Published online Jun 24, 2022. doi: 10.5306/wjco.v13.i6.429
Peer-review started: April 18, 2021
First decision: July 16, 2021
Revised: September 5, 2021
Accepted: May 22, 2022
Article in press: May 22, 2022
Published online: June 24, 2022
The treatment of small cell lung cancer (SCLC) is a challenge for all specialists involved. New treatments have been added to the therapeutic armamentarium in recent months, but efforts must continue to improve both survival and quality of life. Advances in surgery and radiotherapy have resulted in prolonged survival times and fewer complications, while more careful patient selection has led to increased staging accuracy. Developments in the field of systemic therapy have resulted in changes to clinical guidelines and the management of patients with advanced disease, mainly with the introduction of immunotherapy. In this article, we describe recent improvements in the management of patients with SCLC, review current treatments, and discuss future lines of research.
Core Tip: The treatment of small cell lung cancer (SCLC) continues to be a challenge. Recent studies have described survival benefits achieved by new treatments or combinations of treatments that are both safe and effective. Immunotherapy has a new role in SCLC. Nevertheless, continued research efforts are needed. Here, we review the current management of SCLC and discuss recent improvements and future lines of research.
- Citation: Pangua C, Rogado J, Serrano-Montero G, Belda-Sanchís J, Álvarez Rodríguez B, Torrado L, Rodríguez De Dios N, Mielgo-Rubio X, Trujillo JC, Couñago F. New perspectives in the management of small cell lung cancer. World J Clin Oncol 2022; 13(6): 429-447
- URL: https://www.wjgnet.com/2218-4333/full/v13/i6/429.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i6.429
Small cell lung cancer (SCLC) accounts for 14% of all lung cancers[1,2], and most cases are associated with tobacco use[3]. Although the global incidence of SCLC is falling, the ratio of male to female cases is currently 1:1[1,2]. SCLC is a fast-growing cancer, and most patients have extensive disease when diagnosed. In approximately one-third of cases, the cancer is limited to the thorax and can be treated with concurrent chemotherapy and radiotherapy. Just a small percentage of patients are amenable to surgery and adjuvant therapy. The goal of treatment in patients with extensive disease is to alleviate symptoms and prolong survival, although long-term survivorship in this setting is rare[4].
Early-stage SCLC, stage I and IIA (T1-2N0M0) SCLC in the American Joint Committee on Cancer/International Union Against Cancer classification[5-7], accounts for 7% of all SCLCs and 0.29% of all lung cancers[8]. Numerous studies have shown excellent survival rates in patients with SCLC cT1-2N0M0 treated with surgery as part of a multimodal approach[6,9-28] (Table 1).
| Ref. | Study type & time period. LoE | Inclusion criteria | Number of patients | Neoadjuvant/adjuvant treatments | PCI | Survival data |
| Jin et al[9], 2018 | RS; SEER 2004-2013; 3A | cI-II | n = 2129; S: 387; RT 1032; S + RT: 154; No S or RT: 556 | - | 5-yr OS T1N0: 46.0% S vs 23.8% RT; 5-yr OS T2N0: 42.6% S vs 24.7% RT; T3N0 or T1-2N1 (stage IIB) patients treated with S did not have higher 5-yr OS rates than those treated with RT | |
| Yang et al[10], 2018 | RS; NCDB 2003-2011; Propensity score match S + AC vs CRT; 3A | cT1-2N0M0 | S + AC: 501; CRT: 501 | S + AC: 501 | - | 5-yr OS: 47.6% S + AC vs 29.8% CRT (P < 0.01) |
| Ahmed et al[11], 2017 | RS; SEER 2007-2013; 3A | Stage I SCLC | n = 1902; S: 427; S + RT: 115 | - | - | MST: 50 mo (S); MST: 60 + mo (S + RT) |
| Wakeam et al[12], 2017 | RS; NCDB 2004-2013; 3A | cT1-2N0M0 | n = 5079 | - | MST: 25.3 mo | |
| Wakeam et al[13], 2017 | RS; NCDB 2004-2013; Stage-specific propensity score match S vs NST; 3A | cI-III | n = 2619 | No AD treatment 24% NC or NR 4%; AC 27%; AR 1%; ACR 32%; NC or NR and AC or AR 2%; Other 10% | - | MST cI 38.6 vs 22.9 mo S vs NST; MST cII 23.4 vs 20.7 mo S vs NST; MST cIIIA 21.7 vs 16.0 mo S vs NST |
| Combs et al[14], 2015 | RS; NCDB 1998-2011; 3A | cT1-3N0-2 SCLC | n = 2476; S 841 cIA, 168 cIB | All; S: 68% | - | 5-yr OS: 54% (cIA); 36% (cIB) |
| Ogawa et al[15], 2012 | RS; Institutional 1995-2008; 4 | cI-III; pI-III SCLC | n = 28 (23 SCLC before S); S 21 cI, 5 cII, 7 cIII2 | NC 8; AC 19, ACR 2 | - | 5-yr OS 47% |
| Ju et al[16], 2012 | RS; Institutional 1990-2009; 4 | pI-III | n = 34 | NC 3; AC 1, AR 19, 10 CRT | - | 5-yr OS 66% |
| Vallières et al[6], 2009 | RS; IASLC 1990-2000; 3A | Resected SCLC | n = 349 (68 pIA, 91 pIB) | - | - | 5-yr OS: 53% (pIA); 44% (pIB) |
| Lim et al[17], 2008 | RS; Institutional 1980-2007; 4 | cI-cIIIB | n = 59 | AC 13; AR 2; ACR 1 | - | 5-yr OS for all patients 52%; No difference in 5-yr survival across; cT and cN categories; No difference in 5-yr survival across; cI to cIII stages |
| Wang et al[18], 2007 | RS; Institutional; 4 | pI-III | n = 122 | QT & CRT (not specified) | - | MST 50 mo; 5-yr OS 66% |
| Veronesi et al[19], 2007 | RS; Institutional; 4 | cI-IIIA | n = 23 | AC all | - | MST 24 mo |
| Tsuchiya et al[20], 2005 | Prospective phase II trial; 1991-1996; 2B | cI-IIIA | n = 62 | AC 42 (69%) | - | MST not reached in pI; MST 449 d for pII; MST 712 d for pIIIA; 3-yr OS 61%; 3-yr survival rate cI, cII, cIIIA 68%, 56% and 13% respectively |
| Brock et al[21], 2005 | RS; Institutional 1976-2002; 4 | Resected SCLC | n = 82 (24 stage I, S + AC) | AC 55% | 23% | 5-yr OS: 86% (platinum AC); 42% (non-platinum AC) |
| Nakamura et al[22], 2004 | RS; Institutional; 4 | cI-III SCLC | n = 69 | S 37, NC 32, AC 41, ACR 7 | - | 5-yr survival 48.9 % cI, 33.3 % cII, 20.2 % cIIIA, 0 % cIIIB |
| Badzio et al[23], 2004 | Comparative RS; Institutional 1984-1996; 4 | cI-III balanced in both, S and NST groups | n = 134 | S 67 (all AC); NST 67 (all QT) | 34% only S group | MST 22 mo (S); MST 11 mo (NST); 5-yr OS S 27%, NST 4% |
| Lewiński et al[24], 2001 | R; Institutional 1976-2002; 4 | cI-IIIA SCLC | n = 75 | NC all | If CR to NC | MST N0+1 25 mo; MST N2 14 mo; MST resected 18 mo; 5-yr OS resected 29% |
| Cataldo et al[25], 2000 | RS; Institutional 1982-1992; 4 | cI-III SCLC | n = 60 | AC 88%; pII AR (11%); pIII AR (21%) | 41% | 5-yr survival rate 40% pI, 36% pII and 15% pIII |
| Inoue et al[26], 2000 | RS; Institutional 1975-1994; 4 | Resected SCLC | n = 91 (32 cIA, 30 cIB) | All 78% | 5.5% | MST 53 mo, 5-yr OS 49% (cIA); MST 25 mo, 5-yr OS 47% (cIB) |
| Kobayashi et al[27], 2000 | RS; Institutional 1982-1992; 4 | cI-III SCLC | n = 59 | NC 71% | - | 5-yr survival rate 55% pI, 33% pII, 23% pIII |
| Eberhardt et al[28], 1999 | Prospective phase II trial; Institutional 1991-1995; 2B | cIB-cIIIB | n = 46 | IB/IIA had NC + S; IIB/IIIA had NCR + S | - | MST all patients 36 mo; MST R0 patients 68 mo; 5-yr survival rate all patients 46%; 5-yr survival rate R0 patients 63% |
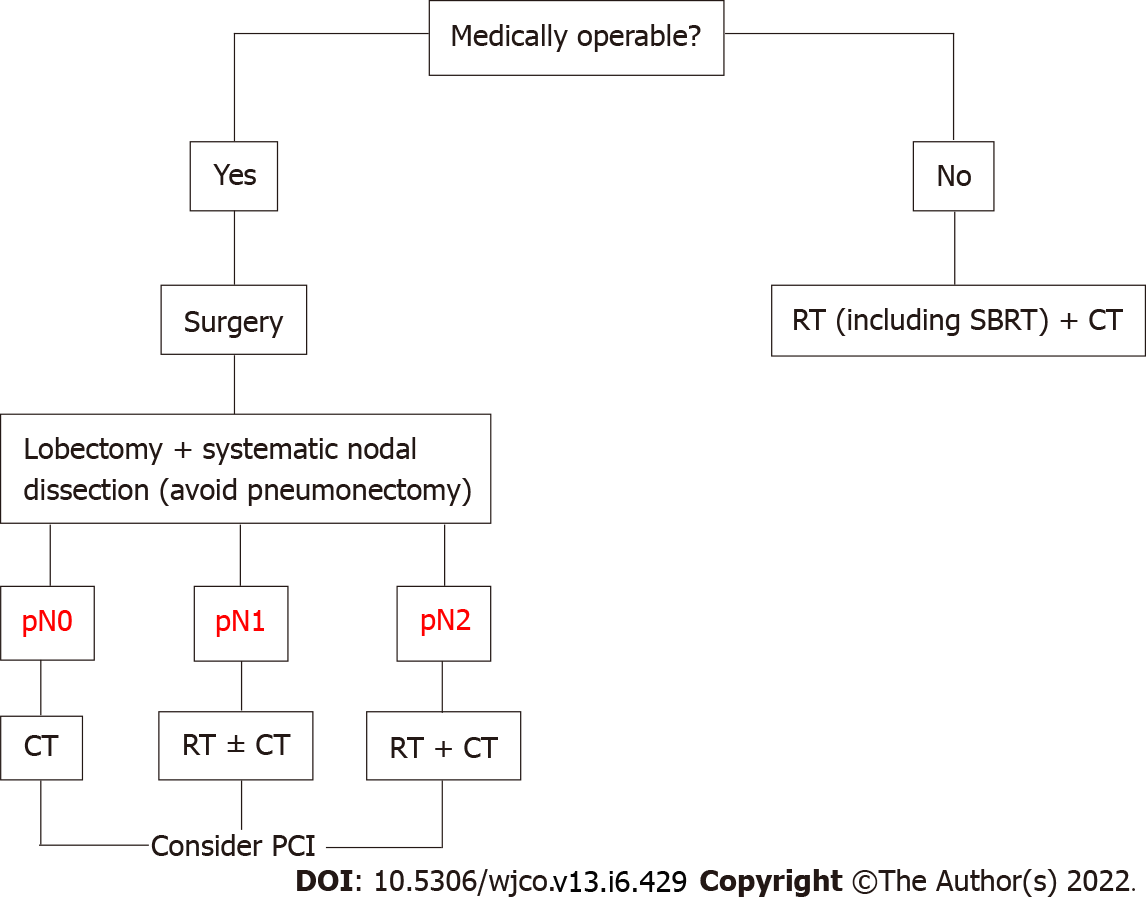
Surgical resection followed by adjuvant therapy is currently recommended by most clinical guidelines for operable stage I and IIA SCLC. Choice of adjuvant treatment varies according to pathologic tumor-node-metastasis stage: Chemotherapy for pN0, chemotherapy ± radiotherapy for pN1 and chemoradiotherapy for pN2[29-32] (Figure 1). The indications for the surgical treatment of SCLC can be summarized as follows: (1) Intraoperative diagnosis of a pulmonary SCLC nodule. Between 3% and 5% of SCLCs present as a pulmonary nodule. Multidisciplinary treatment involving surgical resection, systematic nodal dissection, and adjuvant chemotherapy or chemoradiotherapy can achieve survival rates comparable to those seen in non-SCLC[8]; (2) Diagnosis of stage I or IIA SCLC. Local or regional recurrence[33-39] (tumor and/or hilar-mediastinal lymph nodes) is the most common form of disease in patients who relapse after complete remission with chemoradiotherapy[40-45]. Surgery as part of a multimodal approach achieves better local disease control[46-50] than chemoradiotherapy[51-54]; (3) Mixed histology (SCLC with a non-SCLC component). Between 2% and 28% of patients have mixed SCLC/non-SCLC[55-59]. Recurrence or failure to respond to first-line chemotherapy is likely to be due to the non-SCLC component; and (4) Salvage surgery for local chemo-resistant SCLC or exclusively local recurrence after response to chemoradiotherapy. Selected patients in this setting might benefit from surgical resection[60-62].
Lobectomy is the preferred procedure for surgical resection, as it is associated with significantly better survival than sublobar resection[40,45,49,54,63]. The significant discrepancies observed between clinical and pathologic stages (mainly due to undetected lymph node metastasis before surgery) highlight the importance of accurate clinical nodal staging and systematic lymph node dissection[47,64]. The recommendations for ruling out hilar and mediastinal lymph node involvement are very similar across the different guidelines. Ideally, clinical staging should be performed using semi-invasive techniques that enable biopsy and the pathologic study of lymph nodes (e.g., transbronchial ultrasound and esophageal echoendoscopy) and invasive techniques such as video mediastinoscopy, anterior mediastinotomy, and videothoracoscopy.
Thoracic radiotherapy and stereotactic body radiotherapy in early-stage SCLC: SCLC is usually classified as limited-stage (LS) or extensive-stage (ES) disease[65]. With adequate treatment, overall survival (OS) is 16-22 mo in patients with LS-SCLC and 8-13 mo in those with ES-SCLC. The corresponding 5-year survival rates are < 20% and < 2%[66]. Radiotherapy is associated with better OS when given in the first few weeks after the start of chemotherapy (ideally during cycle 1 and never later than cycle 3), and the shorter the duration the better[67].
Hypofractionated radiotherapy is well tolerated and produces similar response rates to standard fractionation. Proposed schedules include 40 Gy in 16 fractions with chemotherapy and prophylactic cranial irradiation (PCI)[68] and 55 Gy in 25 once-daily fractions, also with chemotherapy and PCI[69]. Higher complete response rates and longer OS have been observed for hyperfractionated vs hypofractionated radiotherapy (45 Gy in 30 fractions twice daily vs 42 Gy in 15 fractions twice daily), but the differences were not statistically significant[70].
Treatment must be individualized. Some clinical guidelines recommend surgery and adjuvant chemotherapy for stage I and IIA disease[30,71]. This combination has achieved OS rates of 50%-70%[20,21,72,73]. Nonetheless, stereotactic body radiotherapy (SBRT) should be considered in patients who are unfit for or refuse surgery, as it is not inferior to conventional treatment and has an acceptable safety profile (toxicity < grade 3)[74-80]. Although the evidence is based on small series, SBRT can achieve local control rates > 85%. No clear benefit, however, has been observed for OS (63%-83% at 1 year, 35%-76% at 2 years, and 21%-26% at 3 years) (Table 2). This could have several explanations. First, SCLC is a fast-spreading tumor (associated with distant metastases in 50% of cases), requiring clinicians to consider neoadjuvant or adjuvant chemotherapy (preferably adjuvant in the case of SBRT due to its short treatment time), particularly in the case of tumors > 2 cm[77-81]. Adjuvant chemotherapy can improve OS by up to 25%[82]. Second, the disease may have been initially understaged. Thus, staging with positron emission tomography-computed tomography (CT) and mediastinoscopy/endobronchial ultrasound is recommended before proposing surgery or SBRT. SBRT should be planned using intensity-modulated techniques (e.g., intensity-modulated radiotherapy, volumetric modulated arc therapy) and delivered with image-guided inter- and/or intrafraction monitoring (e.g., Conebeam, ExacTrac) and respiratory control (e.g., four-dimensional CT, deep inspiration breath hold, active breathing control, gating). The number of fractions can vary, but a biologically effective dose of >100 Gy must be delivered to the isocenter of the tumor. Because SCLC is highly radiosensitive, some groups have suggested using a lower dose, particularly in patients with ultracentral tumors[83].
| Ref. | Sample size | Fractionation | QT | Prophylactic cranial irradiation | Local control | Overall survival | Disease-free survival |
| Videtic et al[76], 2013 | n = 6 | 60 Gy (3 fx); 50 Gy (5 fx); 30 Gy (1 fx) | 4/6 | 4/6 | 100% (1 yr) | 63% (1 yr) | 75% (1 yr) |
| Shioyama et al[77], 2015 | n = 64 | 48 Gy (4 fx) | 36/64 | 10/64 | 89% (2 yr) | 76% (2 yr) | |
| Stahl et al[79], 2017 | n = 285 | 48-60 Gy (3-5 fx) | 130/285 | 35% (3 yr). 21.5% (5 yr) | |||
| Verma et al[75], 2017 | n = 74 | 50 Gy (5 fx) | 45/74 | 17/74 | 96% (3 yr) | ||
| Shioyama et al[78], 2018 | n = 43 | 36-60 Gy (3-10 fx) | 8/43 | 8/43 | 80.2% (2 yr) | 72.3% (2 yr) | 44.6% (2 yr) |
| Verma et al[74], 2019 | n = 149 | 45-60 Gy (3-8 fx) | 149/149 | 83.8% (29.2 mo) | |||
| Newman et al[81], 2019 | n = 239 | BED > 100 Gy (max 8 fx) | 84/239 | 27% (5 yr); 36% (5 yr, with QT) | |||
| Singh et al[80], 2019 | n = 21 | BED 105.6 Gy (3-5 fx) | 4/21 | 100% (1, 2, 3 yr) | 73.1% (1 yr); 36.6% (2 yr) | 85.7% (1 yr); 42.9% (2 yr) |
Patients with SCLC are at high risk of brain metastases (BM)[84,85]. Research into the potential of PCI began in the late 1970s[86]. Brain magnetic resonance imaging (MRI) should be performed after chemoradiotherapy or systemic therapy[87], as 21.8%-32.5% of patients who achieve complete response subsequently develop BM[88,89]. A meta-analysis published by Aupérin et al[90] in 1999 showed that PCI was associated with a reduced incidence of BM at 3 years (59% vs 33%) and a 5.4% increase in OS. Subsequent meta-analyses have shown similarly favorable results for PCI in patients who had responded to treatment[91-94]. Most of these studies, however, were published before the introduction of restaging with brain MRI, and therefore the true benefit of PCI in LS-SCLC is not so clear[95,96]. Nonetheless, retrospective studies have described beneficial effects for PCI in patients with a previous negative brain MRI scan[97,98]. Patients who have undergone complete resection should benefit from PCI, except patients with stage I disease, who have a low risk of BM[99-101]. There is a growing interest in the use of brain MRI and stereotactic irradiation rather than PCI in patients with LS-SCLC[102], but prospective randomized trials are needed.
Radical treatment with chemotherapy and concomitant radiotherapy are recommended for patients with stage IIB-IIIC disease in good general health[4,103]. Eighty percent of patients with mediastinal involvement treated exclusively with chemotherapy experience local recurrence[104], but the addition of radiotherapy lowers this rate and increases survival[104,105]. The CONVERT trial, which compared fractionated and unfractionated radiotherapy in patients treated with cisplatin-etoposide, reported an overall response rate (ORR) of 70%-90%, an OS of 24-30 mo, and a 5-year OS rate of 25%-30%[106]. Another two trials investigated the combination of bevacizumab, an angiogenic, with conventional chemoradiotherapy, but had to be discontinued because of a relatively high incidence of severe adverse events (tracheoesophageal fistulae)[107].
Radiotherapy with immunotherapy in LS-SCLC: Three trials are currently analyzing the combined use of radiotherapy and immunotherapy in LS-SCLC: The NRG Oncology and Alliance trial (ClinicalTrials.gov Identifier: NCT03811002) investigating chemoradiotherapy with and without atezolizumab; the phase II STIMULI trial (NCT02046733) analyzing nivolumab and ipilimumab after chemoradiotherapy and PCI; and the phase III ADRIATIC trial (NCT03703297) comparing durvalumab, durvalumab plus tremelimumab, and placebo in patients without progression after chemoradiotherapy.
Hippocampal avoidance to reduce the neurotoxicity of PCI: The role of PCI with hippocampal avoidance (HA) in patients with LS- or ES-SCLS without BM is being investigated in three phase III trials: The Dutch NKI/AVL trial (NCT01780675), the NRG Oncology CC003 trial (NCT02635009), and the Spanish PREMER-TRIAL (NCT02397733)[108]. The Dutch group found no significant differences in recall assessed using the revised version of the Hopkins Verbal Learning Test between patients who received PCI and those who received HA-PCI[109]. Using the Free and Cued Selecting Reminding Test, the Spanish group found a significant decline in 3-mo delayed recall [22.22% vs 5.08%; odds ratio (OR) = 5.33; 95% confidence interval (CI): 1.44-19.65; P = 0.006) and total recall (20.63% vs 6.78%; OR = 3.57; 95%CI: 1.09-11.68; P = 0.02] in the PCI vs HA-PCI group[110]. Another potentially interesting line of research is the use of Alzheimer disease drugs to preserve cognition in patients treated with PCI[111].
Proton beam radiation therapy: In non-SCLC, proton therapy has been used to reduce doses to the heart while maintaining high doses to the tumor[112]. Proton beam radiation therapy (PBRT) is potentially beneficial in SCLC, as patients tend to have bulky central disease at diagnosis. In a study of 30 patients at the University of Pennsylvania, PBRT at a median dose of 63.9 cobalt Gy equivalents achieved a promising median OS of 28.2 mo with low toxicity[113]. These results need to be validated in further studies.
Chemotherapy with platinum compounds and etoposide has been the standard treatment for ES-SCLC for many decades. The COCIS meta-analysis showed that cisplatin- and carboplatin-based chemotherapy produced comparable results in terms of OS (9.6 vs 9.4 mo), progression free survival (PFS) (5.5 vs 5.3 mo), and ORR (67% vs 66% mo)[114]. Other strategies attempted, including maintenance treatments and combinations with antiangiogenics, have produced disappointing results[115-117]. The recently published results of the IMpower 133[118] and CASPIAN[119] trials comparing combinations of chemotherapy and immunotherapy followed by immunotherapy with standard platinum and etoposide chemotherapy in ES-SCLC have shown that the combined use of chemotherapy and immunotherapy prolongs OS.
IMpower133 is a phase III trial in which patients received four cycles of carboplatin and etoposide and either atezolizumab or placebo followed by maintenance atezolizumab[118]. The response rates in both arms were similar, but patients in the atezolizumab arm survived for a median of 2.3 mo longer [hazard ratio (HR) = 0.7; 95%CI: 0.54-0.91; P = 0.007]. The updated trial data presented at the 2019 European Society for Medical Oncology congress showed an increase in OS at both 12 mo (39% to 51.9%) and 18 mo (21% to 34%)[120,121].
The phase III CASPIAN trial has three treatment arms. Treatment with durvalumab plus chemotherapy (4-6 cycles of cisplatin or carboplatin plus etoposide) followed by durvalumab maintenance achieved an OS of 12.9 mo (vs 10.5 mo for standard chemotherapy) (HR = 0.75; 95%CI: 0.62-0.9; P = 0.0032), a 2-year PFS of 11% (vs 2.9%), and a 2-year response rate of 13.5% (vs 3.9%)[119,122]. In the third arm, tremelimumab plus durvalumab vs chemotherapy showed no benefit in antitumor activity and was associated with increased toxicity[123].
Results from other studies evaluating combinations of anti-programmed death 1 (PD-1) antibodies have been disappointing. While the combined use of pembrolizumab and chemotherapy increased PFS, it did not provide any significant improvements in OS[124]. In the phase II ECOG-ACRIN EA5161 trial, chemotherapy plus nivolumab followed by maintenance treatment achieved a non-significant improvement in PFS (5.5 vs 4.7 mo) and OS (11.3 vs 8.5 mo)[125] (Table 3). A systematic review and two meta-analyses published in 2020 concluded that a combination of chemotherapy with atezolizumab or durvalumab was the best first-line treatment for ES-SCLC[126,127]. Other options that have been explored include combinations of ipilimumab and chemotherapy (no benefit and greater toxicity)[128,129] and combinations of different chemotherapy agents, such as irinotecan plus etoposide and cisplatin plus irinotecan (also without benefits)[130-132].
| Study | n | Design | Treatment | RR | PFS | OS |
| NCT01450761 | 1132 | Phase III; Randomized, double-blind; Drug: Ipilimumab | Arm A: PE × 4C + ipilimumab × 4C; Control: PE × 4C + placebo × 4C | PR 62% vs 62%; SD 26% vs 27%; PD 6% vs 9% | 4.6 vs 4.4 mo; HR = 0.85, P = 0.0161 | 11.0 vs 10.9 mo; HR = 0.94, P = 0.3775 |
| Impower 133 | 403 | Phase III. Randomized, double-blind; Drug: Atezolizumab | Arm A: PE + atezolizumab × 4C/atezolizumab; Control: PE + placebo × 4C/placebo | 60% vs 64% | 5.2 vs 4.3 mo; HR = 0.77, P = 0.02 | 12.3 vs 10.3 mo; HR = 0.70, P = 0.007 |
| CASPIAN | 805 | Phase III. Randomized, open-label; Drug: Durvalumab | Arm B (n = 268): Durvalumab + PE × 4C/durvalumab; Control: PE × 4C | 68% vs 58% | 5.1 vs 5.4 mo; HR = 0.78, P not tested | 13.0 vs 10.3 mo; HR = 0.73, P = 0.0047 |
| CASPIAN | 805 | Phase III. Randomized, open-label; Drug: Durvalumab + tremelimumab | Arm A (n = 268): Durvalumab + tremelimumab + PE × 4C/durvalumab + tremelimumab. Control: PE × 4C | 58% both arms | 4.9 vs 5.4 mo; HR = 0.84 | 10.4 vs 10.5 mo; HR = 0.82, P = 0.045 |
| KEYNOTE 604 | 453 | Phase III; Randomized, double-blind; Drug: Pembrolizumab | Arm A: Pembrolizumab + PE; Control: | 71% vs 62% | 4.5 vs 4.3 mo; HR = 0.75, P = 0.0023 | 10.8 vs 9.7 mo; HR = 0.80, P = 0.0164 |
| ECOG-ACRIN | 160 | Phase I. Randomized, open-label; Drug: Nivolumab | Arm A: PE + nivolumab × 4C/nivolumab; Control: PE × 4C | 52.29% vs 47.71% | 5.5 vs 4.6 mo; HR = 0.65, P = 0.012 | 11.3 vs 8.5 mo; HR = 0.67, P = 0.038 |
The results of the first randomized trial to demonstrate a reduction in the risk of symptomatic BM (14.6% vs 40.4% at 1 year) and an improvement in OS (27.1% vs 13.3%) in chemotherapy responders who underwent PCI were published in 2007[133]. The results are supported by data from several meta-analyses[134-136], although as a shortcoming of the trial, pre-PCI brain imaging was not performed[133]. The results of a randomized trial conducted in Japan comparing PCI with close MRI follow-up in patients with ES-SCLC who had responded to chemotherapy and had a negative brain MRI were published in 2017. While they did not show an increase in OS (11.6 mo for PCI vs 13.7 mo for MRI follow-up; HR = 1.27; 95%CI: 0.96-1.68; P = 0.094), they did show a significant decrease in the incidence of BM[137].
A recent meta-analysis showed that PCI was only associated with prolonged OS in studies where brain imaging was not performed between chemotherapy and irradiation (HR = 0.70; 95%CI: 0.57-0.85). In other words, PCI did not offer any significant benefits when preceded by MRI or CT to test for BM (HR = 0.94; 95%CI: 0.74-1.18)[138]. Considering the above results and the neurotoxic effects of PCI[139], it would seem reasonable to consider periodic MRI examination as an alternative to PCI in patients with ES-SCLC. In such cases, a joint evaluation should be made by the medical and radiation oncologists[30]. The recommended dose for PCI is 25 Gy in 10 fractions, as higher doses do not appear to reduce the incidence of BM at 2 years and are associated with higher mortality and chronic neurotoxicity[140].
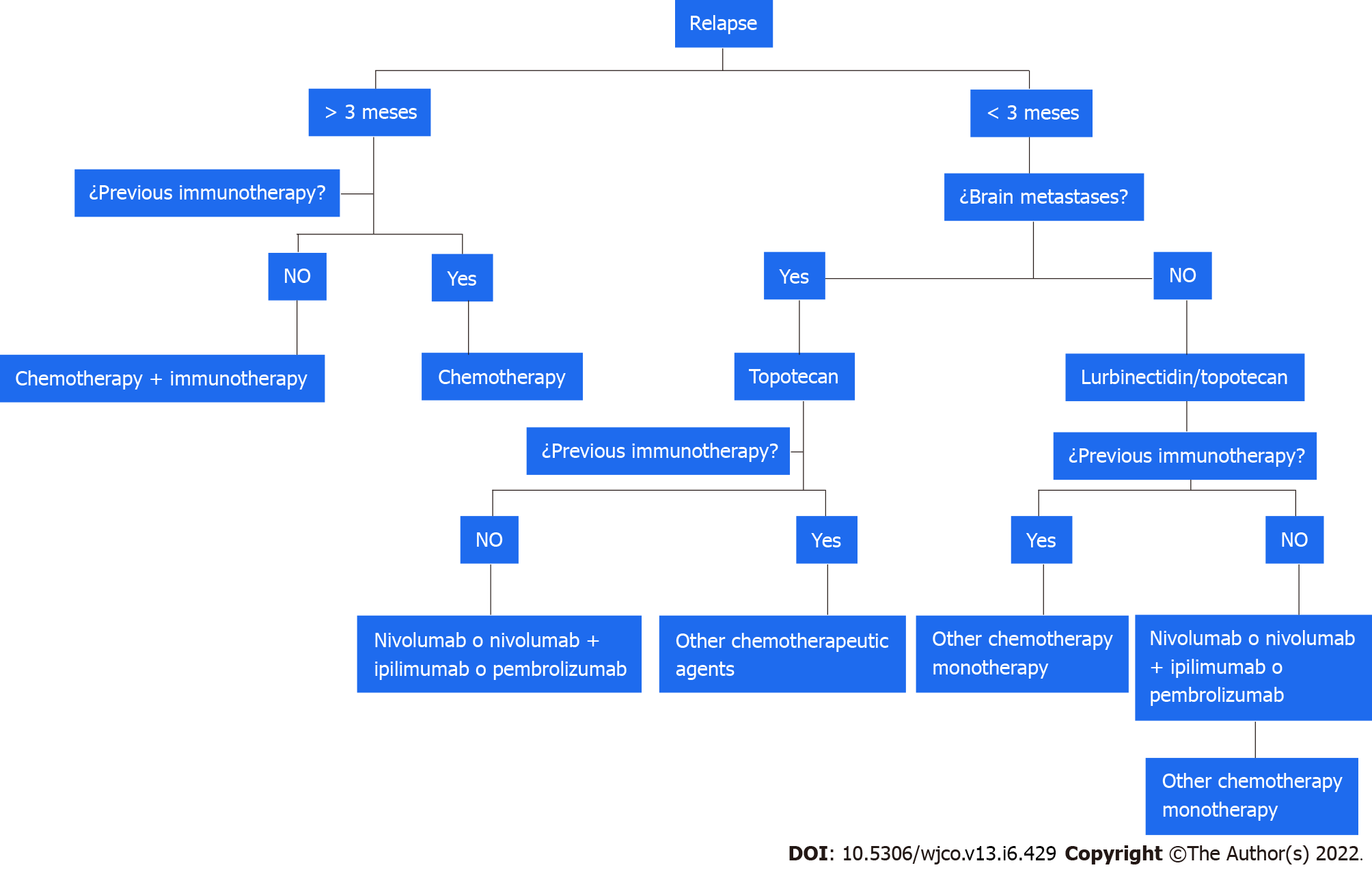
Relapsed SCLC tends to be resistant to treatment and is associated with an OS of 4-5 mo. Response to second-line treatment varies according to PFS and is 10% in patients with a PFS < 3 mo (refractory SCLC) and 25% in those with a PFS of 3-6 mo (sensitive SCLC)[141-143].
Relapse after PFS > 3 mo: Rechallenge treatment with combinations of platinum-based chemotherapy has been investigated in patients with sensitive SCLC. Patients treated with carboplatin and etoposide had a longer PFS than those treated with topotecan, and the greatest benefits were observed for those who relapsed after 6 mo[144,145].
Relapse after PFS of < 3 mo: Until recently, topotecan was the only drug authorized by the US and Food and Drug Administration (FDA) to treat relapsed SCLC. In the 2006 phase III trial that led to its approval, it significantly improved survival compared with best supportive care only[146]. Another phase III trial comparing topotecan and CAV (cyclophosphamide, doxorubicin, and vincristine) reported similar survival and response rates for the two treatments, but found topotecan to be associated with better symptom control and lower toxicity[147]. An additional study evaluating topotecan plus aflibercept, an antiangiogenic, reported an OS of 5 mo[148].
One recent advance in this setting is the recent approval by the FDA of lurbinectedin as a second-line treatment for SCLC. In a study of patients with SCLC without BM, lurbinectedin achieved an ORR of 35%, and a median response duration of 5.1 mo (> 6 mo in 25% of patients)[149]. The combination of lurbinectedin and doxorubicin was investigated in two cohorts in a phase I trial and showed disease control rates of 81% and 70% and a median response duration of 4.5 and 5.2 mo[150]. These findings led to the design of the phase III ATLANTIS trial comparing lurbinectedin plus doxorubicin with topotecan and with CAV; a press release, however, announced no improvement in OS[151] (Figure 2).
Amrubicin is available for the treatment of relapsed SCLC in Japan, but it has not been approved by the FDA. A phase III trial comparing amrubicin with topotecan showed superior symptom control for topotecan but no significant differences in OS[152]. Immune checkpoint inhibitors have also been tested. The CheckMate 032 trial comparing nivolumab alone with nivolumab plus ipilimumab in recurrent SCLC reported improved ORR and OS in both treatment arms regardless of prior treatment or PD-L1 expression[153,154]. With these data, the FDA approved nivolumab for use in previously treated patients.
The phase III CheckMate 331 trial showed similar OS for nivolumab vs standard chemotherapy in the second-line treatment of SCLC[155]. Pembrolizumab has also been tested in SCLC. A pooled analysis of the KEYNOTE-028 (phase Ib)[156] and KEYNOTE-158 (II)[157,158] trials found an ORR of 19.3%, leading to FDA approval. Atezolizumab was also tested in a phase II trial, but the primary endpoint was not met[159]. Paclitaxel every 3 wk for 6 cycles plus pembrolizumab after the second cycle until disease progression achieved a disease control rate of 80% and a median OS of 9.2 mo[160]. Other drugs tested in the setting of relapsed SCLC are temozolomide[161,162], irinotecan[163], paclitaxel[164,165], docetaxel[166], gemcitabine[167,168], and vinorelbine[169]. Finally, a recent phase IIb study showed that belotecan was associated with better OS and disease control than topotecan in patients with sensitive SCLC[170].
New drugs linked to targets with a role in cell proliferation have been developed. These include poly (ADP-ribose) polymerase (PARP) inhibitors, delta-like ligand 3 inhibitors (DLL3), and drugs that selectively inhibit oncogenic transcription. The expression of DNA damage response proteins [especially PARP1/checkpoint kinase 1 (CHK1)] is elevated in SCLC, and in vitro studies have shown an antitumor effect for PARP inhibitors[171]. Monotherapy with PARP inhibitors has also been investigated in different clinical trials, but the results have been disappointing. In an early study, talazoparib showed an ORR of 8.7%[172]. No benefit was observed for maintenance treatment with olaparib after first-line chemotherapy with cisplatin and etoposide[173] or for the addition of veliparib vs placebo to first-line cisplatin and etoposide, with findings showing no significant differences in PFS (6.1 vs 5.5 mo) or OS (10.3 vs 8.9 mo)[174,175].
Discordant results have been reported for combinations of chemotherapy and PARP inhibitors in successive treatment lines. No significant differences were found for PFS or OS in a study comparing temozolomide plus veliparib vs temozolomide only[176]. Temozolomide combined with olaparib, however, was associated with a response rate of 41.7%, a PFS of 4.2 mo, and an OS of 8.5 mo in a phase I/II clinical trial[177]. No benefits have been observed for the combined use of PARP inhibitors and immunotherapy (durvalumab with olaparib, among others)[178]. Future actions targeting this actionable molecular pathway in SCLC will probably involve combinations of PARP inhibitors and chemotherapy agents and immunotherapy, or new molecules. Promising results have been reported for CHK1 (SRA737) combined with low-dose gemcitabine and anti-PD-1/programmed death ligand 1 (PD-L1) immune checkpoint inhibitors[179] and for PARP inhibitors combined with WEE1 inhibitors, which act at the cell-cycle level[180].
Other treatments have also yielded positive results. Lurbinectedin, a selective oncogenic transcription inhibitor, was recently evaluated in combination with irinotecan in pretreated patients in a phase Ib/II basket trial. The results for the SCLC cohort showed an ORR of 62%, a clinical benefit rate of 81%, a disease control rate of 90%, and a PFS of 6.1 mo[181]. Other new molecules with different ligands under investigation include DLL3 inhibitors, such as rovalpituzumab-tesirine. This is a promising drug in pretreated patients expressing DLL3, although recent reports have described greater toxicity and little benefit compared with topotecan[182-184]. AMG 757, a half-life extended DLL3 bispecific T-cell engager, has also shown promising results in pretreated patients in an ongoing phase I trial, with an ORR of 14%, a disease control rate of 37%, and a very promising median duration of 6.2 mo[185].
Numerous questions remain to be answered regarding the role of radiotherapy in ES-SCLC.
Consolidation radiotherapy in extensive SCLC: What is the optimal radiation dose or indication for patients with complete thoracic response or partial distant response? The Chinese phase III trial (NCT02675088) is comparing 45 Gy at 3 Gy/d in 15 fractions vs 10 fractions (CREST trial schedule) with a primary endpoint of OS at 2 years[186]. How can radiotherapy be best combined with immunotherapy? The RAPTOR phase II/III trial (NCT04402788) is evaluating the use of radiotherapy to the chest and distant lesions after 4-6 cycles of carboplatin and etoposide plus atezolizumab.
Stereotactic radiosurgery to treat BM: Stereotactic radiosurgery has not traditionally been investigated in SCLC due to the high incidence of BM and poor prognosis. Nonetheless, there is growing evidence that it may be appropriate[187]. ENCEPHALON, a phase II trial (NCT03297788) is currently comparing stereotactic radiosurgery with whole-brain radiotherapy in patients with SCLC and 1-10 BM.
The treatment of SCLC will continue to be a challenge. Immunotherapy has a new role lung cancer and will be the future treatment standard alone or in combination, as well as the new radiotherapy techniques. As has been occurred in non-SCLC, the future of treatments in both early and advanced stages is through immunotherapy and targeted treatments. Furthermore, the use of different combinations of chemoimmunotherapy in recent months has improved the prognosis of patients with advanced SCLC. Nevertheless, continued research efforts are needed. Different lines of investigation are open and we hope that their findings will continue to improve prognosis and quality of life in this setting.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chen C, China A-Editor: Ma L, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | National Cancer Institute. SEER Cancer Statistics Review, 1975-2017. [cited 20 January 2021]. Available from: https://seer.cancer.gov/archive/csr/1975_2017/. [Cited in This Article: ] |
| 2. | Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539-4544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1197] [Cited by in F6Publishing: 1315] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 3. | Pesch B, Kendzia B, Gustavsson P, Jöckel KH, Johnen G, Pohlabeln H, Olsson A, Ahrens W, Gross IM, Brüske I, Wichmann HE, Merletti F, Richiardi L, Simonato L, Fortes C, Siemiatycki J, Parent ME, Consonni D, Landi MT, Caporaso N, Zaridze D, Cassidy A, Szeszenia-Dabrowska N, Rudnai P, Lissowska J, Stücker I, Fabianova E, Dumitru RS, Bencko V, Foretova L, Janout V, Rudin CM, Brennan P, Boffetta P, Straif K, Brüning T. Cigarette smoking and lung cancer--relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer. 2012;131:1210-1219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 332] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 4. | Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e400S-e419S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 224] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 5. | Detterbeck FC. The eighth edition TNM stage classification for lung cancer: What does it mean on main street? J Thorac Cardiovasc Surg. 2018;155:356-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Vallières E, Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z, Goldstraw P; International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:1049-1059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 321] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 7. | Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Mary Kay Washington, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR: TNM classification of malignant tumours / Description: Eighth edition. | Oxford, United Kingdom; Hoboken, NJ: John Wiley & Sons, Inc., 2017. [Cited in This Article: ] |
| 8. | Varlotto JM, Recht A, Flickinger JC, Medford-Davis LN, Dyer AM, DeCamp MM. Lobectomy leads to optimal survival in early-stage small cell lung cancer: a retrospective analysis. J Thorac Cardiovasc Surg. 2011;142:538-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Jin K, Zhang K, Zhou F, Dai J, Zhang P, Jiang G. Selection of candidates for surgery as local therapy among early-stage small cell lung cancer patients: a population-based analysis. Cancer Commun (Lond). 2018;38:5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Yang CJ, Chan DY, Shah SA, Yerokun BA, Wang XF, D'Amico TA, Berry MF, Harpole DH Jr. Long-term Survival After Surgery Compared With Concurrent Chemoradiation for Node-negative Small Cell Lung Cancer. Ann Surg. 2018;268:1105-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Ahmed Z, Kujtan L, Kennedy KF, Davis JR, Subramanian J. Disparities in the Management of Patients With Stage I Small Cell Lung Carcinoma (SCLC): A Surveillance, Epidemiology and End Results (SEER) Analysis. Clin Lung Cancer. 2017;18:e315-e325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Wakeam E, Byrne JP, Darling GE, Varghese TK Jr. Surgical Treatment for Early Small Cell Lung Cancer: Variability in Practice and Impact on Survival. Ann Thorac Surg. 2017;104:1872-1880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Wakeam E, Acuna SA, Leighl NB, Giuliani ME, Finlayson SRG, Varghese TK, Darling GE. Surgery Versus Chemotherapy and Radiotherapy For Early and Locally Advanced Small Cell Lung Cancer: A Propensity-Matched Analysis of Survival. Lung Cancer. 2017;109:78-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Combs SE, Hancock JG, Boffa DJ, Decker RH, Detterbeck FC, Kim AW. Bolstering the case for lobectomy in stages I, II, and IIIA small-cell lung cancer using the National Cancer Data Base. J Thorac Oncol. 2015;10:316-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Ogawa S, Horio Y, Yatabe Y, Fukui T, Ito S, Hasegawa Y, Mitsudomi T, Hida T. Patterns of recurrence and outcome in patients with surgically resected small cell lung cancer. Int J Clin Oncol. 2012;17:218-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Ju MH, Kim HR, Kim JB, Kim YH, Kim DK, Park SI. Surgical outcomes in small cell lung cancer. Korean J Thorac Cardiovasc Surg. 2012;45:40-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Lim E, Belcher E, Yap YK, Nicholson AG, Goldstraw P. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thorac Oncol. 2008;3:1267-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Wang HJ, Sun KL, Zhang XR, Sun Y, Shi YK. [Combined modality therapy for small cell lung cancer patient with limited stage disease]. Zhonghua Zhong Liu Za Zhi. 2007;29:701-703. [PubMed] [Cited in This Article: ] |
| 19. | Veronesi G, Scanagatta P, Leo F, De Pas T, Pelosi G, Catalano G, Gandini S, De Braud F, Spaggiari L. Adjuvant surgery after carboplatin and VP16 in resectable small cell lung cancer. J Thorac Oncol. 2007;2:131-134. [PubMed] [Cited in This Article: ] |
| 20. | Tsuchiya R, Suzuki K, Ichinose Y, Watanabe Y, Yasumitsu T, Ishizuka N, Kato H. Phase II trial of postoperative adjuvant cisplatin and etoposide in patients with completely resected stage I-IIIa small cell lung cancer: the Japan Clinical Oncology Lung Cancer Study Group Trial (JCOG9101). J Thorac Cardiovasc Surg. 2005;129:977-983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Brock MV, Hooker CM, Syphard JE, Westra W, Xu L, Alberg AJ, Mason D, Baylin SB, Herman JG, Yung RC, Brahmer J, Rudin CM, Ettinger DS, Yang SC. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. J Thorac Cardiovasc Surg. 2005;129:64-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Nakamura H, Kato Y, Kato H. Outcome of surgery for small cell lung cancer -- response to induction chemotherapy predicts survival. Thorac Cardiovasc Surg. 2004;52:206-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Badzio A, Kurowski K, Karnicka-Mlodkowska H, Jassem J. A retrospective comparative study of surgery followed by chemotherapy vs. non-surgical management in limited-disease small cell lung cancer. Eur J Cardiothorac Surg. 2004;26:183-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Lewiński T, Zuławski M, Turski C, Pietraszek A. Small cell lung cancer I--III A: cytoreductive chemotherapy followed by resection with continuation of chemotherapy. Eur J Cardiothorac Surg. 2001;20:391-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Cataldo I, Bidoli P, BregaMassone PP, Conti B, Lequaglie C. Long term survival for resectable small cell lung cancer. Lung Cancer (Amsterdam, Netherlands). 2000;29:130. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Inoue M, Miyoshi S, Yasumitsu T, Mori T, Iuchi K, Maeda H, Matsuda H. Surgical results for small cell lung cancer based on the new TNM staging system. Thoracic Surgery Study Group of Osaka University, Osaka, Japan. Ann Thorac Surg. 2000;70:1615-1619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Kobayashi S, Okada S, Hasumi T, Sato N, Fujimura S. The significance of surgery for bulky N2 small-cell lung cancer: a clinical and in vitro analysis of long-term survivors. Surg Today. 2000;30:978-986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Eberhardt W, Stamatis G, Stuschke M, Wilke H, Müller MR, Kolks S, Flasshove M, Schütte J, Stahl M, Schlenger L, Budach V, Greschuchna D, Stüben G, Teschler H, Sack H, Seeber S. Prognostically orientated multimodality treatment including surgery for selected patients of small-cell lung cancer patients stages IB to IIIB: long-term results of a phase II trial. Br J Cancer. 1999;81:1206-1212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Ganti AKP, Loo BW, Bassetti M, Blakely C, Chiang A, D'Amico TA, D'Avella C, Dowlati A, Downey RJ, Edelman M, Florsheim C, Gold KA, Goldman JW, Grecula JC, Hann C, Iams W, Iyengar P, Kelly K, Khalil M, Koczywas M, Merritt RE, Mohindra N, Molina J, Moran C, Pokharel S, Puri S, Qin A, Rusthoven C, Sands J, Santana-Davila R, Shafique M, Waqar SN, Gregory KM, Hughes M. Small Cell Lung Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:1441-1464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 116] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 30. | Simone CB 2nd, Bogart JA, Cabrera AR, Daly ME, DeNunzio NJ, Detterbeck F, Faivre-Finn C, Gatschet N, Gore E, Jabbour SK, Kruser TJ, Schneider BJ, Slotman B, Turrisi A, Wu AJ, Zeng J, Rosenzweig KE. Radiation Therapy for Small Cell Lung Cancer: An ASTRO Clinical Practice Guideline. Pract Radiat Oncol. 2020;10:158-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 31. | Rudin CM, Giaccone G, Ismaila N. Treatment of Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Oncol Pract. 2016;12:83-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Früh M, De Ruysscher D, Popat S, Crinò L, Peters S, Felip E; ESMO Guidelines Working Group. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi99-v105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 413] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 33. | Shepherd FA, Ginsberg RJ, Feld R, Evans WK, Johansen E. Surgical treatment for limited small-cell lung cancer. The University of Toronto Lung Oncology Group experience. J Thorac Cardiovasc Surg. 1991;101:385-393. [PubMed] [Cited in This Article: ] |
| 34. | Rea F, Callegaro D, Favaretto A, Loy M, Paccagnella A, Fantoni U, Festi G, Sartori F. Long term results of surgery and chemotherapy in small cell lung cancer. Eur J Cardiothorac Surg. 1998;14:398-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Stish BJ, Hallemeier CL, Olivier KR, Harmsen WS, Allen MS, Garces YI. Long-Term Outcomes and Patterns of Failure After Surgical Resection of Small-Cell Lung Cancer. Clin Lung Cancer. 2015;16:e67-e73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Xu L, Zhang G, Song S, Zheng Z. Surgery for small cell lung cancer: A Surveillance, Epidemiology, and End Results (SEER) Survey from 2010 to 2015. Medicine (Baltimore). 2019;98:e17214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Lüchtenborg M, Riaz SP, Lim E, Page R, Baldwin DR, Jakobsen E, Vedsted P, Lind M, Peake MD, Mellemgaard A, Spicer J, Lang-Lazdunski L, Møller H. Survival of patients with small cell lung cancer undergoing lung resection in England, 1998-2009. Thorax. 2014;69:269-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Takei H, Kondo H, Miyaoka E, Asamura H, Yoshino I, Date H, Okumura M, Tada H, Fujii Y, Nakanishi Y, Eguchi K, Dosaka-Akita H, Kobayashi H, Sawabata N, Yokoi K; Japanese Joint Committee of Lung Cancer Registry. Surgery for small cell lung cancer: a retrospective analysis of 243 patients from Japanese Lung Cancer Registry in 2004. J Thorac Oncol. 2014;9:1140-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 39. | Weksler B, Nason KS, Shende M, Landreneau RJ, Pennathur A. Surgical resection should be considered for stage I and II small cell carcinoma of the lung. Ann Thorac Surg. 2012;94:889-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 40. | Yu JB, Decker RH, Detterbeck FC, Wilson LD. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol. 2010;5:215-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 41. | Liu T, Chen Z, Dang J, Li G. The role of surgery in stage I to III small cell lung cancer: A systematic review and meta-analysis. PLoS One. 2018;13:e0210001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Hou SZ, Cheng ZM, Wu YB, Sun Y, Liu B, Yuan MX, Wang XD. Evaluation of short-term and long-term efficacy of surgical and non-surgical treatment in patients with early-stage small cell lung cancer: A comparative study. Cancer Biomark. 2017;19:249-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Lucchi M, Mussi A, Chella A, Janni A, Ribechini A, Menconi GF, Angeletti CA. Surgery in the management of small cell lung cancer. Eur J Cardiothorac Surg. 1997;12:689-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Engelhardt KE, Coughlin JM, DeCamp MM, Denlinger CE, Meyerson SL, Bharat A, Odell DD. Survival after adjuvant radiation therapy in localized small cell lung cancer treated with complete resection. J Thorac Cardiovasc Surg. 2019;158:1665-1677.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Iwata T, Nishiyama N, Nagano K, Izumi N, Mizuguchi S, Tsukioka T, Morita R, Chung K, Hanada S, Inoue K. Role of pulmonary resection in the diagnosis and treatment of limited-stage small cell lung cancer: revision of clinical diagnosis based on findings of resected specimen and its influence on survival. Gen Thorac Cardiovasc Surg. 2012;60:43-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Schneider BJ, Saxena A, Downey RJ. Surgery for early-stage small cell lung cancer. J Natl Compr Canc Netw. 2011;9:1132-1139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Thomas CR Jr, Giroux DJ, Janaki LM, Turrisi AT 3rd, Crowley JJ, Taylor SA, McCracken JD, Shankir Giri PG, Gordon W Jr, Livingston RB, Gandara DR. Ten-year follow-up of Southwest Oncology Group 8269: a phase II trial of concomitant cisplatin-etoposide and daily thoracic radiotherapy in limited small-cell lung cancer. Lung Cancer. 2001;33:213-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Takenaka T, Takenoyama M, Inamasu E, Yoshida T, Toyokawa G, Nosaki K, Hirai F, Yamaguchi M, Shimokawa M, Seto T, Ichinose Y. Role of surgical resection for patients with limited disease-small cell lung cancer. Lung Cancer. 2015;88:52-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 49. | Schreiber D, Rineer J, Weedon J, Vongtama D, Wortham A, Kim A, Han P, Choi K, Rotman M. Survival outcomes with the use of surgery in limited-stage small cell lung cancer: should its role be re-evaluated? Cancer. 2010;116:1350-1357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 50. | Zhong L, Suo J, Wang Y, Han J, Zhou H, Wei H, Zhu J. Prognosis of limited-stage small cell lung cancer with comprehensive treatment including radical resection. World J Surg Oncol. 2020;18:27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 51. | Shepherd FA, Ginsberg RJ, Evans WK, Feld R, Cooper JD, Ilves R, Todd TR, Pearson FG, Waters PF, Baker MA. Reduction in local recurrence and improved survival in surgically treated patients with small cell lung cancer. J Thorac Cardiovasc Surg. 1983;86:498-506. [PubMed] [Cited in This Article: ] |
| 52. | Granetzny A, Boseila A, Wagner W, Krukemeyer G, Vogt U, Hecker E, Koch OM, Klinke F. Surgery in the tri-modality treatment of small cell lung cancer. Stage-dependent survival. Eur J Cardiothorac Surg. 2006;30:212-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Yin K, Song D, Zhang H, Cai F, Chen J, Dang J. Efficacy of surgery and prophylactic cranial irradiation in stage II and III small cell lung cancer. J Cancer. 2018;9:3500-3506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Che K, Shen H, Qu X, Pang Z, Jiang Y, Liu S, Yang X, Du J. Survival Outcomes for Patients with Surgical and Non-Surgical Treatments in Stages I-III Small-Cell Lung Cancer. J Cancer. 2018;9:1421-1429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Babakoohi S, Fu P, Yang M, Linden PA, Dowlati A. Combined SCLC clinical and pathologic characteristics. Clin Lung Cancer. 2013;14:113-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Zhang C, Yang H, Zhao H, Lang B, Yu X, Xiao P, Zhang X. Clinical outcomes of surgically resected combined small cell lung cancer: a two-institutional experience. J Thorac Dis. 2017;9:151-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Lei Y, Feng H, Qiang H, Shang Z, Chang Q, Qian J, Zhang Y, Zhong R, Fan X, Chu T. Clinical characteristics and prognostic factors of surgically resected combined small cell lung cancer: a retrospective study. Lung Cancer. 2020;146:244-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | Mangum MD, Greco FA, Hainsworth JD, Hande KR, Johnson DH. Combined small-cell and non-small-cell lung cancer. J Clin Oncol. 1989;7:607-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 104] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Men Y, Hui Z, Liang J, Feng Q, Chen D, Zhang H, Xiao Z, Zhou Z, Yin W, Wang L. Further understanding of an uncommon disease of combined small cell lung cancer: clinical features and prognostic factors of 114 cases. Chin J Cancer Res. 2016;28:486-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 60. | Shepherd FA, Ginsberg R, Patterson GA, Feld R, Goss PE, Pearson FG, Todd TJ, Winton T, Rubinger M, Johansen E. Is there ever a role for salvage operations in limited small-cell lung cancer? J Thorac Cardiovasc Surg. 1991;101:196-200. [PubMed] [Cited in This Article: ] |
| 61. | Yamada K, Saijo N, Kojima A, Ohe Y, Tamura T, Sasaki Y, Eguchi K, Shinkai T, Goya T, Kondou H. A retrospective analysis of patients receiving surgery after chemotherapy for small cell lung cancer. Jpn J Clin Oncol. 1991;21:39-45. [PubMed] [Cited in This Article: ] |
| 62. | Nakanishi K, Mizuno T, Sakakura N, Kuroda H, Shimizu J, Hida T, Yatabe Y, Sakao Y. Salvage surgery for small cell lung cancer after chemoradiotherapy. Jpn J Clin Oncol. 2019;49:389-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Du X, Tian D, Liu L, Tang Z, Xiao J, Liu W, Yuan S, Cao X, Zhou H, Zhang J. Surgery in patients with small cell lung cancer: A period propensity score matching analysis of the Seer database, 2010-2015. Oncol Lett. 2019;18:4865-4881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Yang H, Xu J, Yao F, Liang S, Zhao H. Analysis of unexpected small cell lung cancer following surgery as the primary treatment. J Cancer Res Clin Oncol. 2018;144:2441-2447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Micke P, Faldum A, Metz T, Beeh KM, Bittinger F, Hengstler JG, Buhl R. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer--what limits limited disease? Lung Cancer. 2002;37:271-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 240] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 66. | de Castro Carpeño J, Dols MC, Gomez MD, Gracia PR, Crama L, Campelo MRG. Survival outcomes in stage IV small-cell lung cancer (IV-SCLC): Analysis from SEER database. Ann Oncol. 2019;30:xi30. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | De Ruysscher D, Lueza B, Le Péchoux C, Johnson DH, O'Brien M, Murray N, Spiro S, Wang X, Takada M, Lebeau B, Blackstock W, Skarlos D, Baas P, Choy H, Price A, Seymour L, Arriagada R, Pignon JP; RTT-SCLC Collaborative Group. Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: usefulness of the individual patient data meta-analysis. Ann Oncol. 2016;27:1818-1828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 68. | Turgeon GA, Souhami L, Kopek N, Hirsh V, Ofiara L, Faria SL. Thoracic irradiation in 3weeks for limited-stage small cell lung cancer: Is twice a day fractionation really needed? Cancer Radiother. 2017;21:89-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Xia B, Hong LZ, Cai XW, Zhu ZF, Liu Q, Zhao KL, Fan M, Mao JF, Yang HJ, Wu KL, Fu XL. Phase 2 study of accelerated hypofractionated thoracic radiation therapy and concurrent chemotherapy in patients with limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2015;91:517-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 70. | Grønberg BH, Halvorsen TO, Fløtten Ø, Brustugun OT, Brunsvig PF, Aasebø U, Bremnes RM, Tollåli T, Hornslien K, Aksnessæther BY, Liaaen ED, Sundstrøm S; Norwegian Lung Cancer Study Group. Randomized phase II trial comparing twice daily hyperfractionated with once daily hypofractionated thoracic radiotherapy in limited disease small cell lung cancer. Acta Oncol. 2016;55:591-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 71. | Johnson BE, Crawford J, Downey RJ, Ettinger DS, Fossella F, Grecula JC, Jahan T, Kalemkerian GP, Kessinger A, Koczywas M, Langer CJ, Martins R, Marymont MH, Niell HB, Ramnath N, Robert F, Williams CC Jr; National Comprehensive Cancer Network (NCCN). Small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4:602-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Davis S, Crino L, Tonato M, Darwish S, Pelicci PG, Grignani F. A prospective analysis of chemotherapy following surgical resection of clinical stage I-II small-cell lung cancer. Am J Clin Oncol. 1993;16:93-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Yang CF, Chan DY, Speicher PJ, Gulack BC, Wang X, Hartwig MG, Onaitis MW, Tong BC, D'Amico TA, Berry MF, Harpole DH. Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J Clin Oncol. 2016;34:1057-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 74. | Verma V, Hasan S, Wegner RE, Abel S, Colonias A. Stereotactic ablative radiation therapy versus conventionally fractionated radiation therapy for stage I small cell lung cancer. Radiother Oncol. 2019;131:145-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 75. | Verma V, Simone CB 2nd, Allen PK, Gajjar SR, Shah C, Zhen W, Harkenrider MM, Hallemeier CL, Jabbour SK, Matthiesen CL, Braunstein SE, Lee P, Dilling TJ, Allen BG, Nichols EM, Attia A, Zeng J, Biswas T, Paximadis P, Wang F, Walker JM, Stahl JM, Daly ME, Decker RH, Hales RK, Willers H, Videtic GM, Mehta MP, Lin SH. Multi-Institutional Experience of Stereotactic Ablative Radiation Therapy for Stage I Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2017;97:362-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 76. | Videtic GM, Stephans KL, Woody NM, Pennell NA, Shapiro M, Reddy CA, Djemil T. Stereotactic body radiation therapy-based treatment model for stage I medically inoperable small cell lung cancer. Pract Radiat Oncol. 2013;3:301-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Shioyama Y, Nagata Y, Komiyama T, Takayama K, Shibamoto Y. Ueki N, Yamada K, Kozuka T, Kimura T, Matsuo Y. Multi-institutional Retrospective study of Stereotactic Body Radiation Therapy for stage I Small Cell Lung Cancer: Japan Radiation Oncology Study Group (JROSG). Int J Radiat Oncol Biol Phys. 2015;93:S101. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 78. | Shioyama Y, Onishi H, Takayama K, Matsuo Y, Takeda A, Yamashita H, Miyakawa A, Murakami N, Aoki M, Matsushita H, Matsumoto Y, Shibamoto Y; Japanese Radiological Society Multi-Institutional SBRT Study Group (JRS-SBRTSG). Clinical Outcomes of Stereotactic Body Radiotherapy for Patients With Stage I Small-Cell Lung Cancer: Analysis of a Subset of the Japanese Radiological Society Multi-Institutional SBRT Study Group Database. Technol Cancer Res Treat. 2018;17:1533033818783904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 79. | Stahl JM, Corso CD, Verma V, Park HS, Nath SK, Husain ZA, Simone CB 2nd, Kim AW, Decker RH. Trends in stereotactic body radiation therapy for stage I small cell lung cancer. Lung Cancer. 2017;103:11-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 80. | Singh R, Ansinelli H, Sharma D, Jenkins J, Davis J, Vargo JA, Sharma S. Clinical Outcomes Following Stereotactic Body Radiation Therapy (SBRT) for Stage I Medically Inoperable Small Cell Lung Carcinoma: A Multi-Institutional Analysis From the RSSearch Patient Registry. Am J Clin Oncol. 2019;42:602-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Newman NB, Sherry AD, Byrne DW, Osmundson EC. Stereotactic body radiotherapy versus conventional radiotherapy for early-stage small cell lung cancer. J Radiat Oncol. 2019;8:239-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 82. | Verma V, Simone CB 2nd, Allen PK, Lin SH. Outcomes of Stereotactic Body Radiotherapy for T1-T2N0 Small Cell Carcinoma According to Addition of Chemotherapy and Prophylactic Cranial Irradiation: A Multicenter Analysis. Clin Lung Cancer. 2017;18:675-681.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 83. | Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, Yamashita T, Niibe Y, Karasawa K, Hayakawa K, Takai Y, Kimura T, Hirokawa Y, Takeda A, Ouchi A, Hareyama M, Kokubo M, Hara R, Itami J, Yamada K. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101:1623-1631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 687] [Cited by in F6Publishing: 645] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 84. | Ichinose Y, Hara N, Ohta M, Motohiro A, Hata K, Yagawa K. Brain metastases in patients with limited small cell lung cancer achieving complete remission. Correlation with TNM staging. Chest. 1989;96:1332-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 85. | Arriagada R, Le Chevalier T, Rivière A, Chomy P, Monnet I, Bardet E, Santos-Miranda JA, Le Péhoux C, Tarayre M, Benhamou S, Laplanche A. Patterns of failure after prophylactic cranial irradiation in small-cell lung cancer: analysis of 505 randomized patients. Ann Oncol. 2002;13:748-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 86. | Arriagada R, Le Chevalier T, Borie F, Rivière A, Chomy P, Monnet I, Tardivon A, Viader F, Tarayre M, Benhamou S. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87:183-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 314] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 87. | Seute T, Leffers P, ten Velde GP, Twijnstra A. Detection of brain metastases from small cell lung cancer: consequences of changing imaging techniques (CT versus MRI). Cancer. 2008;112:1827-1834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 88. | Manapov F, Klautke G, Fietkau R. Prevalence of brain metastases immediately before prophylactic cranial irradiation in limited disease small cell lung cancer patients with complete remission to chemoradiotherapy: a single institution experience. J Thorac Oncol. 2008;3:652-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 89. | Chu X, Li S, Xia B, Chu L, Yang X, Ni J, Zou L, Li Y, Xie C, Lin J, Zhu Z. Patterns of brain metastasis immediately before prophylactic cranial irradiation (PCI): implications for PCI optimization in limited-stage small cell lung cancer. Radiat Oncol. 2019;14:171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | Aupérin A, Arriagada R, Pignon JP, Le Péchoux C, Gregor A, Stephens RJ, Kristjansen PE, Johnson BE, Ueoka H, Wagner H, Aisner J. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1224] [Cited by in F6Publishing: 1094] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 91. | Meert AP, Paesmans M, Berghmans T, Martin B, Mascaux C, Vallot F, Verdebout JM, Lafitte JJ, Sculier JP. Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer. 2001;1:5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 186] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 92. | Viani GA, Boin AC, Ikeda VY, Vianna BS, Silva RS, Santanella F. Thirty years of prophylactic cranial irradiation in patients with small cell lung cancer: a meta-analysis of randomized clinical trials. J Bras Pneumol. 2012;38:372-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Zhang W, Jiang W, Luan L, Wang L, Zheng X, Wang G. Prophylactic cranial irradiation for patients with small-cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer. 2014;14:793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 94. | Yang Y, Zhang D, Zhou X, Bao W, Ji Y, Sheng L, Cheng L, Chen Y, Du X, Qiu G. Prophylactic cranial irradiation in resected small cell lung cancer: A systematic review with meta-analysis. J Cancer. 2018;9:433-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 95. | Mamesaya N, Wakuda K, Omae K, Miyawaki E, Kotake M, Fujiwara T, Kawamura T, Kobayashi H, Nakashima K, Omori S, Ono A, Kenmotsu H, Naito T, Murakami H, Mori K, Harada H, Endo M, Nakajima T, Takahashi T. Efficacy of prophylactic cranial irradiation in patients with limited-disease small-cell lung cancer who were confirmed to have no brain metastasis via magnetic resonance imaging after initial chemoradiotherapy. Oncotarget. 2018;9:17664-17674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 96. | Farris MK, Wheless WH, Hughes RT, Soike MH, Masters AH, Helis CA, Chan MD, Cramer CK, Ruiz J, Lycan T, Petty WJ, Ahmed T, Leyrer CM, Blackstock AW. Limited-Stage Small Cell Lung Cancer: Is Prophylactic Cranial Irradiation Necessary? Pract Radiat Oncol. 2019;9:e599-e607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 97. | Qiu G, DU X, Zhou X, Bao W, Chen L, Chen J, Ji Y, Wang S. Prophylactic cranial irradiation in 399 patients with limited-stage small cell lung cancer. Oncol Lett. 2016;11:2654-2660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 98. | Eze C, Roengvoraphoj O, Niyazi M, Hildebrandt G, Fietkau R, Belka C, Manapov F. Treatment Response and Prophylactic Cranial Irradiation Are Prognostic Factors in a Real-life Limited-disease Small-cell Lung Cancer Patient Cohort Comprehensively Staged With Cranial Magnetic Resonance Imaging. Clin Lung Cancer. 2017;18:e243-e249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 99. | Le Péchoux C, Al Mohkles H, Dhermain F. Place actuelle de l'irradiation prophylactique cérébrale [Present role of prophylactic cranial irradiation]. Bull Cancer. 2013;100:35-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 100. | Wu AJ, Gillis A, Foster A, Woo K, Zhang Z, Gelblum DY, Downey RJ, Rosenzweig KE, Ong L, Perez CA, Pietanza MC, Krug L, Rudin CM, Rimner A. Patterns of failure in limited-stage small cell lung cancer: Implications of TNM stage for prophylactic cranial irradiation. Radiother Oncol. 2017;125:130-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 101. | Lou Y, Zhong R, Xu J, Qiao R, Teng J, Zhang Y, Zhang X, Chu T, Zhong H, Han B. Does surgically resected small-cell lung cancer without lymph node involvement benefit from prophylactic cranial irradiation? Thorac Cancer. 2020;11:1239-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Ozawa Y, Omae M, Fujii M, Matsui T, Kato M, Sagisaka S, Asada K, Karayama M, Shirai T, Yasuda K, Nakamura Y, Inui N, Yamada K, Yokomura K, Suda T. Management of brain metastasis with magnetic resonance imaging and stereotactic irradiation attenuated benefits of prophylactic cranial irradiation in patients with limited-stage small cell lung cancer. BMC Cancer. 2015;15:589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 103. | Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? J Clin Oncol. 1992;10:890-895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 585] [Cited by in F6Publishing: 509] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 104. | Cohen MH, Ihde DC, Bunn PA Jr, Fossieck BE Jr, Matthews MJ, Shackney SE, Johnston-Early A, Makuch R, Minna JD. Cyclic alternating combination chemotherapy for small cell bronchogenic carcinoma. Cancer Treat Rep. 1979;63:163-170. [PubMed] [Cited in This Article: ] |
| 105. | Pignon JP, Arriagada R, Ihde DC, Johnson DH, Perry MC, Souhami RL, Brodin O, Joss RA, Kies MS, Lebeau B. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327:1618-1624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 940] [Cited by in F6Publishing: 853] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 106. | Faivre-Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, Bezjak A, Cardenal F, Fournel P, Harden S, Le Pechoux C, McMenemin R, Mohammed N, O'Brien M, Pantarotto J, Surmont V, Van Meerbeeck JP, Woll PJ, Lorigan P, Blackhall F; CONVERT Study Team. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116-1125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 328] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 107. | Spigel DR, Hainsworth JD, Yardley DA, Raefsky E, Patton J, Peacock N, Farley C, Burris HA 3rd, Greco FA. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol. 2010;28:43-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 245] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 108. | Rodríguez de Dios N, Couñago F, López JL, Calvo P, Murcia M, Rico M, Vallejo C, Luna J, Trueba I, Cigarral C, Farre N, Manero RM, Durán X, Samper P. Treatment Design and Rationale for a Randomized Trial of Prophylactic Cranial Irradiation With or Without Hippocampal Avoidance for SCLC: PREMER Trial on Behalf of the Oncologic Group for the Study of Lung Cancer/Spanish Radiation Oncology Group-Radiation Oncology Clinical Research Group. Clin Lung Cancer. 2018;19:e693-e697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 109. | Belderbos JSA, De Ruysscher DKM, De Jaeger K, Koppe F, Lambrecht MLF, Lievens YN, Dieleman EMT, Jaspers JPM, Van Meerbeeck JP, Ubbels F, Kwint MH, Kuenen MA, Deprez S, De Ruiter MB, Boogerd W, Sikorska K, Van Tinteren H, Schagen SB. Phase 3 Randomized Trial of Prophylactic Cranial Irradiation With or Without Hippocampus Avoidance in SCLC (NCT01780675). J Thorac Oncol. 2021;16:840-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 110. | Rodríguez de Dios N, Murcia M, Couñago F, López JL, Rico M, Samper PM, Vallejo C, Luna FJ, Trueba I, Cigarral CC, Sotoca A, Gispert JD, Farré N, Manero RM, Capellades J, Jiménez M, Durán X, Ordoñez C, Rognoni T, Blanco M, Bacaicoa MC, Torrente M, Montero M, Alonso A, Escribano J, Martínez J, Calvo PP. Phase III trial of prophylactic cranial irradiation with or without hippocampal avoidance for small-cell lung cancer on behalf of GOECP/SEOR-GICOR. Int J Radiat Oncol Biol Phys. 2019;105:S35-S36. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 111. | Brown PD, Gondi V, Pugh S, Tome WA, Wefel JS, Armstrong TS, Bovi JA, Robinson C, Konski A, Khuntia D, Grosshans D, Benzinger TLS, Bruner D, Gilbert MR, Roberge D, Kundapur V, Devisetty K, Shah S, Usuki K, Anderson BM, Stea B, Yoon H, Li J, Laack NN, Kruser TJ, Chmura SJ, Shi W, Deshmukh S, Mehta MP, Kachnic LA; for NRG Oncology. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients With Brain Metastases: Phase III Trial NRG Oncology CC001. J Clin Oncol. 2020;38:1019-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 436] [Cited by in F6Publishing: 406] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 112. | Liao Z, Lee JJ, Komaki R, Gomez DR, O'Reilly MS, Fossella FV, Blumenschein GR Jr, Heymach JV, Vaporciyan AA, Swisher SG, Allen PK, Choi NC, DeLaney TF, Hahn SM, Cox JD, Lu CS, Mohan R. Bayesian Adaptive Randomization Trial of Passive Scattering Proton Therapy and Intensity-Modulated Photon Radiotherapy for Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36:1813-1822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 205] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 113. | Rwigema JM, Verma V, Lin L, Berman AT, Levin WP, Evans TL, Aggarwal C, Rengan R, Langer C, Cohen RB, Simone CB 2nd. Prospective study of proton-beam radiation therapy for limited-stage small cell lung cancer. Cancer. 2017;123:4244-4251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 114. | Rossi A, Di Maio M, Chiodini P, Rudd RM, Okamoto H, Skarlos DV, Früh M, Qian W, Tamura T, Samantas E, Shibata T, Perrone F, Gallo C, Gridelli C, Martelli O, Lee SM. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol. 2012;30:1692-1698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 327] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 115. | Rossi A, Garassino MC, Cinquini M, Sburlati P, Di Maio M, Farina G, Gridelli C, Torri V. Maintenance or consolidation therapy in small-cell lung cancer: a systematic review and meta-analysis. Lung Cancer. 2010;70:119-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 116. | Tiseo M, Boni L, Ambrosio F, Camerini A, Baldini E, Cinieri S, Brighenti M, Zanelli F, Defraia E, Chiari R, Dazzi C, Tibaldi C, Turolla GM, D'Alessandro V, Zilembo N, Trolese AR, Grossi F, Riccardi F, Ardizzoni A. Italian, Multicenter, Phase III, Randomized Study of Cisplatin Plus Etoposide With or Without Bevacizumab as First-Line Treatment in Extensive-Disease Small-Cell Lung Cancer: The GOIRC-AIFA FARM6PMFJM Trial. J Clin Oncol. 2017;35:1281-1287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 117. | Pujol JL, Lavole A, Quoix E, Molinier O, Souquet PJ, Barlesi F, Le Caer H, Moro-Sibilot D, Fournel P, Oster JP, Chatellain P, Barre P, Jeannin G, Mourlanette P, Derollez M, Herman D, Renault A, Dayen C, Lamy PJ, Langlais A, Morin F, Zalcman G; French Cooperative Thoracic Intergroup (IFCT). Randomized phase II-III study of bevacizumab in combination with chemotherapy in previously untreated extensive small-cell lung cancer: results from the IFCT-0802 trial†. Ann Oncol. 2015;26:908-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 118. | Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, Reck M, Mok T, Lam S, Shames DS, Liu J, Ding B, Lopez-Chavez A, Kabbinavar F, Lin W, Sandler A, Liu SV; IMpower133 Study Group. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379:2220-2229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1567] [Cited by in F6Publishing: 1868] [Article Influence: 311.3] [Reference Citation Analysis (0)] |
| 119. | Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, Voitko O, Poltoratskiy A, Ponce S, Verderame F, Havel L, Bondarenko I, Kazarnowicz A, Losonczy G, Conev NV, Armstrong J, Byrne N, Shire N, Jiang H, Goldman JW; CASPIAN investigators. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929-1939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 831] [Cited by in F6Publishing: 1056] [Article Influence: 211.2] [Reference Citation Analysis (0)] |
| 120. | Reck M, Liu SV, Mansfield AS, Mok TSK, Scherpereel A, Reinmuth N, Garassino MC, CastroDe Carpeno J, Califano R, Nishio M, Orlandi F, Alatorre Alexander AJ, Leal TA, Cheng Y, Lee JS, Lam S, McCleland M, Deng Y, Phan S, Horn L. IMpower133: updated overall survival (OS) analysis of first-line (1L) atezolizumab (atezo) + carboplatin + etoposide in extensive-stage SCLC (ES-SCLC). Ann Oncol. 2019;30 Suppl 9:v710-v717. [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 121. | Ortega-Franco A, Ackermann C, Paz-Ares L, Califano R. First-line immune checkpoint inhibitors for extensive stage small-cell lung cancer: clinical developments and future directions. ESMO Open. 2021;6:100003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 122. | Paz-Ares LG, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair M, Özgüroğlu M, Ji JH, Voitko O, Poltoratskiy A, Verderame F, Havel L, Bondarenko I, Armstrong J, Byrne N, Jiang H, Wade Goldman J. Durvalumab ± tremelimumab + platinum-etoposide in first-line extensive-stage SCLC (ES-SCLC): Updated Results from the phase III CASPIAN study. J Clin Oncol. 2020;38:9002. [Cited in This Article: ] |
| 123. | Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, Garassino MC, Voitko O, Poltoratskiy A, Ponce S, Verderame F, Havel L, Bondarenko I, Każarnowicz A, Losonczy G, Conev NV, Armstrong J, Byrne N, Thiyagarajah P, Jiang H, Paz-Ares L; CASPIAN investigators. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22:51-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 297] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 124. | Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, Cheema PK, Rodriguez-Abreu D, Wollner M, Yang JC, Mazieres J, Orlandi FJ, Luft A, Gümüş M, Kato T, Kalemkerian GP, Luo Y, Ebiana V, Pietanza MC, Kim HR; KEYNOTE-604 Investigators. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol. 2020;38:2369-2379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 368] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 125. | Leal T, Wang Y, Dowlati A, Andrew Lewis D, Chen Y, Ramesh Mohindra A, Razaq M, Ahuja HG, Liu J, King DM, Sumey CJ, Ramalingam SS. Randomized phase II clinical trial of cisplatin/carboplatin and etoposide (CE) alone or in combination with nivolumab as frontline therapy for extensive-stage small cell lung cancer (ES-SCLC): ECOG-ACRIN EA5161. J Clin Oncol. 2020;38:9000. [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 126. | Zhou T, Zhang Z, Luo F, Zhao Y, Hou X, Liu T, Wang K, Zhao H, Huang Y, Zhang L. Comparison of First-Line Treatments for Patients With Extensive-Stage Small Cell Lung Cancer: A Systematic Review and Network Meta-analysis. JAMA Netw Open. 2020;3:e2015748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 127. | Facchinetti F, Di Maio M, Tiseo M. Adding PD-1/PD-L1 Inhibitors to Chemotherapy for the First-Line Treatment of Extensive Stage Small Cell Lung Cancer (SCLC): A Meta-Analysis of Randomized Trials. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 128. | Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Lu H, Cuillerot JM, Lynch TJ. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24:75-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 413] [Cited by in F6Publishing: 479] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 129. | Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, Pietanza MC, Wu YL, Zielinski C, Thomas M, Felip E, Gold K, Horn L, Aerts J, Nakagawa K, Lorigan P, Pieters A, Kong Sanchez T, Fairchild J, Spigel D. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J Clin Oncol. 2016;34:3740-3748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 356] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 130. | Zatloukal P, Cardenal F, Szczesna A, Gorbunova V, Moiseyenko V, Zhang X, Cisar L, Soria JC, Domine M, Thomas M. A multicenter international randomized phase III study comparing cisplatin in combination with irinotecan or etoposide in previously untreated small-cell lung cancer patients with extensive disease. Ann Oncol. 2010;21:1810-1816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 131. | Lara PN Jr, Natale R, Crowley J, Lenz HJ, Redman MW, Carleton JE, Jett J, Langer CJ, Kuebler JP, Dakhil SR, Chansky K, Gandara DR. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27:2530-2535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 366] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 132. | Hanna N, Bunn PA Jr, Langer C, Einhorn L, Guthrie T Jr, Beck T, Ansari R, Ellis P, Byrne M, Morrison M, Hariharan S, Wang B, Sandler A. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038-2043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 469] [Cited by in F6Publishing: 463] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 133. | Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, Postmus P, Collette L, Musat E, Senan S; EORTC Radiation Oncology Group and Lung Cancer Group. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 768] [Cited by in F6Publishing: 702] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 134. | Rule WG, Foster NR, Meyers JP, Ashman JB, Vora SA, Kozelsky TF, Garces YI, Urbanic JJ, Salama JK, Schild SE. Prophylactic cranial irradiation in elderly patients with small cell lung cancer: findings from a North Central Cancer Treatment Group pooled analysis. J Geriatr Oncol. 2015;6:119-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 135. | Ge W, Xu H, Yan Y, Cao D. The effects of prophylactic cranial irradiation versus control on survival of patients with extensive-stage small-cell lung cancer: a meta-analysis of 14 trials. Radiat Oncol. 2018;13:155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 136. | Wen P, Wang TF, Li M, Yu Y, Zhou YL, Wu CL. Meta-analysis of prophylactic cranial irradiation or not in treatment of extensive-stage small-cell lung cancer: The dilemma remains. Cancer Radiother. 2020;24:44-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 137. | Takahashi T, Yamanaka T, Seto T, Harada H, Nokihara H, Saka H, Nishio M, Kaneda H, Takayama K, Ishimoto O, Takeda K, Yoshioka H, Tachihara M, Sakai H, Goto K, Yamamoto N. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:663-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 305] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 138. | Yin X, Yan D, Qiu M, Huang L, Yan SX. Prophylactic cranial irradiation in small cell lung cancer: a systematic review and meta-analysis. BMC Cancer. 2019;19:95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 139. | Gondi V, Paulus R, Bruner DW, Meyers CA, Gore EM, Wolfson A, Werner-Wasik M, Sun AY, Choy H, Movsas B. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys. 2013;86:656-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 140. | Le Péchoux C, Dunant A, Senan S, Wolfson A, Quoix E, Faivre-Finn C, Ciuleanu T, Arriagada R, Jones R, Wanders R, Lerouge D, Laplanche A; Prophylactic Cranial Irradiation (PCI) Collaborative Group. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol. 2009;10:467-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 141. | Hurwitz JL, McCoy F, Scullin P, Fennell DA. New advances in the second-line treatment of small cell lung cancer. Oncologist. 2009;14:986-994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 142. | Schneider BJ. Management of recurrent small cell lung cancer. J Natl Compr Canc Netw. 2008;6:323-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 143. | Owonikoko TK, Behera M, Chen Z, Bhimani C, Curran WJ, Khuri FR, Ramalingam SS. A systematic analysis of efficacy of second-line chemotherapy in sensitive and refractory small-cell lung cancer. J Thorac Oncol. 2012;7:866-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 144. | Baize N, Monnet I, Greillier L, Geier M, Lena H, Janicot H, Vergnenegre A, Crequit J, Lamy R, Auliac JB, Letreut J, Le Caer H, Gervais R, Dansin E, Madroszyk A, Renault PA, Le Garff G, Falchero L, Berard H, Schott R, Saulnier P, Chouaid C; Groupe Français de Pneumo-Cancérologie 01–13 investigators. Carboplatin plus etoposide versus topotecan as second-line treatment for patients with sensitive relapsed small-cell lung cancer: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2020;21:1224-1233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 145. | Naito Y, Yamada K, Imamura Y, Ishii H, Matsuo N, Tokito T, Kinoshita T, Azuma K, Hoshino T. Rechallenge treatment with a platinum-based regimen in patients with sensitive relapsed small-cell lung cancer. Med Oncol. 2018;35:61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 146. | O'Brien ME, Ciuleanu TE, Tsekov H, Shparyk Y, Cuceviá B, Juhasz G, Thatcher N, Ross GA, Dane GC, Crofts T. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24:5441-5447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 410] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 147. | von Pawel J, Schiller JH, Shepherd FA, Fields SZ, Kleisbauer JP, Chrysson NG, Stewart DJ, Clark PI, Palmer MC, Depierre A, Carmichael J, Krebs JB, Ross G, Lane SR, Gralla R. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 669] [Cited by in F6Publishing: 632] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 148. | Allen JW, Moon J, Redman M, Gadgeel SM, Kelly K, Mack PC, Saba HM, Mohamed MK, Jahanzeb M, Gandara DR. Southwest Oncology Group S0802: a randomized, phase II trial of weekly topotecan with and without ziv-aflibercept in patients with platinum-treated small-cell lung cancer. J Clin Oncol. 2014;32:2463-2470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 149. | Trigo J, Subbiah V, Besse B, Moreno V, López R, Sala MA, Peters S, Ponce S, Fernández C, Alfaro V, Gómez J, Kahatt C, Zeaiter A, Zaman K, Boni V, Arrondeau J, Martínez M, Delord JP, Awada A, Kristeleit R, Olmedo ME, Wannesson L, Valdivia J, Rubio MJ, Anton A, Sarantopoulos J, Chawla SP, Mosquera-Martinez J, D'Arcangelo M, Santoro A, Villalobos VM, Sands J, Paz-Ares L. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020;21:645-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 143] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 150. | Calvo E, Moreno V, Flynn M, Holgado E, Olmedo ME, Lopez Criado MP, Kahatt C, Lopez-Vilariño JA, Siguero M, Fernandez-Teruel C, Cullell-Young M, Soto Matos-Pita A, Forster M. Antitumor activity of lurbinectedin (PM01183) and doxorubicin in relapsed small-cell lung cancer: results from a phase I study. Ann Oncol. 2017;28:2559-2566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 151. | Farago AF, Drapkin BJ, Lopez-Vilarino de Ramos JA, Galmarini CM, Núñez R, Kahatt C, Paz-Ares L. ATLANTIS: a Phase III study of lurbinectedin/doxorubicin versus topotecan or cyclophosphamide/doxorubicin/vincristine in patients with small-cell lung cancer who have failed one prior platinum-containing line. Future Oncol. 2019;15:231-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 152. | von Pawel J, Jotte R, Spigel DR, O'Brien ME, Socinski MA, Mezger J, Steins M, Bosquée L, Bubis J, Nackaerts K, Trigo JM, Clingan P, Schütte W, Lorigan P, Reck M, Domine M, Shepherd FA, Li S, Renschler MF. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol. 2014;32:4012-4019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 153. | Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, Jäger D, Pietanza MC, Le DT, de Braud F, Morse MA, Ascierto PA, Horn L, Amin A, Pillai RN, Evans J, Chau I, Bono P, Atmaca A, Sharma P, Harbison CT, Lin CS, Christensen O, Calvo E. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883-895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 901] [Cited by in F6Publishing: 902] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 154. | Ready NE, Ott PA, Hellmann MD, Zugazagoitia J, Hann CL, de Braud F, Antonia SJ, Ascierto PA, Moreno V, Atmaca A, Salvagni S, Taylor M, Amin A, Camidge DR, Horn L, Calvo E, Li A, Lin WH, Callahan MK, Spigel DR. Nivolumab Monotherapy and Nivolumab Plus Ipilimumab in Recurrent Small Cell Lung Cancer: Results From the CheckMate 032 Randomized Cohort. J Thorac Oncol. 2020;15:426-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 164] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 155. | Spigel DR, Vicente D, Ciuleanu TE, Gettinger S, Peters S, Horn L, Audigier-Valette C, Pardo Aranda N, Juan-Vidal O, Cheng Y, Zhang H, Shi M, Luft A, Wolf J, Antonia S, Nakagawa K, Fairchild J, Baudelet C, Pandya D, Doshi P, Chang H, Reck M. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331☆. Ann Oncol. 2021;32:631-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 137] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 156. | Ott PA, Elez E, Hiret S, Kim DW, Morosky A, Saraf S, Piperdi B, Mehnert JM. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. J Clin Oncol. 2017;35:3823-3829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 339] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 157. | Chung HC, Lopez-Martin JA, Kao SC, Miller WH, Ros W, Gao B, Marabelle A, Gottfried M, Zer A, Delord JP, Penel N, Jalal SI, Xu L, Zeigenfuss S, Pruitt SK, Piha-Paul SA. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J Clin Oncol. 2018;36:8506-8506. [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 158. | Chung HC, Piha-Paul SA, Lopez-Martin J. -Pembrolizumab after two or more lines od prior therapy in patients with advance small-cell lung cancer (SCLC). Proceedings of the AACR Annual Meeting; 2019; Leeds, GA. Atlanta 2019: Abstract CT073. [Cited in This Article: ] |
| 159. | Pujol JL, Greillier L, Audigier-Valette C, Moro-Sibilot D, Uwer L, Hureaux J, Guisier F, Carmier D, Madelaine J, Otto J, Gounant V, Merle P, Mourlanette P, Molinier O, Renault A, Rabeau A, Antoine M, Denis MG, Bommart S, Langlais A, Morin F, Souquet PJ. A Randomized Non-Comparative Phase II Study of Anti-Programmed Cell Death-Ligand 1 Atezolizumab or Chemotherapy as Second-Line Therapy in Patients With Small Cell Lung Cancer: Results From the IFCT-1603 Trial. J Thorac Oncol. 2019;14:903-913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 160. | Kim YJ, Keam B, Ock CY, Song S, Kim M, Kim SH, Kim KH, Kim JS, Kim TM, Kim DW, Lee JS, Heo DS. A phase II study of pembrolizumab and paclitaxel in patients with relapsed or refractory small-cell lung cancer. Lung Cancer. 2019;136:122-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 161. | Pietanza MC, Kadota K, Huberman K, Sima CS, Fiore JJ, Sumner DK, Travis WD, Heguy A, Ginsberg MS, Holodny AI, Chan TA, Rizvi NA, Azzoli CG, Riely GJ, Kris MG, Krug LM. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res. 2012;18:1138-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 162. | Zauderer MG, Drilon A, Kadota K, Huberman K, Sima CS, Bergagnini I, Sumner DK, Travis WD, Heguy A, Ginsberg MS, Holodny AI, Riely GJ, Kris MG, Krug LM, Pietanza MC. Trial of a 5-day dosing regimen of temozolomide in patients with relapsed small cell lung cancers with assessment of methylguanine-DNA methyltransferase. Lung Cancer. 2014;86:237-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 163. | Masuda N, Fukuoka M, Kusunoki Y, Matsui K, Takifuji N, Kudoh S, Negoro S, Nishioka M, Nakagawa K, Takada M. CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol. 1992;10:1225-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 296] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 164. | Yamamoto N, Tsurutani J, Yoshimura N, Asai G, Moriyama A, Nakagawa K, Kudoh S, Takada M, Minato Y, Fukuoka M. Phase II study of weekly paclitaxel for relapsed and refractory small cell lung cancer. Anticancer Res. 2006;26:777-781. [PubMed] [Cited in This Article: ] |
| 165. | Smit EF, Fokkema E, Biesma B, Groen HJ, Snoek W, Postmus PE. A phase II study of paclitaxel in heavily pretreated patients with small-cell lung cancer. Br J Cancer. 1998;77:347-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 124] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 166. | Smyth JF, Smith IE, Sessa C, Schoffski P, Wanders J, Franklin H, Kaye SB. Activity of docetaxel (Taxotere) in small cell lung cancer. The Early Clinical Trials Group of the EORTC. Eur J Cancer. 1994;30A:1058-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 77] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 167. | Masters GA, Declerck L, Blanke C, Sandler A, DeVore R, Miller K, Johnson D; Eastern Cooperative Oncology Group. Phase II trial of gemcitabine in refractory or relapsed small-cell lung cancer: Eastern Cooperative Oncology Group Trial 1597. J Clin Oncol. 2003;21:1550-1555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 168. | Hoang T, Kim K, Jaslowski A, Koch P, Beatty P, McGovern J, Quisumbing M, Shapiro G, Witte R, Schiller JH. Phase II study of second-line gemcitabine in sensitive or refractory small cell lung cancer. Lung Cancer. 2003;42:97-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 169. | Furuse K, Kubota K, Kawahara M, Takada M, Kimura I, Fujii M, Ohta M, Hasegawa K, Yoshida K, Nakajima S, Ogura T, Niitani H. Phase II study of vinorelbine in heavily previously treated small cell lung cancer. Japan Lung Cancer Vinorelbine Study Group. Oncology. 1996;53:169-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 170. | Kang JH, Lee KH, Kim DW, Kim SW, Kim HR, Kim JH, Choi JH, An HJ, Kim JS, Jang JS, Kim BS, Kim HT. A randomised phase 2b study comparing the efficacy and safety of belotecan vs. topotecan as monotherapy for sensitive-relapsed small-cell lung cancer. Br J Cancer. 2021;124:713-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 171. | Byers LA, Wang J, Nilsson MB, Fujimoto J, Saintigny P, Yordy J, Giri U, Peyton M, Fan YH, Diao L, Masrorpour F, Shen L, Liu W, Duchemann B, Tumula P, Bhardwaj V, Welsh J, Weber S, Glisson BS, Kalhor N, Wistuba II, Girard L, Lippman SM, Mills GB, Coombes KR, Weinstein JN, Minna JD, Heymach JV. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012;2:798-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 392] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 172. | de Bono J, Ramanathan RK, Mina L, Chugh R, Glaspy J, Rafii S, Kaye S, Sachdev J, Heymach J, Smith DC, Henshaw JW, Herriott A, Patterson M, Curtin NJ, Byers LA, Wainberg ZA. Phase I, Dose-Escalation, Two-Part Trial of the PARP Inhibitor Talazoparib in Patients with Advanced Germline BRCA1/2 Mutations and Selected Sporadic Cancers. Cancer Discov. 2017;7:620-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 300] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 173. | Penella W, Gaunt P, Steele N, Ahmed S, Mulatero C, Shah R, Danson S, Hodgkinson E, James K, Watkins B, Fletcher P, Billingham L. P1.07-015 STOMP: A UK National Cancer Research Network Randomised, Double Blind, Multicentre Phase II Trial of Olaparib as Maintenance Therapy in SCLC. J Thorac Oncol. 2017;12:S704-S705. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 174. | Owonikoko TK, Dahlberg SE, Sica GL, Wagner LI, Wade JL 3rd, Srkalovic G, Lash BW, Leach JW, Leal TB, Aggarwal C, Ramalingam SS. Randomized Phase II Trial of Cisplatin and Etoposide in Combination With Veliparib or Placebo for Extensive-Stage Small-Cell Lung Cancer: ECOG-ACRIN 2511 Study. J Clin Oncol. 2019;37:222-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 175. | Poirier JT, George J, Owonikoko TK, Berns A, Brambilla E, Byers LA, Carbone D, Chen HJ, Christensen CL, Dive C, Farago AF, Govindan R, Hann C, Hellmann MD, Horn L, Johnson JE, Ju YS, Kang S, Krasnow M, Lee J, Lee SH, Lehman J, Lok B, Lovly C, MacPherson D, McFadden D, Minna J, Oser M, Park K, Park KS, Pommier Y, Quaranta V, Ready N, Sage J, Scagliotti G, Sos ML, Sutherland KD, Travis WD, Vakoc CR, Wait SJ, Wistuba I, Wong KK, Zhang H, Daigneault J, Wiens J, Rudin CM, Oliver TG. New Approaches to SCLC Therapy: From the Laboratory to the Clinic. J Thorac Oncol. 2020;15:520-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 176. | Pietanza MC, Waqar SN, Krug LM, Dowlati A, Hann CL, Chiappori A, Owonikoko TK, Woo KM, Cardnell RJ, Fujimoto J, Long L, Diao L, Wang J, Bensman Y, Hurtado B, de Groot P, Sulman EP, Wistuba II, Chen A, Fleisher M, Heymach JV, Kris MG, Rudin CM, Byers LA. Randomized, Double-Blind, Phase II Study of Temozolomide in Combination With Either Veliparib or Placebo in Patients With Relapsed-Sensitive or Refractory Small-Cell Lung Cancer. J Clin Oncol. 2018;36:2386-2394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 256] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 177. | Farago AF, Yeap BY, Stanzione M, Hung YP, Heist RS, Marcoux JP, Zhong J, Rangachari D, Barbie DA, Phat S, Myers DT, Morris R, Kem M, Dubash TD, Kennedy EA, Digumarthy SR, Sequist LV, Hata AN, Maheswaran S, Haber DA, Lawrence MS, Shaw AT, Mino-Kenudson M, Dyson NJ, Drapkin BJ. Combination Olaparib and Temozolomide in Relapsed Small-Cell Lung Cancer. Cancer Discov. 2019;9:1372-1387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 178. | Thomas A, Vilimas R, Trindade C, Erwin-Cohen R, Roper N, Xi L, Krishnasamy V, Levy E, Mammen A, Nichols S, Chen Y, Velcheti V, Yin F, Szabo E, Pommier Y, Steinberg SM, Trepel JB, Raffeld M, Young HA, Khan J, Hewitt S, Lee JM. Durvalumab in Combination with Olaparib in Patients with Relapsed SCLC: Results from a Phase II Study. J Thorac Oncol. 2019;14:1447-1457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 179. | Sen T, Della Corte CM, Milutinovic S, Cardnell RJ, Diao L, Ramkumar K, Gay CM, Stewart CA, Fan Y, Shen L, Hansen RJ, Strouse B, Hedrick MP, Hassig CA, Heymach JV, Wang J, Byers LA. Combination Treatment of the Oral CHK1 Inhibitor, SRA737, and Low-Dose Gemcitabine Enhances the Effect of Programmed Death Ligand 1 Blockade by Modulating the Immune Microenvironment in SCLC. J Thorac Oncol. 2019;14:2152-2163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 180. | Lallo A, Frese KK, Morrow CJ, Sloane R, Gulati S, Schenk MW, Trapani F, Simms N, Galvin M, Brown S, Hodgkinson CL, Priest L, Hughes A, Lai Z, Cadogan E, Khandelwal G, Simpson KL, Miller C, Blackhall F, O'Connor MJ, Dive C. The Combination of the PARP Inhibitor Olaparib and the WEE1 Inhibitor AZD1775 as a New Therapeutic Option for Small Cell Lung Cancer. Clin Cancer Res. 2018;24:5153-5164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 181. | Ponce S, Coté GM, Falcon A, Jimenez-Aguilar E. Eficacy and safety profile of lurbinectedin-irinotecan in patients with relapsed SCLC. Results from a phase Ib/II trial. Proceedings of the 2020 World Conference on Lung Cancer; 2021 Jan 28-31; Leeds, Singapore. [Cited in This Article: ] |
| 182. | Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, Byers LA, Johnson ML, Burris HA 3rd, Robert F, Han TH, Bheddah S, Theiss N, Watson S, Mathur D, Vennapusa B, Zayed H, Lally S, Strickland DK, Govindan R, Dylla SJ, Peng SL, Spigel DR; SCRX16-001 investigators. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017;18:42-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 357] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 183. | Morgensztern D, Besse B, Greillier L, Santana-Davila R, Ready N, Hann CL, Glisson BS, Farago AF, Dowlati A, Rudin CM, Le Moulec S, Lally S, Yalamanchili S, Wolf J, Govindan R, Carbone DP. Efficacy and Safety of Rovalpituzumab Tesirine in Third-Line and Beyond Patients with DLL3-Expressing, Relapsed/Refractory Small-Cell Lung Cancer: Results From the Phase II TRINITY Study. Clin Cancer Res. 2019;25:6958-6966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 184] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 184. | Blackhall F, Jao K, Greillier L, Cho BC, Penkov K, Reguart N, Majem M, Nackaerts K, Syrigos K, Hansen K, Schuette W, Cetnar J, Cappuzzo F, Okamoto I, Erman M, Langer SW, Kato T, Groen H, Sun Z, Luo Y, Tanwani P, Caffrey L, Komarnitsky P, Reinmuth N. Efficacy and Safety of Rovalpituzumab Tesirine Compared With Topotecan as Second-Line Therapy in DLL3-High SCLC: Results From the Phase 3 TAHOE Study. J Thorac Oncol. 2021;16:1547-1558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 185. | Giffin MJ, Cooke K, Lobenhofer EK, Estrada J, Zhan J, Deegen P, Thomas M, Murawsky CM, Werner J, Liu S, Lee F, Homann O, Friedrich M, Pearson JT, Raum T, Yang Y, Caenepeel S, Stevens J, Beltran PJ, Canon J, Coxon A, Bailis JM, Hughes PE. AMG 757, a Half-Life Extended, DLL3-Targeted Bispecific T-Cell Engager, Shows High Potency and Sensitivity in Preclinical Models of Small-Cell Lung Cancer. Clin Cancer Res. 2021;27:1526-1537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 186. | Slotman BJ, van Tinteren H, Praag JO, Knegjens JL, El Sharouni SY, Hatton M, Keijser A, Faivre-Finn C, Senan S. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015;385:36-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 334] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 187. | Rusthoven CG, Yamamoto M, Bernhardt D, Smith DE, Gao D, Serizawa T, Yomo S, Aiyama H, Higuchi Y, Shuto T, Akabane A, Sato Y, Niranjan A, Faramand AM, Lunsford LD, McInerney J, Tuanquin LC, Zacharia BE, Chiang V, Singh C, Yu JB, Braunstein S, Mathieu D, Touchette CJ, Lee CC, Yang HC, Aizer AA, Cagney DN, Chan MD, Kondziolka D, Bernstein K, Silverman JS, Grills IS, Siddiqui ZA, Yuan JC, Sheehan JP, Cordeiro D, Nosaki K, Seto T, Deibert CP, Verma V, Day S, Halasz LM, Warnick RE, Trifiletti DM, Palmer JD, Attia A, Li B, Cifarelli CP, Brown PD, Vargo JA, Combs SE, Kessel KA, Rieken S, Patel S, Guckenberger M, Andratschke N, Kavanagh BD, Robin TP. Evaluation of First-line Radiosurgery vs Whole-Brain Radiotherapy for Small Cell Lung Cancer Brain Metastases: The FIRE-SCLC Cohort Study. JAMA Oncol. 2020;6:1028-1037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |