Revised: December 5, 2012
Accepted: December 15, 2012
Published online: February 28, 2013
AIM: To evaluate the complications and clinical outcomes of transcatheter arterial embolization (TAE) for symptoms related to severe arterioportal fistulas (APFs).
METHODS: Six patients (3 males, 3 females; mean age, 63.8 years; age range, 60-71 years) with chronic liver disease and severe APFs due to percutaneous intrahepatic treatment (n = 5) and portal vein (PV) tumor thrombosis of hepatocellular carcinoma (n = 1) underwent TAE for symptoms related to severe APFs [refractory ascites (n = 4), hemorrhoidal hemorrhage (n = 1), and hepatic encephalopathy (n = 1)]. Control of symptoms related to APFs and complications were evaluated during the follow-up period (range, 4-57 mo).
RESULTS: In all patients, celiac angiography revealed immediate retrograde visualization of the main PV before TAE, indicating severe APF. Selective TAE for the hepatic arteries was performed using metallic coils (MC, n = 4) and both MCs and n-butyl cyanoacrylate (n = 2). Three patients underwent repeated TAEs for residual APFs and ascites. Four patients developed PV thrombosis after TAE. During the follow-up period after TAE, APF obliteration and symptomatic improvement were obtained in all patients.
CONCLUSION: Although TAE for severe APFs may sometimes be complicated by PV thrombosis, TAE can be an effective treatment to improve clinical symptoms related to severe APFs.
- Citation: Hirakawa M, Nishie A, Asayama Y, Ishigami K, Ushijima Y, Fujita N, Honda H. Clinical outcomes of symptomatic arterioportal fistulas after transcatheter arterial embolization. World J Radiol 2013; 5(2): 33-40
- URL: https://www.wjgnet.com/1949-8470/full/v5/i2/33.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i2.33
Arterioportal fistulas (APFs) are characterized by anomalous communication between arteries and the portal vein (PV) system caused by congenital vascular malformations, blunt or penetrating trauma, rupture of the hepatic artery, iatrogenic causes (especially liver biopsy), hepatitis, liver cirrhosis, neoplasms, and infections[1-4]. The clinical spectrum of presentation ranges from asymptomatic individuals to patients with severe portal hypertension. Patients with severe APFs can develop various complications of portal hypertension, including gastrointestinal bleeding, refractory ascites, and diarrhea.
In the past, the majority of patients with symptoms related to APFs required surgical treatment[5]. However, some cases in which symptoms related to APF were controlled by transcatheter arterial embolization (TAE) have been sporadically reported. Still, no large series has been well documented. The aim of this study was to evaluate the complications and clinical outcomes of TAE for severe APFs.
Between April 2000 and March 2008, six patients (age range, 60-71 years old; mean age, 63.8 years; three male and three female patients) were treated with TAE for symptoms related to severe APFs in our institution. Our institution did not require institutional review board approval for this type of retrospective study, and the requirements for informed consent were waived because it is a retrospective study. Clinical data recorded before TAE are summarized in Table 1. All patients had chronic liver disease, and hepatitis C viral antibodies were positive in all patients. The major symptoms of six patients at presentation were refractory ascites (n = 4), hemorrhoidal hemorrhage (n = 1), and hepatic encephalopathy (n = 1). These clinical symptoms related to portal hypertension could not be controlled with conservative treatment before TAE. Severe APFs were diagnosed on enhanced computed tomography (CT) and Doppler ultrasound (US). Therefore, the clinical symptoms were thought to be related mainly to severe APFs. The underlying causes of the severe APFs were percutaneous hepatic interventions (n = 5) and portal thrombosis of hepatocellular carcinoma (HCC) (n = 1). Follow-up imaging using CT or US was performed (median follow-up period, 12 mo; range, 4-57 mo).
| Case No. | Age (yr)/gender | Child-Pugh class | Etiology | Clinical symptoms related to APF | Admission from previous intrahepatic intervention (yr) |
| 1 | 70/M | C | MCT for hepatocellular carcinoma | Hemorrhage from hemorrhoid refractory ascites | 2 |
| 2 | 63/F | A | Liver biopsy | Refractory ascites | 5 |
| 3 | 71/M | C | PEIT for hepatocellular carcinoma | Refractory ascites | 1 |
| 4 | 67/M | A | PVTT (hepatocellular carcinoma) | Refractory ascites | NA |
| 5 | 70/F | B | MCT for hepatocellular carcinoma | Hepatic encephalopathy | 1 |
| 6 | 60/F | A | Liver biopsy | Refractory ascites | 6 |
The potential risks and benefits were explained to the patients, and their written, informed consent was obtained before treatment.
Under sterile technique and local anesthesia, all TAE procedures were performed from the right common femoral artery approach. A 4-F sheath (Supersheath; Medikit, Tokyo, Japan) was placed in the femoral artery, followed by a 4-F shepherd hook catheter (Clinical Supply, Gifu, Japan) that was positioned in the celiac artery. By celiac, proper hepatic, and selective arteriography using a 4-F shepherd hook catheter and a coaxial microcatheter (Renegade STC-18, Boston Scientific, United States; Sniper 2, Clinical Supply, Gifu, Japan; or Meistercath, Medikit, Tokyo, Japan), the presence of an APF was determined based on opacification of the PV during the arterial phase of angiography, and the accurate anatomical location and the site of communication between hepatic artery branches and PV branches were defined.
For embolization procedures, microcoils (Tornado embolization microcoils; coil diameters = 3-4 mm, length of introducer = 40-60 mm, Cook, Bloomington, IN, United States) were deployed at both the distal and proximal sites of the APF. However, if the microcatheter could not be inserted into the distal sites of APFs, microcoils were deployed only at the proximal site. A mixture of lipiodol (Lipiodol Ultra-Fluide; TERUMO, Tokyo, Japan) and n-butyl cyanoacrylate (NBCA: Histoacryl-Blue; B. Braun, Melsungen, Germany) was used when many minute feeders into the APF were involved. An NBCA-lipiodol mixture was infused from the proximal portion of the APF. NBCA was diluted with lipiodol at a ratio of 1:4-7 in order to delay the polymerization of NBCA. The NBCA-Lipiodol mixture was infused until delayed APF flow could be seen under fluoroscopy. The procedure was continued until APF flow was decreased or completely occluded on hepatic arteriography.
Complications, TAE technique, control of symptoms related to APFs, and APF flow were evaluated with a review of the patients’ medical records and periodic CT or US imaging.
TAE procedure and clinical outcomes are summarized in Table 2. In all patients, immediate retrograde visualization of the main trunk of the PV and marked enlarged, tortuous branches of the hepatic arteries were seen on celiac and selective hepatic arteriography before TAE. In all patients, superior mesenteric artery (SMA) portography could not detect the main trunk of the PV. Furthermore, reflux to the gastric vein with extensive collateral circulation through the esophageal, superior mesenteric, and perisplenic vascular bed was prominent in all patients before TAE.
| Case No. | Location of APF | Embolic agents | Isolation of APF | TAE (n) | Hepatopedal flow after TAE on SMA portography | Complications after TAE | Control of symptom | Hepatopetal flow/arterial pulse on last US | Follow (M)/outcome | Outcome |
| 1 | A3-LPV | MC | - | 2 | + | HE, PVT | Resolved | +/- | 13/alive good | Alive good |
| 2 | A7 and A8-RPV | MC | + | 2 | + | PVT | Resolved | +/- | 57/alive good | Alive good |
| 3 | A3-LPV | MC | + | 1 | + | None | Resolved | +/- | 14/alive good | Alive good |
| 4 | A4-LPV A7-RPV A8-RPV | MC | - | 3 | + | None | Improved | +/- | 4/alive fair | Alive fair |
| 5 | A7-RPV A8-RPV | MC + glue | + | 1 | + | PVT | Resolved | +/- | 12/alive good | Alive good |
| 6 | A7-RPV | MC + glue | + | 1 | + | PVT | Resolved | +/- | 5/alive good | Alive good |
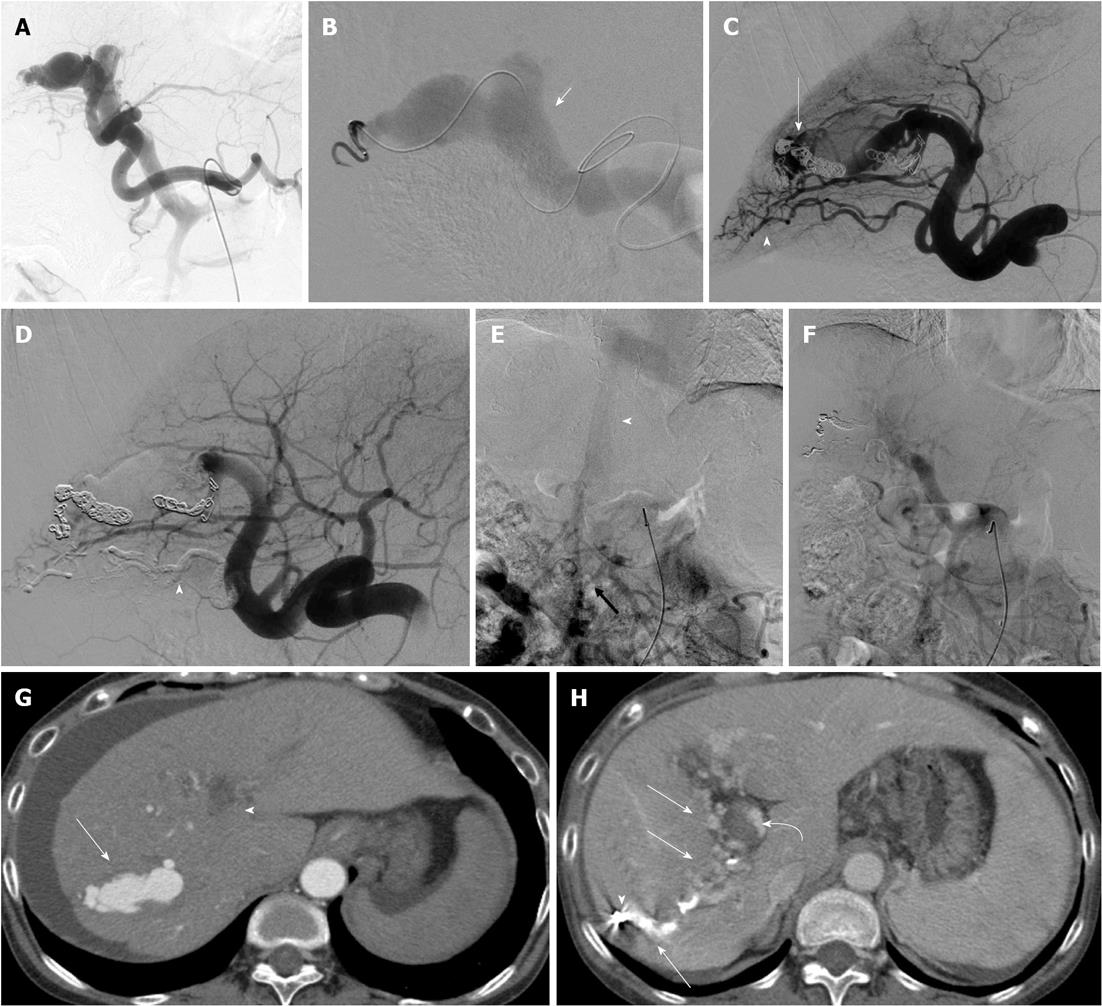
TAE using microcoils alone was performed in four patients. TAE using both microcoils and NBCA was performed in two patients. In these two patients, the major feeders of the APFs could be embolized both proximal and distal to the sites of fistulas using microcoils; however, many minute feeders into the APFs were embolized using NBCA. Embolizations both proximal and distal to the sites of fistulas were successfully performed in four patients. In the other two patients, because of very tortuous hepatic arteries, superselective insertion of a microcatheter into the distal site of the fistula could not be performed. In three of the patients, additional TAEs were required because of residual APF flow and residual ascites. In two of these three patients, complete occlusion of the APF was successfully obtained with additional TAE using metallic coils (MC). In the other patient, complete occlusion of the APF and control of ascites could not be obtained with two TAEs using MC. In this patient, the etiology of APF was identified as idiopathic or iatrogenic before TAE; however, the etiology was diagnosed as portal vein tumor thrombosis (PVTT) of diffuse HCC on final follow-up imaging. In this patient, complete occlusion of the APF was successfully obtained on angiography with embolization of both right and left hepatic arteries using microcoils. In all patients, SMA portography detected the main trunk of the PV after the final TAE. There were no technical failures related to the migration of embolic agents. In all patients, hepatopetal portal flow was seen on color Doppler US two or three days after the final TAE. Final follow-up imaging showed complete APF obliteration in all but the one patient with APFs due to PVTT.
In five of the six patients, the clinical symptoms resolved during follow-up after the final TAE. In the one patient who had severe APFs due to PVTT, the refractory ascites and APFs improved, but they could not be completely controlled by three TAEs.
Transient liver dysfunction after TAE was observed in all patients, which was controlled gradually by conservative treatment.
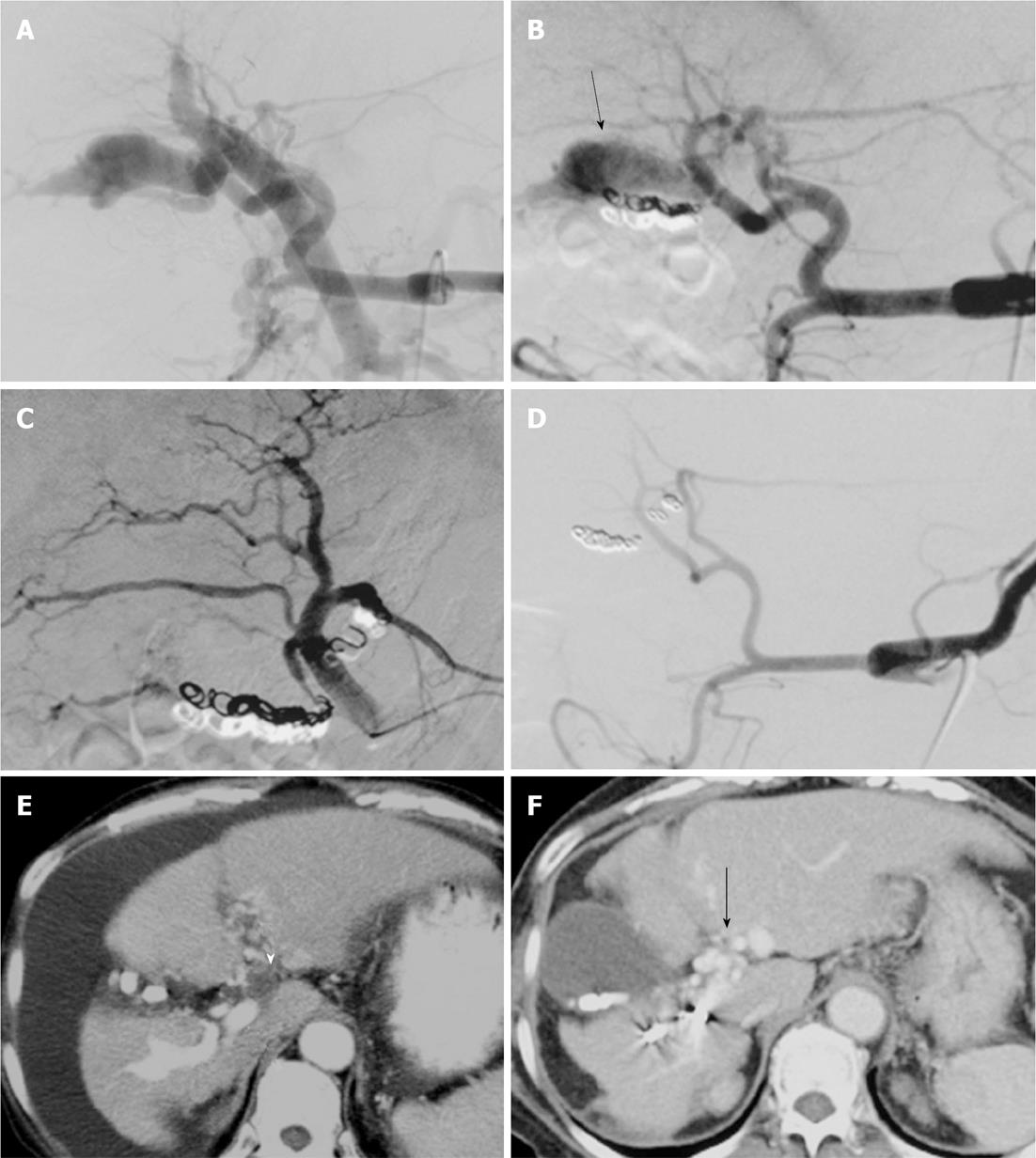
In four patients, a portal vein thrombosis (PVT) recognized on CT taken before TAE was observed to have deteriorated after TAE (Figures 1 and 2). In all four of these patients, the initial clinical symptoms were controlled despite progression of PVT (Cases 1, 2, 5, and 6). In only one (Case 1) of these four patients, hepatic encephalopathy developed with deteriorated PVT. In the patient with hepatic encephalopathy (Case 1), decreased ascites and improved hemorrhoids were seen despite PVT progression; however, this patient experienced disorientation and unconsciousness 2 mo after TAE. Further imaging studies including abdominal US disclosed PVT extending from the main PV to the superior mesenteric vein. Anticoagulation therapy using warfarin was started; the PVT, clinical symptoms, and laboratory data improved gradually. Follow-up color Doppler US did not show PVT and arterial pulsation, and it demonstrated a hepatopetal flow signal in the PV. This patient was well with a normal serum ammonia level eight months after TAE.
Though another three patients presenting with deteriorated PVT had no symptoms related to PVT or portal hypertension, two patients received anticoagulation therapy (Cases 5 and 6). In these three patients, PVT was not controlled, and final follow-up enhanced CT demonstrated obliteration of the APF and hepatopetal flow via hepatic hilar cavernous transformation. Gastric endoscopy demonstrated partial regression of the gastro-esophageal varices in all patients.
APFs are a rare cause of portal hypertension[4,6]. Intrahepatic APFs are mostly caused by abdominal trauma, iatrogenic injury, or congenital vascular malformations such as telangiectatic disorders[7,8].
In a large, multi-center, retrospective study, the frequency of APF following liver biopsy was 10% if angiography was delayed to 3 wk after the biopsy[9]. If the APF is small and peripherally located, it is likely to resolve spontaneously within 3 mo[10]. If the APF is large and centrally located, it is unlikely to resolve spontaneously and is likely to be a symptomatic fistula. Typically, 80% of cases with intrahepatic APF is diagnosed within 2 years after blunt hepatic trauma[10]. The present patients with APFs due to past percutaneous hepatic intervention showed clinical symptoms 2.7 years (mean) after the hepatic intervention, and their APFs were centrally located. In the present study, because all patients had chronic liver disease and some patients had HCC, all patients underwent periodic enhanced CT. With the deterioration of APFs on periodic enhanced CT, which shows simultaneous enhancement of the aorta and main PV in the hepatic arterial phase, the clinical symptoms related to APFs could not be controlled with conservative treatment. Therefore, we believe that the indications for TAE of APFs should include intractable symptoms related to portal hypertension and enhanced CT showing severe APF.
The clinical presentation of intrahepatic APF, ranging from asymptomatic cases to syndromes variously combining portal hypertension and other changes in cardiovascular hemodynamics, which cause massive variceal bleeding, intestinal ischemia, refractory ascites, and even heart failure, depends mainly on the size and location of the fistula, shunt volume, and the resistance of the liver. Except for one case that showed hemorrhaging from a hemorrhoid, no variceal bleeding occurred; however, there were mild to moderate esophageal varices before TAE in all patients. These varices subsided following successful embolotherapy of the APF, which improved the portal hypertension.
Symptomatic APFs have been reported to be occluded using steel coils, microcoils, detachable coils, or a combination with NBCA, polyvinyl alcohol, and/or microspheres[8,11-14]. The embolic agents should be chosen according to the size of the vascular communication. Proper selection of the embolic material is very important for the success of TAE. For high-flow APFs, MC are frequently selected as the embolic agent; however, when a fistula develops in a large vessel, arterial embolization with MC is controversial due to the potential risk of migration of the metallic coil into the portal venous system. This is especially true if the fistula is larger than 8 mm and there is a high-flow state[15,16]. In the literature, detachable balloons have been shown to be the most efficient choice of embolic material for especially large, high-flow fistulas, because they are able to totally occlude the feeder and the fistula[17]. However, the detachable balloon is presently unavailable in Japan.
Controlled-release detachable coils, such as the interlocking detachable coil (IDC) or guglielmi detachable coil (GDC), can avoid the risk of coil migration to an undesirable distal site or PV in TAE for large APFs. However, because the controlled-release detachable coils were not able to cause thrombosis of the high-flow fistulous connection, the placement of other fibered coils proximal to the initial controlled-release detachable coils may have caused complete occlusion of the APF. Ishigami et al[18] suggested that TAE using classic stainless coils and IDCs under balloon occlusion may be useful when the APF shunt flow is too fast. In the present series, two patients had large, high-flow APFs that were embolized with IDC as a first anchoring coil at the proper distal and proximal sites of the fistula and with subsequent fibered coils at the proximal site of the APF. This technique can avoid undesirable coil migration to the PV, and it successfully achieved complete occlusion of the APF. In these two patients, the main feeders of the APFs were embolized superselectively with microcoils; however, the considerably tortuous other feeders were also present concurrently. Because superselective placement of a microcatheter at the site of the APFs via tortuous feeders was impossible, an NBCA-lipiodol mixture was administered from the proximal site of the feeders. Finally, complete occlusion of APFs was obtained without any migration of glue. Therefore, we believe that the combination use of controlled-release detachable coils, fibered coils, and glue can be very effective for the TAE of APFs.
In the literature, PVTT of HCC may cause severe APF symptoms including ascites, hepatic encephalopathy, or variceal bleeding from the fistula itself, and these APFs and symptoms could be controlled with TAE using microcoils in most patients[19]. However, APFs due to PVTT should be embolized carefully, because TAE may cause liver failure.
Some other large series reported that TAE using microcoils for APF was useful for improving the symptoms related to APFs[4,20,21]. In all of the present patients, obliteration of APF flow and restoration of normal hepatopetal portal flow were observed on radiological examination during the follow-up period after APF closure. An objective improvement in clinical findings related to APFs was obtained in all patients. Therefore, we also think that TAE using MC and glue is effective for symptomatic APF.
In the literature reporting on TAE for APFs, no severe complications have been described, and the liver function has gradually returned to normal after embolization. Some previous studies reported the migration of embolic agents to the portal system[4]. Coils and other embolic agents can easily pass into the portal system because APFs are usually in a high-flow state. Migration of embolic agents may cause the blockage of PV branches, leading to subsequent thrombosis. Although no cases of failure related to migration occurred, this should be considered when embolizing a large or high-flow APF.
Chen et al[22] reported a patient presenting with PVT observed on follow-up CT 2 d after TAE for APF using GDC, Nester coils, and NBCA glue (50% concentration). In this patient, three platinum coils inadvertently migrated to the PV. Chen et al[22] proposed that the sequelae of coil migration might cause portal thrombus formation and its associated complications. However, the patient remained in remission with no symptoms related to PVT or portal hypertension at 3 mo after treatment. In the present four patients, PVT deteriorated after TAE without the migration of embolic agents to the portal system. In only one of these four patients, hepatic encephalopathy was likely to have been a result of portal hypertension related to the PVT.
Factors contributing to PVT formation after TAE include sluggish blood flow in the PV and the migration of embolic agents to the PV. In the present study, hepatofugal portal flow was recognized on angiography before TAE, while hepatopetal portal flow was shown after TAE. Therefore, sluggish blood flow in the PV might have occurred and caused deterioration of the PVT. In the present study, migrated coils and glue were not seen; however, tiny amounts of glue can migrate to the PV and cause deterioration of the PVT.
Three of the four patients who developed PVT after TAE received anticoagulation therapy using warfarin; however, PVT improved in only one patient. In the other three patients who were treated with/without anticoagulation therapy, a chronically thrombosed main trunk of the native PV and a patent intrahepatic PV via cavernous transformation in the hepatic hilum were seen on follow-up CT. These three patients maintained liver function, and no recurrent findings related to APF were seen on the final follow-up examinations.
Cavernous transformation is a sequela of long-term PVT in which the etiology determines the outcome. The formation of these vessels is necessary in order to drain splenic and mesenteric venous flow into the intrahepatic portal branches and to maintain sufficient hepatic blood flow and normal hepatic function.
Although TAE for APF caused the deterioration of PVT, portal hypertension related to APF was improved if hepatopetal flow via cavernous transformation could be reconstituted. Anticoagulant therapy may be required to prevent clot propagation in the intrahepatic PV; however, it may also prevent clot propagation of an embolized APF. Anticoagulant therapy in this type of clinical scenario should be performed carefully[23].
The present study had a limitation. The long-term clinical outcomes of TAE for APFs were evaluated in only a small number of patients. Therefore, no conclusions about long-term outcomes could be drawn from this study. Percutaneous hepatic intervention therapy, such as radiofrequency ablation and percutaneous ethanol injection, is frequently undertaken today in patients with liver tumors, and symptomatic APF may be on the increase in the future[11,12].
In conclusion, although TAE for severe APF may sometimes result in PV thrombosis, TAE is an effective treatment for improving clinical symptoms related to APF. The proper technique and embolic materials should be selected according to the flow velocity through the fistula.
Patients with severe arterioportal fistulas (APFs) can develop various complications of portal hypertension, including gastrointestinal bleeding, refractory ascites, and diarrhea. In the past, the majority of patients with symptoms related to APFs required surgical treatment. However, some cases in which symptoms related to APF were controlled by transcatheter arterial embolization (TAE) have been sporadically reported.
In the past, the majority of patients with symptoms related to APFs have required surgical treatment. However, some cases in which symptoms related to APF were controlled by TAE have been sporadically reported. In this study, the authors demonstrate the complications and clinical outcome of TAE for severe APFs.
Recent reports have highlighted the clinical improvement of TAE for symptomatic APFs. This is the first study to report that some patients were complicated with portal vein thrombosis (PVT) after TAE. Furthermore, their study would suggest that TAE can be an effective treatment to improve clinical symptoms related to severe APFs, although TAE for severe APFs may sometimes complicate PVT.
By understanding the complications and clinical outcomes after TAE, this study may represent a future strategy for TAE for symptoms related to severe APFs.
Although TAE for severe APFs may sometimes complicate PV thrombosis, TAE can be an effective treatment to improve clinical symptoms related to severe APFs.
Currently, percutaneous hepatic intervention therapy such as radiofrequency ablation and percutaneous ethanol injection is frequently applied to patients with liver tumors, and symptomatic APF may be on the increase in the future. The authors examined the expression of the complications and clinical outcomes of TAE for symptoms related to severe APFs; although TAE for severe APF may sometimes complicate PV thrombosis, TAE is an effective treatment for improving clinical symptoms related to APF. The results are interesting and may represent that the proper technique and embolic materials should be selected according to the flow velocity through the fistula.
P- Reviewer Chen MJ S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Gallego C, Miralles M, Marín C, Muyor P, González G, García-Hidalgo E. Congenital hepatic shunts. Radiographics. 2004;24:755-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Guzman EA, McCahill LE, Rogers FB. Arterioportal fistulas: introduction of a novel classification with therapeutic implications. J Gastrointest Surg. 2006;10:543-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Okuda K, Musha H, Nakajima Y, Takayasu K, Suzuki Y, Morita M, Yamasaki T. Frequency of intrahepatic arteriovenous fistula as a sequela to percutaneous needle puncture of the liver. Gastroenterology. 1978;74:1204-1207. [PubMed] [Cited in This Article: ] |
| 4. | Vauthey JN, Tomczak RJ, Helmberger T, Gertsch P, Forsmark C, Caridi J, Reed A, Langham MR, Lauwers GY, Goffette P. The arterioportal fistula syndrome: clinicopathologic features, diagnosis, and therapy. Gastroenterology. 1997;113:1390-1401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 159] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Linder F. Acquired arterio-venous fistulas. Report of 223 operated cases. Ann Chir Gynaecol. 1985;74:1-5. [PubMed] [Cited in This Article: ] |
| 6. | Redmond PL, Kumpe DA. Embolization of an intrahepatic arterioportal fistula: case report and review of the literature. Cardiovasc Intervent Radiol. 1988;11:274-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Graham WP, Eiseman B, Pryor R. Hepatic artery aneurysm with portal vein fistula in a patient with familial hereditary telangiectasia. Ann Surg. 1964;159:362-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Korula J, Fried J, Weissman M, Greaney G, Liew CT, Finck E. Fatal hemorrhage from an arterio-portal-peritoneal fistula after percutaneous liver biopsy. Gastroenterology. 1989;96:244-246. [PubMed] [Cited in This Article: ] |
| 9. | Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 850] [Cited by in F6Publishing: 769] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 10. | Tanaka H, Iwai A, Sugimoto H, Yoshioka T, Sugimoto T. Intrahepatic arterioportal fistula after blunt hepatic trauma: case reports. J Trauma. 1991;31:143-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Chavan A, Harms J, Pichlmayr R, Galanski M. Transcatheter coil occlusion of an intrahepatic arterioportal fistula in a transplanted liver. Bildgebung. 1993;60:215-218. [PubMed] [Cited in This Article: ] |
| 12. | Agha FP, Raji MR. Successful transcatheter embolic control of significant arterioportal fistula: a serious complication of liver biopsy. Br J Radiol. 1983;56:277-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Heaton ND, Davenport M, Karani J, Mowat AP, Howard ER. Congenital hepatoportal arteriovenous fistula. Surgery. 1995;117:170-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Cheung YF, Leung MP. Coil embolization of hepatoportal arteriovenous fistula in a neonate. Clin Cardiol. 1999;22:675-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Isik FF, Greenfield AJ, Guben J, Birkett D, Menzoian JO. Iatrogenic arterioportal fistulae: diagnosis and management. Ann Vasc Surg. 1989;3:52-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Deitrick J, McNeill P, Posner MP, Kellum J, Cho S, Tisnado J, Lee HM. Traumatic superior mesenteric artery--portal vein fistula. Ann Vasc Surg. 1990;4:72-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Akpek S, Ilgit ET, Cekirge S, Yücel C. High-flow arterioportal fistula: treatment with detachable balloon occlusion. Abdom Imaging. 2001;26:277-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Ishigami K, Yoshimitsu K, Honda H, Kuroiwa T, Irie H, Aibe H, Tajima T, Hashizume M, Masuda K. Coil embolization of arterioportal fistula that developed after partial gastrectomy. Cardiovasc Intervent Radiol. 1999;22:328-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Furuse J, Iwasaki M, Yoshino M, Konishi M, Kawano N, Kinoshita T, Ryu M, Satake M, Moriyama N. Hepatocellular carcinoma with portal vein tumor thrombus: embolization of arterioportal shunts. Radiology. 1997;204:787-790. [PubMed] [Cited in This Article: ] |
| 20. | Tasar M, Gulec B, Bozlar U, Saglam M, Ugurel MS, Ucoz T. Intrahepatic arterioportal fistula and its treatment with detachable balloon and transcatheter embolization with coils and microspheres. Clin Imaging. 2005;29:325-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Tarazov PG. Intrahepatic arterioportal fistulae: role of transcatheter embolization. Cardiovasc Intervent Radiol. 1993;16:368-373. [PubMed] [Cited in This Article: ] |
| 22. | Chen Q, Tack C, Morcos M, Ruggiero M, Schlossberg P, Fogel J, Weng LJ, Farkas J. Embolotherapy of an arterioportal fistula. Cardiovasc Intervent Radiol. 2007;30:1047-1051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Condat B, Pessione F, Hillaire S, Denninger MH, Guillin MC, Poliquin M, Hadengue A, Erlinger S, Valla D. Current outcome of portal vein thrombosis in adults: risk and benefit of anticoagulant therapy. Gastroenterology. 2001;120:490-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 358] [Article Influence: 15.6] [Reference Citation Analysis (0)] |