Published online Mar 24, 2022. doi: 10.5306/wjco.v13.i3.200
Peer-review started: April 9, 2021
First decision: July 29, 2021
Revised: August 16, 2021
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: March 24, 2022
Celiac disease (CeD) is a chronic autoimmune disorder that is triggered by gluten in genetically susceptible individuals, and that is characterized by CeD-specific antibodies, HLA-DQ2 and/or HLA-DQ8 haplotypes, enteropathy and different clinical pictures related to many organs. Intestinal lymphoma may develop as a result of refractory CeD. If a patient diagnosed with CeD is symptomatic despite a strict gluten-free diet for at least 12 months, and does not improve with severe villous atrophy, refractory CeD can be considered present. The second of the two types of refractory CeD has abnormal monoclonal intraepithelial lymphocytes and can be considered as pre-lymphoma, and the next picture that will emerge is enteropathy-associated T-cell lymphoma. This manuscript addresses "CeD and malignancies" through a review of current literature and guidelines.
Core Tip: Malignancies are among the leading consequence of celiac disease (CeD), and intestinal lymphoma and adenocarcinomas in particular. Enteropathy-associated T-cell lymphoma type 1 has been shown to develop from refractory CeD type 2, while the association of CeD with other cancer types is controversial. Decades of reported studies suggest that a non-delayed diagnosis of CeD and strict adherence to a gluten-free diet significantly reduces the rate of cancer development associated with CeD.
- Citation: Demiroren K. Possible relationship between refractory celiac disease and malignancies. World J Clin Oncol 2022; 13(3): 200-208
- URL: https://www.wjgnet.com/2218-4333/full/v13/i3/200.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i3.200
Celiac disease (CeD) is a chronic autoimmune disorder that is triggered by gluten in genetically susceptible persons with HLA-DQ2 and/or HLA-DQ8 haplotypes, and is characterized by CeD-specific antibodies and enteropathy[1-3]. The prevalence of CeD in the general population is approximately 1% on serological screening, and 0.6% as histologically confirmed[3].
CeD can affect many organs, and can cause or trigger, or be associated with different clinical pictures, including growth retardation, short stature, chronic diarrhea, constipation[1], iron deficiency anemia[4], dermatitis herpetiformis[5], dental enemal defects[6], aphthous stomatitis[7], rickets, osteoporosis[8,9], arthralgia, arthritis[10], idiopathic epilepsy[11], peripheral neuropathy[12], ataxia[13], abnormal liver tests, autoimmune hepatitis[14], type1 diabetes mellitus[15], IgA deficiency[16], psychiatric comorbidities[17], intestinal lymphoma[3], etc. It is not known exactly why these clinical pictures emerge as different manifestations in different patients, as there are complex underlying mechanisms. Although the relationship between CeD and intestinal lymphoma is known, there have been many studies and case reports suggesting its association with other malignancies. For the present manuscript, a systematic literature search of PubMed/MEDLINE was carried out using the search terms “Celiac disease AND guideline, and Celiac disease AND malignancy” and a review was made on the subject of "CeD and malignancies" in current literature and guidelines in line with the following structure: (1) Pathogenesis of CeD; (2) Refractory CeD; (3) Enteropathy-associated T-cell lymphoma (EATL); (4) CeD and malignancies; and (5) Conclusion.
Although the pathogenesis of CeD is not fully understood, it is considered to be attributable to the coaction of genetic, environmental and immunologic factors. The HLA-DQ2 and/or HLA-DQ8 haplotypes are necessary for CeD development. Studies have shown that around 4% of HLA-DQ2 + cases develop CeD, and that HLA-DQ2 and HLA-DQ8 negative CeD development is extremely rare[18]. It is evident that environmental factors are at the core of the CeD pathogenesis, of which gluten is the sine qua non trigger. The gliadin proteins found in gluten are composed of glutamine and prolamine residues, and cannot be fully digested, even in a healthy person. HLA-DQ2 and HLA-DQ8 proteins are located on the surface of intestinal antigen-presenting cells. Undigested gliadin peptides in the intestinal lumen pass through the intestinal epithelium and undergo cross-linking and deamination through tissue transglutaminase (tTG) in the lamina propria. The glutamine contained within gliadin is converted to glutamic acid, bound to HLA-DQ2 and HLA-DQ8 and presented to CD4+ T cells. The cross-linking of gliadin and tTG results in the formation of tTG antibodies that impair the function of tTG. Activated CD4+ T cells cause the production of pro-inflammatory cytokines like interferon-γ that contain T-helper cells that worsen the inflammatory effect in the process. Matrix metalloproteinases cause the degradation of the extracellular matrix and damage to the basement membranes, resulting in an increase in natural killer (NK) T lymphocytes within the epithelial cell. Gliadins also upregulate the expression of the zonulin protein by increasing intestinal permeability in both CeD patients and healthy people. Increased anti-tTG levels are also known to inhibit tTG and make gliadin harder to digest, which in turn increases tTG activity, resulting in a vicious cycle. Intraepithelial lymphocytes (IELs) include T cell receptor (TCR)αβ+ and -γδ+ T cells, and NK cells. Most of these TCR+ IELs express a variety of NK cell receptors, and in addition, the number of CD8+ TCRαβ+ and TCRγδ+ increases. Consequently, characteristic lesions of CeD develop by apoptosis[2,18-22].
CeD is in general similar to other autoimmune diseases, but has a very clear and indispensable trigger: gluten. Gluten-induced intestinal lesions and autoantibodies begin to improve in the absence of gluten. Anti-tTG antibodies increase to protect against the disease, and are at the center of the pathogenesis. They may appear before villous atrophy develops and can induce CeD[21].
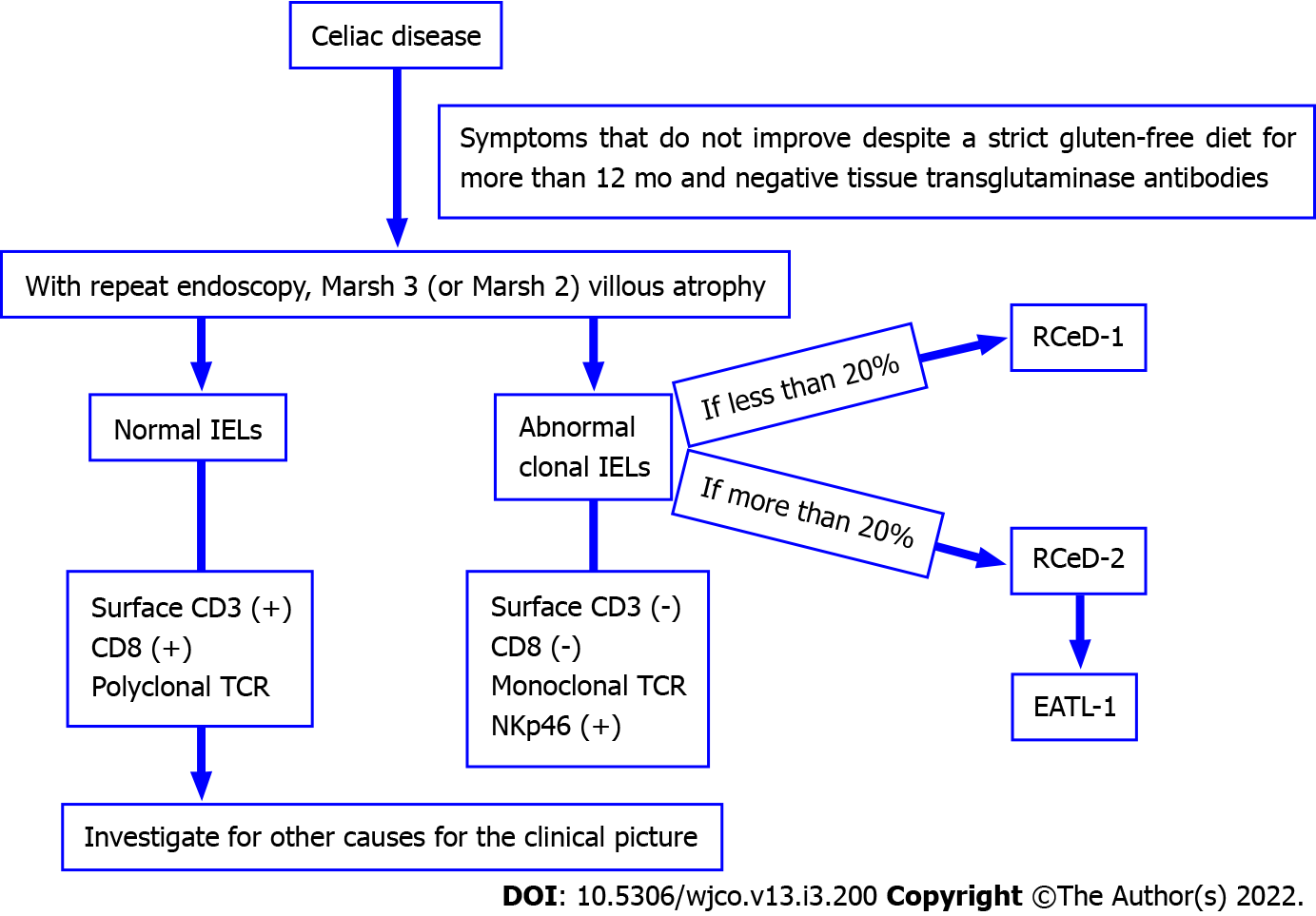
Refractory CeD (RCeD) patients are those with a pre-existing diagnosis of CeD whose CeD-related symptoms fail to improve, and in whom villous atrophy develops despite a strict gluten-free diet for more than 12 months[23-25]. RCeD is mostly diagnosed after the age of 50 years, but younger cases have been identified. The incidence for both types of RCeD is in the 0.04%-1.5% range[3].
When RCeD is suspected, a second endoscopy and several biopsies are mandatory. Duodenal biopsies show Marsh type III, and sometimes Marsh type II[3]. The presence of subepithelial collagen extending to the lamina propria in the duodenal second part, chronic inflammation and crypt hypoplasia (not hyperplasia) with villous atrophy are common microscopic findings of RCeD[23].
Refractory CeD is divided histologically into two subgroups according to the immunophenotype of IELs: type 1 (RCeD-1) and type 2 (RCeD-2). RCeD-1 has a normal intraepithelial lymphocyte phenotype while RCeD2 has an abnormal clonal lymphocyte population[25]. In RCeD-1, the symptoms are less severe, and the endoscopic and histological features are similar to active uncomplicated CeD. RCeD-1 shows the same normal immunophenotype as CeD, often leading to difficulties in differential diagnosis from CeD, although differentiating between RCeD-1 and RCeD-2 is mandatory due to the different treatment strategies and prognosis[3].
The immunophenotype of abnormal IELs in RCeD-2 is different to that of RCeD-1. It has been reported that interleukin-15 and somatic mutations in JAK1 or STAT3 in the proliferation of aberrant T cells play an important role in the formation of RCeD-2[24]. Cording et al[26] identified a complex mutational profile of JAK1 and STAT3 that activated the NF-κB pathway in CeD-associated lymphomagenesis.
While most lymphocytes express CD3, CD8 and polyclonal TCRβ, RCeD-2 is characterized by abnormal T cells that do not express surface CD3 or CD8, but instead express intracellular CD3 by a TCR gamma rearrangement[23-25], and these cells also express NK surface markers[24,27]. RCeD-1 becomes involved when abnormal T cells account for less than 20%, and RCeD-2 for more than 20%. RCeD-2 may be referred to as pre-lymphoma or low grade lymphoma due to the high risk of conversion to EATL[3,28]. Verbeek et al[29] suggest that the quantification of abnormal T cells using flow cytometry is preferable to T cell clonality analyses in differentiating RCeD patients. The use of a cut-off value of 20% for the classification of patients can also support the selection of long-term follow-up and treatment.
Figure 1 summarizes the properties of RCeD-1, RCeD-2 and EATL.
The goal of treatment is to prevent RCeD-1 patients from converting to RCeD-2, and then to EATL, in that a total of 52% of RceD-2 patients have been reported to develop EATL within 4–6 years of diagnosis of RCEeD-2[30]. Immunosuppressive drugs are used together with nutritional support for the treatment of RCeD-1. Although similar therapies have been applied for RCeD-2, their usefulness is limited. In such patients, autologous hematopoietic stem cell transplantation following high-dose chemotherapy is an alternative treatment[3,31].
Enteropathy-associated T-cell lymphoma accounts for less than 1% of all non-Hodgkin lymphomas, and as such is considered a rare GI lymphoma[3]. Approximately 50% of RCeD-2 patients are thought to develop overt lymphoma within 5 years of diagnosis[18]. EATL occurs predominantly in patients in the sixth and seventh decades, and usually develops in those diagnosed with CeD[25,32,33]. EATL is thought to be derived from IELs, and the abnormal immune phenotype of IELs seen in RCeD-2 indicates early-stage lymphoma development. To date, two histologically subtypes of EATL have been described[23].
A microscopic examination of type I EATL (EATL-1) reveals transmural infiltration including pleomorphic medium- to large-size neoplastic lymphocytes, histiocytes and eosinophils. Mitotic figures and necrosis are common, and enteropathic changes such as villous atrophy, crypt hyperplasia and intraepithelial lymphocytosis may be seen in the non-tumor gastrointestinal tract mucosa[25,33]. Tumor cells in EATL-1 have a pattern of CD2+, CD3+, CD5-, CD4-, CD7+, CD8-, CD56-, TCR- (usually), CD103+ and CD30+ (often), and a high Ki-67 proliferative index and p53 expression. Epstein-Barr virus is negative[33]. In some cases, tumor cells may show pronounced pleomorphism reminiscent of anaplastic large cell lymphoma or Hodgkin lymphoma[23]. The IELs in the non-neoplastic mucosa have the same immunophenotype as in RCeD-2. Type 2 EATL (EATL-2) is rare, and is generally not associated with a previous diagnosis of CeD[3]. While the features of non-tumoral mucosa resemble those of CeD, the tumor cells in EATL-2 have a CD3+, CD8+, CD56+ or CD4- pattern. NKp46, indicating progression from RCeD-2, has also been reported in EATL[23].
The increased risk of malignant lymphomas in CeD is correlated to small bowel histopathology, and so no increased risk of lymphoma is expected in CeD patients with improved intestinal mucosal changes and with a gluten-free diet, or in potential CeD patients with an already normal intestinal mucosa[34]. Goerres et al[35] found intestinal UDP-glucuronosyltransferases, which are involved in the detoxification of ingested toxins and carcinogens, to be decreased in CeD, and suggested that this could potentially pose a risk of cancer. Kamycheva et al[36] reported the leukocyte telomere length to be shorter in CeD seropositive patients, which may indicate genomic instability – a well-known predisposing factor of genetic changes and eventual carcinogenesis.
Ferguson et al[37] reported a 1.9 times greater risk of mortality in 653 CeD patients after a mean follow-up of 13.5 years, with the most common causes of death being lymphoproliferative disease and esophageal cancer. Freeman[38] identified 8.4% lymphoma, 1.4% small bowel carcinoma and 0.5% hypopharyngeal carcinoma in 214 patients with CeD, and reported the risk of lymphoma and small bowel adenocarcinoma to be increased especially in patients diagnosed with CeD after the age of 60 years, suggesting that risk increases the longer the diagnosis of CeD is delayed. Howdle et al[32] reported 13% of adenocarcinoma cases and 39% of lymphomas to have CeD.
Grainge et al[39] reported in their cohort study that the risk of any malignancy in CeD patients was 40% greater than in the general population, with an average follow-up of 25 years. They reported the highest risk in those with non-Hodgkin's lymphomas, with an overall incidence of 1.3 per 1000 person-years, but that the overall malignancy risk did not increase significantly 15 years after the diagnosis of CeD. Eigner et al[40] identified RCeD in 2.6% of 1,138 CeD patients, and reported that in 29 RCeD patients followed for 25 years, RCeD-1 developed in 1.3%, RCeD- 0.6%, EATL in 0.6% and small intestine adenocarcinoma in 0.4%, with a mortality rate of 48%. They noted further that in the preceding five years, there had been no patients diagnosed with RCeD-2, EATL or small bowel adenocarcinoma, which could be related to the increased awareness of CeD and strict adherence to a gluten-free diet.
Green et al[41] reported detecting small bowel adenocarcinoma in two (0.2%) and non-Hodgkin’s lymphoma in five (0.4%) of 1,612 CeD patients, with EATL being found in three patients (relative risk was 300). In a meta-analysis Han et al[42] reported a pooled odds ratio (OR) for the risk of all malignancies of 1.25, and 1.60 for GI malignancy in CeD patients. Of the GI malignancies, esophageal cancer (pooled OR= 3.72) and small intestinal carcinoma (pooled OR = 14.41) were associated with a greater risk. Ilus et al[43] reported that the standardized incidence ratio (SIR) did not increase for the series as a whole in 32,439 CeD patients, but reported a decrease in breast and lung cancers, and an increase in NHL (SIR: 1.94) and small bowel cancers (SIR: 4.29) 5 years after the CeD diagnosis. In a recent study, Koskinen et al[44] reported that although the overall mortality in adult CeD diagnosed in 2005–2014 had not increased, mortality associated with lymphoproliferative diseases had increased, but to a lesser degree than previously reported.
Table 1 provides details of studies of malignancies in CeD patients, including those identifying and not identifying an increased risk. The malignancies associated with CeD in the case reports are presented in Table 2.
| Ref. | Study design | Increased risk | No increased risk |
| Eigner et al[40] | Retrospective cohort | EATL | - |
| Small bowel adenocarcinoma | |||
| Freeman[38] | Retrospective cohort | Lymphoma | - |
| Small bowel carcinoma | |||
| Hypopharyngeal carcinoma | |||
| Grainge et al[39] | Cohort | All malignancies | - |
| Non-Hodgkin’s lymphoma | |||
| Howdle et al[32] | Survey | Small bowel adenocarcinoma | - |
| Small bowel lymphoma | |||
| van Gils et al[47] | Case-control | T-cell lymphoma, predominantly EATL | Other types of lymphomas |
| Small bowel adenocarcinoma | GI carcinomas | ||
| Esophageal squamous cell carcinoma | |||
| Anderson et al[48] | Retrospective cohort | Non-Hodgkin's lymphoma (but not statistically significant) | - |
| Green et al[41] | National survey | Small bowel adenocarcinoma | - |
| Non-Hodgkin’s lymphoma | |||
| Han et al[42] | Meta-analysis | All malignancies | Other GI cancers |
| Small intestinal cancers | |||
| Esophageal cancer | |||
| Ilus et al[43] | Retrospective cohort | Non-Hodgkin lymphoma | Decreased risk of lung, pancreatic, bladder, renal and breast cancer |
| Small intestinal cancer | |||
| Colon cancer | |||
| Basal cell carcinoma of the skin | |||
| Kent et al[49] | Cohort | Papillary thyroid cancer | - |
| Lebwohl et al[50] | Population-based setting | - | Cutaneous malignant melanoma |
| Volta et al[51] | Cohort | - | Colon carcinoma |
| Ref. | Diagnosis of malignancies (age in years) |
| Ahluwalia et al[52] | Burkitt-like lymphoma of colon (75) |
| Buess et al[53] | EATL causing obstructive jaundice (54) |
| Cankurtaran et al[54] | Plasma cell dyscrasia (65) |
| Cereda et al[55] | 1st patient: Burkitt lymphoma of the small bowel (5) |
| 2nd patient: Ependymoma (4) | |
| 3rd patient: Ewing sarcoma (6) | |
| Zunguo et al[56] | Large B-cell lymphoma and enteropathy-type T-cell lymphoma (65) |
| Fallah et al[57] | Adenocarcinoma of the small intestine (89) |
| Jafroodi et al[58] | Hodgkin’s lymphoma (11) |
| Naderi et al[59] | Two patients: germ cell tumor (3.5 and 5) |
| 3rd patient: Wilm’s tumor (6) | |
| 4th patient: Acute lymphobolastic lymphoma (4.5) | |
| 5th patient: Astrocytoma (8) | |
| Sahin et al[60] | Intestinal adenocarcinoma (58) |
| Zullo et al[61] | Intestinal adenocarcinoma (77) |
A causal relationship between CeD and EATL2 has been proven. Although its relationship with other cancer types is controversial, considering the pathogenesis of CeD, such a possibility can be considered. Studies have suggested that this risk is gradually decreasing[38,39] due to the increased awareness of CeD over the years, and the widespread use of diagnostic tests and endoscopy, which have made diagnosis easier and more common. Furthermore, the increase in the availability of commercial gluten-free products has facilitated stricter compliance with gluten-free diets. Today, the follow-up of CeD patients at certain periods is recommended in CeD guidelines[1,45]. In the event of suspected non-compliance with a gluten-free diet, or when presented with symptoms, the patient is re-evaluated with CeD-specific antibodies and the presence of RCeD is investigated. The major limitation of most of the above-mentioned studies is the lack of reporting on the compliance of CeD patients with the diet "assessed from year to year" based on CeD-specific tests. Indeed, in some of the studies, the CeD diagnosis was made either together or recently in some of the patients diagnosed with lymphoma at elderly ages. For this reason, objective evaluations (monitoring with CeD-specific antibodies or measurement of gluten immunogenic peptides in urine and feces[46]) of CeD patients diagnosed in childhood will yield better results. In addition to the above, since intestinal villous atrophy improves with a gluten-free diet, an early diagnosis of CeD and a lifelong gluten-free diet are very important in preventing the formation of intestinal lymphoma and adenocarcinoma. Regular follow-ups can support patients in their compliance with a gluten-free diet.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Toma A, Netherlands S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C, Lelgeman M, Mäki M, Ribes-Koninckx C, Ventura A, Zimmer KP; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1708] [Cited by in F6Publishing: 1692] [Article Influence: 141.0] [Reference Citation Analysis (1)] |
| 2. | Kupfer SS, Jabri B. Pathophysiology of celiac disease. Gastrointest Endosc Clin N Am. 2012;22:639-660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Al-Toma A, Volta U, Auricchio R, Castillejo G, Sanders DS, Cellier C, Mulder CJ, Lundin KEA. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7:583-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 413] [Article Influence: 82.6] [Reference Citation Analysis (1)] |
| 4. | Karaman K, Akbayram S, Kar S, Demirören K. Prevalence of Celiac Disease in Children With Iron Deficiency Anemia in Van Lake Region of Turkey. J Pediatr Hematol Oncol. 2016;38:143-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Reunala T, Salmi TT, Hervonen K, Kaukinen K, Collin P. Dermatitis Herpetiformis: A Common Extraintestinal Manifestation of Coeliac Disease. Nutrients. 2018;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Souto-Souza D, da Consolação Soares ME, Rezende VS, de Lacerda Dantas PC, Galvão EL, Falci SGM. Association between developmental defects of enamel and celiac disease: A meta-analysis. Arch Oral Biol. 2018;87:180-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Nieri M, Tofani E, Defraia E, Giuntini V, Franchi L. Enamel defects and aphthous stomatitis in celiac and healthy subjects: Systematic review and meta-analysis of controlled studies. J Dent. 2017;65:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Stazi AV, Trecca A, Trinti B. Osteoporosis in celiac disease and in endocrine and reproductive disorders. World J Gastroenterol. 2008;14:498-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Capriles VD, Martini LA, Arêas JA. Metabolic osteopathy in celiac disease: importance of a gluten-free diet. Nutr Rev. 2009;67:599-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Dima A, Jurcut C, Jinga M. Rheumatologic manifestations in celiac disease: what should we remember? Rom J Intern Med. 2019;57:3-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Bashiri H, Afshari D, Babaei N, Ghadami MR. Celiac Disease and Epilepsy: The Effect of Gluten-Free Diet on Seizure Control. Adv Clin Exp Med. 2016;25:751-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Cicarelli G, Della Rocca G, Amboni M, Ciacci C, Mazzacca G, Filla A, Barone P. Clinical and neurological abnormalities in adult celiac disease. Neurol Sci. 2003;24:311-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Mearns ES, Taylor A, Thomas Craig KJ, Puglielli S, Leffler DA, Sanders DS, Lebwohl B, Hadjivassiliou M. Neurological Manifestations of Neuropathy and Ataxia in Celiac Disease: A Systematic Review. Nutrients. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Mirzaagha F, Azali SH, Islami F, Zamani F, Khalilipour E, Khatibian M, Malekzadeh R. Coeliac disease in autoimmune liver disease: a cross-sectional study and a systematic review. Dig Liver Dis. 2010;42:620-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Mahmud FH, Clarke ABM, Joachim KC, Assor E, McDonald C, Saibil F, Lochnan HA, Punthakee Z, Parikh A, Advani A, Shah BR, Perkins BA, Zuijdwijk CS, Mack DR, Koltin D, De Melo EN, Hsieh E, Mukerji G, Gilbert J, Bax K, Lawson ML, Cino M, Beaton MD, Saloojee NA, Lou O, Gallego PH, Bercik P, Houlden RL, Aronson R, Kirsch SE, Paterson WG, Marcon MA. Screening and Treatment Outcomes in Adults and Children With Type 1 Diabetes and Asymptomatic Celiac Disease: The CD-DIET Study. Diabetes Care. 2020;43:1553-1556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Wang N, Truedsson L, Elvin K, Andersson BA, Rönnelid J, Mincheva-Nilsson L, Lindkvist A, Ludvigsson JF, Hammarström L, Dahle C. Serological assessment for celiac disease in IgA deficient adults. PLoS One. 2014;9:e93180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Slim M, Rico-Villademoros F, Calandre EP. Psychiatric Comorbidity in Children and Adults with Gluten-Related Disorders: A Narrative Review. Nutrients. 2018;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Tjon JM, van Bergen J, Koning F. Celiac disease: how complicated can it get? Immunogenetics. 2010;62:641-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Guandalini S, Assiri A. Celiac disease: a review. JAMA Pediatr. 2014;168:272-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 20. | Lindfors K, Mäki M, Kaukinen K. Transglutaminase 2-targeted autoantibodies in celiac disease: Pathogenetic players in addition to diagnostic tools? Autoimmun Rev. 2010;9:744-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Di Sabatino A, Vanoli A, Giuffrida P, Luinetti O, Solcia E, Corazza GR. The function of tissue transglutaminase in celiac disease. Autoimmun Rev. 2012;11:746-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Kumar J, Kumar M, Pandey R, Chauhan NS. Physiopathology and Management of Gluten-Induced Celiac Disease. J Food Sci. 2017;82:270-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Villanacci V, Ciacci C, Salviato T, Leoncini G, Bonetti LR, Ragazzini T, Limarzi F, Saragoni L. Histopathology of Celiac Disease. Position Statements of the Italian Group of Gastrointestinal Pathologists (GIPAD-SIAPEC). Transl Med UniSa. 2020;23:28-36. [PubMed] [Cited in This Article: ] |
| 24. | Hujoel IA, Murray JA. Refractory Celiac Disease. Curr Gastroenterol Rep. 2020;22:18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Ondrejka S, Jagadeesh D. Enteropathy-Associated T-Cell Lymphoma. Curr Hematol Malig Rep. 2016;11:504-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Cording S, Lhermitte L, Malamut G, Berrabah S, Trinquand A, Guegan N, Villarese P, Kaltenbach S, Meresse B, Khater S, Dussiot M, Bras M, Cheminant M, Tesson B, Bole-Feysot C, Bruneau J, Molina TJ, Sibon D, Macintyre E, Hermine O, Cellier C, Asnafi V, Cerf-Bensussan N; CELAC network. Oncogenetic landscape of lymphomagenesis in coeliac disease. Gut. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 27. | Cheminant M, Bruneau J, Malamut G, Sibon D, Guegan N, van Gils T, Cording S, Trinquand A, Verkarre V, Lhermitte L, Brousse N, Jannot AS, Khater S, Frenzel L, Delarue R, Suarez F, Marçais A, Mulder CJ, Macintyre E, Asnafi V, Pouyet L, Bonnafous C, Lhospice F, Molina TJ, Meresse B, Cellier C, Cerf-Bensussan N, Hermine O; CELAC network°. NKp46 is a diagnostic biomarker and may be a therapeutic target in gastrointestinal T-cell lymphoproliferative diseases: a CELAC study. Gut. 2019;68:1396-1405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | van Wanrooij RL, Müller DM, Neefjes-Borst EA, Meijer J, Koudstaal LG, Heideman DA, Bontkes HJ, von Blomberg BM, Bouma G, Mulder CJ. Optimal strategies to identify aberrant intra-epithelial lymphocytes in refractory coeliac disease. J Clin Immunol. 2014;34:828-835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Verbeek WH, Goerres MS, von Blomberg BM, Oudejans JJ, Scholten PE, Hadithi M, Al-Toma A, Schreurs MW, Mulder CJ. Flow cytometric determination of aberrant intra-epithelial lymphocytes predicts T-cell lymphoma development more accurately than T-cell clonality analysis in Refractory Celiac Disease. Clin Immunol. 2008;126:48-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Al-Toma A, Verbeek WH, Hadithi M, von Blomberg BM, Mulder CJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut. 2007;56:1373-1378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 246] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 31. | Al-toma A, Visser OJ, van Roessel HM, von Blomberg BM, Verbeek WH, Scholten PE, Ossenkoppele GJ, Huijgens PC, Mulder CJ. Autologous hematopoietic stem cell transplantation in refractory celiac disease with aberrant T cells. Blood. 2007;109:2243-2249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Howdle PD, Jalal PK, Holmes GK, Houlston RS. Primary small-bowel malignancy in the UK and its association with coeliac disease. QJM. 2003;96:345-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 33. | Foukas PG, Bisig B, de Leval L. Recent advances upper gastrointestinal lymphomas: molecular updates and diagnostic implications. Histopathology. 2021;78:187-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Elfström P, Granath F, Ekström Smedby K, Montgomery SM, Askling J, Ekbom A, Ludvigsson JF. Risk of lymphoproliferative malignancy in relation to small intestinal histopathology among patients with celiac disease. J Natl Cancer Inst. 2011;103:436-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Goerres M, Roelofs HM, Jansen JB, Peters WH. Deficient UDP-glucuronosyltransferase detoxification enzyme activity in the small intestinal mucosa of patients with coeliac disease. Aliment Pharmacol Ther. 2006;23:243-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Kamycheva E, Goto T, Camargo CA Jr. Celiac disease autoimmunity is associated with leukocyte telomere shortening in older adults: The U.S. National Health and Nutrition Examination Survey. Exp Gerontol. 2017;89:64-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Ferguson A, Kingstone K. Coeliac disease and malignancies. Acta Paediatr Suppl. 1996;412:78-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Freeman HJ. Lymphoproliferative and intestinal malignancies in 214 patients with biopsy-defined celiac disease. J Clin Gastroenterol. 2004;38:429-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Grainge MJ, West J, Solaymani-Dodaran M, Card TR, Logan RF. The long-term risk of malignancy following a diagnosis of coeliac disease or dermatitis herpetiformis: a cohort study. Aliment Pharmacol Ther. 2012;35:730-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Eigner W, Bashir K, Primas C, Kazemi-Shirazi L, Wrba F, Trauner M, Vogelsang H. Dynamics of occurrence of refractory coeliac disease and associated complications over 25 years. Aliment Pharmacol Ther. 2017;45:364-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Green PHR, Stavropoulos SN, Panagi SG, Goldstein SL, Mcmahon DJ, Absan H, Neugut AI. Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol. 2001;96:126-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 358] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 42. | Han Y, Chen W, Li P, Ye J. Association Between Coeliac Disease and Risk of Any Malignancy and Gastrointestinal Malignancy: A Meta-Analysis. Medicine (Baltimore). 2015;94:e1612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 43. | Ilus T, Kaukinen K, Virta LJ, Pukkala E, Collin P. Incidence of malignancies in diagnosed celiac patients: a population-based estimate. Am J Gastroenterol. 2014;109:1471-1477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Koskinen I, Virta LJ, Huhtala H, Ilus T, Kaukinen K, Collin P. Overall and Cause-Specific Mortality in Adult Celiac Disease and Dermatitis Herpetiformis Diagnosed in the 21st Century. Am J Gastroenterol. 2020;115:1117-1124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Husby S, Murray JA, Katzka DA. AGA Clinical Practice Update on Diagnosis and Monitoring of Celiac Disease-Changing Utility of Serology and Histologic Measures: Expert Review. Gastroenterology. 2019;156:885-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 46. | Ruiz-Carnicer Á, Garzón-Benavides M, Fombuena B, Segura V, García-Fernández F, Sobrino-Rodríguez S, Gómez-Izquierdo L, Montes-Cano MA, Rodríguez-Herrera A, Millán R, Rico MC, González-Naranjo C, Bozada-García JM, Díaz J, Coronel-Rodríguez C, Espín B, Romero-Gómez M, Cebolla Á, Sousa C, Comino I, Argüelles F, Pizarro Á. Negative predictive value of the repeated absence of gluten immunogenic peptides in the urine of treated celiac patients in predicting mucosal healing: new proposals for follow-up in celiac disease. Am J Clin Nutr. 2020;112:1240-1251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 47. | van Gils T, Nijeboer P, Overbeek LI, Hauptmann M, Castelijn DA, Bouma G, Mulder CJ, van Leeuwen FE, de Jong D. Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: Consequences for follow-up: Celiac disease, lymphoma and GI carcinoma. United European Gastroenterol J. 2018;6:1485-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Anderson LA, McMillan SA, Watson RG, Monaghan P, Gavin AT, Fox C, Murray LJ. Malignancy and mortality in a population-based cohort of patients with coeliac disease or "gluten sensitivity". World J Gastroenterol. 2007;13:146-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 59] [Cited by in F6Publishing: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | Kent L, McBride R, McConnell R, Neugut AI, Bhagat G, Green PH. Increased risk of papillary thyroid cancer in celiac disease. Dig Dis Sci. 2006;51:1875-1877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Lebwohl B, Eriksson H, Hansson J, Green PH, Ludvigsson JF. Risk of cutaneous malignant melanoma in patients with celiac disease: a population-based study. J Am Acad Dermatol. 2014;71:245-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Volta U, Vincentini O, Quintarelli F, Felli C, Silano M; Collaborating Centres of the Italian Registry of the Complications of Celiac Disease. Low risk of colon cancer in patients with celiac disease. Scand J Gastroenterol. 2014;49:564-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Ahluwalia M, Gotlieb V, Damerla V, Saif MW. Aggressive Burkitt-like lymphoma of colon in a patient with prior celiac disease. Yale J Biol Med. 2006;79:173-175. [PubMed] [Cited in This Article: ] |
| 53. | Buess M, Steuerwald M, Wegmann W, Rothen M. Obstructive jaundice caused by enteropathy-associated T-cell lymphoma in a patient with celiac sprue. J Gastroenterol. 2004;39:1110-1113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Cankurtaran M, Ulger Z, Doğan S, Balam Yavuz B, Halil M, Güllü I, Gedikoğlu G, Anioğul S. Complications due to late diagnosis of celiac disease with co-existing plasma cell dyscrasia in an elderly patient. Aging Clin Exp Res. 2006;18:75-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Cereda S, Cefalo G, Spreafico F, Catania S, Meazza C, Podda M, Terenziani M. Celiac disease and childhood cancer. J Pediatr Hematol Oncol. 2006;28:346-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 56. | Du Z, Chen J, Zhou X, Zhang T, Chen B, Tang F. Composite lymphoma with relapse of enteropathy-type T-cell lymphoma. Leuk Lymphoma. 2009;50:749-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Fallah J, Afari ME, Cordova AC, Olszewski AJ, Minami T. Small Bowel Adenocarcinoma as the Cause of Gastrointestinal Bleeding in Celiac Disease: A Rare Malignancy in a Common Disease. Case Rep Oncol Med. 2015;2015:865383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 58. | Jafroodi M, Zargari O, Hoda S. Concomitant Hodgkin's lymphoma and atopic dermatitis in a child with Celiac disease. Arch Iran Med. 2009;12:317-319. [PubMed] [Cited in This Article: ] |
| 59. | Naderi M, Shahramian I, Delaramnasab M, Bazi A. Coincidence of celiac disease with nongastrointestinal tumors in children. Pediatr Hematol Oncol. 2017;34:478-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 60. | Sahin C, Ozseker B, Sagiroglu T, Cullu N. Intestinal invagination secondary to intestinal adenocarcinoma in coeliac disease. BMJ Case Rep. 2015;2015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Zullo A, De Francesco V, Manta R, Ridola L, Lorenzetti R. A Challenging Diagnosis of Jejunal Adenocarcinoma in a Celiac Patient: Case Report and Systematic Review of the Literature. J Gastrointestin Liver Dis. 2017;26:411-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |