Published online Jul 28, 2017. doi: 10.4329/wjr.v9.i7.312
Peer-review started: March 29, 2017
First decision: April 18, 2017
Revised: May 20, 2017
Accepted: June 19, 2017
Article in press: June 20, 2017
Published online: July 28, 2017
To investigate rates of distant metastases (DM) detected with [18]fluorodeoxyglucose-positron emission tomography/computed tomography (18FDG-PET/CT) in early stage invasive breast cancer.
We searched the English language literature databases of PubMed, EMBASE, ISI Web of Knowledge, Web of Science and Google Scholar, for publications on DM detected in patients who had 18FDG-PET/CT scans as part of the staging for early stages of breast cancer (stage I and II), prior to or immediately following surgery. Reports published between 2011 and 2017 were considered. The systematic review was conducted according to the PRISMA guidelines.
Among the 18 total studies included in the analysis, the risk of DM ranged from 0% to 8.3% and 0% to 12.9% for stage I and II invasive breast cancer, respectively. Among the patients with clinical stage II, the rate of occult metastases diagnosed by 18FDG-PET/CT was 7.2% (range, 0%-19.6%) for stage IIA and 15.8% (range, 0%-40.8%) for stage IIB. In young patients (< 40-year-old), 18FDG-PET/CT demonstrated a higher prevalence of DM at the time of diagnosis for those with aggressive histology (i.e., triple-negative receptors and poorly differentiated grade).
Young patients with poorly differentiated tumors and stage IIB triple-negative breast cancer may benefit from 18FDG-PET/CT at initial staging to detect occult DM prior to surgery.
Core tip: This systematic review identifies groups of patients with early stage breast cancer who might benefit most from [18]fluorodeoxyglucose-positron emission tomography/computed tomography (commonly known as 18FDG-PET/CT) scan at initial staging, prior to surgery.
- Citation: Vinh-Hung V, Everaert H, Farid K, Djassemi N, Baudin-Veronique J, Bougas S, Michailovich Y, Joachim-Contaret C, Cécilia-Joseph E, Verschraegen C, Nguyen NP. Preoperative [18]fluorodeoxyglucose-positron emission tomography/computed tomography in early stage breast cancer: Rates of distant metastases. World J Radiol 2017; 9(7): 312-320
- URL: https://www.wjgnet.com/1949-8470/full/v9/i7/312.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i7.312
Breast cancer is the most common cancer in women worldwide[1]. Mortality of breast cancer has declined notably in the United States, with death rates in 2012 decreasing 36% from peak rates as a result of improvements in early detection and treatment[2]. Yet, there remains considerable heterogeneity in the outcomes of early stage breast cancer[3]. The rate of death at 7 year due to stage I breast cancer was 2.1% in women aged 40 years or younger (as compared to 1.6% in women aged over 50) and was 3.8% in women with negative estrogen receptor status (as compared to 1.1% in those with positive estrogen receptor status)[3].
There is a large consensus that imaging should be limited to patients with apparent advanced disease or clinical suspicion of metastases[4-7]. Accordingly, staging scans are seldom performed[6,8]. The question arises, then, as to whether the excess mortality observed in “early stage” patients[3] is due to unfavorable biological factors or instead to the initial misclassification as “early stage”. We hypothesize that some clinically early stage breast cancer patients could benefit from a formal staging workup.
[18]fluorodeoxyglucose-positron emission tomography (18FDG-PET) scan is a valuable, well established tool for diagnostic staging in numerous cancer sites[9-12], as well as for locally advanced breast cancer to detect distant metastases (DM)[13-15]. Even though PET imaging is more sensitive for detection of loco-regional spread and metastatic disease in breast cancer compared to computed tomography (CT) scan alone, its high cost precludes the routine use of PET scan in clinical practice. Thus, a review of the literature is necessary for future guidelines about the benefit of PET scan in early stage breast cancer.
Standard-of-care for early stage breast cancer is surgery, either alone or followed by adjuvant radiotherapy and/or systemic therapy, depending on the pathologic stage and the type of surgery to be performed. The presence or absence of axillary lymph node metastases in patients with clinically non-palpable lymph nodes is routinely assessed through sentinel lymph node sampling or axillary lymph node dissection. Alternatively, PET scan could be most helpful in assessing the presence of DM in early stage breast cancer, which would preclude first-line surgery[16]. The prevalence of occult DM diagnosed by PET scan in patients with early stage breast cancer has not been analyzed and was the topic of this literature review. In particular, we sought to identify subsets of early stage breast cancer patients who might benefit most from PET scan, prior to surgery.
Electronic searches were performed in the following databases: PubMed, EMBASE, ISI Web of Knowledge (Web of Science), and Google Scholar. The following terms were explored and used in each database search: “Breast cancer”, “surgery”, “PET scan”, “distant metastases”, and “stage I (T1N0M0) and II (T0-2N1M0, T3N0M0)”. All relevant articles were accessed in full-text. The reference lists of relevant papers were then searched for additional publications.
Eligible studies over the past 6 year (2011-2017) in the present review included those in which patients had 18FDG-PET/CT scan as part of their workup prior to or immediately after surgery for histologically-proven breast cancer, regardless of age or sex, and in which the rates of DM were reported by 18FDG-PET/CT scan. All patients had clinical stage I or stage II breast cancer. Only studies reported in English were considered. Duplicated studies were excluded.
Prevalence of DM was extracted from each study and correlated to the disease stage. The influence of age, histology (e.g., lobular vs ductal), tumor grade (e.g., well differentiated vs poorly differentiated), and receptor status on the rate of DM (if reported) was also analyzed using descriptive summaries.
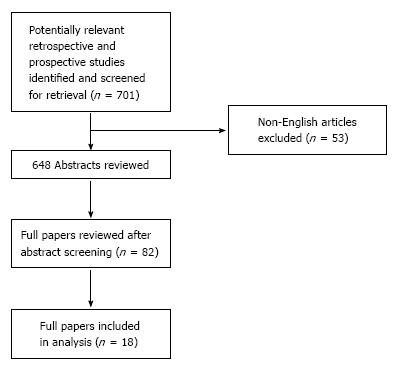
Figure 1 summarizes the search strategy. A total of 701 reports published between 2011 and 2017 were considered. Out of the 82 full papers that were assessed according to their potential for consisting of information relevant to the review, 18 were found to match the selection criteria and were selected for study inclusion. 18FDG-PET/CT scanning had been performed in addition to the clinical staging with or without conventional imaging in those 18 studies, either through a retrospective review or within a prospective protocol. As none of the studies was randomized, bias could not be excluded. Publications reviewing patients with early stage invasive breast cancer were included.
Clinical stage: The rates of DM ranged from 0% to 30%[17-34] for the entire group of reported patients. However, only 9 of the studies correlated DM rates detected by 18FDG-PET/CT with the clinical stage[17,22-24,27,29,31,32,34]. The rate of DM was lowest among studies of patients with invasive lobular cancer compared to studies that had included a mixture of other histologies, such as invasive ductal carcinoma[18] (Tables 1 and 2).
| Ref. | Subjects, n | Stage | Age, median | Histology | Tumor grade | Tumor receptors | Distant metastases | 2nd primaries |
| Groheux et al[17] | 131 | II: 84 | NS | IDC: 114 | 1: 9 | ER+: 82 | 5.90% (II) | 1% (II) |
| III: 47 | ILC: 8 | 2: 5 | HER2+: 30 | |||||
| T1: 2 | Other: 9 | 3: 53 | ||||||
| T2: 71 | NS: 4 | |||||||
| T3: 58 | ||||||||
| N0: 50 | ||||||||
| N1: 59 | ||||||||
| N2: 18 | ||||||||
| Bernsdorf et al[18] | 103 | T2 or | 55 | IDC: 83 | 1: 11 | ER+: 74 | 8% | 1.90% |
| higher | (24-81) | ILC: 14 | 2: 54 | HER+: 22 | ||||
| Other: 6 | 3: 37 | TN: 13 | ||||||
| NS: 1 | ||||||||
| Choi et al[19] | 154 | I: 69 | 52 | IDC: 141 | NS: 154 | NS | 8.40% | NS |
| II: 51 | (30-81) | ILC: 4 | ||||||
| III: 21 | Other: 9 | |||||||
| IV: 13 | ||||||||
| T1: 89 | ||||||||
| T2: 51 | ||||||||
| T3: 14 | ||||||||
| Garami et al[20] | 115 | T1: 56 | 55.7 | IDC: 92 | 1: 16 | ER+: 89 | 6.90% | 2.60% |
| T2: 48 | ILC: 11 | 2: 50 | ER-: 26 | |||||
| NS: 11 | Other: 12 | 3: 48 | ||||||
| N0: 57 | NS: 1 | |||||||
| N+: 46 | ||||||||
| NS: 12 | ||||||||
| Groves et al[21] | 70 | T1: 34 | 61 | IDC: 45 | 1: 02 | ER+: 64 | 2.80% | NS |
| T2: 30 | ILC: 10 | 2: 33 | HER+: 15 | |||||
| N1: 24 | Other: 5 | 3: 25 | ||||||
| Gunalp et al[22] | 141 | I: 19 | 47 | NS | 2 + 3: 141 | NS | 5% (I) | NS |
| II: 100 | (28-78) | 30% (II) | ||||||
| III: 14 | ||||||||
| Pritchard et al[23] | 325 | T1: 207 | 56 | IDC: 290 | 1: 68 | NS | 1.50% | NS |
| T2: 110 | (28-83) | ILC: 35 | 2: 158 | |||||
| T3: 8 | 3: 92 | |||||||
| N0: 325 | ||||||||
| Cochet et al[24] | 142 | II: 79 | 51 | IDC: 128 | 1+2: 81 | ER+/HER2-: 63 | 7.5% (II) | NS |
| III: 46 | (25-85) | ILC: 11 | 3: 56 | HER2+: 33 | ||||
| IV: 17 | Other: 3 | NS: 3 | TN: 31 | |||||
| T2 or | ||||||||
| Higher | ||||||||
| Jeong et al[25] | 178 | N0: 178 | 54.9 | IDC: 145 | NS | NS | 0% | 2.80% |
| T1: 108 | (33-82) | ILC: 11 | ||||||
| T2: 64 | DCIS: 12 | |||||||
| T3: 6 | Other: 10 | |||||||
| Koolen et al[26] | 62 | I: 35 | 59.8 | IDC: 58 | 1: 21 | ER+/HER2-: 48 | 16% | 3% |
| II: 25 | (26-75) | ILC: 1 | 2: 29 | TN: 7 | ||||
| III: 2 | Other: 3 | 3: 09 | HER2+: 7 | |||||
| T1: 62 | NS: 3 | |||||||
| Riedl et al[27] | 134 | I: 20 | 36.2 | IDC: 124 | 1: 01 | ER+/HER2-: 75 | 5% (I) | 4% |
| II: 91 | (22-39) | ILC: 1 | 2: 23 | HER2+: 26 | 10.9% (II) | |||
| III: 19 | Other: 9 | 3: 110 | ||||||
| Zhang et al[28] | 164 | T1: 127 | 45 | IDL: 150 | 1: 23 | ER+: 140 | 4.80% | NS |
| T2: 35 | (21-70) | ILC: 14 | 2-3: 141 | HER2+: 18 | ||||
| T3: 2 | ||||||||
| N0: 123 | ||||||||
| N1: 29 | ||||||||
| N2: 9 | ||||||||
| N3: 3 | ||||||||
| Hogan et al[29] | 146 | I: 8 | 57 | ILC: 146 | NS | ER+/HER2-: 132 | 0% (I) | NS |
| II: 50 | (34-92) | HER2+: 8 | 4% (II) | |||||
| III: 88 | TN: 5 | |||||||
| Krammer et al[30] | 101 | II: 75 | 54 | IDC: 80 | 1: 05 | ER+: 67 | 15.80% | NS |
| III: 15 | ILC: 15 | 2: 48 | HER2+: 56 | |||||
| IV: 11 | Other: 9 | 3: 45 | ||||||
| T1: 7 | NS: 6 | |||||||
| T2: 69 | ||||||||
| T3: 4 | ||||||||
| T4: 5 | ||||||||
| Nursal et al[31] | 419 | I: 104 | 51.5 | IDC: 305 | NS | NS | 2.9% (I) | NS |
| II: 315 | ILC: 29 | 12.4% (II) | ||||||
| T1: 127 | Other: 85 | |||||||
| T2: 270 | ||||||||
| T3: 20 | ||||||||
| Ulaner et al[32] | 232 | I: 23 | 51 | IDC: 217 | 2: 8 | TN: 232 | 0% (I) | NS |
| II: 169 | (25-93) | ILC: 2 | 3: 217 | 10% (II) | ||||
| III: 40 | NS: 7 | |||||||
| Lebon et al[33] | 214 | I: 24 | 45.2 | IDC: 181 | 1: 13 | HR+/HER2-: 89 | 8.3% (I) | NS |
| II: 124 | ILC: 10 | 2: 68 | HER2+: 61 | 12.9% (II) | ||||
| III: 66 | Other: 23 | 3: 133 | TN: 63 | |||||
| NS: 1 | ||||||||
| Ulaner et al[34] | 483 | I: 36 | 52.7 | IDC: 414 | 1: 5 | ER+: 402 | 2.8% (I) | 1.40% |
| II: 331 | (23.6-89.5) | ILC: 41 | 2: 55 | HER2+: 245 | 9.7% (II) | |||
| III: 116 | Other: 28 | 3: 400 | TN: 0 | 24.1% (III) | ||||
| NS: 23 |
| Ref. | Subjects, n | Age, median | Distant metastases rate | ||
| IIA | IIB | All | |||
| Groheux et al[17] | 84 | NS | 2.80% (1/36) | 8.30% (4/48) | 5.95% |
| Gunalp et al[22] | 100 | 51 | 19.60% (10/51) | 40.80% (20/49) | 30% |
| Cochet et al[24] | 142 | 51 | 9.10% (2/22) | 7.00% (4/57) | 7.60% |
| Jeong et al[25] | 70 | 54.9 | 0% (0/64) | 0% (0/6) | 0% |
| Riedl et al[27] | 91 | 36.2 | 5% (2/44) | 17% (8/47) | 10.90% |
| Nursal et al[31] | 315 | 51.5 | 9.50% (19/199) | 17.20% (20/116) | 12.40% |
| Ulaner et al[32] | 169 | 51 | 5% (4/82) | 15% (13/87) | 9.50% |
| Lebon et al[33] | 124 | 45.2 | 11% (7/64) | 15% (9/60) | 12.90% |
| Ulaner et al[34] | 483 | 52.7 | 4.20% (6/143) | 13.80% (26/188) | 9.70% |
| All | 1578 | 47.8 | 7.20% (0%-19.6%) | 15.80% (0%-40.8%) | 11.40% (0%-12.9%) |
Overall, the rate of DM for presumed stage I was low for all cancer types but non-negligible, ranging from 0% to 8.3%[22,27,29,31,32,34]. Among the 9 studies that reported the rate of DM in patients with stage II breast cancer separately, the prevalence ranged from 0% to 12.4%[17,22,24,27,29,31,32,34]. Patients with large tumors and/or axillary lymph node metastases appeared to be at increased risk of DM; specifically, the rate of DM was 7.2% (range, 0%-19.6%) and 15.8% (range, 0%-40.8%) for stage IIA and stage IIB, respectively[17,22,24,27,31,32,34].
Tumor size: Among studies that included a significant proportion of patients with large tumors (T2 and/or T3), the DM rate was higher and ranged from 8% to 8.4%[18,19], as compared to the range of 1.5% to 4.8% in studies including patients with smaller tumors[21,23,28]. However, since those latter studies also included a small proportion of patients with stage III disease and did not analyze the metastatic rate in relation to the clinical stage, the correlation between tumor size and DM rate remains unclear.
Nodal status: Patients who presented with N1 disease also presented with a higher risk of having DM. The rate of DM was 6% and 20% for N0 and N1 disease, respectively[31].
Receptor status: Among the 232 patients with triple-negative breast cancer, the DM rate was 0% and 10.9% for clinical stage I and stage II diseases, respectively, but there was no comparison performed with receptor-positive cases[32]. Other studies did not report the rates of DM according to receptor status.
Age: Two studies reported the influence of age on DM rate[27,33]. In the first study, among 134 young patients (< 40-year-old), the DM rate was 5% and 10.9% for clinical stage I and stage II, respectively[27]. In the second study, among 214 stage I-III patients, the DM rates did not differ significantly between the age groups of < 40-year-old and ≥ 40-year-old[33]. However, the DM rates in the younger age group were 8% in stage I, 9% in stage IIA and 17% in stage IIB, equating to 2x’s higher than those found in the first study.
Histologic grade: Histologic grade of the tumor may also be associated with increased risk of developing DM. Among 141 patients with moderate to poorly differentiated invasive breast cancers, the rate of DM was 30% for stage II patients[22]. However, correlation between tumor histologic grade and DM risk was not investigated in other studies[17-21,23,24,26-33].
This article reviews the role of 18FDG-PET/CT scan in the detection of DM in patients with early stages (i.e., I and II) of invasive breast cancer. The findings might represent important information applicable to discussions with patients about the utility of the scan. In contrast to stage III breast cancer, the role of 18FDG-PET/CT scan in identifying patients with clinical stages I and II who are at high risk of DM is controversial. Even though 18FDG-PET/CT scan may also be capable of identifying a second primary cancer, its main role in patients with breast cancer is the detection of DM, which could preclude upfront surgery[16]. Furthermore, the detection of DM could be of critical importance for the correct classification of patients and in the evaluation of treatment outcomes.
As the risk of DM is low in “early stage” asymptomatic breast cancer patients, an expensive imaging study, such as with the 18FDG-PET/CT scan, is not justified for the staging workup of all patients[6,35]. However, breast cancer is a heterogeneous disease, with some subgroups of patients at risk of developing DM even at the early stage. Subgroups of breast cancer patients with worse outcome include younger patients[36] and patients that have tumors with a more aggressive biological profile[37]. Rare histologic subtypes, such as metaplastic carcinoma of breast and invasive micropapillary carcinoma, are also more frequently associated with poor prognosis because of the high rate of axillary lymph node involvement and DM[38,39]. Genomic classification of risk, such as the oncotype DX and Perou’s studies, also identified the risk of distant recurrence[40-42]. Thus, those patients at high risk of systemic spread may benefit from early diagnosis of DM, for which chemotherapy may be initiated in a timely manner and unnecessary surgery may be avoided. The benefit of 18FDG-PET scan may outweigh its cost in those circumstances.
In patients with clinical stage I breast cancer, regardless of age, tumor grade or aggressive histology, the risk of DM as diagnosed by 18FDG-PET scan ranged from 0% to 8.3%[22,27,29,31-34]. This low, though not negligible, metastatic rate has been corroborated in studies with a high proportion of patients with T1 and N0 disease[21,23]. Even though the number of patients with stage I disease in those studies was small, preliminary evidence suggested that 18FDG-PET scan may not be cost effective for clinical stage I patients.
In patients with clinical stage II breast cancer, the prevalence of occult DM detected through 18FDG-PET scan ranged from 0% to 12.4%[17,22,24,27,29,31,32,34]. As stage II breast cancer patients also comprise a heterogeneous group, the risk of DM is higher for patients with stage IIB disease (T3N0M0, T2N1M0) than for those with stage IIA (T1N1M0, T2N0M0) disease. Discounting the one study that included only 6 patients with stage IIB disease[25], the risk of unsuspected DM diagnosed by 18FDG-PET scan ranged from 2.8% to 19.6% for stage IIA and 9.1% to 40% for stage IIB, respectively[17,22,27,31,32,34] (Table 2).
Patients with stage IIB have larger tumors than those with stage IIA. As tumor size has been reported to be correlated with an increased risk of DM, this may be one of the reasons underlying the higher rate of DM at diagnosis[43]. Other studies have corroborated the increased prevalence of DM diagnosed with 18FDG-PET scan for patients with large tumors compared to those with smaller tumors[18-21,23,28]. It is likely that other factors, like axillary lymph node metastases and tumor biology, may also lead to a high rate of DM at diagnosis[22,24,27,29,31].
Patients with triple-negative breast cancer frequently have a worse prognosis than their counterparts who harbor other subtypes because of the high rate of DM[44]. A 10% rate of unsuspected DM was seen on 18FDG-PET scan compared to conventional imaging for patients with clinical stage II breast cancer[32]. However, even among those triple-negative breast cancer patients, the rate of DM remained low for stage IIA disease. Specifically, the DM rate was 5% and 15% for stage IIA and IIB triple-negative breast cancers, respectively.
Another prognostic factor that has been reported in the literature is the patient age at diagnosis. Young patients (< 40-year-old) may have a more aggressive tumor biology that translates to a lower survival rate compared to older patients[36]. Among young patients with breast cancer, those with stage IIB disease had a 17% rate of DM compared to 5% for stage IIA. The incidence of DM in patients with clinical stage IIA with moderate to poorly differentiated grade carcinoma climbs to 19.6% after 18FDG-PET scanning[22]. Tumor biology needs to be taken into account beyond the conventional TNM staging. In patients with invasive micropapillary carcinoma, for example, a high rate of DM detected by 18FDG-PET scan before surgery has been reported. Among 16 patients with invasive micropapillary carcinoma who underwent 18FDG-PET scan when the tumor was diagnosed, axillary lymph node metastases and DM were observed in 12 (75% of cases)[45].
To date, this is the first study looking at the impact of 18FDG-PET on the management of invasive micropapillary carcinoma, a rare tumor with a high rate of axillary lymph node invasion and DM, even in the case of a relatively small tumor. Moreover, no study has been performed yet to investigate the role of 18FDG-PET scan for the diagnosis of occult DM in patients who had surgery for metaplastic carcinoma of the breast, another rare tumor with a poor survival rate associated with a high propensity to metastasize to distant sites.
Our study was restricted by the limited availability of the data correlating clinical stages and biology with the risk of DM diagnosed with 18FDG-PET/CT scan in patients with early stage breast cancer. Ki-67 is a known prognostic marker[46] but was not reported in any of the recent and largest studies[31-34]. Most studies were retrospective. The classification of patients into stages was usually done after the 18FDG-PET/CT image acquisition, which might have affected the selection of patients. Some studies included the more advanced stages, stage III and IV, and a few studies included post-operative patients. Many issues of importance are relevant for breast cancer, notably the emerging role of PET/MRI and its comparison with PET/CT[47], the use of PET in the monitoring of neoadjuvant therapy[48], the use for staging and restaging[49], the standardized uptake values (commonly known as SUVs) and how they relate to lymph node status[50], the prognostic role of FDG-PET[51] and the suitability for treatment planning[52]; all these represent immensely exciting domains of breast cancer research, but would have confused the scope of the present study, namely the rates of DM.
In summary, the current review suggests a need for future prospective studies looking at subgroups of patients who would most likely benefit from PET scan before surgery-stage IIB, poorly differentiated tumors, rare tumors with aggressive biology, such as invasive micropapillary carcinoma, and young age. These patients would most likely receive systemic therapy. Detection of DM could help in selecting the optimal sequence of therapies and the monitoring thereof. Incorporating biomarkers such as c-erbB2 and genetic arrays in those studies may further help the clinician to define the risk of DM at diagnosis for patients with early stage breast cancer.
In patients with clinical stage I breast cancer, the systematic use of 18FDG-PET/CT scan for staging is not cost effective because the yield of 18FDG-PET/CT-detected DM in clinical stage I is low. In young patients with stage IIB triple-negative and/or poorly differentiated breast cancer, 18FDG-PET/CT scan identifies a substantial rate of DM and should therefore be considered for these patients. Finally, the role of 18FDG-PET for stage II breast cancer and for rare tumors with aggressive biology needs to be defined in future prospective studies.
The authors would like to express their heartfelt gratitude to Carl Leak, for revising the language of this manuscript, to Jessica Malki, Olga Morgan, Brentwood Oftedal, Yeoshina Pillay and Andrew Westfall of the RUSM Oncology Society, Ross University School of Medicine, Dominica, West Indies for their enthusiastic interest and partaking in the discussion and the writing.
Staging of cancer is the process of identifying and classifying the extent of the disease. Staging is important to aid the clinician in planning treatment, to inform the patient on prognosis, to evaluate the results of treatment, and to facilitate the exchange of information between treatment centers. Initial staging is based on all evidence acquired before treatment. The evidence arises from physical examination, imaging, pathology, and/or endoscopic or surgical exploration.
In early breast cancer (small tumor and no symptom), previous diagnostic studies rarely detected metastases. The contentious issue is that the earlier studies were based on the use of conventional imaging with poor detection performance. Metastatic disease might have been missed.
[18]fluorodeoxyglucose-positron emission tomography/computed tomography (18FDG-PET/CT) combines metabolic and anatomic imaging. It requires a dual competence in radiology and in nuclear medicine. Negative reviews of its role in breast cancer confounded it with 18FDG-PET alone, did not have the joint nuclear-radiologist’s expertise to analyze the images, or focused only on the detection of regional lymph node involvement. There has been no pooled evaluation of the rates of distant metastases detected with 18FDG-PET/CT. This study fills the gap.
The present review identifies groups of patients with early breast cancer, who are at high risk for distant metastases, notably those with stage IIB or aggressive histologies, in whom it might be prudent to reconsider the role of 18FDG-PET/CT.
18FDG is a radioactively labeled glucose analog. It allows the detection of tissues that have a high glucose uptake, such as tumors with a high metabolic activity. Imaging with 18FDG, the 18FDG-PET, shows areas of high activity. The 18FDG-PET imaging combined with CT imaging shows where the areas of high activity are distributed in the body; N1 disease: Cancer that has spread to regional lymph nodes; Distant metastases: Cancer that has spread beyond the breast and regional lymph nodes to distant organs or distant lymph nodes; Triple-negative breast cancer: Breast tumor that tested negative for the estrogen receptor, the progesterone receptor, and the human epidermal growth receptor HER2. Triple negative tumors might respond to chemotherapy but will not to receptor targeted treatments.
A well-written review article, summarising important information to the field.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Martinique
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Bilir C, Wang L, Wang SK S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Schnitt SJ, Lakhani SR. Breast Cancer in: World Cancer Report 2014. Lyon, France: International Agency for Research on Cancer 2014; 362-373. [Cited in This Article: ] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12135] [Cited by in F6Publishing: 12702] [Article Influence: 1587.8] [Reference Citation Analysis (2)] |
| 3. | Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. 2015;313:165-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 425] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 4. | Barrett T, Bowden DJ, Greenberg DC, Brown CH, Wishart GC, Britton PD. Radiological staging in breast cancer: which asymptomatic patients to image and how. Br J Cancer. 2009;101:1522-1528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v8-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1055] [Cited by in F6Publishing: 1015] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 6. | Merrill SA, Stevens P, Verschraegen C, Wood ME. Utility and Costs of Routine Staging Scans in Early-Stage Breast Cancer. Am J Hematol Oncol. 2016;12:4. [Cited in This Article: ] |
| 7. | Schnipper LE, Smith TJ, Raghavan D, Blayney DW, Ganz PA, Mulvey TM, Wollins DS. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol. 2012;30:1715-1724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 447] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 8. | Chand N, Cutress RI, Oeppen RS, Agrawal A. Staging Investigations in Breast Cancer: Collective Opinion of UK Breast Surgeons. Int J Breast Cancer. 2013;2013:506172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Zhang Y, Feng B, Zhang GL, Hu M, Fu Z, Zhao F, Zhang XL, Kong L, Yu JM. Value of 18F-FDG PET-CT in surveillance of postoperative colorectal cancer patients with various carcinoembryonic antigen concentrations. World J Gastroenterol. 2014;20:6608-6614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Tantiwongkosi B, Yu F, Kanard A, Miller FR. Role of (18)F-FDG PET/CT in pre and post treatment evaluation in head and neck carcinoma. World J Radiol. 2014;6:177-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 42] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 11. | Sun L, Wan Y, Lin Q, Sun YH, Zhao L, Luo ZM, Wu H. Multiple primary malignant tumors of upper gastrointestinal tract: a novel role of 18F-FDG PET/CT. World J Gastroenterol. 2010;16:3964-3969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 6] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Abuodeh Y, Naghavi AO, Ahmed KA, Venkat PS, Kim Y, Kis B, Choi J, Biebel B, Sweeney J, Anaya DA. Prognostic value of pre-treatment F-18-FDG PET-CT in patients with hepatocellular carcinoma undergoing radioembolization. World J Gastroenterol. 2016;22:10406-10414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Groheux D, Giacchetti S, Delord M, Hindié E, Vercellino L, Cuvier C, Toubert ME, Merlet P, Hennequin C, Espié M. 18F-FDG PET/CT in staging patients with locally advanced or inflammatory breast cancer: comparison to conventional staging. J Nucl Med. 2013;54:5-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Champion L, Lerebours F, Cherel P, Edeline V, Giraudet AL, Wartski M, Bellet D, Alberini JL. 18F-FDG PET/CT imaging versus dynamic contrast-enhanced CT for staging and prognosis of inflammatory breast cancer. Eur J Nucl Med Mol Imaging. 2013;40:1206-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Liu Y. Role of FDG PET-CT in evaluation of locoregional nodal disease for initial staging of breast cancer. World J Clin Oncol. 2014;5:982-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 33] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Badwe R, Hawaldar R, Nair N, Kaushik R, Parmar V, Siddique S, Budrukkar A, Mittra I, Gupta S. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol. 2015;16:1380-1388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 346] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 17. | Groheux D, Giacchetti S, Espié M, Vercellino L, Hamy AS, Delord M, Berenger N, Toubert ME, Misset JL, Hindié E. The yield of 18F-FDG PET/CT in patients with clinical stage IIA, IIB, or IIIA breast cancer: a prospective study. J Nucl Med. 2011;52:1526-1534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Bernsdorf M, Berthelsen AK, Wielenga VT, Kroman N, Teilum D, Binderup T, Tange UB, Andersson M, Kjær A, Loft A. Preoperative PET/CT in early-stage breast cancer. Ann Oncol. 2012;23:2277-2282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Choi YJ, Shin YD, Kang YH, Lee MS, Lee MK, Cho BS, Kang YJ, Park JS. The Effects of Preoperative (18)F-FDG PET/CT in Breast Cancer Patients in Comparison to the Conventional Imaging Study. J Breast Cancer. 2012;15:441-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Garami Z, Hascsi Z, Varga J, Dinya T, Tanyi M, Garai I, Damjanovich L, Galuska L. The value of 18-FDG PET/CT in early-stage breast cancer compared to traditional diagnostic modalities with an emphasis on changes in disease stage designation and treatment plan. Eur J Surg Oncol. 2012;38:31-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Groves AM, Shastry M, Ben-Haim S, Kayani I, Malhotra A, Davidson T, Kelleher T, Whittaker D, Meagher M, Holloway B. Defining the role of PET-CT in staging early breast cancer. Oncologist. 2012;17:613-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Gunalp B, Ince S, Karacalioglu AO, Ayan A, Emer O, Alagoz E. Clinical impact of (18)F-FDG PET/CT on initial staging and therapy planning for breast cancer. Exp Ther Med. 2012;4:693-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Pritchard KI, Julian JA, Holloway CM, McCready D, Gulenchyn KY, George R, Hodgson N, Lovrics P, Perera F, Elavathil L. Prospective study of 2-[18F]fluorodeoxyglucose positron emission tomography in the assessment of regional nodal spread of disease in patients with breast cancer: an Ontario clinical oncology group study. J Clin Oncol. 2012;30:1274-1279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Cochet A, Dygai-Cochet I, Riedinger JM, Humbert O, Berriolo-Riedinger A, Toubeau M, Guiu S, Coutant C, Coudert B, Fumoleau P. 18F-FDG PET/CT provides powerful prognostic stratification in the primary staging of large breast cancer when compared with conventional explorations. Eur J Nucl Med Mol Imaging. 2014;41:428-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Jeong YJ, Kang DY, Yoon HJ, Son HJ. Additional value of F-18 FDG PET/CT for initial staging in breast cancer with clinically negative axillary nodes. Breast Cancer Res Treat. 2014;145:137-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Koolen BB, van der Leij F, Vogel WV, Rutgers EJ, Vrancken Peeters MJ, Elkhuizen PH, Valdés Olmos RA. Accuracy of 18F-FDG PET/CT for primary tumor visualization and staging in T1 breast cancer. Acta Oncol. 2014;53:50-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Riedl CC, Slobod E, Jochelson M, Morrow M, Goldman DA, Gonen M, Weber WA, Ulaner GA. Retrospective analysis of 18F-FDG PET/CT for staging asymptomatic breast cancer patients younger than 40 years. J Nucl Med. 2014;55:1578-1583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Zhang X, Wu F, Han P. The role of (18)F-FDG PET/CT in the diagnosis of breast cancer and lymph nodes metastases and micrometastases may be limited. Hell J Nucl Med. 2014;17:177-183. [PubMed] [Cited in This Article: ] |
| 29. | Hogan MP, Goldman DA, Dashevsky B, Riedl CC, Gönen M, Osborne JR, Jochelson M, Hudis C, Morrow M, Ulaner GA. Comparison of 18F-FDG PET/CT for Systemic Staging of Newly Diagnosed Invasive Lobular Carcinoma Versus Invasive Ductal Carcinoma. J Nucl Med. 2015;56:1674-1680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Krammer J, Schnitzer A, Kaiser CG, Buesing KA, Sperk E, Brade J, Wasgindt S, Suetterlin M, Schoenberg SO, Sutton EJ. (18) F-FDG PET/CT for initial staging in breast cancer patients - Is there a relevant impact on treatment planning compared to conventional staging modalities? Eur Radiol. 2015;25:2460-2469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Nursal GN, Nursal TZ, Aytac HO, Hasbay B, Torun N, Reyhan M, Yapar AF. Is PET/CT Necessary in the Management of Early Breast Cancer? Clin Nucl Med. 2016;41:362-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Ulaner GA, Castillo R, Goldman DA, Wills J, Riedl CC, Pinker-Domenig K, Jochelson MS, Gönen M. (18)F-FDG-PET/CT for systemic staging of newly diagnosed triple-negative breast cancer. Eur J Nucl Med Mol Imaging. 2016;43:1937-1944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Lebon V, Alberini JL, Pierga JY, Diéras V, Jehanno N, Wartski M. Rate of Distant Metastases on 18F-FDG PET/CT at Initial Staging of Breast Cancer: Comparison of Women Younger and Older Than 40 Years. J Nucl Med. 2017;58:252-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Ulaner GA, Castillo R, Wills J, Gönen M, Goldman DA. (18)F-FDG-PET/CT for systemic staging of patients with newly diagnosed ER-positive and HER2-positive breast cancer. Eur J Nucl Med Mol Imaging. 2017; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Debald M, Wolfgarten M, Kreklau P, Abramian A, Kaiser C, Höller T, Leutner C, Keyver-Paik MD, Braun M, Kuhn W. Staging of primary breast cancer is not indicated in asymptomatic patients with early tumor stages. Oncol Res Treat. 2014;37:400-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Ribnikar D, Ribeiro JM, Pinto D, Sousa B, Pinto AC, Gomes E, Moser EC, Cardoso MJ, Cardoso F. Breast cancer under age 40: a different approach. Curr Treat Options Oncol. 2015;16:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Braunstein LZ, Niemierko A, Shenouda MN, Truong L, Sadek BT, Abi Raad R, Wong JS, Punglia RS, Taghian AG, Bellon JR. Outcome following local-regional recurrence in women with early-stage breast cancer: impact of biologic subtype. Breast J. 2015;21:161-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Chen HL, Ding A. Comparison of invasive micropapillary and triple negative invasive ductal carcinoma of the breast. Breast. 2015;24:723-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Lai HW, Tseng LM, Chang TW, Kuo YL, Hsieh CM, Chen ST, Kuo SJ, Su CC, Chen DR. The prognostic significance of metaplastic carcinoma of the breast (MCB)--a case controlled comparison study with infiltrating ductal carcinoma. Breast. 2013;22:968-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869-10874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7772] [Cited by in F6Publishing: 7651] [Article Influence: 332.7] [Reference Citation Analysis (0)] |
| 41. | Reis-Filho JS, Pusztai L. Gene expression profiling in breast cancer: classification, prognostication, and prediction. Lancet. 2011;378:1812-1823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 522] [Cited by in F6Publishing: 491] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 42. | Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, Pierga JY, Brain E, Causeret S, DeLorenzi M. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med. 2016;375:717-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1123] [Cited by in F6Publishing: 1143] [Article Influence: 142.9] [Reference Citation Analysis (0)] |
| 43. | Fei F, Messina C, Slaets L, Chakiba C, Cameron D, Bogaerts J, Bonnefoi H. Tumour size is the only predictive factor of distant recurrence after pathological complete response to neoadjuvant chemotherapy in patients with large operable or locally advanced breast cancers: a sub-study of EORTC 10994/BIG 1-00 phase III trial. Eur J Cancer. 2015;51:301-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Kast K, Link T, Friedrich K, Petzold A, Niedostatek A, Schoffer O, Werner C, Klug SJ, Werner A, Gatzweiler A. Impact of breast cancer subtypes and patterns of metastasis on outcome. Breast Cancer Res Treat. 2015;150:621-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 45. | Yun SU, Choi BB, Shu KS, Kim SM, Seo YD, Lee JS, Chang ES. Imaging findings of invasive micropapillary carcinoma of the breast. J Breast Cancer. 2012;15:57-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | de Azambuja E, Cardoso F, de Castro G, Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96:1504-1513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 587] [Cited by in F6Publishing: 651] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 47. | Tabouret-Viaud C, Botsikas D, Delattre BM, Mainta I, Amzalag G, Rager O, Vinh-Hung V, Miralbell R, Ratib O. PET/MR in Breast Cancer. Semin Nucl Med. 2015;45:304-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 48. | Rousseau C, Devillers A, Sagan C, Ferrer L, Bridji B, Campion L, Ricaud M, Bourbouloux E, Doutriaux I, Clouet M. Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2006;24:5366-5372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 218] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 49. | Groheux D, Cochet A, Humbert O, Alberini JL, Hindié E, Mankoff D. 18F-FDG PET/CT for Staging and Restaging of Breast Cancer. J Nucl Med. 2016;57 Suppl 1:17S-26S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 50. | Futamura M, Asano T, Kobayashi K, Morimitsu K, Nawa M, Kanematsu M, Morikawa A, Mori R, Yoshida K. Prediction of macrometastasis in axillary lymph nodes of patients with invasive breast cancer and the utility of the SUV lymph node/tumor ratio using FDG-PET/CT. World J Surg Oncol. 2015;13:49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Vinh-Hung V, Everaert H, Lamote J, Voordeckers M, van Parijs H, Vanhoeij M, Verfaillie G, Fontaine C, Vees H, Ratib O. Diagnostic and prognostic correlates of preoperative FDG PET for breast cancer. Eur J Nucl Med Mol Imaging. 2012;39:1618-1627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Bral S, Vinh-Hung V, Everaert H, De Coninck P, Storme G. The use of molecular imaging to evaluate radiation fields in the adjuvant setting of breast cancer: a feasibility study. Strahlenther Onkol. 2008;184:100-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |