Published online Feb 15, 2016. doi: 10.4291/wjgp.v7.i1.160
Peer-review started: March 31, 2015
First decision: June 18, 2015
Revised: September 25, 2015
Accepted: November 3, 2015
Article in press: November 4, 2015
Published online: February 15, 2016
AIM: To investigate recruitment, retention, and estimates for effects of formula supplementation with Lactobacillus rhamnosus GG (LGG) on inflammatory biomarkers and fecal microbial community in infants with colic.
METHODS: A prospective, double-blind, placebo-controlled trial was conducted in otherwise healthy infants with colic. We screened 74 infants and randomized and analyzed results in 20 infants [9 receiving LGG (LGG+) and 11 not receiving LGG (LGG-)]. LGG was incorporated in the formula (Nutramigen®) (minimum of 3 × 107 CFU/d) in the LGG+ group. Fecal microbiota and inflammatory biomarkers, including fecal calprotectin (FC), plasma cytokines, circulating regulatory T cells (Tregs), and crying + fussing time were analyzed to determine optimal time points and effect sizes for a larger trial.
RESULTS: Recruitment in this population was slow, with about 66% of eligible infants willing to enroll; subject retention was better (75%). These rates were influenced by parents’ reluctance to volunteer their infant for a clinical trial and by their tendency to change formulas. The maximal difference of crying + fussing time was observed at day 14, comparing the 2 groups, with a mean difference of -91 (95%CI: -76, 259) min (P = NS). FC showed no significant difference, but the optimal time to determine a potential effect was at day 90 [with a mean difference of 121 (95%CI: -48, 291) μg/g stool], observing a lower level of FC in the LGG+ group. The fecal microbial communities were chaotic, as determined by Shannon’s diversity index and not apparently influenced by the probiotic. No significant change was observed in plasma inflammatory cytokines or Tregs, comparing LGG+ to LGG- groups.
CONCLUSION: Designing future colic trials involving a probiotic-supplemented formula for infants in the United States will require consideration for difficult enrollment. Infants with colic have major variations in feal microbiota and calprotectin, both of which improve with time, with optimal time points for measurement at days 14 and 90 after treatment.
Core tip: The “dysbiosis” theory proposes that newborns with abnormal colonization are predisposed to having gut inflammation and colic. Probiotics may reduce crying and diversify the fecal microbiota. A prospective, double-blind, placebo-controlled trial was conducted in healthy infants with colic. After 75% screen failure or dropouts, 20 infants were analyzed (9 receiving formula with Lactobacillus GG and 11 not receiving Lactobacillus rhamnosus GG in their formula). We found that: (1) recruitment/retention indicate future randomized controlled trials should enroll 80 patients with an optimal timepoint for observing a potential difference in crying at 14 d; (2) microbial communities were chaotic in infants with colic, even more so than reported in Dutch infants; and (3) our study was the first to analyze cytokine levels and circulating Tregs in infants with colic.
- Citation: Fatheree NY, Liu Y, Ferris M, Van Arsdall M, McMurtry V, Zozaya M, Cai C, Rahbar MH, Hessabi M, Vu T, Wong C, Min J, Tran DQ, Navarro F, Gleason W, Gonzalez S, Rhoads JM. Hypoallergenic formula with Lactobacillus rhamnosus GG for babies with colic: A pilot study of recruitment, retention, and fecal biomarkers. World J Gastrointest Pathophysiol 2016; 7(1): 160-170
- URL: https://www.wjgnet.com/2150-5330/full/v7/i1/160.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v7.i1.160
Colic has been defined as inconsolable crying and fussing, for greater than 3 h daily for more than 3 d per week in infants from 3 wk to 3 mo of age[1]. There have been many theories to explain the occurrence of colic, including bacterial overgrowth[1], the “fourth trimester” theory in which the baby is wishes to remain in utero[2], parental depression[3], excessive intestinal gas[1], and milk protein allergy[4]. Savino was the first to propose that an abnormal microbiota (“dysbiosis”) might be an important pathophysiological mechanism, by demonstrating a reduced abundance of Lactobacilli and increased abundance of E. coli in the stools of infants with colic[5].
Our previous studies showed that intestinal inflammation was a feature of colic[6]. This led to the hypothesis that there may be an association of dysbiosis and intestinal inflammation, both of which may improve with probiotic treatment. The probiotic Lactobacillus rhamnosus GG (LGG) has been shown to reduce diarrhea in children with acute infectious enteritis[7] and to facilitate the development of a more diverse fecal microbiota[8]. Others have hypothesized that early colonization of the immature small intestine with lactobacillus would reduce gut inflammation and symptoms in infants with colic. However, previous studies have focused on breast-fed babies.
In the current studies, our two major aims were: (1) to investigate the feasibility of recruitment and retention of babies with colic randomized to receive a probiotic-containing formula; and (2) to determine effect size of LGG-supplemented formula on crying + fussing time, the intestinal microbiota, and the inflammatory biomarker calprotectin in infants with colic.
This protocol was approved by the Institutional Review Board of the University of Texas Health Science Center at Houston. Every 10 patients, a Data Safety Monitoring Board convened to review safety. A consort checklist is available as supporting information. The trial protocol was registered in http://www.clinicaltrials.gov NCT01279265.
This study was a prospective, double-blind, placebo-controlled trial in otherwise healthy infants with colic, designed as a pilot study for determining potential effects of treatment with casein-hydrolysate formula with LGG versus no LGG on selected biomarkers in infants with established colic. This formula (Nutramigen) was chosen because at the time it was the only formula that could be obtained in liquid form with or without probiotic, because it has been suggested to be beneficial in infants with colic[4], and because Food and Drug Administration-monitored safety and biomarker trials of direct probiotic supplementation in infants with colic had not been completed at this point. Partially of fully formula-fed infants age 3-13 wk old born full-term (> 37 wk gestation) were included if they fulfilled the colic definition of crying and fussing more than 3 h per day for at least 3 d weekly, documented at enrollment by at least 2 abnormal Barr diaries over a 3-d period[9]. Patients were actively recruited through university community clinics, 4 pediatric practices affiliated with our university, the pediatric gastroenterology clinic, and other local pediatricians via mailings, television coverage, and a website. Infants were excluded if they had failure to thrive, chronic lung disease, diarrhea, fever, and if they took a probiotic prior to enrollment.
Infants were randomly assigned by block randomization (groups of 4) to one of two formulas, either casein hydrolysate (Nutramigen®) with LGG (Enflora™) or casein hydrolysate without LGG (Nutramigen A+®). Initially, the protocol was to enroll children with or without colic to receive the above 2 formulas in white, unlabeled containers and to measure crying + fussing time and the biomarkers, but the protocol had to be changed because they were uncomfortable with this type of label. We changed the protocol so that containers were partially covered with a sticky label to ensure blinding. The randomization schedule was computer-generated, prepared by the study biostatistician and implemented by pharmacists in Department of Investigational Drugs Services at Memorial Hermann Hospital. Infants with partial breast-feeding were required to take at least 240 mL of formula per day to ensure at least 3.6 × 107 CFU’s of LGG (if they were randomized to the LGG+ group). However, most infants were completely formula-fed (as shown in Table 1). The infants were required to take study formula for the entire 90 d of observation, with research visits on days 1, 14, 42, and 90. Patients were followed by telephone on a weekly basis. During each clinical visit, the medical history and clinical condition of each infant was evaluated by a pediatric gastroenterologist. Stool and blood were collected at baseline and follow up visits.
| LGG+ group | LGG- group | ||||
| Continuous variables | n | mean ±SD | n | mean ± SD | P |
| Age at the time randomized (d) | 9 | 57 ± 30 | 11 | 68 ± 28 | 0.341 |
| Gestational age (wk) | 9 | 38 ± 2 | 11 | 37 ± 2 | 0.201 |
| Birth weight (kg) | 9 | 3.1 ± 0.8 | 11 | 2.9 ± 1.1 | 0.471 |
| Birth height (cm) | 7 | 51.6 ± 1.8 | 9 | 47.2 ± 5.3 | 0.041 |
| Discrete variables | n | mean ± SD | n | mean ± SD | |
| Gender | 9 | 11 | |||
| Female | 4 (44.4) | 4 (36.4) | 1.0 | ||
| Male | 5 (55.6) | 7 (63.6) | |||
| Race | 9 | 11 | 1.0 | ||
| African-american | 3 (33.3) | 4 (36.4) | |||
| Caucasian | 6 (66.7) | 7 (63.6) | |||
| Ethnicity | 9 | 11 | 1.0 | ||
| Hispanic or latino | 2 (22.2) | 2 (18.2) | |||
| Not hispanic or latino | 7 (77.8) | 9 (81.8) | |||
| Partial breast feed | 9 | 11 | 1.0 | ||
| Yes | 3 (33.3) | 3 (27.3) | |||
| No | 6 (66.7) | 8 (72.7) | |||
| Formula type | 9 | 11 | |||
| Earth’s best | 1 (11.1) | 0 (0) | |||
| Enfamil | 1 (11.1) | 0 (0) | |||
| Enfamil gentleease | 1 (11.1) | 0 (0) | |||
| Gerber good start | 0 (0) | 1 (9.1) | |||
| Isomil | 1 (11.1) | 1 (9.1) | |||
| Neocate | 0 (0) | 1 (9.1) | |||
| Nutramigen ready mix | 0 (0) | 1 (9.1) | |||
| Similac | 2 (22.2) | 0 (0) | |||
| Similac advance | 1 (11.1) | 0 (0) | |||
| Similac senstive | 0 (0) | 5 (45.5) | |||
| Breast milk | 2 (22.2) | 2 (18.2) | |||
Infant crying and fussing time was quantified using the Barr Diary, a well-validated instrument, as previously described[9] at each study visit.
Parents/guardians were asked to have the infant fast for a minimum of 3 h before the baseline visit and before visit 2. After two baseline samples were collected (separated by 15 min), the infant was fed 60 mL of glucose water, and at time = 45 min, exhaled air was collected and breath hydrogen and methane were measured using the Quintron Model SC Microlyzer™ (Quintron Instrument Co., Inc., Milwaukee, Wisconsin). In all infants, breath methane was negligible. A breath test was considered positive if the baseline hydrogen level was ≥ 20 parts per million (ppm) or if there was an increase from baseline of ≥ 12 ppm[6].
Fecal calprotectin (FC). Stool samples was prepared and analyzed by using a quantitative calprotectin ELISA kit according to manufacturer’s instructions, as previously described[10]. The level of calprotectin was expressed as μg/g of stool weight.
Plasma cytokines and percentage of Tregs: Plasma cytokines were detected by using MSD Human ProInflammatory 7-Plex Ultra-Sensitive Kit (Meso Scale Discovery®, Gaithersburg, MD) which measures human IFN-γ, IL-1β, IL-6, IL-8, IL-10, IL-12p70, and TNF-α[10]. This inflammatory panel was chosen because LGG has been shown to prevent enterocyte apoptosis induced by IFN-γ, IL-1β, IL-6, IL-8, IL-10, IL-12p70, and TNF-α[11] Isolated peripheral blood mononuclear cells were stained with surface CD4 and intracellular FOXP3 antibodies and analyzed by using flow cytometry[10].
Fecal pyrosequencing analysis: Parents were instructed to collect a stool sample within 48 h of the visit and to store stools frozen. In the lab, stool samples were subdivided and stored at -80 °C until analyzed. DNA extraction, polymerase chain reaction-amplification, pyrosequencing and taxonomic identification of 16S rRNA gene sequences in stool specimens were performed as previously described[12], using QIIME[13] to analyze microbial communities. A total of 185373 reads with an average length of 460 ± 62 bases were included in this study. The average number of reads per sample was 3783.
Sample size and power: This pilot study aimed to determine recruitment, retention, adverse events, and biomarkers but was not powered to detect differences in crying time between the two study arms. (Based on our previous study[6] showing 297 ± 142 min/d as a mean crying+fussing time in colicky infants, we determined that a sample size of 60 colicky infants (30 infants per study arm) would have been required to detect a mean difference of 100 min/d of crying + fussing time, with a power of 0.80 at the 5% level).
Baseline characteristics were compared between two groups using the two sample t-test or Wilcoxon rank sum test for continuous variables and the Fisher’s exact test for categorical variables. For estimating the effect size of Barr diary crying time and FC, we used generalized Estimating Equation method with autoregressive covariance structure to account for potential correlation between measures at multiple visits during the follow up period. Time variable (i.e., visits), treatment group indicator, as well as their interactions are included as covariates to estimate the differences in the outcomes between the two study arms (i.e., LGG- values minus LGG+ values) over time. The adjusted means and the mean differences between LGG- vs LGG+ groups as well as their 95%CI were calculated. The Wilcoxon rank sum test was applied to compare the cytokines at baseline and at each of the follow up visits. All the above analyses were conducted using statistical software SAS 9.3 (SAS Institute, Cary, NC). Microbiota data were analyzed using Mann-Whitney U-test and one-way ANOVA using GraphPad Prism v5.0 for windows (GraphPad Software, San Diego, CA). Shannon’s Diversity Index was calculated using a locally developed pyrosequencing pipeline[12].
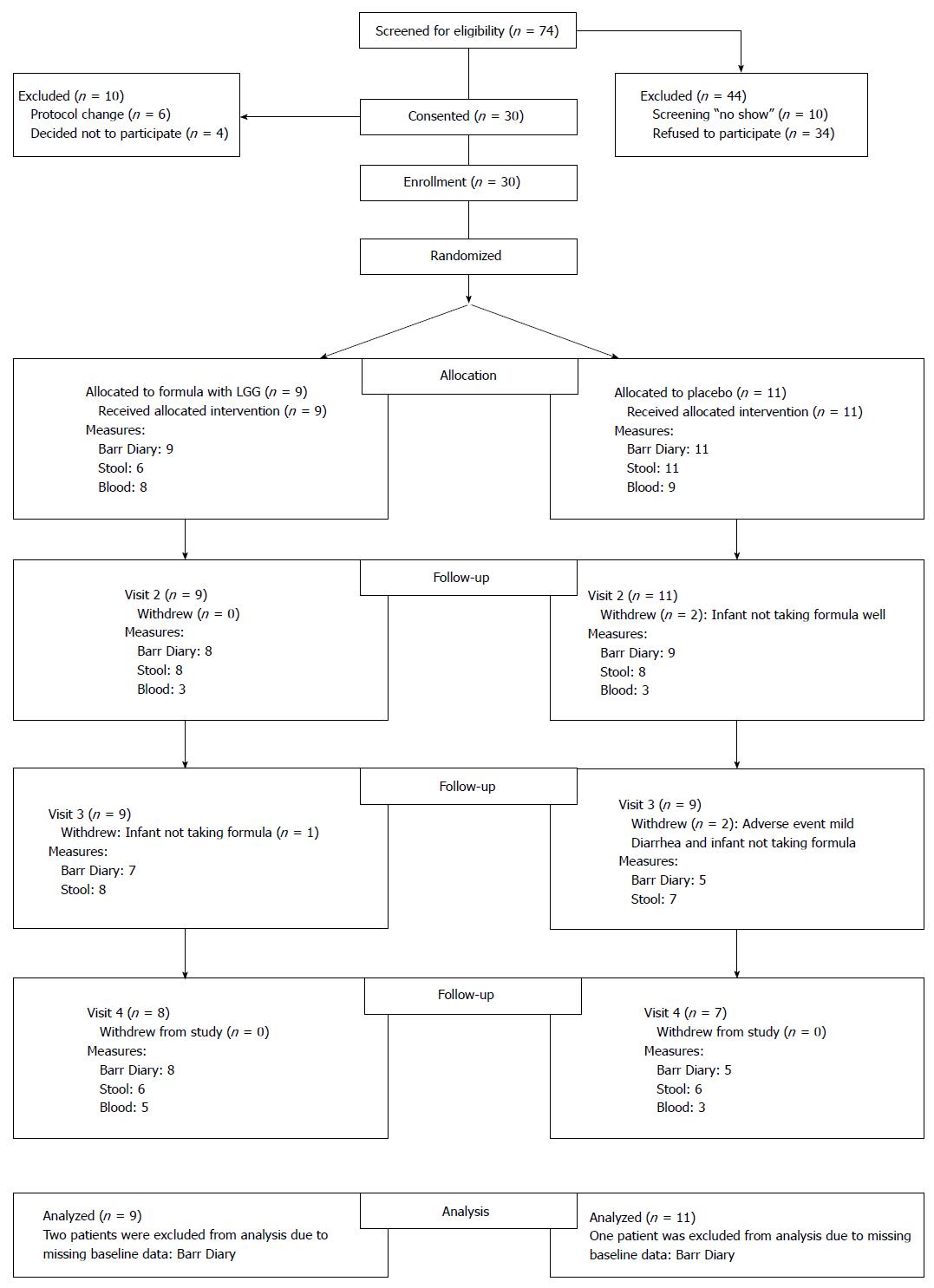
Enrollment took place from September 2011 to January 2013. Seventy-four infants with colic were screened based on the above inclusion and exclusion criteria. Forty-four (59%) infants were not included in the study because they did not come to the scheduled first clinic visit or because the parents/guardians chose not to participate. Thirty were enrolled, but 4 changed their mind after signing consent, and 6 were uncomfortable when they received cans of formula with a white label. Subsequently, we changed by taping over the label to hide only the probiotic part of the label (The study initially was designed to include 30 infants in each group, but it was closed because of lack of funds to continue). Twenty infants randomized to either the LGG+ group (n = 9) or to the placebo (LGG- group) (n = 11) group were able to be analyzed (Figure 1, Consort Diagram). Baseline characteristics including mean gestational age, age at enrollment, weight, and length showed no differences between the two groups (Table 1). Feeding method was exclusive formula feeding in 70%, although 3 in each group were receiving limited supplementation with breast milk. A wide range of formulas were given prior to enrollment, none of which contained a probiotic. Almost 5-h daily of crying and fussing provided evidence of severe symptoms in these infants.
Longitudinal analysis of outcome variables indicated that total crying + fussing times at baseline were comparable, and at each of the three treatment visits crying + fussing time decreased (Table 2). The maximal difference of crying + fussing time was observed at visit 2 (day 14) comparing the 2 groups, with a mean difference of -91 (95%CI: -76, 259) min, trending toward a shorter crying+fussing time in the LGG+ group. This difference was entirely the result of improved fussing time. After the immediate dropout following written consent of 4 infants, we observed a loss of 5 of the 20 children during follow-up. These children dropped out because of mild diarrhea (n = 1) or their parents’ decision to change the formula (n = 4). No adverse events during the period of observation were deemed attributable to the study product (LGG). These findings indicate that in studies of babies with colic, dropout is a common problem, with formula-changing being the major reason.
| Adjusted means (95%CI) | P value | |||
| LGG+ group | LGG- group | Mean differences(95%CI) | ||
| Crying + fussing time (min) | ||||
| Visit 1 (Baseline) | 296 (210, 381) | 337 (251, 422) | 41 (-80, 161) | 0.51 |
| Visit 2 | 197 (117, 278) | 289 (142, 436) | 91 (-76, 259) | 0.29 |
| Visit 3 | 144 (54, 234) | 199 (69, 328) | 55 (-104, 213) | 0.50 |
| Visit 4 | 111 (65, 157) | 133 (60, 205) | 22 (-64, 107) | 0.62 |
| Fecal calprotectin (μg/g) | ||||
| Visit 1 (Baseline) | 285 (199, 371) | 294 (184, 404) | 9 (-131, 149) | 0.90 |
| Visit 2 | 226 (182, 270) | 305 (186, 423) | 79 (-48, 205) | 0.22 |
| Visit 3 | 229 (113, 345) | 250 (154, 347) | 21 (-130, 172) | 0.78 |
| Visit 4 | 211 (80, 342) | 332 (225, 440) | 121 (-48, 291) | 0.16 |
We previously demonstrated an elevated FC in babies with colic, compared to age-matched babies without colic[6]. Longitudinal analysis of FC at baseline and at follow up visits showed that the values were similar at baseline (Table 2), while the maximal mean difference in FC between the LGG+ and LGG- groups was seen at visit 4 (90 d of probiotic formula treatment), with a difference of -121 (-48, 291) μg/g stool, observing a statistically nonsignificant lower level of FC in the LGG+ group.
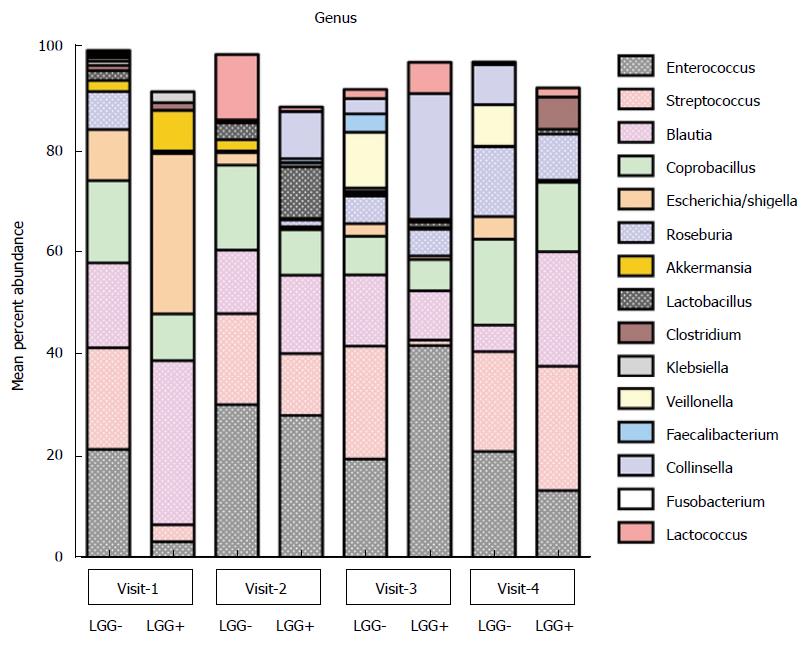
Distribution of predominant bacterial taxa in colicky infants: No differences in fecal diversity at any of the visits were observed between the infants with LGG+ and LGG- formulae. At baseline, the most abundant bacterial phylum was Firmicutes (72%); followed by Proteobacteria (24%). Enterobacteriales was the most abundant order and Enterobacteriaceae the most abundant family in these infants. The genus level analysis at enrollment showed the major genera to be Blautia, Escherichia/Shigella, Enterococcus, Streptococcus, and Coprobacillus (Figure 2). There was no significant difference in averaged genus level microbial composition distribution between (LGG+)-fed colicky babies compared to (LGG-)-fed babies with regard to the major taxa. Minor differences might have been missed because of small sample size.
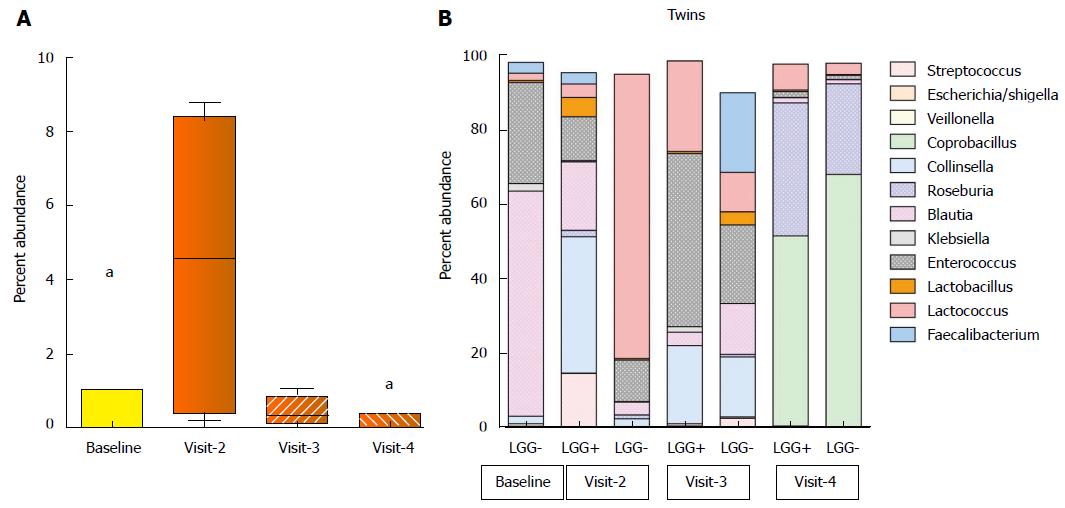
Abundance of L. rhamnosus in stool specimens: Previously, investigators reported that a 2 wk trial of LGG at similar doses resulted in positive LGG cultures of the stool of 85% of healthy infants during its administration and in half when measured 28 d later[14]. Here, L. rhamnosus abundance increased to about 5% of total bacteria after 14 d of LGG+ formula treatment (P = 0.006), which was significantly higher than baseline, and also higher than on visit 3 or visit 4 (P < 0.05) (Figure 3A).
Diversity of microbiota and changes over time: We had the opportunity to analyze the evolution of microbial communities of a set of dizygotic twins at the genus level, as shown in Figure 3B. One infant was randomized to the LGG- group, while the other was randomized to LGG+. Note that we were unable to obtain baseline sequence data for one twin despite two attempts, but data were available for both at each follow-up visit. At each of the treatment visits, we were able to compare the microbiota of the two infants, and we found that Lactobacillus was present in the twin babies’ stools at all follow-up visits. It was most abundant in the LGG+ infant at the 2nd visit. However, lactobacillus abundance declined to low levels in both infants by visit 4. Comparing visits 2, 3 and 4 in these two infants, the stool composition fluctuated greatly. However, comparison of the microbiota of twin A and B revealed remarkable convergence of the community profile among the twins, most evident at visit 4.
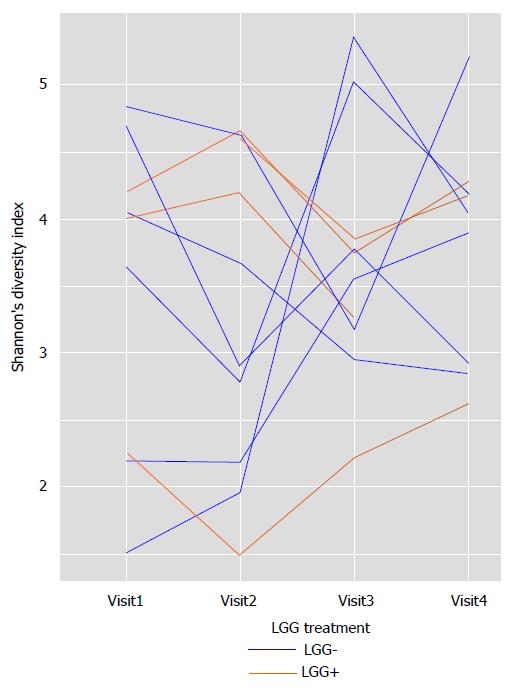
Shannon’s diversity index: varied wildly in all infants over time. Figure 4 shows data for all infants whose parents brought stools at each of the 4 clinic visits. There were no major differences noted between LGG+ and LGG- infants, because in both groups diversity fluctuated greatly from visit to visit. Thus, fecal microbial community was “chaotic” and did not show stabilization by the end of the 3-mo study, when the infants were 4-5 mo old.
There were no major differences between the LGG+ and LGG- groups with respect to breath hydrogen levels (data not shown), plasma cytokine levels, or percentage of circulating Tregs (Table 3). With respect to breath hydrogen, 5 of the 20 infants (25%) had breath hydrogen increases of ≥ 12 ppm above baseline at their initial sampling, while two infants (10%) had increased breath hydrogen at visit 2. However, there was no significant difference between the LGG+ and placebo groups with respect to breath hydrogen changes, and there was also no correlation between changes in breath hydrogen and crying time.
| Adjusted geometric means (95%CI) | P | ||
| LGG+ group | LGG- group | ||
| Cytokines | |||
| IFN-γ (pg/mL) | |||
| Visit 1 (Baseline) | 0.7 (0.4, 1.3) | 0.6 (0.3, 1.2) | 0.84 |
| Visit 2 | 1.2 (0.7, 2.0) | 0.5 (0.3, 0.9) | 0.04 |
| Visit 4 | 0.4 (0.3, 0.6) | 0.2 (0.1, 0.4) | 0.07 |
| IL-10 (pg/mL) | |||
| Visit 1 (Baseline) | 3.7 (2.3, 6.0) | 4.2 (2.2, 8.1) | 0.78 |
| Visit 2 | 4.3 (3.2, 5.7) | 3.8 (2.3, 6.4) | 0.72 |
| Visit 4 | 2.7 (1.7, 4.2) | 2.0 (1.1, 3.7) | 0.47 |
| IL-12p70 (pg/mL) | |||
| Visit 1 (Baseline) | 0.2 (0.1, 0.4) | 0.2 (0.1, 0.4) | 0.85 |
| Visit 2 | 0.3 (0.2, 0.4) | 0.4 (0.3, 0.6) | 0.29 |
| Visit 4 | 0.2 (0.1, 0.5) | 0.3 (0.2, 0.4) | 0.57 |
| IL-1β (pg/mL) | |||
| Visit 1 (Baseline) | 0.08 (0.05, 0.13) | 0.19 (0.08, 0.43) | 0.09 |
| Visit 2 | 0.27 (0.08, 0.88) | 0.15 (0.06, 0.40) | 0.45 |
| Visit 4 | 0.11 (0.05, 0.21) | 0.16 (0.05, 0.49) | 0.52 |
| IL-6 (pg/mL) | |||
| Visit 1 (Baseline) | 0.3 (0.2, 0.5) | 0.4 (0.3, 0.6) | 0.36 |
| Visit 2 | 0.3 (0.2, 0.6) | 0.3 (0.2, 0.4) | 0.66 |
| Visit 4 | 0.2 (0.1, 0.4) | 0.2 (0.1, 0.3) | 0.63 |
| IL-8 (pg/mL) | |||
| Visit 1 (Baseline) | 6.5 (4.8, 8.7) | 7.0 (5.3, 9.4) | 0.69 |
| Visit 2 | 5.4 (4.1, 7.3) | 9.5 (6.6, 13.7) | 0.02 |
| Visit 4 | 4.9 (3.2, 7.5) | 4.6 (2.8, 7.8) | 0.88 |
| TNF-α (pg/mL) | |||
| Visit 1 (Baseline) | 4.5 (3.8, 5.3) | 4.8 (3.7, 6.2) | 0.69 |
| Visit 2 | 5.4 (4.4, 6.5) | 4.2 (3.2, 5.5) | 0.15 |
| Visit 4 | 4.4 (3.3, 5.9) | 5.7 (4.7, 6.9) | 0.14 |
| Treg (%) | |||
| Visit 1 (Baseline) | 7.2 (6.5, 8.0) | 6.7 (5.9, 7.5) | 0.37 |
| Visit 2 | 7.5 (6.8, 8.2) | 8.5 (7.0, 10.3) | 0.23 |
| Visit 4 | 7.5 (6.1, 9.3) | 9.2 (8.1, 10.5) | 0.10 |
Recently published in JAMA Pediatrics, Sung’s systematic review of probiotics for colic emphasized that there remains “insufficient evidence to support probiotics to manage colic, especially in formula-fed infants”[15]. Our study which focused on this group, was not powered to determine efficacy of LGG+ formula, but we aimed to identify key information for future prospective randomized, controlled trials of treatments for infants with colic in the United States. Although not focused on colic, a recent double blind placebo-controlled trial focused on preterm infants (gestational age 32-36 wk.) who were randomized to receive LGG (> 109 CFU/d), a combination prebiotic or placebo. Authors reported that these infants were more likely to be contented (as opposed to “excessive criers”) during the first 2 mo of life if they received prebiotic or LGG as compared to placebo[16]. Barr diaries were not used in this study. In addition to Sung’s trial, our results may be included in meta-analyses of probiotic supplementation for formula-fed infants with colic.
One key finding was that recruitment of babies with colic in this country is difficult. During the consent process, two-thirds of parents we interviewed declined to particiate in this trial. Possible reasons included the FDA- and IRB-mandated consent form (which contained the statement “very rare cases of blood infection, acidosis (acid in the blood), endocarditis (heart valve infection), and meningitis (swelling and irritation of the covering tissue around the brain) in patients who are already ill have been reported”. Parents who declined to participate said that they preferred to try formulas, herbal remedies, and probiotics that are advertised to be beneficial for babies with colic or “sensitive” intestines. To the authors, this indicated their uncertainty associated with clinical research and the informed consent process.
After recruitment, retention was about 75%, with dropouts related to infants not taking the formula or to the observation of loose stools. Based on our previous study of crying + fussing time in colicky infants[6], we determined that a sample size of 60 colicky infants (30 infants per study arm) would detect a mean difference of 100 min/d of crying + fussing time between the 2 study groups. We attempted to recruit this number over 2 years, but were unable to do so. Although we cannot be certain that enrollment and dropout rates would be the same for similar trials, we can estimate that a future probiotic formula trial conducted in the United States would require recruitment of > 80 enrollees for 60 infants to complete the trial. With our recruitment numbers in the 4th largest United States city, with 5300 pediatric gastroenterology visits annually, we suggest that a 3-center, 2-year trial would be optimal. Note that smaller differences in crying + fussing time were found to be significant in the Savino et al[5] and Szajewska et al[17] studies, but they required numbers of infants similar to the 30 in each group that we herein recommend.
We found no major side effects or safety problems contributing to product concerns or patient dropouts.
We reported previously in infants with colic elevated FC, suggesting a contribution of gut inflammation to this condition[18]. In the current study, we found that FC levels were high at virtually all time-points in the infants with colic, in both LGG+ and LGG- infants. The values of FC were consistently above the normal clinical range reported in adults (0-162 μg/g). An elevated FC in infants with colic and in normal infants at this age may reflect low-grade intestinal inflammation during this period of aggressive microbial colonization[19]. In addition, some of the infants were partially breast-fed, a condition arguably associated with a higher FC level[20], although there were only 3 such infants in each group. The difference in FC between groups was not significant. There could be a downward trend as a consequence of normal colonization, because FC levels in older children are substantially lower than those in infants[21]. Savino et al[22] recently published in abstract form a similar increase in FC in infants with colic.
While FC may be a helpful biomarker during the evolution of colic, plasma cytokines appear not to be informative. All of the cytokine levels in the infant plasma were detectable above the minimal detection level of the assay, and values were similar to those previously reported in other studies[23-25].
Our study provides evidence that excessive intestinal H2 gas production is less prevalent in our population than was reported previously[1]. Glucose breath testing and/or elevated fasting breath H2 in our study was abnormal in only 25% of our infants with colic. A previous study, in which infants with colic received the nonabsorbable sugar lactulose which raised breath H2 values, showed that most babies did not have any symptomatic response to lactulose[26]. It is possible that excessive gas could be a contributing factor to colic in a subset of infants.
Pyrosequencing of 16S rRNA in the stool showed several interesting findings. First, longitudinally hyper-variable microbiota profiles during the 3-mo study (very evident in the twin pair shown in Figure 1B) support the concept of a “chaotic” pattern of colonization described by Palmer et al[27] early in life. This chaotic pattern has been challenged, with Koenig et al[28] suggesting a revised concept of population shifts attributable to major changes in life events. However in our study, patients were followed by telephone on a weekly basis for 3 mo. There were no major changes in life events, such as diet, diarrhea, antibiotic administration, or probiotic ingestion that were reported (Figure 1B).
One unexpected observation of our study was that inclusion of LGG in the formula had little impact on the overall composition or microbial diversity of the stool. This finding was particularly evident comparing the microbiota of the twin infants. The twins’ fecal microbial composition at 3 time-points fluctuated greatly, and there were Lactobacilli in both infants’ stools at visits 2 (day 14) to 4 (day 90), even though only one received LGG. Previous studies have shown that among adult monozyotic twins, the average microbiota similarity between twins is significantly higher than between unrelated subjects[29]. We suggest that factors other than L. rhamnosus were responsible for shifts in the fecal microbiota pattern of the infants. Such factors may include exposure to other people, animals, or environments.
In summary, our study showed that future trials of probiotics in formula for infants with colic at concentrations similar to those of Nutramigen with LGG should aim to recruit around 80 infants and should focus on determining efficacy on crying time at 14 d and on FC at 90 d. Changes in the microbiota (and/or their metabolic products) might be optimally observed at 2-4 wk of treatment.
The authors thank Drs. Mary Kay Koenig, Kuojen Tsao, and Cynthia Bell, for serving on the Data Safety Monitoring Committee. We would also like to acknowledge the University of Texas Pediatric Clinic’s physicians and nursing staff, as well as the Memorial Hermann and Kelsey-Seybold clinics, and other local Houston pediatric clinics in Houston that provided referrals for this study. We thank Dr. Yen Pham for her assistance in project planning and IRB approval, Deborah Lake and Meredith Rayne in Media Relations for helping us recruit patients, and the pediatric phlebotomist, Shantel Beasley. Finally, we thank Elizabeth Donnachie from Gulf States Hemophilia Center at the University of Texas Health Science Center at Houston, who assisted in operating BD FACSCalibur.
Colic has been defined as inconsolable crying plus fussing time equaling greater than 3 h daily for more than 3 d per week in infants from 3 wk to 3 mo of age. There have been many theories to explain the occurrence of colic, including bacterial overgrowth, the “fourth trimester” theory in which the baby would prefer to stay in utero, parental depression, excessive intestinal gas, and milk protein allergy. The probiotic Lactobacillus rhamnosus GG (LGG) has been shown to reduce diarrheal volume and duration in children with acute infectious enteritis; it has also been shown to facilitate the development of more diverse fecal microbiota. Savino was the first to propose that an abnormal microbiota (“dysbiosis”) might be an important pathophysiological mechanism.
Colic has begun to be studied as an example of possible dysbiosis with inflammation of the gut. Recent studies have centered on possible gut developmental issues, including the transition of environment from the intrauterine “bubble” to the external world and exposure to many new bacterial communities. Manipulation of the gut microbiota could be a major advance toward reducing the crying and fussing times of these infants.
This study is one of the first to investigate the impact on a probiotic on the microbiota of colicky babies and fecal calprotectin, as a marker of inflammation, during early life.
To summarize the practical applications of your research findings, so that readers may understand the perspectives by which this study will affect the field and future research. Future trials of probiotic-supplemented formulas may benefit from our pilot trial. The authors demonstrated robust numbers for crying + fussing time, fecal calprotectin, and microbial diversity in this population.
LGG is a health-promoting bacterial species which may be capable of reducing inflammation and regulating gut function. Microbiota is the term for bacterial community found in various locations such as skin and gut. The authors’ focus is on the intestine, which has been shown to regulate diverse functions in the human body.
The topic is interesting and the manuscript well presented.
P- Reviewer: Bourke B, Bian ZX, Silva MA S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
| 1. | Moore DJ, Robb TA, Davidson GP. Breath hydrogen response to milk containing lactose in colicky and noncolicky infants. J Pediatr. 1988;113:979-984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 72] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Karp H. The fourth trimester and the calming reflex: novel ideas for nurturing young infants. Midwifery Today Int Midwife. 2012;25-26, 67. [PubMed] [Cited in This Article: ] |
| 3. | van den Berg MP, van der Ende J, Crijnen AA, Jaddoe VW, Moll HA, Mackenbach JP, Hofman A, Hengeveld MW, Tiemeier H, Verhulst FC. Paternal depressive symptoms during pregnancy are related to excessive infant crying. Pediatrics. 2009;124:e96-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Lucassen PL, Assendelft WJ. Systematic review of treatments for infant colic. Pediatrics. 2001;108:1047-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Savino F, Cordisco L, Tarasco V, Palumeri E, Calabrese R, Oggero R, Roos S, Matteuzzi D. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics. 2010;126:e526-e533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 6. | Rhoads JM, Fatheree NY, Norori J, Liu Y, Lucke JF, Tyson JE, Ferris MJ. Altered fecal microflora and increased fecal calprotectin in infants with colic. J Pediatr. 2009;155:823-828.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr. 2001;33 Suppl 2:S17-S25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 263] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 8. | Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Sears MR, Becker AB, Scott JA, Kozyrskyj AL. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Immunol. 2013;9:15. [PubMed] [Cited in This Article: ] |
| 9. | Barr RG, Rotman A, Yaremko J, Leduc D, Francoeur TE. The crying of infants with colic: a controlled empirical description. Pediatrics. 1992;90:14-21. [PubMed] [Cited in This Article: ] |
| 10. | Mangalat N, Liu Y, Fatheree NY, Ferris MJ, Van Arsdall MR, Chen Z, Rahbar MH, Gleason WA, Norori J, Tran DQ. Safety and tolerability of Lactobacillus reuteri DSM 17938 and effects on biomarkers in healthy adults: results from a randomized masked trial. PLoS One. 2012;7:e43910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959-50965. [PubMed] [Cited in This Article: ] |
| 12. | Gupta RW, Tran L, Norori J, Ferris MJ, Eren AM, Taylor CM, Dowd SE, Penn D. Histamine-2 receptor blockers alter the fecal microbiota in premature infants. J Pediatr Gastroenterol Nutr. 2013;56:397-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23810] [Cited by in F6Publishing: 22357] [Article Influence: 1596.9] [Reference Citation Analysis (0)] |
| 14. | Petschow BW, Figueroa R, Harris CL, Beck LB, Ziegler E, Goldin B. Effects of feeding an infant formula containing Lactobacillus GG on the colonization of the intestine: a dose-response study in healthy infants. J Clin Gastroenterol. 2005;39:786-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Sung V, Collett S, de Gooyer T, Hiscock H, Tang M, Wake M. Probiotics to prevent or treat excessive infant crying: systematic review and meta-analysis. JAMA Pediatr. 2013;167:1150-1157. [PubMed] [Cited in This Article: ] |
| 16. | Pärtty A, Luoto R, Kalliomäki M, Salminen S, Isolauri E. Effects of early prebiotic and probiotic supplementation on development of gut microbiota and fussing and crying in preterm infants: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2013;163:1272-1277.e1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Szajewska H, Gyrczuk E, Horvath A. Lactobacillus reuteri DSM 17938 for the management of infantile colic in breastfed infants: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2013;162:257-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 18. | Kapel N, Campeotto F, Kalach N, Baldassare M, Butel MJ, Dupont C. Faecal calprotectin in term and preterm neonates. J Pediatr Gastroenterol Nutr. 2010;51:542-547. [PubMed] [Cited in This Article: ] |
| 19. | Schelonka RL, Maheshwari A, Carlo WA, Taylor S, Hansen NI, Schendel DE, Thorsen P, Skogstrand K, Hougaard DM, Higgins RD. T cell cytokines and the risk of blood stream infection in extremely low birth weight infants. Cytokine. 2011;53:249-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Savino F, Castagno E, Calabrese R, Viola S, Oggero R, Miniero R. High faecal calprotectin levels in healthy, exclusively breast-fed infants. Neonatology. 2010;97:299-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Olafsdottir E, Aksnes L, Fluge G, Berstad A. Faecal calprotectin levels in infants with infantile colic, healthy infants, children with inflammatory bowel disease, children with recurrent abdominal pain and healthy children. Acta Paediatr. 2002;91:45-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Savino F, De MA, Ceratto S, and Mostert M. Fecal Calprotectin During Treatment of Severe Infantile Colic With Lactobacillus reuteri DSM 17938: A Randomized, Double-Blind, Placebo-Controlled Trial. Pediatrics. 2015;135:S5-S6. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Keir AK, McPhee AJ, Andersen CC, Stark MJ. Plasma cytokines and markers of endothelial activation increase after packed red blood cell transfusion in the preterm infant. Pediatr Res. 2013;73:75-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Mellgren K, Hedegaard CJ, Schmiegelow K, Müller K. Plasma cytokine profiles at diagnosis in pediatric patients with non-hodgkin lymphoma. J Pediatr Hematol Oncol. 2012;34:271-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Rovira-Vallbona E, Moncunill G, Bassat Q, Aguilar R, Machevo S, Puyol L, Quintó L, Menéndez C, Chitnis CE, Alonso PL. Low antibodies against Plasmodium falciparum and imbalanced pro-inflammatory cytokines are associated with severe malaria in Mozambican children: a case-control study. Malar J. 2012;11:181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Treem WR, Hyams JS, Blankschen E, Etienne N, Paule CL, Borschel MW. Evaluation of the effect of a fiber-enriched formula on infant colic. J Pediatr. 1991;119:695-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1965] [Cited by in F6Publishing: 1886] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 28. | Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4578-4585. [PubMed] [Cited in This Article: ] |
| 29. | Tims S, Derom C, Jonkers DM, Vlietinck R, Saris WH, Kleerebezem M, de Vos WM, Zoetendal EG. Microbiota conservation and BMI signatures in adult monozygotic twins. ISME J. 2013;7:707-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 246] [Article Influence: 20.5] [Reference Citation Analysis (0)] |