INTRODUCTION
According to the Atlanta Symposium held in 1992, acute pancreatitis (AP) is defined as an acute inflammatory process of the pancreas that frequently spreads to peripancreatic tissues and/or remote organ systems[1].
Alcohol abuse and gallstone migration are the established risk factors for the development of AP. Moreover, in recent years, genetic factors and obesity have also been identified as risk factors for the development of this disease[2]. AP normally runs a benign course causing minimum organ dysfunction and an uneventful recovery in most patients. However, 10%-20% of patients develop severe acute pancreatitis (SAP) and suffer systemic inflammatory response syndrome (SIRS) and/or pancreatic necrosis[3]. Despite improvements in clinical care, SAP causes death in 10%-25% of patients[4,5].
AP occurs when pancreatic enzymes are prematurely activated inside the pancreas leading to autodigestion of the gland and local inflammation[6]. These enzymes can also reach the bloodstream, stimulating the production of inflammatory cytokines and tumor necrosis factor-α (TNF-α) from leukocytes. The release of those substances triggers an inflammatory cascade, which leads to the SIRS[7].
Morbidity of SAP takes place in two phases: the first phase normally characterizes the first 14 d of the disease. It is related to organ or multiorgan failure, secondary to SIRS, and is not necessarily related to the presence of pancreatic necrosis[8]. The late phase, starts 14 d after the onset of the disease and is marked by infected necrosis of the gland, septic systemic complications and multiorgan failure syndrome, causing a significant increase in mortality[9,10]. Reports from various countries have shown an increase in AP incidence[11,12], perhaps in relation to rising obesity rates, which would increase the development of gallstone pancreatitis[13]. Moreover, although fatalities associated with AP have decreased over time, the population mortality rate has remained unchanged[10,14].
The lack of advances in the management of AP reflects both the limited understanding of the early AP pathophysiological mechanisms and the difficulty in identifying the patients who are at risk of developing severe disease[3,10]. Accurate diagnosis of SAP on admission to the hospital is of paramount importance[15] and there is, therefore, agreement about the need for finding predictors of severe disease to identify patients who are at risk of morbidity and death.
The main aim of this article is to review the set of events, first localized in the pancreas, that contribute to pancreatic inflammation, and then explain how this local reaction spreads to other organs and contributes to multiorganic shock.
INITIAL PATHOPHYSIOLOGICAL EVENTS IN AP
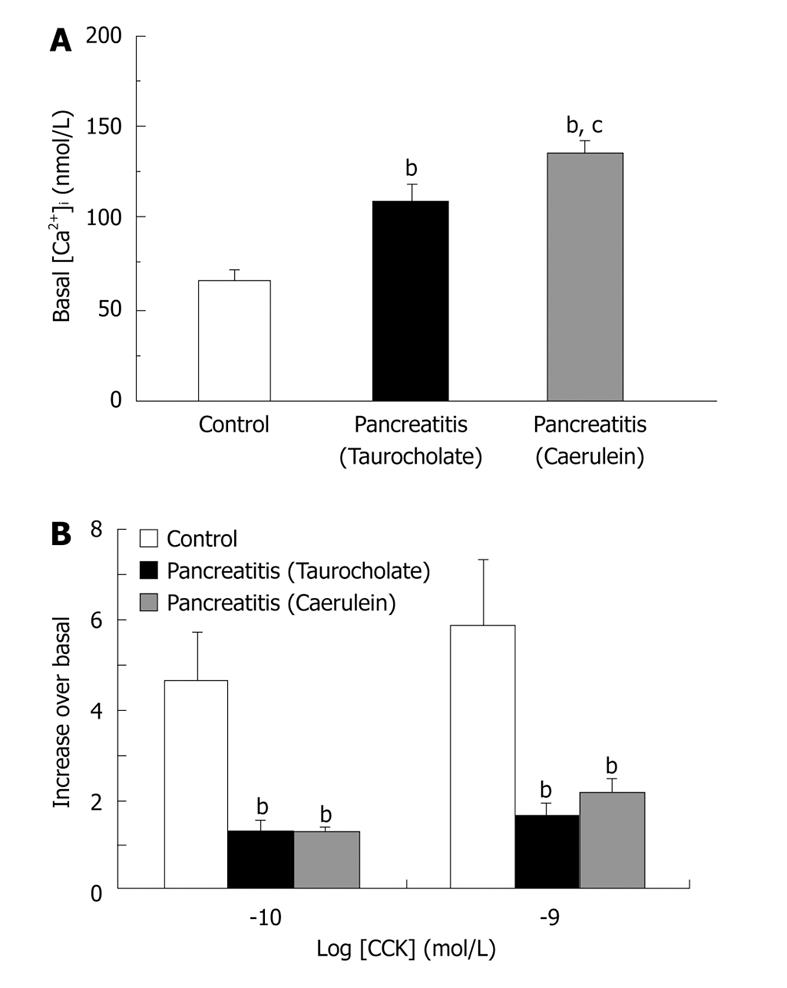
AP is caused by the premature intracellular activation of trypsinogen and other zymogens within the pancreatic acinar cells, accompanied by disruption of normal signal transduction and secretion[6,16,17]. Unregulated activation of pancreatic enzymes is related to an impaired cytosolic Ca2+ homeostasis[18,19]. Thus, we have demonstrated, by using two different AP experimental models[20,21], that basal levels of cytosolic Ca2+ are significantly increased in pancreatic acini isolated from pancreatitic rats with respect to control animals (Figure 1A). Moreover, an important reduction in calcium response to cholecystokinin is also observed in rats with AP (Figure 1B)[20,21]. Calcium signalling alteration seems to be one of the earliest events in AP development since it is observed previous to enzymatic secretion impairment in pancreatic acini[21].
Figure 1 Basal and CCK-stimulated cytosolic calcium homeostasis are impaired in two different models of experimental AP.
Experimental AP was induced in rats by infusing 5% (wt/vol) sodium taurocholate through the pancreatic duct (Taurocholate) or by subcutaneous injections of caerulein at a dose of 20 μg/kg
Sustained elevation of cytosolic Ca2+, described as occurring during the onset of AP, depend on both release of Ca2+ from intracellular stores and uptake from the extracellular milieu[22-24]. Store-operated channels have been recently involved in Ca2+ influx in AP[25], while the acid granular calcium stores, regulated by inositol 1,4,5-trisphosphate, as well as stores regulated by the ryanodine receptor, could be involved in intracellular Ca2+ release[23,26]. These mechanisms, and those previously described[27], may be involved in the pathological Ca2+ increase in the early phase of AP.
The effect of Ca2+ on zymogen activation within acinar cells seems to be mediated by calcineurin, a Ca2+/calmodulin-dependent serine/threonine phosphatase, which could be activated in the early phase of AP leading to formation of active trypsin[28,29].
In addition to cytosolic calcium impairment, oxidative stress has also been involved in abnormal enzyme activation during the early phase of AP[30]. Excessive oxygen radical formation from activated leukocytes[31] leads to oxidative stress and pathological processes. In experimental AP, superoxide radical accumulation could lead to cell cytoskeleton dysfunction leading to intracellular transport impairment and premature activation of digestive enzymes[30]. Moreover, increased oxidative stress causes an antioxidative capacity reduction and increases lipid peroxidation, and both effects could also play an important role in acinar cell damage[30].
Other early events that take place in the onset of AP is cytoskeletal proteins breakdown, probably mediated by cytosolic proteases activation[32] and disruption of acinar cell membranes, which could allow the influx of calcium and the exit of molecules, such as enzymes, from acinar cells[33].
Trypsin activation, which is a consequence of the prolonged elevated cytosolic Ca2+ and also of the deficient lysosomal degradation[34], occurs in post-exocytotic endocytic vacuoles[35]. After this event, several enzymes such as elastase and phospholipases and other mediators are activated[36], with increased leukocyte migration to the pancreas.
NEUROGENIC INFLAMMATION IN AP
Initial events, described above, take place within the pancreas and lead to the damage of gland tissue and pain arising in the damaged area. The detection and transmission of painful stimuli depend on stimulation of sensory neurons. These neurons have their bodies located at dorsal root ganglia, their axonal projections running centrally to the spinal chord and their dendrite projections innervating specific organs, including the pancreas. Stimulation of these primary sensory neurons activates second neurons in the spinal chord, relaying the signal pain, but this can also lead to neurotransmitter release from their ends in the peripheral tissues. These neurotransmitters exert bioactive actions, especially vasodilator effects, which could lead to neurogenic inflammation[37].
Substance P (SP) and calcitonin gene-related peptide (CGRP) are neurotransmitters that are released from these sensitive neurons, which seem to account for neurogenic inflammation in the pancreas. They can interact with endothelial cells, arterioles, mast cells and other immune cells to induce vasodilation, edema and inflammatory cell infiltration[38].
Concerning SP, it has been shown to play an important role in many inflammatory states[38]. SP has been detected within the pancreas and its levels are increased in caerulein-induced AP[38]. Moreover, we have recently shown that both neurotransmitters, SP and CGRP, are massively released from sensitive fibers within the pancreas of rats with taurocholate-induced SAP[21] suggesting that neurogenic inflammation is important in SAP development.
Vasodilator peptides release is supposed to be a protective mechanism since these molecules could improve pancreatic blood perfusion, avoiding ischemic damage. However, in a way that is not completely understood, they can also lead to deleterious effects in AP, which contribute to later SAP damage. During the last years, a set of experimental studies have confirmed the hypothesis that microcirculatory derangement plays a pivotal role in the pathogenesis of SAP[39,40].
Both SP and CGRP, and possibly other vasodilators, could be involved in this microcirculatory impairment. It has been recently shown that SP mediates pancreatic microcirculatory dysfunction during the development of experimental AP[41]. Thus, SP, through the neurokinin 1 receptor, would lead to vasodilation and plasma extravasation causing leukocyte adhesion, infiltration, and edema[42].
However, actions of these vasodilator neurotransmitters can go further. SP can specifically stimulate infiltrated leukocytes promoting inflammatory mediators release[43]. Pro-inflammatory molecules, such as cytokines, histamine or TNF-α, produced by lymphocytes, monocytes, macrophages and mast cells, would enhance tissue damage and further increase leukocyte recruitment. All these mechanisms contribute to amplify the inflammatory and systemic manifestation of AP[44].
Furthermore, SP could mediate the inflammatory response by directly activating tachykinin receptors in pancreatic acinar cells, leading to release of both enzyme and inflammatory mediators from these cells[45,46].
In this early phase of AP, acinar cells would be the primary source of inflammatory mediators and the relative imbalance between pro-inflammatory and anti-inflammatory responses could be the crucial issue for the progression and severity of the disease[44]. Damage, which is first confined in the pancreas, can finally be disseminated throughout the body and may cause the feared multiorganic failure.
SAP progression includes different events that lead to the vascular endothelial barrier dysfunction with an increased permeability and transendothelial migration of leukocytes and harmful enzymes to various tissues[47,48]. In the following section, we will describe the different pathways that can lead to this injury.
FROM LOCAL PANCREATIC DAMAGE TO SYSTEMIC EFFECTS
Despite all the research on pathophysiological mechanisms involved in SAP development, the explanation for how the inflammatory process extends and affects different organs is unknown. SIRS, which is the main life-threatening complication in patients with SAP[7,8], is characterized by pulmonary, cardiovascular and renal insufficiency[49]. Moreover, SIRS can be associated with an impaired cardiovascular function characterized by hypotension, which has been considered as a risk factor for death on admission at the hospital[50].
A recent review focusing on the reasons for multiple organ dysfunction in SAP concluded that injury incidence is lower in organs located farther from the pancreas[51]. The authors suggested that enzymatic proteases, especially trypsin, are the main culprits in damage spread. In mild cases, trypsin remains confined to the pancreas, whereas in SAP it enters the blood circulation and could affect other tissues[51].
Moreover, the overwhelming production of pro-inflammatory mediators, as well as oxidative injury, has also been involved in extending the inflammation to other organs different from the pancreas. Thus, inflammatory mediators, such as ICAM-1, could contribute to the exit of activated pancreatic enzymes from the circulation by increasing capillary permeability[52] and a reduction of hepatic damage after antioxidant agentsadministration has been shown during experimental AP[53].
As we have mentioned above, inflammation of different organs and multiorganic failure is usually associated with a dramatic fall of arterial blood pressure. Although the mechanisms underlying hypotension associated with SAP are still not completely understood, a failure in the physiological equilibrium between vasodilator and vasoconstrictor mediators has been proposed[54]. In two recent studies, we have analyzed whether an overwhelming production of vasodilators or a diminished response to vasoconstrictors occur in SAP-associated hypotension[55,56] and in the following paragraphs we will summarize our main conclusions.
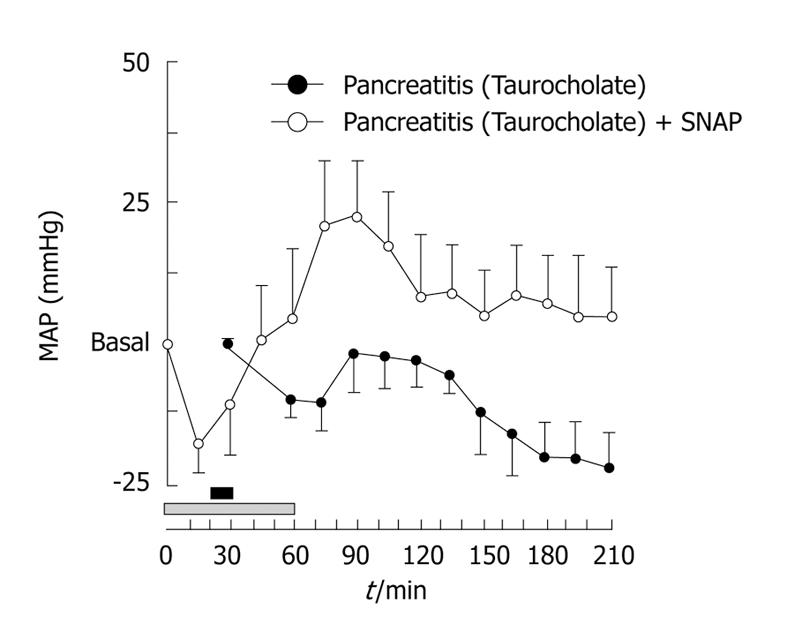
Nitric oxide (NO), an important vasodilator, has been implicated in the pathophysiology of AP. However, there is not agreement about its beneficial or detrimental role in this disease[57]; moreover, an increase in NO has been suggested in SAP in humans[58], but this issue is not yet clear[59]. We have demonstrated, by using an experimental model of necrotizing AP in rats, that NO and other vasodilators may exert a beneficial effect on SAP when they are administered before induction of pancreatitis[55]. Thus, retrograde infusion of 5% sodium taurocholate through the pancreatic duct caused SAP associated with a fall of approximately 25 mmHg in mean arterial pressure in rats 2 or 3 h after pancreatitis induction (Figure 2). Treatment with a NO donor, S-nitroso-N-acetylpenicillamine (SNAP), previously to pancreatitis induction, led to a stabilization of arterial pressure in pancreatitic animals (Figure 2). Since enhancement of capillary blood flow protects ischemic areas in the pancreas from becoming necrotic[60], we postulated that NO, as well as other vasodilators, would reduce the systemic circulatory derangement derived from the development of SAP by increasing blood flow in the gland[55]. Moreover, we have observed that in later phases of SAP, when hemodynamic impairment is established, NO still plays an important role in regulating the vascular tone[56] and controlling arterial pressure in this condition. But, what is the physiological role of vasodilators and vasoconstrictors once the hypotensive phase associated with AP has been reached?
Figure 2 Hypotension associated with severe acute pancreatitis (SAP) is prevented with previous infusion of S-nitroso-N-acetylpenicillamine (SNAP).
Mean arterial pressure (MAP) values along the experimental time in rats that were infused with 5% taurocholate through the pancreatic duct for 10 min (black horizontal bar) (Pancreatitis) or 5% taurocholate through the pancreatic duct for 10 min and intravenously infused with SNAP (200 μg/kg per hour) for 1 h (gray horizontal bar) (Pancreatitis + SNAP). Results are mean ± SE of changes above or below the basal MAP value (n = 6 for each treatment).
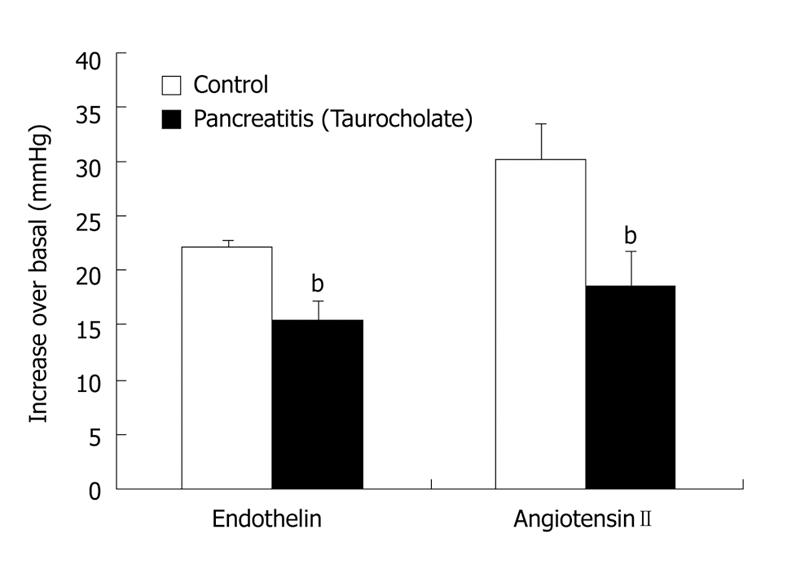
Our work has demonstrated that hypotension associated with SAP could be related to a marked decrease in the response to vasoconstrictor substances, such as endothelin and angiotensin II[56]. Both endothelin and renin-angiotensin system (RAS) are increased in SAP[61,62]. The hypertensive response to exogenously administered vasoconstrictors is lower in animals with pancreatitis than in control rats (Figure 3), perhaps due to the fact that their receptors are occupied by the endogenous endothelin and angiotensin II released during AP. Although the pressor responsiveness to angiotensin II is reduced in animals with pancreatitis, we have demonstrated that RAS plays a very important role in maintaining arterial pressure in these animals, since angiotensin II synthesis inhibition aggravates the physiological homeostatic response of the cardiovascular system in SAP[56], which could finally lead to the animal’s death. This demonstrates the importance of RAS in the hypotensive status.
Figure 3 Response of arterial blood pressure to endothelin and angiotensin II is impaired in pancreatitic rats.
Taurocholate was retrogradely infused through the pancreatic duct. Once mean arterial pressure in animals with pancreatitis diminished to around 20 mmHg with respect to basal values or at an equivalent time in controls, both endothelin (4 nmol/kg) and angiotensin II (225 ng/kg)
In conclusion, AP is a common inflammatory disease with increasing incidence. Although the majority of patients have a mild episode of AP, some of them develop a severe course and suffer SIRS. It seems that once hypotension is fully established, opportunities to ameliorate the disease state and reverse the situation are limited. This means that therapeutic efforts should be directed towards earlier phases of the disease. It is particularly important to understand the pathophysiological mechanisms that occur at the first phases of AP to avoid damage spreading out of the pancreas. Moreover, efforts should also be directed to know why AP develops to become a severe disease in some patients but not in others, and to identify the patients who are at risk for developing severe disease in the earliest phases of AP.
Peer reviewer: Mukaddes Eşrefoğlu, Professor, Department of Histology and Embryology, Inonu University Medical Faculty, Malatya 44280, Turkey
S- Editor Li LF L- Editor Lutze M E- Editor Yang C