.2 years. Tumour size was ≤ 2 cm in 48% of patients and > 2 cm in 52%. The most common histology was invasive ductal carcinoma (86%), followed by lobular invasive carcinoma (14%). Thirty-five per cent of patients were premenopausal. The majority of patients had tumours of stage II/III (60%). Fifty-three per cent of patients underwent BCS, while 47% underwent a mastectomy. Axillary lymph node status was positive in 37% of cases and negative in 63%. Regarding the immunohistochemical classification, the most frequent subtype was HER2-enriched (54%), followed by luminal B (23%), triple-negative (13%), and luminal A (10%) tumours. The study variables did not differ significantly between patients of the ESAS Group and the no-ESAS Group (mean age P = 0.524; age ≤ 60 years P = 0.880; breast cancer stage P = 0.56; cancer histology P = 0.415; tumour size P = 0.258; axillary lymph node status P = 0.883; immunohistochemical classification P = 0.754; type of surgery P = 0.157), except for premenopausal status, which was more frequent in the ESAS Group (P = 0.015). There were there 8 additional unplanned visits for 6 patients in the ESAS Group, and 18 additional visits for 12 patients in the no-ESAS Group (P = 0.035) Six patients of the ESAS Group and 12 of the no-ESAS Group needed one or more unplanned visit during the AC duration, for a total of 8 and 18 visits, respectively (P = 0.057). Grade 3-4 toxicity occurred in two and three patients of the ESAS Group and the no-ESAS Group, respectively (P = 0.652). Forty-eight patients of the ESAS Group received an additional visit due to an ESAS score > 3. Globally, the mean number of medical visits was 4.38 ± 0.51 in the ESAS Group and 16.18 ± 1.82 in the no-ESAS Group (P < 0.001) (Table 2).
| Characteristic | Group A (ESAS) (n = 100) | Group B (No-ESAS) (n = 100) | P value |
| Age (mean ± SD) | 57.7 ± 11.5 | 56.6 ± 12.4 | 0.524 |
| Age groups, n (%) | 0.880 | ||
| ≤ 60 yr | 64 (64) | 62 (62) | |
| > 60 yr | 36 (36) | 38 (38) | |
| Premenopausal status | 51 (51) | 34 (34) | 0.015 |
| Breast cancer stage, n (%) | |||
| I | 44 (44) | 36 (36) | 0.506 |
| II | 28 (28) | 31 (31) | |
| III | 28 (28) | 33 (33) | |
| Cancer histology, n (%) | |||
| Ductal | 84 (84) | 88 (88) | 0.415 |
| Lobular | 16 (16) | 12 (12) | |
| Tumour size (mean ± SD) , n (%) | 0.258 | ||
| ≤ 2 cm | 52 (52) | 44 (44) | |
| > 2 cm | 48 (48) | 56 (56) | |
| Lymph node status, n (%) | 0.883 | ||
| N0 | 66 (66) | 59 (59) | |
| N+ | 34 (34) | 41 (41) | |
| Himmunohistochemical classification, n (%) | 0.754 | ||
| Luminal A | 12 (12) | 8 (8) | |
| Luminal B | 21 (21) | 25 (25) | |
| HER2 positive | 55 (55) | 54 (54) | |
| TNBC | 12(12) | 13 (13) | |
| Type of surgery, n (%) | 0.157 | ||
| BCS | 58 (58) | 48 (48) | |
| Mastectomy | 42 (42) | 52 (52) |
| Variable | Group A (ESAS) | Group B (No ESAS) |
| N of doctor visit scheduled for each patient | 3 | 16 |
| Total No. of scheduled doctor visits | 300 | 1600 |
| N of patients requiring adjunctive visit on the bases of ESAS score > 3 | 48 | - |
| N of adjunctive visits on the bases of ESAS score > 3 | 130 | - |
| N of patients requiring unplanned doctor visits | 6 | 12 |
| N of unplanned doctor visit | 8 | 18 |
| Effective total No. of doctor visitsa | 438 | 1618 |
| N of visits for each patient (mean ± SD)b | 4.38 ± 0.51 | 16.18 ± 1.82 |
| Adverse effects during chemotherapy treatment | 2 | 3 |
Based on multivariate analysis, women of the ESAS Group were more likely to undergo additional visits before chemotherapy for an ESAS score > 3 if they were aged > 60 years, received a mastectomy, or had tumour stage II/III (Table 3). We did not find any association between additional visits and immunohistochemical tumour classification or lymph node status. Age > 60 years was the strongest predictor of receiving additional medical visits before chemotherapy (OR 4.93, 95% confidence interval 1.26-19.25).
| Variable | Odds ratio | St. Error | Z-score | 95%CI | P value |
| Age | |||||
| > 60 (n = 36) | 4.93 | 0.695 | 1.596 | 1.26-19.25 | 0.022a |
| ≤ 60 (n = 64) | Ref. | ||||
| Lymph node status | |||||
| Positive (n = 44) | 0.50 | 0.662 | 0.691 | 0.13-1.83 | 0.297 |
| Negative (n = 66) | Ref. | ||||
| Type of surgery | |||||
| Mastectomy (n = 42) | 0.15 | 0.726 | -1.895 | 0.03-0.62 | 0.009b |
| BCS (n = 58) | Ref. | ||||
| IHC classification | |||||
| Luminal (n = 33) | 1.96 | 0.699 | 0.674 | 0.49-7.73 | 0.335 |
| Non-Luminal (n = 67) | Ref. | ||||
| Tumour stage | |||||
| I (n = 44) | 0.86 | 0.880 | 0.149 | 1.12-35.44 | 0.036c |
| II/III (n = 56) | Ref. |
Various chemotherapy regimens, which can be associated with either minor or major toxicity, are commonly used for AC in patients undergoing surgery for breast cancer[4,14]. However, no recognised guidelines exist regarding some aspects of this important part of the multidisciplinary treatment. The main result of this case-matched analysis is that ESAS screening may safely reduce the frequency of medical visits in the setting of AC in patients with breast cancer. This finding may have some advantageous implications in oncological practice, especially in the current scenario, where an increase in coronavirus pandemic 2019 (COVID-19) cases throughout the world has imposed measures for minimising the risk of infection among patients and health care providers.
Pre-chemotherapy assessment varies among oncology services. On a general basis, during the medical visit before chemotherapy, relevant information to manage any possible treatment side effect are collected, and a physical examination might be carried out. In the present study, we have used the ESAS score as a patient-reported outcomes tool. The ESAS is one of the first multidimensional assessment tools that has been used in clinical practice. The scale was created for the clinical assessment of the increase and modification of symptoms in patients with advanced cancers admitted to palliative care units[11,15,16]. The ESAS score has subsequently been validated in various studies and used as a tool for the detection of symptoms divided by clusters, favouring the implementation of interventions for symptom management[17]. In patients with breast cancer, correct symptom assessment and management still represent a challenge for medical oncologists[18]. Specifically, in the early setting of the disease, the correct assessment and management of symptoms is essential to improve quality of life and patient adherence to treatments and, therefore, the effectiveness of adjuvant therapies.
Several studies have explored the role of the ESAS to predict patient-related outcomes in patients with breast cancer, especially in the setting of advanced disease[19]. In a recent review including nine articles, the authors reported that the ESAS score is a promising tool for predictive modelling of time to death in patients with breast cancer receiving palliative care[19]. However, few studies have investigated the role of the ESAS in the setting of breast cancer. In patients with non-metastatic breast cancer who received radiotherapy, the ESAS score has been used to identify significant symptoms linked to a worse overall quality of life[20].
In the series described herein, we found that the patients who completed the ESAS questionnaire received significantly fewer medical visits during chemotherapy period compared with patients of the control group. In the series described herein, we found that the use of the ESAS questionnaire allowed to identify patients who required additional medical visits before a chemotherapy cycle. To note, the reduction in the number of scheduled visits based on the ESAS score, did not affect the occurrence of complications from chemotherapy, and was associated to a reduced number of unplanned medical visits. In fact, patients of the ESAS Group were scheduled to receive only three visits; additional visits were deemed necessary only when the ESAS score was > 3. These findings are consistent with the experience of Barbera et al[14], who demonstrated that screening with the ESAS was associated with decreased emergency department visits by patients with breast cancer receiving AC. It has been suggested that screening of routine symptoms, using tailored patient-reported outcomes tools, could be useful for improving patient/physician communication, helping to monitor the treatment response and identifying unrecognised problems[20-23].
In this study, we hypothesised that the ESAS score in the setting of AC would be able to safely reduce the number of medical visits. We used the occurrence of grade 3-4 chemotherapy toxicity as a surrogate of safety; this measure did not differ between the two study groups. The need for medical visits in patients undergoing AC for breast cancer depends on many tumour- and patient-related factors[4,14]. In our experience, patients aged > 60 years had a fourfold increased risk of receiving additional visits based on the ESAS score, reflecting the importance of patient age regarding anticancer treatments. Of note, the number of unplanned medical visits due to acute toxicity experienced by patients was lower in the ESAS Group. In another study involving a cohort of 2541 patients with stage I-III breast cancer, women undergoing chemotherapy for breast cancer screened with the ESAS had a 43% lower rate of emergency department visits than those who were not screened with the ESAS[14].
Medical visits for pre-chemotherapy assessment represent a significant burden on the oncological care system. There are several potential advantages of reducing the number of medical visits in patients receiving AC. First, although we did not calculate the time spent on every visit, we can assume that the reduced number of medical visits does translate to a significant sparing of time in oncology departments; hence, oncologists and nurses may spend their time on other clinical activities. This may have important implications especially in high-volume oncology centres. Second, the ESAS score permits patients to take an active role in deciding the course of their AC treatment. Generally, patient-reported outcomes have been gaining importance for describing subjective symptoms and improving quality of life[4,23,24]. Studies have compared the description of toxicity and adverse effects by using patient-related-outcome tools in comparison with physician-reported findings. A possible underestimation of the incidence and the entity of symptoms reported by physicians has been evidenced[25,26]. Baratelli et al demonstrated, in a cohort of 211 patients receiving active anticancer treatment, that these tools produced high patient satisfaction and a significant quality-of-life improvement, compared with the traditional modality of a medical visit[23]. Third, in the current scenario, where contact restrictions are encouraged, use of the ESAS questionnaire may reduce the risk of COVID-19 infections among oncologic patients. In fact, the decrease in medical visits could reduce both personal contacts and the duration of stay in oncology units among patients with chemotherapy-induced immunosuppression. At the time of writing, the world is experiencing a new wave of the pandemic due to the delta and omicron variants of severe acute respiratory syndrome coronavirus 2.
Several studies have investigated the role of the ESAS score on quality-of-life perception, supportive care needs and symptom assessment in patients with cancer; however, to the best of our knowledge, this is the first study focussing on its impact on medical visits in the setting of AC. We recognise that this work has some limitations, the main one being the small sample size. Furthermore, we arbitrarily decided to set the ESAS score cut-off point for patients to receive additional medical visits for AC administration as 3. Regarding this matter, the optimal cut-off points for the symptoms and quality indicators of the ESAS remain ill defined[27,28].
In summary, our work provides evidence that the use of the ESAS score may safely reduce the number of medical visits in patients undergoing AC. Moreover, it implies that ESAS may help to identify patients who do not need to visit a doctor during each course of chemotherapy, as well as to identify a group of patients with a high risk of complications in whom a treatment adjustment is needed. This may result in several advantages for both patients and health care providers, especially in the current COVID-19 pandemic. Additional studies are needed to gain new insights into the role of patient-reported outcome strategies in the management of AC in the setting of breast cancer.
Adjuvant chemotherapy (AC) represents a fundamental part of multidisciplinary treatment of women with high-risk breast cancer, since it has been associated to a reduced risk of developing cancer recurrence, as well as to an increased survival. However, no standardised guidelines that regulate the pre-treatment assessment of patients candidates for AC exist. In common practice, a pre-chemotherapy medical visit before every cycle of AC is scheduled, and this represents a time- and resource-demanding practice.
Accurate use of the Edmonton Symptom Assessment Scale (ESAS) may lead to identify patients who do not need to visit a doctor during each course of AC.
To evaluate the value of the ESAS in safely reduce the number of medical visits prior adjuvant chemotherapy.
One-hundred breast cancer women candidates to AC were administered the ESAS score (ESAS Group), and were scheduled to receive a total of three medical visits for the entire AC duration. They were prospectively compared to a to a matched-pair group of 100 patients who received adjuvant chemotherapy without ESAS (no-ESAS Group) and were scheduled to receive 16 medical visits for the entire AC duration. Study endpoints were the number of medical visits, occurrence of severe complications, and the number of unplanned visits.
The mean number of medical visits was 4.38 ± 0.51 in the ESAS Group and 16.18 ± 1.82 in the no-ESAS group (P < 0.001). Unplanned visits during the entire duration of chemotherapy were 8 in the ESAS Group and 18 in the no-ESAS Group visits (P = 0.035). Grade 3-4 toxicity did not differ between the study groups (P = 0.652). Forty-eight patients of the ESAS Group received additional visits due to an ESAS score > 3. With multivariate analysis, women of the ESAS group were more likely to undergo additional visits for an ESAS score > 3 if they were aged 60 or older, received a mastectomy, or had tumour stage II/III.
Our results suggest that the ESAS score may be used for selecting a group of breast cancer patients for whom it is safe to reduce the number of medical visits in the setting of AC. This may permit a more rational utilization of human resources and a possible reduction of coronavirus pandemic 2019 infection risk in oncologic patients.
Additional studies are needed to gain new insights into the role of patient-reported outcome strategies in the management of AC in the setting of breast cancer.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kapritsou M, Greece; Peng XC, China; Senchukova M, Russia; Xu X, China S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Saini KS, Taylor C, Ramirez AJ, Palmieri C, Gunnarsson U, Schmoll HJ, Dolci SM, Ghenne C, Metzger-Filho O, Skrzypski M, Paesmans M, Ameye L, Piccart-Gebhart MJ, de Azambuja E. Role of the multidisciplinary team in breast cancer management: results from a large international survey involving 39 countries. Ann Oncol. 2012;23:853-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Shao J, Rodrigues M, Corter AL, Baxter NN. Multidisciplinary care of breast cancer patients: a scoping review of multidisciplinary styles, processes, and outcomes. Curr Oncol. 2019;26:e385-e397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Fancellu A, Sanna V, Sedda ML, Delrio D, Cottu P, Spanu A, Giuliani G, Conti M, Piras R, Crivelli P, Porcu A. Benefits of Organized Mammographic Screening Programs in Women Aged 50 to 69 years: A Surgical Perspective. Clin Breast Cancer. 2019;19:e637-e642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Enright K, Grunfeld E, Yun L, Moineddin R, Ghannam M, Dent S, Eisen A, Trudeau M, Kaizer L, Earle C, Krzyzanowska MK. Population-based assessment of emergency room visits and hospitalizations among women receiving adjuvant chemotherapy for early breast cancer. J Oncol Pract. 2015;11:126-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Wang Y, Yin W, Lin Y, Zhou L, Du Y, Yin K, Lu J. Early breast cancer patients benefit more from longer course chemotherapy: a matched-pair analysis. Future Oncol. 2019;15:1781-1789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Trudeau M, Charbonneau F, Gelmon K, Laing K, Latreille J, Mackey J, McLeod D, Pritchard K, Provencher L, Verma S. Selection of adjuvant chemotherapy for treatment of node-positive breast cancer. Lancet Oncol. 2005;6:886-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Bastedo SJ, Krzyzanowska MK, Moineddin R, Yun L, Enright KA, Grunfeld E. A population-based assessment of primary care visits during adjuvant chemotherapy for breast cancer. Curr Oncol. 2017;24:90-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Hassett MJ, O'Malley AJ, Pakes JR, Newhouse JP, Earle CC. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98:1108-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 9. | Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6-9. [PubMed] [Cited in This Article: ] |
| 10. | Diplock BD, McGarragle KMC, Mueller WA, Haddad S, Ehrlich R, Yoon DA, Cao X, Al-Allaq Y, Karanicolas P, Fitch MI, Myers J, Mitchell AJ, Ellis JWM. The impact of automated screening with Edmonton Symptom Assessment System (ESAS) on health-related quality of life, supportive care needs, and patient satisfaction with care in 268 ambulatory cancer patients. Support Care Cancer. 2019;27:209-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Hui D, Bruera E. The Edmonton Symptom Assessment System 25 Years Later: Past, Present, and Future Developments. J Pain Symptom Manage. 2017;53:630-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 406] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 12. | Moro C, Brunelli C, Miccinesi G, Fallai M, Morino P, Piazza M, Labianca R, Ripamonti C. Edmonton Symptom Assessment Scale: Italian validation in two palliative care settings. Support Care Cancer. 2006;14:30-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Edmonton Symptom Assessment System (ESAS). [cited 20 April 2022]. Available from: https://www.eoc.ch/dms/site-eoc/documenti/pallclick/strumenti/ESAS20Spiegazione20e20Esempi202D20i2Dcurpal2D004.pdf. [Cited in This Article: ] |
| 14. | Barbera L, Sutradhar R, Howell D, Sussman J, Seow H, Dudgeon D, Atzema C, Earle C, Husain A, Liu Y, Krzyzanowska MK. Does routine symptom screening with ESAS decrease ED visits in breast cancer patients undergoing adjuvant chemotherapy? Support Care Cancer. 2015;23:3025-3032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Chasen M, Bhargava R, Dalzell C, Pereira JL. Attitudes of oncologists towards palliative care and the Edmonton Symptom Assessment System (ESAS) at an Ontario cancer center in Canada. Support Care Cancer. 2015;23:769-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Cummings G, Biondo PD, Campbell D, Stiles C, Fainsinger R, Muise M, Hagen N. Can the global uptake of palliative care innovations be improved? Palliat Med. 2011;25:71-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Pirl WF, Fann JR, Greer JA, Braun I, Deshields T, Fulcher C, Harvey E, Holland J, Kennedy V, Lazenby M, Wagner L, Underhill M, Walker DK, Zabora J, Zebrack B, Bardwell WA. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120:2946-2954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 18. | Laugsand EA, Sprangers MA, Bjordal K, Skorpen F, Kaasa S, Klepstad P. Health care providers underestimate symptom intensities of cancer patients: a multicenter European study. Health Qual Life Outcomes. 2010;8:104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 19. | Milton L, Behroozian T, Coburn N, Trudeau M, Razvi Y, McKenzie E, Karam I, Lam H, Chow E. Prediction of breast cancer-related outcomes with the Edmonton Symptom Assessment Scale: A literature review. Support Care Cancer. 2021;29:595-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Chow S, Wan BA, Pidduck W, Zhang L, DeAngelis C, Chan S, Yee C, Drost L, Leung E, Sousa P, Lewis D, Lam H, Chow R, Lock M, Chow E. Symptoms Predictive of Overall Quality of Life Using the Edmonton Symptom Assessment Scale in Breast Cancer Patients Receiving Radiotherapy. Clin Breast Cancer. 2019;19:405-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Montgomery N, Howell D, Ismail Z, Bartlett SJ, Brundage M, Bryant-Lukosius D, Krzyzanowska M, Moody L, Snyder C, Barbera L; Cancer Care Ontario Patient Reported Outcome Advisory Committee. Selecting, implementing and evaluating patient-reported outcome measures for routine clinical use in cancer: the Cancer Care Ontario approach. J Patient Rep Outcomes. 2020;4:101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013;13:211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 448] [Cited by in F6Publishing: 503] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 23. | Baratelli C, Turco CGC, Lacidogna G, Sperti E, Vignani F, Marino D, Zichi C, De Luca E, Audisio M, Ballaminut D, Bellezza A, Chiotto P, Ciriolo G, Comite R, Codegone F, Florio S, Fusco L, Polimeno L, Pozzi D, Zilio E, Terzolo S, Di Maio M. The role of patient-reported outcomes in outpatients receiving active anti-cancer treatment: impact on patients' quality of life. Support Care Cancer. 2019;27:4697-4704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, Schrag D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA. 2017;318:197-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1430] [Cited by in F6Publishing: 1306] [Article Influence: 186.6] [Reference Citation Analysis (0)] |
| 25. | Fromme EK, Eilers KM, Mori M, Hsieh YC, Beer TM. How accurate is clinician reporting of chemotherapy adverse effects? J Clin Oncol. 2004;22:3485-3490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 417] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 26. | Di Maio M, Gallo C, Leighl NB, Piccirillo MC, Daniele G, Nuzzo F, Gridelli C, Gebbia V, Ciardiello F, De Placido S, Ceribelli A, Favaretto AG, de Matteis A, Feld R, Butts C, Bryce J, Signoriello S, Morabito A, Rocco G, Perrone F. Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J Clin Oncol. 2015;33:910-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 308] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 27. | Yamaguchi T, Morita T, Nitto A, Takahashi N, Miyamoto S, Nishie H, Matsuoka J, Sakurai H, Ishihara T, Tarumi Y, Ogawa A. Establishing Cutoff Points for Defining Symptom Severity Using the Edmonton Symptom Assessment System-Revised Japanese Version. J Pain Symptom Manage. 2016;51:292-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Oldenmenger WH, de Raaf PJ, de Klerk C, van der Rijt CC. Cut points on 0-10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: a systematic review. J Pain Symptom Manage. 2013;45:1083-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |