Published online Oct 24, 2022. doi: 10.5306/wjco.v13.i10.853
Peer-review started: April 11, 2022
First decision: May 31, 2022
Revised: June 12, 2022
Accepted: October 11, 2022
Article in press: October 11, 2022
Published online: October 24, 2022
Retrorectal hamartomas or tailgut cysts (TCs) are rare. In most cases, they are asymptomatic and benign; however, rarely, they undergo malignant trans
A 55-year-old woman presented to our hospital with lower back pain. On magnetic resonance imaging, a large pelvic mass was found, which was located on the right of the ischiorectal fossa, extending to the minor pelvis. The patient underwent extensive surgical resection of the lesion through the right buttock. Histological examination confirmed the diagnosis of a retrorectal mucinous adenocarcinoma originating from a TC. Surgical resection of the tumour was complete, and the patient recovered without complications. The pilonidal sinus was then excised. One year later, semi-annual positron emission tomography-computed tomography and magnetic resonance imaging scans did not reveal any evidence of local recurrence or metastatic disease.
Preoperative recognition, histological diagnosis, and treatment of TCs pose significant challenges. In addition, the possibility of developing invasive mucin
Core Tip: Retrorectal hamartomas or tailgut cysts are extremely rare. In certain cases, they undergo malignant transformation, predominantly in the form of adenocarcinomas. Mucinous adenocarcinomas are rare forms of carcinoma arising from tailgut cysts, with only 18 cases reported in the literature from 1988 to 2021. Furthermore, to our knowledge, coexistence of a pilonidal tract and mucinous adenocarcinoma is extremely rare; this being the second reported case in the literature. We present the case of a 55-year-old woman with a large pelvic mass on the right of the ischiorectal fossa and a pilonidal cyst. Surgical resection of the tumour and cyst was completed and the patient recovered well.
- Citation: Malliou P, Syrnioti A, Koletsa T, Karlafti E, Karakatsanis A, Raptou G, Apostolidis S, Michalopoulos A, Paramythiotis D. Mucinous adenocarcinoma arising from a tailgut cyst: A case report. World J Clin Oncol 2022; 13(10): 853-860
- URL: https://www.wjgnet.com/2218-4333/full/v13/i10/853.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i10.853
Retrorectal hamartomas or tailgut cysts (TCs) are very rare, with an incidence rate of approximately 1/40000[1]. TCs are believed to be embryologically derived from a remnant of the posterior intestine[2]. Alternative terminologies such as ‘cyst of postanal intestine’, ‘retrorectal cystic hamartoma’, ‘tailgut vestiges’, ‘myoepithelial hamartoma of the rectum’, and ‘rectal cyst’ have been used in the literature to describe these lesions[3]. These tumours are thin-walled, multi-layered structures lined by various glandular or transitional epithelia[4].
TCs occur more often in middle-aged women, whereas they are rare in children[5]. Forty percent of TCs occurring in children and new-borns are teratomas. Moreover, 10% of teratomas coexist with developmental disorders of the midline such as encephalocele[2,5]. In this age group, tumours could be benign, whereas malignant tumours are more common in older children[5]. Most TCs in adults are benign; however, malignant transformation has been reported in the literature, particularly in symptomatic cases[6].
TCs are mostly asymptomatic prior to clinical recognition. Symptoms are often associated either with a growing tumour mass and may include lower abdominal pain, rectal tenesmus and constipation or with infectious complications, even including fistulas[5].
Considering the rarity of this developmental anomaly, we present an interesting case of invasive mucinous adenocarcinoma originating from a TC associated with a pilonidal cyst that was managed in the Emergency Surgical Department of University Hospital.
A 55-year-old woman presented to the Emergency Surgical Department of the University Hospital with lower back pain.
The patient complained of pain in the previous 6 mo.
The patient had a history of ductal breast cancer, which was diagnosed 10 years ago and treated with lobectomy and adjuvant therapy. She also underwent hip arthroplasty 1.5 years ago and was under no medication and in good physical condition with good nutrition, according to her age.
No pathological conditions were found.
The arterial blood pressure was 130/85 mmHg, temperature was 36.7 °C, and oxygen saturation level was 98%. Physical examination revealed a large, palpable gluteal mass.
On her admission to our department, the routine laboratory test and carcinoembryonic antigen (CEA) results were within normal limits.
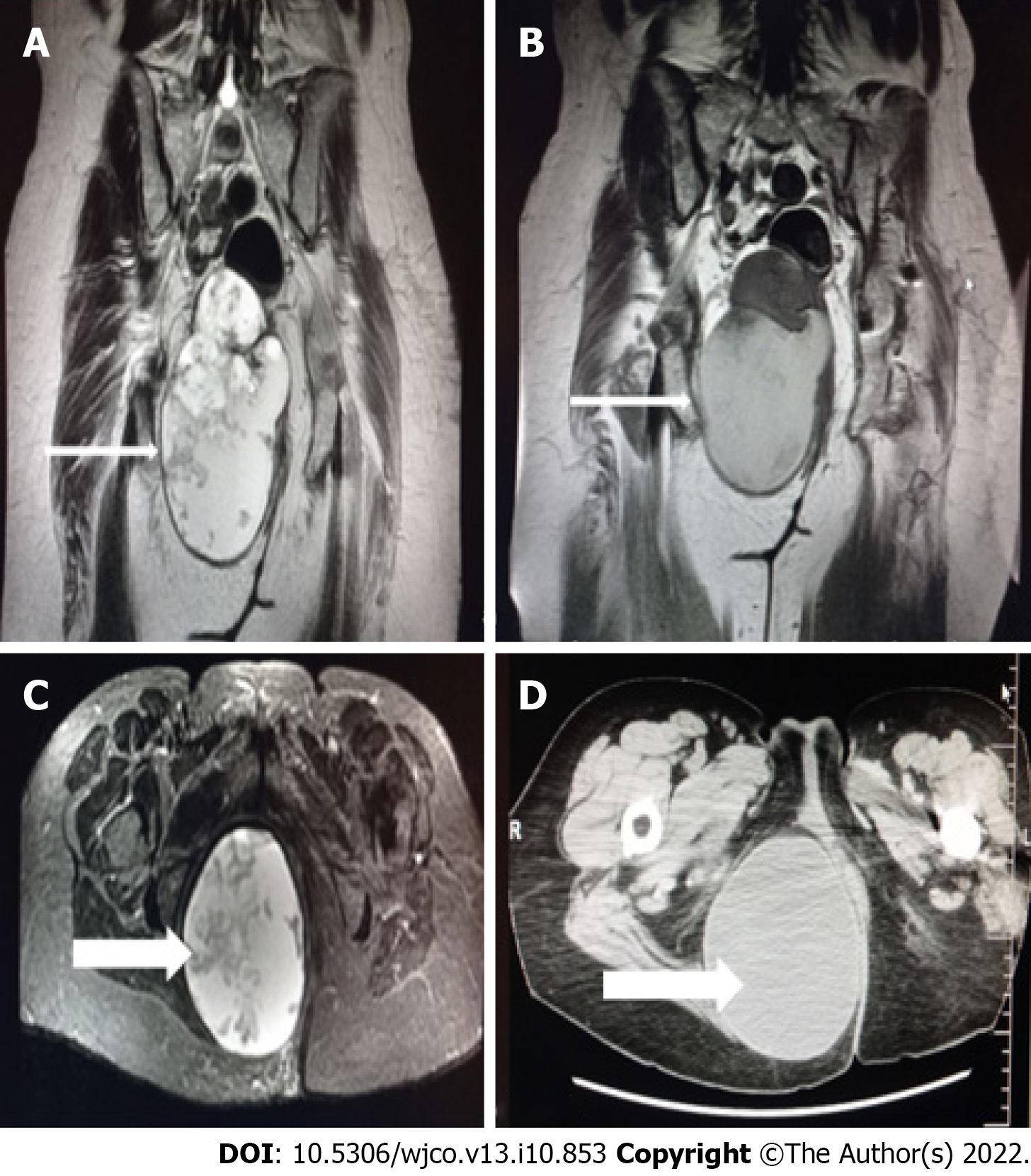
Magnetic resonance imaging (MRI) of the pelvis (Figure 1) revealed a pelvic mass that was located to the right of the rectus fossa, in contact with the uterus and rectum, which seemed to apply pressure on the adjacent structures and possibly on the sciatic nerve, and extended to the minor pelvis. The dimensions of the mass were 11 cm × 10 cm and 6 cm × 16.2 cm, and neural derivation was initially suspected.
Chest X-ray and computed tomography (CT) scans showed no abnormal findings. The abdominal CT scan revealed a large multifaceted formation located on the right side of the rectum, between the urinary bladder and coccyx and up to the fatty tissue of the buttocks, with enriched diaphragms. The appendix and ovaries were normal.
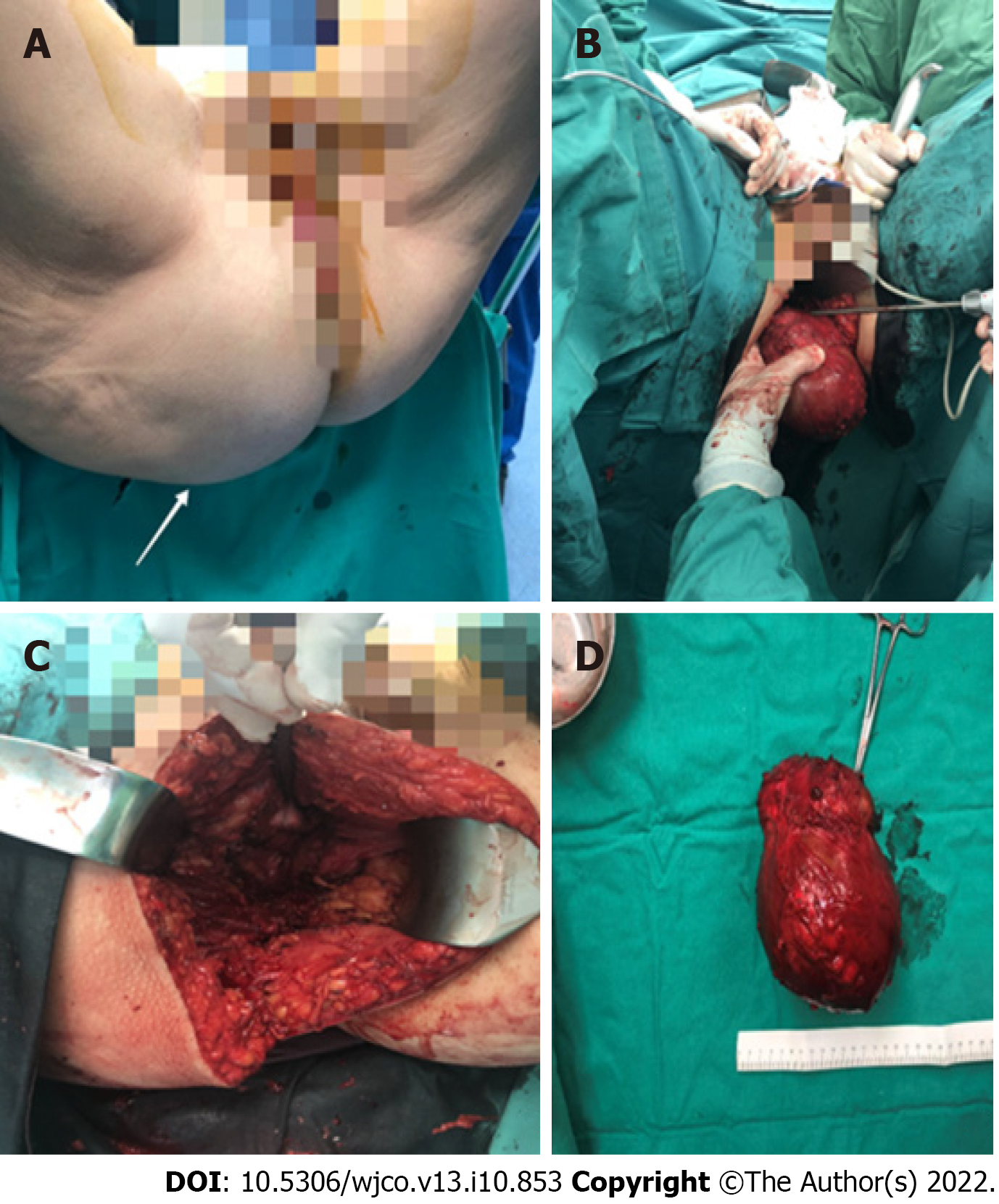
The patient underwent extensive surgical resection of the lesion through the right buttock (Figure 2).
An incision approximately 20 cm long was made, and sharp dissection was performed to carry the incision down directly into the midline until the presacral fascia was found. The medial gluteal fibres were then divided bilaterally to expose the attached mass which pushed the rectum and uterus away without infiltrating these structures. During dissection, it was crucial to avoid injury to the rectal wall, vagina, sciatic nerve, and urethra. This was facilitated by the use of rectoscopy during surgery, along with preoperative bowel preparation. A Foley catheter was used as a guide for the urethra. The lesion was resected, and the gluteal muscles were returned to the midline. The remaining layers of the incision were reapproximated and closed. Simultaneously, a pilonidal sinus was found and removed.
Preoperative planning concerned proper positioning of the patient. Lithotomy positioning was preferred because of the direct approach to the mass, rectum, and vagina and the potential need for a combined transabdominal incision.
Concerns were also raised about the contingent need for other specialists such as gynaecologists and urologists if the lesion was found to infiltrate the vagina or urinary tract. On that ground, these specialists stood by during the surgery.
The recuperation of the patient was uneventful, and she was discharged from the hospital on the seventh post-operative day because of delayed bowel movement.
Wound care was performed as usual, and the skin sutures were removed 2 wk later, without any complications.
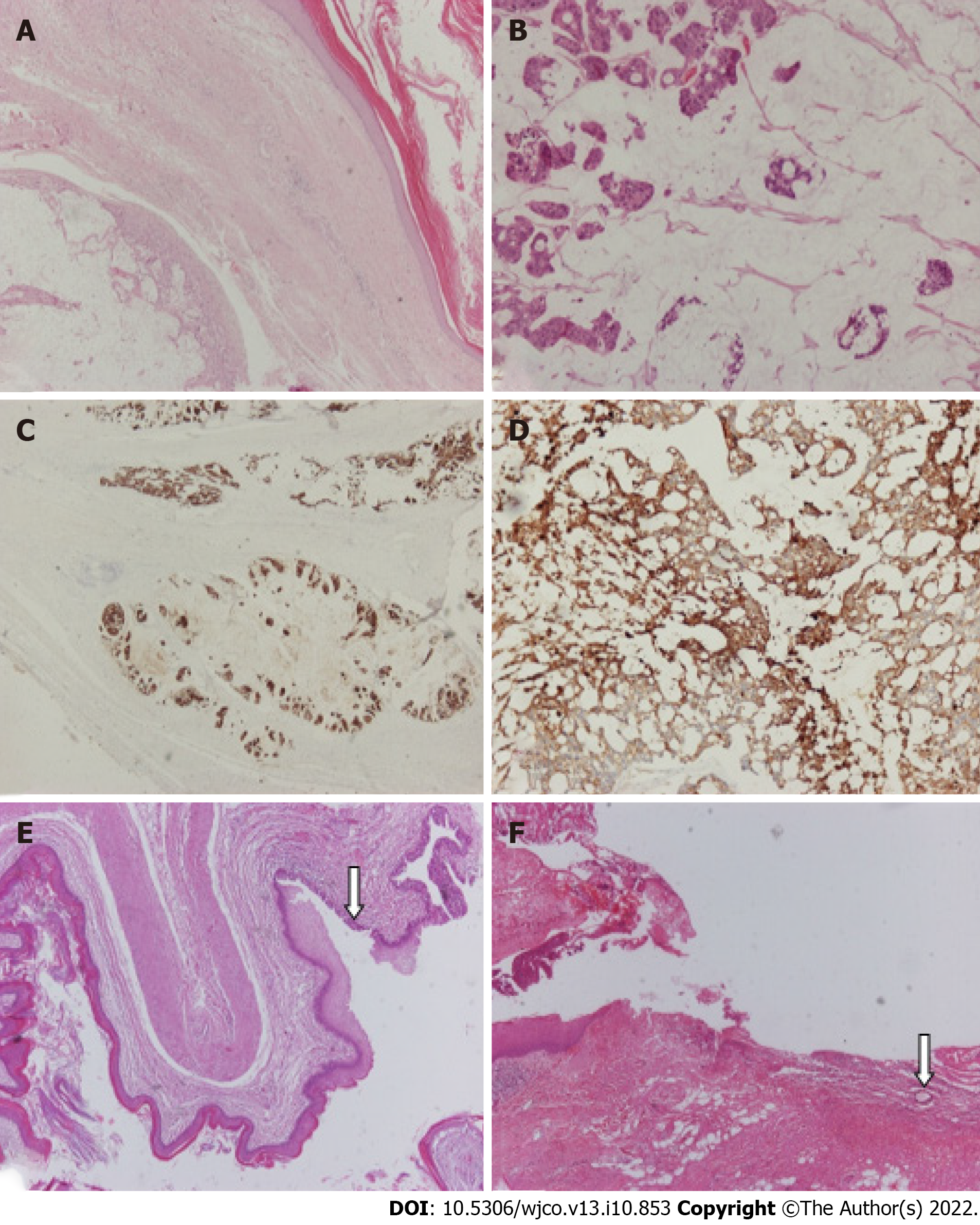
Both the mass and the pilonidal sinus were sent separately for histopathological examination. Upon grossing, a large mass was found to be cystic and filled with mucohaemorrhagic material. In a peripheral location, two smaller cystic spaces were identified, which were also filled with mucus and an amorphous material. Microscopic examination confirmed the presence of a cystic mass comprising thick fibrous bands that divided it into three cystic spaces, the largest of which corresponded to mucinous adenocarcinoma (Figure 3A). The neoplastic cells were medium to large in size, with roundish or irregular hyperchromatic atypical nuclei surrounded by an eosinophilic or pale cytoplasm (Figure 3B). Few “signet ring” cells were also observed. Tumour cells were arranged in glandular or cribriform structures, trabeculae, variably sized solid groups, and within large “lakes” of mucin. Rarely, isolated neoplastic cells floating in the mucin were identified. A large number of mitoses was observed. Regions of tumour necrosis and calcification were also observed. On immunohistochemical evaluation, the neoplastic cells exhibited the following immunophenotypes: CK20+ (Figure 3C), CDX2+, CK7+, GATA3-, ER-, PR-, and calretinin-.
Most current and similar published cases reported positivity, even partially, of TC or adenocarcinoma arising on the cyst to CK7 antibody.
Embryologically, the rectum is the last part of the tailgut, and both normal rectal mucosa and rectal adenocarcinomas present CK7 positivity in almost one-fifth of cases[7].
HER2 immunostaining showed faint, segmental, and membranous positivity in a small number of tumour cells (HER2 1+). The two other cystic spaces were lined with keratinising squamous or pseudostratified ciliated columnar or metaplastic squamous epithelia (Figure 3E). The mass was circumscribed with bundles of connective tissue at the periphery, and the surgical margins were tumour-free. Based on these findings, the diagnosis of an invasive mucinous adenocarcinoma, possibly on the grounds of the presence of a posterior rectal cyst sinus (TC), was established.
Gross examination of the sacrococcygeal pilonidal cyst revealed an elliptical skin-excision specimen. On the skin’s surface, a hole measuring 0.1 cm in the greatest diameter was identified, which upon parallel sectioning was found to be continuous with a sinus tract that terminated in a brownish grey-coloured area. Microscopic examination revealed that the sinus tract was lined mainly by stratified squamous epithelium and partially by granulation tissue. Hair shafts were also focally identified around the sinus tract (Figure 3F). The latter extended to the deep tissue resection margin. No communication between the sinus tract and TC was found, albeit multiple sections.
The recuperation of the patient was uneventful, and she was discharged from the hospital on seventh post-operative day. According to the histopathology report, the oncology council recommended 22 sessions of radiotherapy while the patient completed the treatment. After 1 year, follow-up of the patient with semi-annual positron emission tomography-CT and MRI, did not show any evidence of local or metastatic recurrent disease.
Herein, we present an interesting case of mucinous adenocarcinoma arising on a TC. Mucinous adenocarcinoma is a rare type of carcinoma occurring on TCs, with only 18 cases reported in the English literature from 1988 to 2021. Furthermore, to our knowledge, coexistence of a pilonidal tract is extremely rare, this being the second reported case in the literature. A connection between the pilonidal sinus and TC was not established using imaging, intraoperatively, or on pathological examination.
Primary retrorectal tumours include congenital (55%-65% of all tumours in this region), neurogenic (10%-12%), osteogenic (5%-11%), inflammatory (5%), and other tumour types (5%-11%). According to their embryonic origin, cysts are classified into epidermal, dermal, neural, teratoma, enteric, rectal duplication, mucous-secreting, enterogenous, simplex, gland anal, rectal, hamartoma, and TCs[5,8]. TCs are found in the presacral space and are typically thin-walled cysts that may be single or multiloculated, branched, and may contain green opalescent colloid fluid[2].
In 1885, Middeldorpf et al[9] reported the first case of a cystic mass in the retrorectal space in a 1-year-old girl, which was most likely a rectal duplication cyst. Hjermstad and Helwig reported the largest case series of TCs, which included 53 patients with an age range of 4 days to 73 years and average age of 36 years[10]. Based on the current literature, TCs may be asymptomatic or present with non-specific symptoms owing to the large size of the pelvic mass[2,5,8]. They can also lead to several complications including a neurogenic bladder, haemorrhage, faecal incontinence, faecal fistula, intestinal obstruction, infections, or malignant transformation as observed in the present case[11,12].
The diagnosis of TCs may be delayed because of the absence of typical symptoms[6]. Often, TCs are discovered incidentally through imaging tests during the investigation of other entities[5]. A CT scan typically shows a well-defined homogeneous retrorectal mass of water to soft-tissue density)[12]. A more solid appearance could also be described because of the keratinous or inflammatory debris within a cyst[3,5]. Higher-resolution scans may identify most TCs as multiloculated cysts[3]. On T1-weighted images, MRI scans reveal a hypointense lesion, whereas lesions are homogeneously hyperintense on T2-weighted images. However, MRI is not the gold standard for discriminating benign and malignant lesions[3].
A definitive diagnosis is established by histopathological examination[14]. TCs are congenital lesions that develop from the residual posterior remnant of the intestine, which retains its structure and architecture regarding the mature ectodermal, endodermal, and mesodermal tissue elements. The lining epithelia may vary, including squamous, ciliated columnar, pseudostratified, columnar, transitional, goblet columnar, and cuboidal epithelia[8,14]. Additionally, it is characterised by the presence of a smooth muscle layer and connective tissue, which may be disarrayed and do not encompass the nerve plexus or differentiated neuronal cells[14]. The immunophenotype of the mucinous adenocarcinoma in this case was that described in similar previously reported cases and is characterised by CK7, CK20, and CDX-2 positivity[15].
Most TCs are benign; nevertheless, rare cases of malignancies have been reported, including the present case[6]. Apart from adenocarcinomas, neuroendocrine, endometrioid, adenosquamous, and squamous cell carcinomas and sarcomas have also been described[13]. Although the option of needle biopsy seems attractive, it is not broadly recommended because of the possibility of false-negative results and the risk of tumour seeding[16].
Once a presacral tumour is diagnosed, the treatment of choice is extensive surgical removal due to the possibility of malignant transformation. The surgical approach depends on tumour location. Complete excision could be achieved with a posterior approach for tumours extending below the sacral spinal nerve 4 (S4), which is effective at a rate of 95%. For tumours that extend above S4, the abdominal or abdominal-perineal approach is suggested[16]. When TC is malignant, many studies suggest that treatment should include adjuvant radiation therapy alone or in combination with chemotherapy[6,17-19]. Martins et al[20] suggest both radiation therapy and chemotherapy. Liang et al[6] argue that the mainstream treatment method for TCs with adenocarcinoma is surgical resection followed by chemotherapy. Baverez et al[21] suggest that neoadjuvant chemoradiotherapy, similar to locally advanced rectal adenocarcinoma, decreases the risk of post-operative recurrence. Supplemental treatment can be administered as it is believed to contribute to the prevention of tumour recurrence. However, there is no clear evidence that it would improve the prognosis as there is no general consensus on treatment standards for TC-associated adenocarcinoma owing to its very low incidence rate[22]. Factors that determine prognosis include the stage during diagnosis, tumour histology and grade, and completeness of resection[3]. Compared with neuroendocrine tumours, adenocarcinomas arising from TCs have a poorer prognosis and carry a risk of local recurrence and metastasis[8]. Follow-up of the patient is also recommended, including monitoring for signs of recurrence with periodic positron emission tomography-CT and MRI scans in addition to serum CEA levels that serve as an indicator of the tumour’s response to treatment and development of recurrence[19]. According to Chhabra et al[3], once a TC malignancy has been diagnosed and is associated with an elevated CEA level, CEA levels may be used as a simple measure to assess the tumour’s response to treatment or development of recurrence. In our case, the patient did not have elevated CEA levels; therefore, this measure was not monitored after surgery. Di Nuzzo et al[23] reported the use of combined MRI and endoscopy for post-operative follow-up.
TCs are rare clinical and pathological entities. The novelties of this case include the presence of a mucinous adenocarcinoma arising from a TC and that it is the second reported case of an association between TC and pilonidal cyst. Generally, TCs constitute both diagnostic and treatment challenges. Imaging tests may be helpful; however, a definitive diagnosis is usually established after complete surgical excision and histopathological examination. Guidelines for appropriate therapeutic management are required for TC-associated adenocarcinomas, although timely and extensive surgical resection along with adjuvant radiation therapy with or without chemotherapy have been used with good outcomes.
Conflict of interest statement: All authors declare that they have no conflicts of interest.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elkady N, Egypt; Rosen SA, United States S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Baek SK, Hwang GS, Vinci A, Jafari MD, Jafari F, Moghadamyeghaneh Z, Pigazzi A. retrorectal tumors: a comprehensive literature review. World J Surg. 2016;40:2001-2015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Broccard SP, Colibaseanu DT, Behm KT, Mishra N, Davis P, Maimone KL, Mathis KL, Stocchi L, Dozois EJ, Merchea A. Risk of malignancy and outcomes of surgically resected presacral tailgut cysts: A current review of the Mayo Clinic experience. Colorectal Dis. 2022;24:422-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Chhabra S, Wise S, Maloney-Patel N, Rezac C, Poplin E. Adenocarcinoma associated with tail gut cyst. J Gastrointest Oncol. 2013;4:97-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 11] [Reference Citation Analysis (0)] |
| 4. | Moreira AL, Scholes JV, Boppana S, Melamed J. p53 Mutation in adenocarcinoma arising in retrorectal cyst hamartoma (tailgut cyst): report of 2 cases--an immunohistochemistry/immunoperoxidase study. Arch Pathol Lab Med. 2001;125:1361-1364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Rachel F, Guzik A, Szczerba D, Kozieł K, Gutterch K. Tailgut cyst and a very rare case of a tailgut cyst with mucinous adenocarcinoma in a 73 year old woman treated for buttock abscess with fistula. Pol Przegl Chir. 2019;91:38-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Liang F, Li J, Yu K, Zhang K, Liu T. Tailgut cysts with malignant transformation: features, diagnosis, and treatment. Med Sci Monit. 2020;26:e919803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Saad RS, Silverman JF, Khalifa MA, Rowsell C. CDX2, cytokeratins 7 and 20 immunoreactivity in rectal adenocarcinoma. Appl Immunohistochem Mol Morphol. 2009;17:196-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Mastoraki A, Giannakodimos I, Panagiotou K, Frountzas M, Chrysikos D, Kykalos S, Theodoropoulos GE, Schizas D. Epidemiology, diagnostic approach and therapeutic management of tailgut cysts: A systematic review. Int J Clin Pract. 2021;75:e14546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Middeldorpf K. Zur kenntniss der angebornen sacralgeschwulste. Virchows Arch (Pathol Anat). 1885;101:37-44. [Cited in This Article: ] |
| 10. | Hjermstad BM, Helwig EB. Tailgut cysts. Report of 53 cases. Am J Clin Pathol. 1988;89:139-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 228] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Böhm B, Milsom JW, Fazio VW, Lavery IC, Church JM, Oakley JR. Our approach to the management of congenital presacral tumors in adults. Int J Colorectal Dis. 1993;8:134-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Wang JY, Hsu CH, Changchien CR, Chen JS, Hsu KC, You YT, Tang R, Chiang JM. Presacral tumor: a review of forty-five cases. Am Surg. 1995;61:310-315. [PubMed] [Cited in This Article: ] |
| 13. | Krivokapic Z, Dimitrijevic I, Barisic G, Markovic V, Krstic M. Adenosquamous carcinoma arising within a retrorectal tailgut cyst: report of a case. World J Gastroenterol. 2005;11:6225-6227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Kanamori Y, Watanabe T, Ogawa K, Tomonaga K, Takezoe T, Ohno M, Tahara K, Miyazaki O, Yoshioka T. Tailgut cyst in a female infant with a skin dimple at the coccygeal region. J Pediatr Surg. 2016;14:38-41. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Wang M, Liu G, Mu Y, He H, Wang S, Li J. Tailgut cyst with adenocarcinoma transition: A rare case report. Medicine (Baltimore). 2020;99:e20941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Messick CA, Hull T, Rosselli G, Kiran RP. Lesions originating within the retrorectal space: a diverse group requiring individualized evaluation and surgery. J Gastrointest Surg. 2013;17:2143-2152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Almeida Costa NA, Rio G. Adenocarcinoma within a tailgut cyst. BMJ Case Rep. 2018;2018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Demirel AH, Cetin E, Temiz A. Squamous cell carcinoma arising in a sacrococcygeal tailgut cyst. An Bras Dermatol. 2018;93:733-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Maruyama A, Murabayashi K, Hayashi M, Nakano H, Isaji S, Uehara S, Kusuda T, Miyahara S, Kondo A, Yabana T. Adenocarcinoma arising in a tailgut cyst: report of a case. Surg Today. 1998;28:1319-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Martins P, Canotilho R, Peyroteo M, Afonso M, Moreira A, de Sousa A. Tailgut cyst adenocarcinoma. Autops Case Rep. 2020;10:e2019115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Baverez M, Thibaudeau E, Libois V, Kerdraon O, Senellart H, Raoul JL. Retrorectal mucinous adenocarcinoma arising from a tailgut cyst: a case report. Case Rep Oncol. 2021;14:147-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Jarboui S, Jarraya H, Mihoub MB, Abdesselem MM, Zaouche A. Retrorectal cystic hamartoma associated with malignant disease. Can J Surg. 2008;51:E115-E116. [PubMed] [Cited in This Article: ] |
| 23. | Di Nuzzo MM, De Werra C, Pace M, Franca RA, D'Armiento M, Bracale U, Lionetti R, D'Ambra M, Calogero A. Promoting laparoscopic anterior approach for a very low presacral primary neuroendocrine tumor arising in a tailgut cyst. Healthcare (Basel). 2022;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |