Published online Aug 28, 2016. doi: 10.4329/wjr.v8.i8.743
Peer-review started: January 25, 2016
First decision: February 29, 2016
Revised: May 2, 2016
Accepted: May 31, 2016
Article in press: June 2, 2016
Published online: August 28, 2016
To clarify clinicopathological features of ductal carcinoma in situ (DCIS) visualized on [F-18] fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT).
This study retrospectively reviewed 52 consecutive tumors in 50 patients with pathologically proven pure DCIS who underwent [F-18] FDG-PET/CT before surgery. [F-18] FDG-PET/CT was performed after biopsy in all patients. The mean interval from biopsy to [F-18] FDG-PET/CT was 29.2 d. [F-18] FDG uptake by visual analysis and maximum standardized uptake value (SUVmax) was compared with clinicopathological characteristics.
[F-18] FDG uptake was visualized in 28 lesions (53.8%) and the mean and standard deviation of SUVmax was 1.63 and 0.90. On univariate analysis, visual analysis and the SUVmax were associated with symptomatic presentation (P = 0.012 and 0.002, respectively), palpability (P = 0.030 and 0.024, respectively), use of core-needle biopsy (CNB) (P = 0.023 and 0.012, respectively), ultrasound-guided biopsy (P = 0.040 and 0.006, respectively), enhancing lesion ≥ 20 mm on magnetic resonance imaging (MRI) (P = 0.001 and 0.010, respectively), tumor size ≥ 20 mm on histopathology (P = 0.002 and 0.008, respectively). However, [F-18] FDG uptake parameters were not significantly associated with age, presence of calcification on mammography, mass formation on MRI, presence of comedo necrosis, hormone status (estrogen receptor, progesterone receptor and human epidermal growth factor receptor-2), and nuclear grade. The factors significantly associated with visual analysis and SUVmax were symptomatic presentation (P = 0.019 and 0.001, respectively), use of CNB (P = 0.001 and 0.031, respectively), and enhancing lesion ≥ 20 mm on MRI (P = 0.001 and 0.049, respectively) on multivariate analysis.
Although DCIS of breast is generally non-avid tumor, symptomatic and large tumors (≥ 20 mm) tend to be visualized on [F-18] FDG-PET/CT.
Core tip: Symptomatic tumor or large ductal carcinoma in situ (DCIS) (≥ 20 mm) is often visualized on [F-18] fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT). This evidence suggests that large DCIS (≥ 20 mm) has possibility to be selected as target lesion on [F-18] FDG-PET/CT prior to neoadjuvant chemotherapy.
- Citation: Fujioka T, Kubota K, Toriihara A, Machida Y, Okazawa K, Nakagawa T, Saida Y, Tateishi U. Tumor characteristics of ductal carcinoma in situ of breast visualized on [F-18] fluorodeoxyglucose-positron emission tomography/computed tomography: Results from a retrospective study. World J Radiol 2016; 8(8): 743-749
- URL: https://www.wjgnet.com/1949-8470/full/v8/i8/743.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i8.743
Since widespread of and technical improvements to screening mammography, the frequency of ductal carcinoma in situ (DCIS) has increased substantially. mammography is able to detect even small DCIS if they have suspicious microcalcifications[1]. With the increasing use of ultrasound and magnetic resonance imaging (MRI), occult DCIS that cannot be identified by mammography, such as DCIS without microcalcification and in patients with dense breasts, have been occasionally detected[2-5]. Nowadays, maximally 20%-25% of new breast cancer cases consist of DCIS[6-8].
[F-18] fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) has been recognized as an essential modality for detecting hypermetabolic activity in primary breast tumor, diagnosing and staging local and distant sites, and evaluating the response to therapy[9-13]. It has been reported that the 25%-77% sensitivity of DCIS is lower than that of invasive ductal carcinoma (IDC) on [F-18] FDG-PET/CT[10,11]. However, DCIS often can be detected on [F-18] FDG-PET/CT. The purpose of this study was to clarify clinicopathological features of DCIS visualized on [F-18] FDG-PET/CT with special reference to pathologic specimens obtained by surgery.
This retrospective study was officially approved by the Ethical Commission of our institution (No. 1987). The inclusion criteria for patients with breast cancer were as follows: (1) those who underwent [F-18] FDG-PET/CT and operation at our hospital between March 2008 and May 2014; and (2) those with a pathologically proven pure DCIS, which means DCIS without microinvasion. The decision to perform [F-18] FDG-PET/CT was left to the discretion of the surgeon. In this study, after searching the database of radiology reports and clinical records at our institute, we identified 52 consecutive lesions in 50 patients with a mean age of 56.3 years (range, 33-85 years). All of the patients were not pregnant or breastfeeding. [F-18] FDG-PET/CT was performed after biopsy in all patients. The mean interval from biopsy to [F-18] FDG-PET/CT was 29.2 d (range, 43-133 d) and from biopsy to operation was 78.8 d (range, 34-224 d). The treatment comprised of total mastectomy in 14 lesions, breast conserving surgery in 27, and skin sparing mastectomy in 11.
All patients had physical examinations, mammography, ultrasound, and MRI. Mammography examination (craniocaudal and mediolateral oblique views) was performed using Lorad Selenia (Hologic Inc, Bedford, Massachusetts). EUB-7500 scanner with a EUP-L54MA 9.75-MHz linear probe (Hitachi Medical Systems, Tokyo) or Aplio XG scanner with a PLT-805AT 8.0-MHz linear probe (Toshiba Medical Systems, Tochigi) was used for the ultrasound examinations.
MRI examination was performed with 1.5-T system (Magnetom Vision, Siemens, Erlangen) for 7 patients and 3.0-T system (Signa HDxt, General Electric Medical Systems, Milwaukee, Wisconsin) for 43 patients using a breast coil in the prone position using a breast coil. To evaluate the MRI imaging, the early phase of a contrast enhancement study within 1 and 2 min after intravenous bolus injection of Gd-DTPA (0.2 mL/kg) was acquired. Unilateral coronal T1 weighted sequence [repetition time (TR)/echo time (TE) = 170/4.7, flip angle = 40°, 4 mm thick section, 256 × 256 matrix, 210 mm field of view)] using 1.5-T system and bilateral axial fat suppressed T1 weighted sequence (TR/TE = 6.5/2.4, flip angle = 10°, 2 mm thick section, 512 × 512 matrix, 360 mm field of view) using 3.0-T system were employed.
Percutaneous needle biopsy was done with either ultrasound-guided core-needle biopsy (CNB); 14-gauge Biopsy System (C.R. Bard, Covington, Georgia), ultrasound-guided or stereotactic vacuum-assisted biopsy (VAB); 8- or 11-gauge Mammotome (Ethicon Endo-Surgery, Cincinnati, Ohio) or 11- or 14-gauge Vacora (CR Bard, Murray Hill, New Jersey).
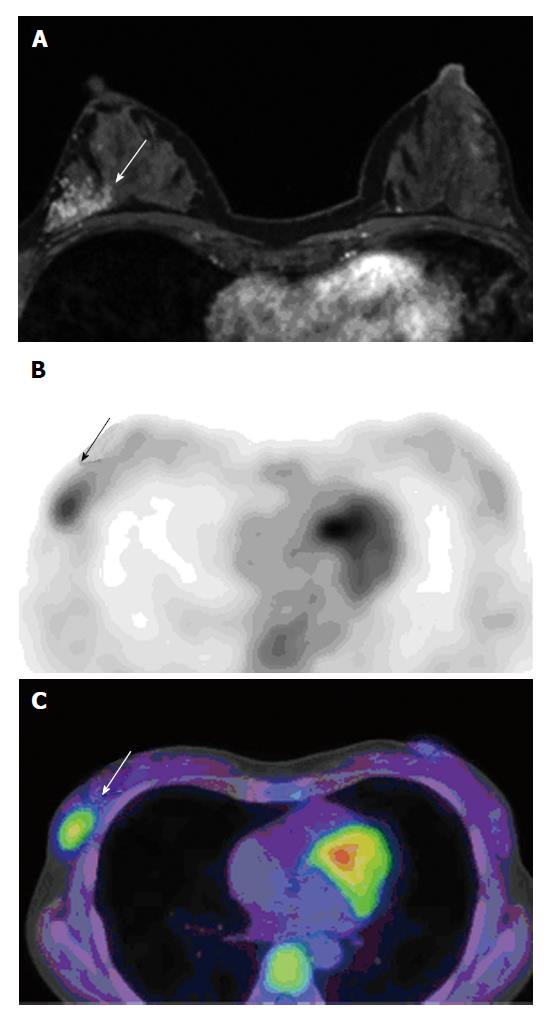
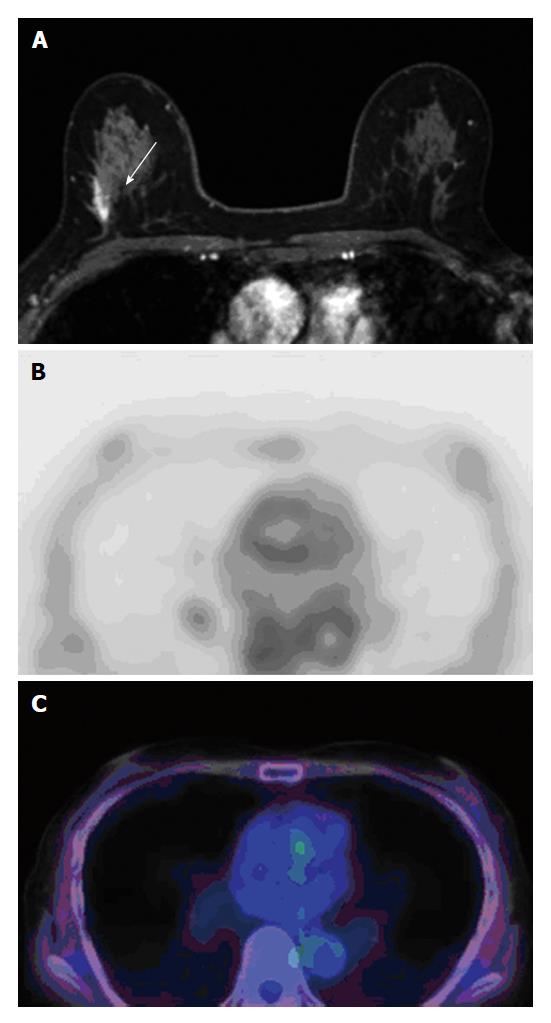
One radiologist had 5 years of experience in breast imaging recorded the reason for presentation (screening-detected or symptomatic lesion), presence of a palpable lesion, use of biopsy device, and image guidance, then evaluated presence of clustered micro calcifications within the tumor on mammography, presence of mass formation, and size (largest diameter) of enhancing lesion on MRI by the American College of Radiology Breast Imaging Reporting and Data System without access to FDG-PET/CT or pathological data[14] (Figure 1A and 2A).
After at least a 4-h fasting period, the patients received an intravenous injection of 3.7 MBq/kg (0.1 mCi/kg) [F-18] FDG. Images were obtained by whole-body mode with a PET/CT system (Aquiduo; Toshiba Medical Systems, Tokyo). CT images were performed by the following parameters: Pitch 0.938; 0.5 s gantry rotation time; 30 mm/s table time; 120 KVp; auto-exposure control (SD 20); and 2.0-mm slice thickness. Contrast agents were not used during our study. Approximately 60 min after the FDG injection, whole-body emission PET scan was obtained with the following parameters: 7-8 bed positions; 2-min emission time per bed position; 3.375-mm slice thickness; and 128 × 128 matrix.
[F-18] FDG-PET/CT images were reviewed by two nuclear medicine physicians (with 5 and 17 years of experience) in consensus; although they knew that the patients had DCIS, they were blind to clinical information including menopausal status and phase of the menstrual cycles, and presence of fibrocystic changes in patients. They performed visual analysis without defining a cutoff point. Lesions showing [F-18] FDG uptake higher than the surrounding background of normal breast tissue were defined as FDG positive. Region of interest (ROI) was placed on the PET images for measuring the maximum standardized uptake values (SUVmax) of the tumor. If the tumor was FDG negative, ROI was drawn on the background breast tissue area, and the SUVmax was established (Figures 1B, 1C, 2B and 2C).
Specimens were cut into 5-10 mm contiguous sections and then stained using hematoxylin and eosin staining. Additional immunohistochemistry of markers for estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor (HER)-2 was used to evaluate the hormone receptor status. All cases were diagnosed by more than two pathologists, and the following histological features were recorded: Presence of comedo necrosis, nuclear grade (1, 2 or 3), hormone receptor status (ER, PgR and HER-2), and tumor size (largest diameter).
The patient population showed normal distribution with the Smirnov-Kolmogorov analysis in this study. The patient characteristics and findings of [F-18] FDG-PET/CT (results of visual analysis and the SUVmax) were compared in each group using univariate analysis of Fisher’s exact test and Mann-Whitney U test. Further, we also performed multiple logistic regression analyses. Statistical calculations were performed with IBM SPSS statistics 22. P values of < 0.05 were regarded as significant.
Visual analysis revealed that [F-18] FDG uptake was seen in 28 lesions (53.8%, Figure 1). All values are provided as mean ± SD. The SUVmax of all lesions was 1.63 ± 0.90 (range, 0.62-5.49), of [F-18] FDG-positive lesions were 2.18 ± 1.16 (range, 1.16-5.49), and of [F-18] FDG-negative lesions were 0.99 ± 0.19 (range, 0.62-1.29, Figure 2).
Clinically, 39 lesions (75.0%) were symptomatic, 13 (25.0%) were detected on screening, and 15 (28.8%) were palpable (Table 1). Percutaneous biopsy was performed using ultrasound-guided CNB in 20 lesions (38.5%), ultrasound-guided VAB in 25 (48.1%), and stereotactic VAB in 7 (13.5%). Moreover, calcification on mammography was found in 27 lesions (52.0%). Eight patients (15.4%) had mass formation; 42 lesions (80.8%) did not have mass formation and 2 (3.8%) were undetected at MRI. The mean size of enhancing lesions at MRI was 31.7 ± 30.0 mm (range, 0-80 mm), of [F-18] FDG-negative lesions was 22.8 ± 18.5 mm (range, 0-60 mm), and of [F-18] FDG-positive lesions was 39.3 ± 18.2 mm (range, 7-80 mm). In 34 lesions (65.4%), sizes of enhancing lesions were ≥ 20 mm.
| Clinical characteristics | n | Pathological characteristics | n | ||
| Symptomatic presentation | Y/N | 13/39 | Comedo necrosis | Y/N | 22/30 |
| Palpability | Y/N | 15/37 | ER | Y/N | 42/10 |
| Biopsy device | CNB/VAB | 20/32 | PgR | Y/N | 38/14 |
| Image guidance | US/ST | 45/7 | HER2 | Y/N | 22/30 |
| Calcification at MG | Y/N | 27/25 | Nuclear grade | 1/2/3 | 42/82 |
| Mass formation at MRI | Mass | 8 | Tumor size at pathology | Mean ± SD, range | 41.4 ± 32.3, 1-150 |
| Non mass | 42 | < 20 mm | 15 | ||
| Undetectable | 2 | ≥ 20 mm | 37 | ||
| Lesion size at MRI | Mean ± SD, range | 31.7 ± 20.0, 0-80.0 | |||
| < 20 mm | 18 | ||||
| ≥ 20 mm | 34 | ||||
Microscopic observation revealed that 22 lesions (42.3%) had comedo necrosis, 42 (80.8%) were ER positive, 38 (73.1%) were PgR positive, and 22 (42.3%) were HER-2 positive. Further, 42 lesions (80.8%) were nuclear grade 1, 8 (15.4%) were nuclear grade 2, and 2 (3.8%) were nuclear grade 3. The mean pathological size of tumors was 41.4 ± 32.2 mm (range, 1-150 mm), of [F-18] FDG-negative lesions was 34.5 ± 39.4 mm (range, 1-150 mm), and of [F-18] FDG-positive lesions was 47.3 ± 24.0 mm (range, 2-85 mm); 37 lesions (71.1%) were ≥ 20 mm in size.
On univariate analysis (Table 2), visual analysis and significant association was found between the SUVmax and symptomatic presentation (P = 0.012 and 0.002, respectively), palpability (P = 0.030 and 0.024, respectively), use of CNB (P = 0.023 and 0.012, respectively), ultrasound-guided biopsy (P = 0.040 and 0.006, respectively), large size (≥ 20 mm) of enhancing lesion on MRI (P = 0.001 and 0.010, respectively), and large tumor size (≥ 20 mm) on histopathology (P = 0.002 and 0.008, respectively). However, visual analysis and SUVmax failed to show association with age, presence of calcification on mammography, mass formation on MRI, presence of comedo necrosis, hormone receptor status (ER, PgR), HER-2 expression, and nuclear grade.
| FDG uptake | SUVmax | ||||||||
| Negative | Positive | Percent | P1 | P2 | mean ± SD | P3 | P4 | ||
| Age (yr) | < 55 | 10 | 13 | 56.5% | 0.785 | 0.373 | 1.88 ± 1.09 | 0.077 | 0.210 |
| ≥ 55 | 14 | 15 | 51.7% | 1.44 ± 0.66 | |||||
| Symptomatic presentation | Y | 2 | 11 | 84.6% | 0.012 | 0.019 | 2.36 ± 1.24 | 0.002 | 0.001 |
| N | 22 | 17 | 43.6% | 1.39 ± 0.59 | |||||
| Palpability | Y | 3 | 12 | 80.0% | 0.030 | 0.083 | 1.99 ± 0.87 | 0.024 | 0.853 |
| N | 21 | 16 | 43.2% | 1.49 ± 0.87 | |||||
| Biopsy device | CNB | 5 | 15 | 75.0% | 0.023 | 0.001 | 1.86 ± 0.68 | 0.012 | 0.031 |
| VAB | 19 | 13 | 40.6% | 1.49 ± 0.99 | |||||
| Image guidance | US | 18 | 27 | 60.0% | 0.040 | 0.545 | 1.73 ± 0.92 | 0.006 | 0.849 |
| ST | 6 | 1 | 14.3% | 0.99 ± 0.20 | |||||
| Calcification at MG | Y | 15 | 12 | 44.4% | 0.177 | 0.323 | 1.44 ± 0.66 | 0.107 | 0.214 |
| N | 9 | 16 | 64.0% | 1.84 ± 1.07 | |||||
| Mass formation at MRI | Y | 3 | 5 | 62.5% | 0.711 | 0.215 | 1.76 ± 0.72 | 0.348 | 0.253 |
| N | 21 | 23 | 52.3% | 1.61 ± 0.93 | |||||
| Lesion size at MRI (mm) | < 20 | 14 | 4 | 22.2% | 0.001 | 0.001 | 1.24 ± 0.57 | 0.010 | 0.049 |
| ≥ 20 | 10 | 24 | 70.6% | 1.84 ± 0.97 | |||||
| Comedo necrosis | Y | 9 | 13 | 59.1% | 0.581 | 0.284 | 1.69 ± 0.85 | 0.634 | 0.301 |
| N | 15 | 15 | 50.0% | 1.59 ± 0.94 | |||||
| ER | Y | 19 | 23 | 54.8% | 1 | 0.249 | 1.67 ± 0.96 | 0.889 | 0.628 |
| N | 5 | 5 | 50.0% | 1.47 ± 056 | |||||
| PgR | Y | 18 | 20 | 52.6% | 1 | 0.608 | 1.64 ± 0.96 | 0.804 | 0.731 |
| N | 6 | 8 | 57.1% | 1.60 ± 0.72 | |||||
| HER2 | Y | 10 | 12 | 54.5% | 1 | 0.681 | 1.65 ± 0.78 | 0.788 | 0.496 |
| N | 14 | 16 | 53.3% | 1.62 ± 0.98 | |||||
| Nuclear grade | 1 | 20 | 22 | 52.4% | 0.736 | 0.510 | 1.64 ± 0.93 | 0.898 | 0.718 |
| 2.3 | 4 | 6 | 60.0% | 1.61 ± 0.79 | |||||
| Tumor size at pathology | < 20 | 12 | 3 | 20.0% | 0.002 | 0.708 | 1.19 ± 0.55 | 0.008 | 0.516 |
| ≥ 20 | 12 | 25 | 67.6% | 1.81 ± 0.97 | |||||
On multivariate analysis (Table 2), the factors significantly associated with visual analysis and SUVmax were symptomatic presentation (P = 0.019 and 0.001, respectively), use of CNB (P = 0.001 and 0.031, respectively), and large size (≥ 20 mm) of enhancing lesion on MRI (P = 0.001 and 0.049, respectively).
This study demonstrated that symptomatic and large DCIS (≥ 20 mm) often can be visualized on [F-18] FDG-PET/CT. The results mirrored those of the previous study showing sensitivity of 25%-77%[10,11]. Although [F-18] FDG uptake depends on cell density in patients with predominant and pure DCIS, the association between [F-18] FDG and clinicopathological features of DCIS has not been fully elucidated. To the best of our knowledge, our study has the largest sample number among studies examining clinicopathological features of DCIS visualized on [F-18] FDG-PET/CT. Our hypothesis is that large DCIS (≥ 20 mm) has possibility to be selected as target lesion on [F-18] FDG-PET/CT prior to neoadjuvant chemotherapy.
In our study, FDG uptake was highly seen in enhancing lesions of ≥ 20 mm in size on MRI (24/34, 70.6%) and in tumors histopathologically measuring ≥ 20 mm on (25/37, 67.6%). These results show that large tumors tend to have [F-18] FDG uptake in DCIS, similar to a previous study that reported that sensitivity of [F-18] FDG-PET and PET/CT is associated to a large tumor size in IDC[10,11].
In this study, FDG uptake was highly detected in symptomatic (11/13, 84.6%) and palpable lesions (12/15, 80.0%); [F-18] FDG-PET/CT findings were markedly associated with symptomatic presentation and palpability. It has been reported that a large tumor tend to be symptomatic and palpable and that most tumors do not become palpable until a size > 10 mm in diameter is reached[10]. These factors may reflect the association between symptomatic presentation, palpability, and [F-18] FDG-PET/CT findings. In contrast, [F-18] FDG uptake was not highly visualized in screening-detected lesions (17/39, 43.6%) and non-palpable lesions (16/37, 43.2%). These results indicate that FDG-PET/CT was not appropriate for the screening of DCIS.
[F-18] FDG-PET/CT findings markedly associated with the use of CNB and ultrasound-guided biopsy in our study. Stereotactic VAB were performed in 7 lesions undetected by ultrasound, and [F-18] FDG uptake was visualized only in 1/7 (14.3%) lesions. It is possible that larger and palpable lesions are more easily detected and effectively performed using ultrasound CNB than stereotactic VAB. These factors may reflect the association between biopsy device, image guidance, and [F-18] FDG-PET/CT findings.
A previous meta-analysis has demonstrated that pooled random-effect risk values of significant predictors for ipsilateral breast tumor recurrence were the presence of symptoms 1.35 (95%CI: 1.12-1.62), presence of comedo necrosis 1.71 (95%CI: 1.36-2.16), high tumor nuclear grade 1.81 (95%CI: 1.53-2.13), and large tumor size 1.63 (95%CI: 1.30-2.06)[15]. Of these factors, [F-18] FDG-PET/CT findings associated with macro factors (presence of symptoms and large tumor size) but not with micro factors (presence of comedo necrosis and high tumor nuclear grade) in this study. Although the detection of [F-18] FDG-PET/CT depends not only on the tumor volume but also on the degree of FDG activity, tumor-to- normal tissue ratio, and respiratory effects, a lesion < 10 mm lesion may not be detected as the resolution of PET/CT imaging is limited[15,16]. These micro factors will not reflect [F-18] FDG uptake for the limitation of resolution of PET/CT imaging. If a more precise examination can be conducted using high-resolution PET/CT system or positron emission mammography with the addition of more cases and longer follow-up, [F-18] FDG uptake can be predictors for ipsilateral breast tumor recurrence in DCIS patients.
Another previous meta-analysis has reported that a random-effects pooled estimate for underestimation in patients with DCIS at needle biopsy was 25.9% (95%CI: 22.5%, 29.5%)[17] and one study has also shown that high SUVmax is a significant predictive factor for underestimation of IDC in patients with DCIS on needle biopsy[18]. Thus, we need to consider that DCIS proven by core-needle biopsy and with SUVmax will have invasive lesions.
We presented clinicopathological features of DCIS visualized on [F-18] FDG-PET/CT with the largest sample number and hypothesized that large DCIS (≥ 20 mm) might be selected as target lesion on [F-18] FDG-PET/CT prior to neoadjuvant chemotherapy. However, this study had several limitations. First, our study was conducted retrospectively. Second, our study could not correlate the findings with menopausal status and phase of the menstrual cycles, and presence of fibrocystic changes in patients, which can influence normal breast parenchymal enhancement at MRI and [F-18] FDG uptake on PET/CT[19,20]. Third, invasive intervention might affect [F-18] FDG uptake of the lesion in our study, because all patients had biopsy prior to [F-18] FDG-PET/CT study and the mean interval from biopsy to [F-18] FDG-PET/CT was 29.2 d (range, 43-133 d). And finally, PET/CT has limitation in DCIS less than 20 mm; however, MRI might be more sensitive in lesion less than 20 mm.
In conclusion, although most DCIS are non-avid on [F-18] FDG-PET/CT, [F-18] FDG uptake of symptomatic tumors or tumors greater than or equal to 20 mm often can be visualized.
Nowadays, the incidence of ductal carcinoma in situ (DCIS) has increased by prevailing mammography, ultrasound, and magnetic resonance imaging and over 20% of newly identified breast cancer consists of DCIS. However, the sensitivity of [F-18] fluorodeoxyglucose-positron emission tomography/computed tomography (FDG PET/CT) for DCIS remains obscure.
[F-18] FDG PET/CT has been an essential modality for detecting metabolic activity in primary breast tumor, diagnosing and staging local and distant sites, and evaluating the response to therapy. Wide range of the sensitivity to detect primary tumor in patients with DCIS exists.
DCIS often can be detected on [F-18] FDG-PET/CT in daily practice. The authors assessed clinicopathological features of tumor visualized on [F-18] FDG-PET/CT using pathologic specimens obtained by surgery.
This study revealed that symptomatic tumor and large DCIS (≥ 20 mm) is often visualized on [F-18] FDG-PET/CT. The results suggest that large DCIS (≥ 20 mm) has possibility to be selected as target lesion on [F-18] FDG-PET/CT prior to neoadjuvant chemotherapy. Devulking after neoadjuvant chemotherapy may affect surgical approach.
DCIS is a group of non-invasive malignant epithelial tumors characterized by non-invasion of adjacent tissues and mostly adenocarcinoma derived from the mammary parenchymal epithelium. [F-18] FDG-PET/CT is one of the hybrid type imaging modality which provides activity of glucose metabolism.
This article mentioned that DCIS of breast with diameter > 20 mm can be visualized on [F-18] FDG-PET/ CT. This result is very useful in clinical diagnosis.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chu JP, Tsikouras PPT, Zafrakas M S- Editor: Gong XM L- Editor: A E- Editor: Zhang FF
| 1. | Barreau B, de Mascarel I, Feuga C, MacGrogan G, Dilhuydy MH, Picot V, Dilhuydy JM, de Lara CT, Bussières E, Schreer I. Mammography of ductal carcinoma in situ of the breast: review of 909 cases with radiographic-pathologic correlations. Eur J Radiol. 2005;54:55-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Berg WA, Gutierrez L, NessAiver MS, Carter WB, Bhargavan M, Lewis RS, Ioffe OB. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233:830-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1011] [Cited by in F6Publishing: 928] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 3. | Schouten van der Velden AP, Schlooz-Vries MS, Boetes C, Wobbes T. Magnetic resonance imaging of ductal carcinoma in situ: what is its clinical application? A review. Am J Surg. 2009;198:262-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Baur A, Bahrs SD, Speck S, Wietek BM, Krämer B, Vogel U, Claussen CD, Siegmann-Luz KC. Breast MRI of pure ductal carcinoma in situ: sensitivity of diagnosis and influence of lesion characteristics. Eur J Radiol. 2013;82:1731-1737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Jin ZQ, Lin MY, Hao WQ, Jiang HT, Zhang L, Hu WH, Zhang M. Diagnostic evaluation of ductal carcinoma in situ of the breast: ultrasonographic, mammographic and histopathologic correlations. Ultrasound Med Biol. 2015;41:47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6112] [Cited by in F6Publishing: 5922] [Article Influence: 348.4] [Reference Citation Analysis (0)] |
| 7. | Li CI, Daling JR, Malone KE. Age-specific incidence rates of in situ breast carcinomas by histologic type, 1980 to 2001. Cancer Epidemiol Biomarkers Prev. 2005;14:1008-1011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Rosen EL, Eubank WB, Mankoff DA. FDG PET, PET/CT, and breast cancer imaging. Radiographics. 2007;27 Suppl 1:S215-S229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 194] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 9. | Rostom AY, Powe J, Kandil A, Ezzat A, Bakheet S, el-Khwsky F, el-Hussainy G, Sorbris R, Sjoklint O. Positron emission tomography in breast cancer: a clinicopathological correlation of results. Br J Radiol. 1999;72:1064-1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Avril N, Rosé CA, Schelling M, Dose J, Kuhn W, Bense S, Weber W, Ziegler S, Graeff H, Schwaiger M. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol. 2000;18:3495-3502. [PubMed] [Cited in This Article: ] |
| 11. | Mavi A, Urhan M, Yu JQ, Zhuang H, Houseni M, Cermik TF, Thiruvenkatasamy D, Czerniecki B, Schnall M, Alavi A. Dual time point 18F-FDG PET imaging detects breast cancer with high sensitivity and correlates well with histologic subtypes. J Nucl Med. 2006;47:1440-1446. [PubMed] [Cited in This Article: ] |
| 12. | Groheux D, Giacchetti S, Delord M, Hindié E, Vercellino L, Cuvier C, Toubert ME, Merlet P, Hennequin C, Espié M. 18F-FDG PET/CT in staging patients with locally advanced or inflammatory breast cancer: comparison to conventional staging. J Nucl Med. 2013;54:5-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Wang Y, Zhang C, Liu J, Huang G. Is 18F-FDG PET accurate to predict neoadjuvant therapy response in breast cancer? A meta-analysis. Breast Cancer Res Treat. 2012;131:357-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA. Breast Imaging Reporting and Data System: BI-RADS Atlas. 5th ed. American College of Radiology, Reston, 2013. . [Cited in This Article: ] |
| 15. | Wang SY, Shamliyan T, Virnig BA, Kane R. Tumor characteristics as predictors of local recurrence after treatment of ductal carcinoma in situ: a meta-analysis. Breast Cancer Res Treat. 2011;127:1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Groheux D, Espié M, Giacchetti S, Hindié E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013;266:388-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 17. | Brennan ME, Turner RM, Ciatto S, Marinovich ML, French JR, Macaskill P, Houssami N. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011;260:119-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 248] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 18. | Shigematsu H, Kadoya T, Masumoto N, Matsuura K, Emi A, Kajitani K, Amioka A, Okada M. Role of FDG-PET/CT in prediction of underestimation of invasive breast cancer in cases of ductal carcinoma in situ diagnosed at needle biopsy. Clin Breast Cancer. 2014;14:358-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Kang SS, Ko EY, Han BK, Shin JH, Hahn SY, Ko ES. Background parenchymal enhancement on breast MRI: influence of menstrual cycle and breast composition. J Magn Reson Imaging. 2014;39:526-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Vranjesevic D, Schiepers C, Silverman DH, Quon A, Villalpando J, Dahlbom M, Phelps ME, Czernin J. Relationship between 18F-FDG uptake and breast density in women with normal breast tissue. J Nucl Med. 2003;44:1238-1242. [PubMed] [Cited in This Article: ] |