Published online Dec 28, 2013. doi: 10.4329/wjr.v5.i12.484
Revised: October 17, 2013
Accepted: December 9, 2013
Published online: December 28, 2013
AIM: To assess the prognostic value and risk classification improvement of metabolic staging (MS) with Initial 2-[18F]-fluoro-2-desoxy-D-glucose positron emission tomography (FDG-PET) in initial staging of Hodgkin’s Lymphoma (HL) patients to predict 5 years overall survival (5y-OS) and event free survival (EFS).
METHODS: A total of 275 patients were included in this retrospective study, 155 patients were staged with conventional anatomical staging (AS), and 120 also submitted to MS (FDG-PET). Prognostic analysis compared 5y-OS and 5y-EFS of patients staged with AS and MS. Risk-adjusted models incorporated clinical risk factors, computed tomography and FDG-PET staging.
RESULTS: During the follow up of 267 evaluated patients, 220 (122 AS and 98 MS) achieved complete remission after first-line therapy (median follow-up: 70 ± 29 mo), treatment failure occurred in 79 patients and 34 died. The 5y-EFS for early vs advanced disease in AS patients was 79.3% and 66.7%, and 85.6% and 53.6% in MS patients, respectively (P < 0.01). The 5y-OS for early and advanced disease with AS was 91.3% and 81.5%, and 97.5% and 80.7% for patients staged with MS, respectively. Cox proportional hazards analysis demonstrated that FDG-PET added significant prognostic information and improved risk prediction (P = 0.02).
CONCLUSION: Initial staging FDG-PET could be used as an accurate and independent predictor of OS and EFS in HL, with impact in 5y-EFS and OS.
Core tip: Initial 2-[18F]-fluoro-2-desoxy-D-glucose positron emission tomography (FDG-PET) has impact in the determination of the event free survival and overall survival (OS) in Hodgkin’s Lymphoma patients, also initial staging with FDG-PET was the strongest predictor of OS and event free survival of the evaluated variables analyzed. In the currently era of tailoring therapy to an individual level, initial staging might play an even more important role.
- Citation: Cerci JJ, Linardi CCG, Pracchia LF, Junior JS, Trindade E, Delbeke D, Cerci RJ, Carr R, Meneghetti JC, Buccheri V. 2-[18F]-fluoro-2-desoxy-D-glucose positron emission tomography initial staging impacts on survival in Hodgkin lymphoma. World J Radiol 2013; 5(12): 484-490
- URL: https://www.wjgnet.com/1949-8470/full/v5/i12/484.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i12.484
The anatomical extent, or stage, of disease is an important predictor of prognosis in Hodgkin’s Lymphoma (HL). The method to determine disease stage at diagnosis has evolved over time. During the 1970s establishing the clinical extent of disease was based on clinical examination and laparotomy to assess abdominal disease[1]. During the 1980s computed tomography (CT) allowed more accurate assessment of the presence of enlarged visceral lymph nodes, increasing the staging precision, and then combining disease stage with other clinical signs to increase the accuracy of prognosis prediction at the time of diagnosis[2,3]. The Ann Arbor staging system, modified in Cotswolds in 1988 is used in the initial staging of Hodgkin lymphoma patients. Stage I indicates involvement of one lymph node chain, stage II indicates involvement of at least two lymph node chains, confined to one side of the diaphragm, stage III indicates involvement of lymph nodes from both sides of the diaphragm and stage IV indicates involvement at least one extra-lymphatic organ. The absence or presence of constitutional symptoms, like fever, weight loss or night sweating are denoted by adding “A” or “B” to the stage; respectively, and the presence of a large tumoral mass with at least 10 cm in the largest diameter is classified as bulky disease[2].
Since the 1990s, the introduction of positron emission tomography with 2-[18F]-fluoro-2-desoxy-D-glucose (FDG-PET) has enabled the detection of metabolically active tumors cells within anatomically normal lymph nodes and extranodal sites. A number of publications demonstrated the increased sensitivity of pre-treatment staging by PET compared to CT[4-8]. At that time, standard treatment for HL was based on the ABVD regimen irrespective of disease stage at diagnosis.
Subsequently, attention has focused on the use of PET to assess response to of treatment assessing the reduction of FDG after 2 or 3 cycles of chemotherapy[9-11]. Clinical interest has been directed to exploring the potential of early “metabolic” response assessment to reduce treatment, and therefore toxicity in rapid responders, or intensify treatment in slow responders to increase cure rates.
More recently, attempts to personalize treatment and increase cure has shifted the emphasis earlier, to focus on disease assessment at diagnosis and stratifying primary treatment intensity to match the better prognosis of early stage, and worse prognosis of late stage disease[12]. Initial treatment stratification is based on the premise that accurate staging, alone or as part of a prognostic index, predicts response to treatment and cure.
The availability of PET remains limited to major referral centers. Scarce evidence demonstrating the increased accuracy of metabolic staging by PET in comparison to anatomical staging by CT has been published. This increased accuracy would translate into greater discrimination between favorable and poor risk HL at the time of diagnosis. Therefore the aim of the current study was to establish whether PET staging translates into more accurate prediction of outcome. This information is not only clinically important, but also relevant to future planning of clinical PET indications.
Patients presenting at the Hematology Division of the São Paulo University Clinics Hospital with newly diagnosed, biopsy proven, classical HL, between July 1999 and December 2007 were included in a this retrospective study comparing disease staging by CT or by PET. Patients included between 1999 and 2003 (Era 1) were imaged by CT, and from 2003 to the end of 2007 (Era 2) by PET in addition to CT. The Ethical Board of the Clinical Hospital of the University of São Paulo approved the study.
All patients underwent clinical staging procedures including physical examination, complete blood cell counts and blood chemistry, CT scans (cervical, thoracic, abdomen and pelvic), and bilateral iliac crest bone marrow biopsy. CT scans were sectioned at a thickness of 1 mm, and oral and intravenous contrast agents were administered to all patients. Conventional anatomical staging (AS) of each patient was assigned according to the Ann Arbor staging system[1] modified during the Cotswold meeting based on CT imaging and results of bone marrow biopsy.
Whole-body PET imaging was acquired after a 60 min uptake period following the intravenous administration of 296-444 MBq (8-12 mCi) of FDG. Imaging was performed using 2-D acquisition in a GE Advance PET scanner (GE Advance; GE Healthcare, Waukesha, Wisconsin, United States). Attenuation correction was performed using 68Ge sources. Two experienced board-certified nuclear medicine physicians interpreted FDG-PET scan. Areas of non-physiological abnormalities with increased FDG uptake over the background were classified as positive for disease. Involvement was defined according to the criteria established by the Consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma[4].
During Era 2 patients underwent CT, PET imaging and bone marrow biopsy. The interval between CT and PET scan was never longer than two weeks. Final stage assignment was based on information from both CT and PET, Metabolic stage (MS).
Treatment planning and outcome analysis were based on assignment of disease stage as determined by conventional AS in Era 1 and metabolic staging in Era 2.
Early stage I and II patients were treated with four to six cycles of chemotherapy using ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine). Advanced stage III patients were treated with six to eight cycles (varying according to anatomical treatment response) of ABVD and all stage IV patients were treated with eight cycles of ABVD. Radiotherapy on involved fields was included in early stage I and II cases, as part of the combined modality therapy, after four cycles of chemotherapy, or when bulky disease was present, regardless of the clinical stage.
This approach to treatment of HL was the same throughout all the period of study, and did not change following the introduction of PET. Treatment was not modified by results of mid treatment scanning during the PET era.
Differences in categorical variables among groups were compared by the χ2 test and differences in continuous variables among groups were compared by the Mann-Whitney test. The overall survival (OS) was calculated from the time of diagnosis to death from any cause or time of censoring and the event free survival (EFS) was calculated from the time of diagnosis to refractory disease, relapse, and death from any cause or time of censoring using the Kaplan-Meyer method. For the purpose of survival analysis patients were divided into Early Stage (I, II) and Advance Stage (III, IV) and the survival curves were compared by log-rank test. The effect of MS and AS on 5-year incidence of treatment failure and mortality risk were also determined using Cox regression models. Patients of Era 1 (AS) were censored if reached a maximum follow up of 5 years for the purpose of comparative analysis with patients of Era 2 (MS).
All statistical analyses were done using Stata Statistical Software, Release 11 (College Station, TX: StataCorp LP). A P value of 0.05 was considered statistically significant.
This study population included 275 patients. Eight (2.8%) patients lost follow-up, therefore 267 patients (5/155 of AS and 3/120 of MS), were included in the final study cohort.
Table 1 shows the baseline clinical characteristics of the MS and AS groups. Baseline and clinical characteristics of patients were similar in both groups, with no statistical differences (Table 1).
| Characteristics | Era 1 150 patients AS | Era 2 117 patients MS | P |
| Gender | |||
| Male | 82 (54.7) | 63 (52.5) | 0.89 |
| Age, median ± SD | 33.4 ± 15.1 | 34.1 ± 16.1 | 0.25 |
| Pathological subtype | |||
| Nodular sclerosis | 88 (58.7) | 71 (59.2) | |
| Non classified | 17 (11.3) | 22 (18.3) | |
| Mixed cellularity | 31 (20.7) | 12 (10.0) | 0.53 |
| Lymphocyte predominance | 12 (8.0) | 9 (7.5) | |
| Lymphocyte depleted | 2 (1.3) | 6 (5.0) | |
| B symptoms | 99 (66.0) | 76 (65.0) | 0.85 |
| Bulky disease (> 7 cm) | 91 (60.3) | 67 (57.3) | 0.74 |
| Clinical stage | 0.12 | ||
| I | 12 (8.0) | 9 (7.7) | |
| II | 61 (40.7) | 45 (38.5) | |
| III | 35 (23.3) | 27 (23.1) | |
| IV | 42 (28.0) | 36 (30.8) | |
| Treatment failure | 43 (28.7) | 36 (30.8) | 0.70 |
| Death | 20 (13.3) | 14 (12.0) | 0.73 |
Twenty six of the 117 Era 2 patients had their disease upstaged by PET, in comparison with anatomical staging (I→II, 5 patients; I→III, 1 patient; I→IV, 1 patient; II→IV, 4 patients, III→IV, 8 patients) and 14 had their disease downstaged (II→I, 3 patients; III→II, 4 patient; IV→I, 1 patient; IV→II, 2 patients; IV→III, 4 patients).
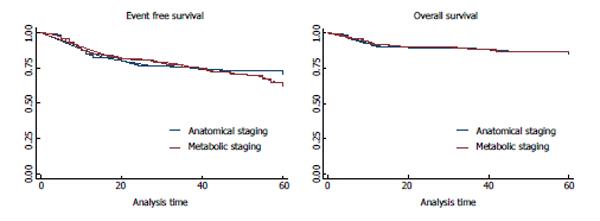
During the follow up, 29.6% (79/267) patients presented treatment failure (refractory disease or relapse), including 28.6% (43/150) patients staged with AS and 30.7% (36/117) patients staged with MS. The median follow-up was 55 ± 16 mo; 45 ± 21 mo for patients staged with AS and 40 ± 18 mo for patients staged with MS. Moreover, 12.7% (34/267) patients died; 13.3% (20/150) patients staged with AS and 12% (14/117) patients staged with MS. All treatment failures were confirmed by biopsy. There were no statistical differences of treatment failure and mortality between the two groups (Table 1, Figure 1).
The hazard ratios of clinical characteristics in the group of patients staged by CT and with FDG-PET are listed in Table 2. The evaluation with FDG-PET dividing patients with early and advance stage was the most important prognostic factor for EFS and OS in the MS group of patients, while international prognosis index was the most important in the AS group of patients.
| Five-year-EFS | Five-year-OS | |||||||
| AS | MS | CS | MS | |||||
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | |
| Age (> 45, ≤ 45) | 1.89 (0.95-3.76) | 0.067 | 1.75 (0.86-3.57) | 0.121 | 2.97 (1.18-7.47) | 0.020 | 6.59 (2.20-19.68) | 0.001 |
| Gender | 1.32 (0.72-2.44) | 0.362 | 0.85 (0.44-1.63) | 0.628 | 2.00 (0.76-5.20) | 0.155 | 0.83 (0.29-2.38) | 0.739 |
| B symptoms | 2.19 (1.05-4.57) | 0.036 | 1.76 (0.82-3.76) | 0.140 | 2.27 (0.76-6.81) | 0.142 | 1.44 (0.45-4.61) | 0.532 |
| Bulky | 0.94 (0.50-1.79) | 0.868 | 0.94 (0.48-1.84) | 0.869 | 1.06 (0.40-2.77) | 0.899 | 0.41 (0.13-1.22) | 0.111 |
| Early vs advance clinical stage | 2.05 (1.09-3.85) | 0.024 | 2.73 (1.19-6.24) | 0.017 | 2.51 (0.96-6.55) | 0.059 | 8.09 (1.05-61.85) | 0.044 |
| Stage IV disease | 2.42 (1.32-4.42) | 0.004 | 2.33 (1.20-4.50) | 0.012 | 3.67 (1.52-8.88) | 0.004 | 6.54 (1.82-23.48) | 0.004 |
| Serum albumin | 1.09 (.057-2.07) | 0.775 | 1.52 (0.71-3.25) | 0.273 | 2.12 (0.71-6.37) | 0.178 | 1.91 (0.53-6.87) | 0.318 |
| Hemoglobin | 1.50 (0.79-2.85) | 0.206 | 1.35 (0.67-2.71) | 0.399 | 2.47 (1.02-5.96) | 0.044 | 1.43 (0.48-4.29) | 0.514 |
| White blood cell count | 2.11 (1.03-4.30) | 0.039 | 1.62 (0.62-4.18) | 0.319 | 2.55 (1.84-8.2) | 0.041 | 1.68 (0.37-7.54) | 0.495 |
| Lymphocyte count | 1.81 (0.95-3.44) | 0.067 | 2.61 (1.27-5.3) | 0.009 | 2.44 (0.99-6.00) | 0.050 | 2.84 (0.95-8.51) | 0.061 |
| IPI (0-2, ≥ 3) | 3.41 (1.80-6.47) | 0.000 | 2.08 (1.07-4.08) | 0.031 | 3.74 (1.43-9.77) | 0.007 | 4.98 (1.38-17.86) | 0.014 |
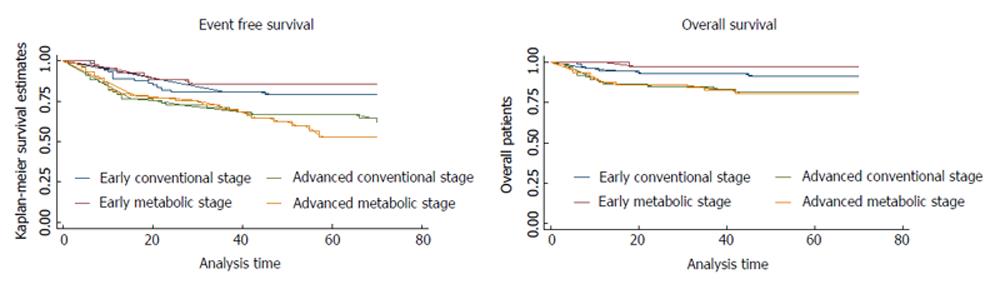
The three- and five-year OS and EFS of AS and MS in initial staging of HL patients are shown in Table 3. The five-year EFS for early and advanced patients staged with AS was 79.3% and 66.7%, respectively; and 85.6% and 53.6% for patients staged with MS (P < 0.01). The five-year OS for early and advanced patients staged with AS was 91.3% and 81.5%, respectively; and 97.5% and 80.7% for patients staged with MS (P = 0.04). Figure 2 shows the Kaplan-Meier plot showing the OS and EFS for early and advance stage patients groups, staged with AS and MS.
| Survival | 150Patients CS | 117Patients MS | 267Total patients |
| Three-year EFS | |||
| Stage I | 91.7% | 100.0% | 93.7% |
| Stage II | 78.7% | 84.4% | 80.9% |
| Early stage | 80.7% | 85.6% | 82.6% |
| Stage III | 79.7% | 76.7% | 78.4% |
| Stage IV | 63.7% | 65.2% | 64.5% |
| Advanced stage | 71.0% | 70.0% | 70.5% |
| Five-year EFS | |||
| Stage I | 91.7% | 100.0% | 93.7% |
| Stage II | 77.0% | 84.4% | 79.8% |
| Early stage | 79.3% | 85.6% | 81.5% |
| Stage III | 76.5% | 69.0% | 73.1% |
| Stage IV | 58.6% | 44.8% | 53.7% |
| Advanced stage | 66.7% | 53.6% | 61.9% |
| Three-year OS | |||
| Stage I | 91.7% | 100.0% | 93.7% |
| Stage II | 93.2% | 97.2% | 94.8% |
| Early stage | 93.0% | 97.5% | 94.6% |
| Stage III | 94.2% | 96.3% | 95.2% |
| Stage IV | 77.2% | 74.2% | 75.7% |
| Advanced stage | 84.9% | 82.8% | 83.9% |
| Five-year OS | |||
| Stage I | 91.7% | 100.0% | 93.7% |
| Stage II | 91.2% | 97.2% | 93.3% |
| Early stage | 91.3% | 97.5% | 93.4% |
| Stage III | 94.2% | 90.9% | 92.9% |
| Stage IV | 71.1% | 74.2% | 72.5% |
| Advanced stage | 81.5% | 80.7% | 81.1% |
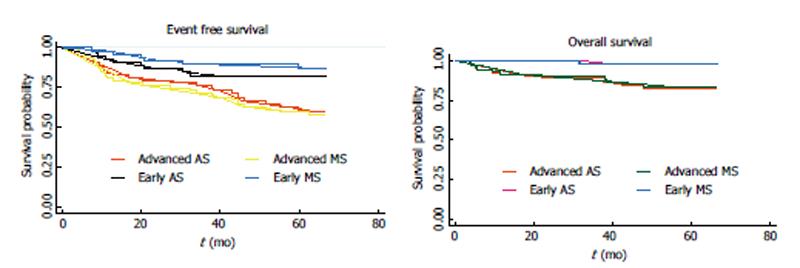
When the EFS was evaluated in Era 2 patients according to their conventional staging, instead of their metabolic staging, the five-year EFS for early and advanced patients was 82.6% and 61.4%, respectively, instead of 85.6% and 53.6%. When the OS was evaluated in Era 2 patients according to their conventional staging, instead of their metabolic staging the five-year OS for early and advanced patients was 97.9% and 82.4%, respectively, instead of 97.5% and 80.7% (Figure 3).
The results of this single center study, with a standardized protocol for treating early and advance stage disease, shows that the 5 years EFS for early and advanced patients staged with AS were 79.3% and 66.7%, and 85.6% and 53.6% for patients staged with MS, respectively (P < 0.01). The 5 years OS for early and advanced patients staged with AS was 91.3% and 81.5%, respectively; and 97.5% and 80.7% for patients staged with MS (P = 0.04). This demonstrates that MS has impact on EFS and OS.
In the last 20 years, FDG-PET has been shown to be more accurate then AS, changing clinical stage in about 30% of patients[5-8,13-16]. Although many studies compared CT and PET stage assignment, little data was presented on how this can be translated into outcomes. Munker et al[17], in a retrospective study evaluating 73 HL patients demonstrated a worse prognosis for the cases in which FDG-PET changed stage from I or II to III or IV. However, due to the limited time of follow-up, no difference in survival could be demonstrated between patients who remained in the same stage and patients who were upstaged by PET. Our results of 267 HL patients, with a longer follow up, shows that when staging takes full account of initial PET data, the separation of outcomes between early and advance stage HL becomes statistically significant regarding EFS, and clinically important with significant higher hazard ratios regarding OS and EFS. These differences are probably related to a better stratification of risk of these patients with FDG-PET, since no differences in therapy, supportive care and follow up were performed in this series of patients.
Also a great importance has been pointed that metabolic treatment response is more important than initial staging especially in HL[11,18-21]. Although current focus is on adapting therapy based on interim PET response, it is perhaps even more important to ensure the most appropriate initial staging, if taken into account the tendency is tailoring therapy with ABVD for early disease and BEACOPP for advance stage[22]. Therefore getting initial staging accuracy is even more important because it might influence primary therapy.
This study has some important limitations. Results are based on two series of cases, one staged conventionally and another subsequent series also staged with FDG-PET. However the spread of Ann Arbor stage and overall treatment outcome data is equivalent between the two series, demonstrating that both are equivalent in terms of patients and treatment. When the EFS of Era 2 patients classified according to metabolic staging was compared to the EFS of the same patients classified according to anatomical staging, it was also possible to observe a higher accuracy of the metabolic staging. Also, a randomized trial would be desirable, however in face of the amount of data showing the importance of MS, denying the FDG-PET evaluation in a group could be an ethical issue. Another limitation of the current study is the analysis of a patient population from a single health center. Several authors have extensively studied the performance of PET and PET/CT compared with CT over the past decades; as such, the compilation of multiple studies with larger series should be recommend.
Standard treatment for Hodgkin’s Lymphoma (HL) was based on the ABVD regimen irrespective of disease stage at diagnosis. The aim of the current study was to establish whether positron emission tomography (PET) staging translates into more accurate prediction of outcome.
This information is not only clinically important, but also relevant to future planning of clinical PET indications.
The results of the authors study indicate that Initial metabolic staging (MS) has impact in the determination of the event free survival (EFS) and overall survival (OS) in HL patients, also initial staging with 2-[18F]-fluoro-2-desoxy-D-glucose positron emission tomography (FDG-PET) is the strongest predictor of OS and EFS of the evaluated variables analyzed.
Initial staging with FDG-PET is the strongest predictor of OS and EFS of the evaluated variables analyzed. In the currently era of tailoring therapy to an individual level, initial staging might play an even more important role.
The author reported the prognostic value and risk classification improvement of using MS with FDG-PET in initial staging of HL patients to predict 5 years OS and EFS, compared to conventional staging with computed tomography (CT). The conclusion is that initial staging of FDG-PET in HL is an accurate and independent predictor of OS and EFS. The difference in EFS and OS observed with two different staging systems may be explained by the fact that PET scan is more accurate in staging. Therefore, some of the early stages as determined by CT scan are upstaged to more advanced stages.
P- Reviewers: Chen F, Pan CX S- Editor: Ma YJ L- Editor: A E- Editor: Liu SQ
| 1. | Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971;31:1860-1861. [PubMed] [Cited in This Article: ] |
| 2. | Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, Rosenberg SA, Coltman CA, Tubiana M. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630-1636. [PubMed] [Cited in This Article: ] |
| 3. | Urba WJ, Longo DL. Hodgkin’s disease. N Engl J Med. 1992;326:678-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 179] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Weihrauch MR, Re D, Bischoff S, Dietlein M, Scheidhauer K, Krug B, Textoris F, Ansén S, Franklin J, Bohlen H. Whole-body positron emission tomography using 18F-fluorodeoxyglucose for initial staging of patients with Hodgkin’s disease. Ann Hematol. 2002;81:20-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Jerusalem G, Beguin Y, Fassotte MF, Najjar F, Paulus P, Rigo P, Fillet G. Whole-body positron emission tomography using 18F-fluorodeoxyglucose compared to standard procedures for staging patients with Hodgkin’s disease. Haematologica. 2001;86:266-273. [PubMed] [Cited in This Article: ] |
| 6. | Hutchings M, Loft A, Hansen M, Pedersen LM, Berthelsen AK, Keiding S, D’Amore F, Boesen AM, Roemer L, Specht L. Position emission tomography with or without computed tomography in the primary staging of Hodgkin’s lymphoma. Haematologica. 2006;91:482-489. [PubMed] [Cited in This Article: ] |
| 7. | Pracchia LF, Chaves AA, Cerci JJ, Soares Junior J, Meneghetti JC, Buccheri V. Metabolic test with fluorine-18-fluorodeoxyglucose in staging and detection of residual tumor or recurrence in Hodgkin lymphoma. Clinics (Sao Paulo). 2007;62:121-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Cerci JJ, Trindade E, Buccheri V, Fanti S, Coutinho AM, Zanoni L, Linardi CC, Celli M, Delbeke D, Pracchia LF. Consistency of FDG-PET accuracy and cost-effectiveness in initial staging of patients with Hodgkin lymphoma across jurisdictions. Clin Lymphoma Myeloma Leuk. 2011;11:314-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Hutchings M, Loft A, Hansen M, Pedersen LM, Buhl T, Jurlander J, Buus S, Keiding S, D’Amore F, Boesen AM. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107:52-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 566] [Cited by in F6Publishing: 603] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 10. | Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, Patti C, Loft A, Di Raimondo F, D’Amore F. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746-3752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 668] [Cited by in F6Publishing: 607] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 11. | Cerci JJ, Pracchia LF, Linardi CC, Pitella FA, Delbeke D, Izaki M, Trindade E, Soares J, Buccheri V, Meneghetti JC. 18F-FDG PET after 2 cycles of ABVD predicts event-free survival in early and advanced Hodgkin lymphoma. J Nucl Med. 2010;51:1337-1343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Uhm J, Kuruvilla J. Treatment of newly diagnosed advanced stage Hodgkin lymphoma. Blood Rev. 2012;26:167-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Cerci JJ, Pracchia LF, Soares Junior J, Linardi Cda C, Meneghetti JC, Buccheri V. Positron emission tomography with 2-[18F]-fluoro-2-deoxy-D-glucose for initial staging of hodgkin lymphoma: a single center experience in Brazil. Clinics (Sao Paulo). 2009;64:491-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Bangerter M, Moog F, Buchmann I, Kotzerke J, Griesshammer M, Hafner M, Elsner K, Frickhofen N, Reske SN, Bergmann L. Whole-body 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) for accurate staging of Hodgkin’s disease. Ann Oncol. 1998;9:1117-1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 190] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Jerusalem G, Hustinx R, Beguin Y, Fillet G. Positron emission tomography imaging for lymphoma. Curr Opin Oncol. 2005;17:441-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Weihrauch MR, Dietlein M, Schicha H, Diehl V, Tesch H. Prognostic significance of 18F-fluorodeoxyglucose positron emission tomography in lymphoma. Leuk Lymphoma. 2003;44:15-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Munker R, Glass J, Griffeth LK, Sattar T, Zamani R, Heldmann M, Shi R, Lilien DL. Contribution of PET imaging to the initial staging and prognosis of patients with Hodgkin’s disease. Ann Oncol. 2004;15:1699-1704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Gallamini A, Kostakoglu L. Interim FDG-PET in Hodgkin lymphoma: a compass for a safe navigation in clinical trials. Blood. 2012;120:4913-4920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Mikhaeel NG, Hutchings M, Fields PA, O’Doherty MJ, Timothy AR. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol. 2005;16:1514-1523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 332] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 20. | Hutchings M, Mikhaeel NG, Fields PA, Nunan T, Timothy AR. Prognostic value of interim FDG-PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann Oncol. 2005;16:1160-1168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 283] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 21. | Kostakoglu L, Gallamini A. Interim 18F-FDG PET in Hodgkin lymphoma: would PET-adapted clinical trials lead to a paradigm shift. J Nucl Med. 2013;54:1082-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Blum KA. Upcoming diagnostic and therapeutic developments in classical Hodgkin’s lymphoma. Hematology Am Soc Hematol Educ Program. 2010;2010:93-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |