Published online Apr 28, 2012. doi: 10.4329/wjr.v4.i4.128
Revised: April 20, 2012
Accepted: April 27, 2012
Published online: April 28, 2012
Lung cancer is the most common cause of death from cancer in males, accounting for more than 1.4 million deaths in 2008. It is a growing concern in China, Asia and Africa as well. Accurate staging of the disease is an important part of the management as it provides estimation of patient’s prognosis and identifies treatment sterategies. It also helps to build a database for future staging projects. A major revision of lung cancer staging has been announced with effect from January 2010. The new classification is based on a larger surgical and non-surgical cohort of patients, and thus more accurate in terms of outcome prediction compared to the previous classification. There are several original papers regarding this new classification which give comprehensive description of the methodology, the changes in the staging and the statistical analysis. This overview is a simplified description of the changes in the new classification and their potential impact on patients’ treatment and prognosis.
- Citation: Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, Beek EJV. The 7th lung cancer TNM classification and staging system: Review of the changes and implications. World J Radiol 2012; 4(4): 128-134
- URL: https://www.wjgnet.com/1949-8470/full/v4/i4/128.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i4.128
Lung cancer is the most common cause of cancer deaths in males, accounting for 13% (1.6 million) of the total cancer cases and 18% (1.4 million) of the cancer deaths in 2008. Male lung cancer death rates are decreasing in the western world and increasing in China and several other countries in Asia and Africa. Female lung cancer death rates are increasing worldwide, with the exception of United States; Canada, United Kindom and Australia[1-3].
Complete resection of lung cancer is associated with significantly longer survival remission but only about 25% of patients are candidates for surgical treatment at the time of diagnosis[4]. Staging of cancer at the time of diagnosis is the most important predictor of survival, and treatments options should be based on the stage. Since the introduction of tumour, node, metastasis (TNM) staging by Pierre Denoix between the years 1943 and 1952, there have been significant changes including the TNM staging for lung cancer. The International Union Against Cancer (UICC) TNM Prognostic Factors Project continued to develop the TNM classification as more data became available. The pocket book, “Livre de Poche”, was the first edition of the TNM classification and was published in 1968 and following several updates, the sixth edition was released in 2002. Like other tumours, lung cancer classification and staging assess the anatomical extension of the tumor which is critical to choosing a therapy and provides information on prognosis[5-9].
The International Association for the Study of Lung Cancer (IASLC) announced a major revision of the TNM staging system for lung cancer[10]. This has been included in the seventh edition of the “TNM classification of malignant tumours” published by the UICC in January 2010 (Table 1). The previous latest update of the classification was based on a predominantly surgical database of 5319 patients from a single centre in the United States from 1972 to 1988[11]. The database was small and old and there was little internal and no external validation of stage groupings relating to these data and given the fact that this is the commonest cause for cancer related death, a major revision of the staging was long overdue and became possible with the availability of multicentre and larger cohorts of patients being treated for lung cancer.
| T: Tumour | |
| TX | Primary tumour cannot be assessed, or tumour proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy |
| T0 | No evidence of primary tumour |
| Tis | Carcinoma in situ |
| T1 | Tumour < 3 cm in greatest dimension, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchus (i.e., not in the main bronchus) |
| T1a | Tumour < 2 cm in greatest dimension |
| T1b | Tumour > 2 cm but < 3 cm in greatest dimension |
| T2 | Tumour > 3 cm but < 7 cm or tumour with any of the following features (T2 tumours with these features are classified T2a if < 5 cm): |
| Involves main bronchus, > 2 cm distal to the carina | |
| Invades visceral pleura | |
| Associated with atelectasis or obstructive pneumonitis that extends to the hilar region but does not involve the entire lung | |
| T2a | Tumour > 3 cm but < 5 cm in greatest dimension |
| T2b | Tumour > 5 cm but < 7 cm in greatest dimension |
| T3 | Tumour > 7 cm or one that directly invades any of the following: |
| Chest wall (including superior sulcus tumours), diaphragm, phrenic nerve, mediastinal pleura, parietal pericardium | |
| Tumour in the main bronchus < 2 cm distal to the carina but without involvement of the carina | |
| Associated atelectasis or obstructive pneumonitis of the entire lung | |
| Separate tumour nodule(s) in the same lobe | |
| T4 | Tumour of any size that invades any of the following: |
| Mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body, carina | |
| Separate tumour nodule(s) in a different ipsilateral lobe | |
| N: Nodes | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension |
| N2 | Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s) |
| N3 | Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s) |
| M: Metastases | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Separate tumour nodule(s) in a contralateral lobe |
| tumour with pleural nodules or malignant pleural/ pericardial effusion | |
| M1b | Distant metastasis |
Traditionally the TNM classification has been used for non-small-cell lung cancer (NSCLC). Even though the TNM classification was applicable to the small-cell lung cancer (SCLC), this was not practiced. SCLC was classified as “local” and “extensive” disease. The new classification is also applicable to both types of lung cancers[12].
Imaging has a fundemental role in staging of the lung cancer. As imaging techniques have improved especially in terms of resolution and speed for CT scans and as newer techniques like positron emission tomography (PET) have become established in the routine clinical practice, consideration needed to be given to revising the classification and staging of lung cancer. The new classification has led to alteration in treatment options and in predicting the prognosis.
The staging of lung cancer can be clinical or pathological (also known as surgical). Clinical staging involves radiological studies (plain radiographs and CT scan).
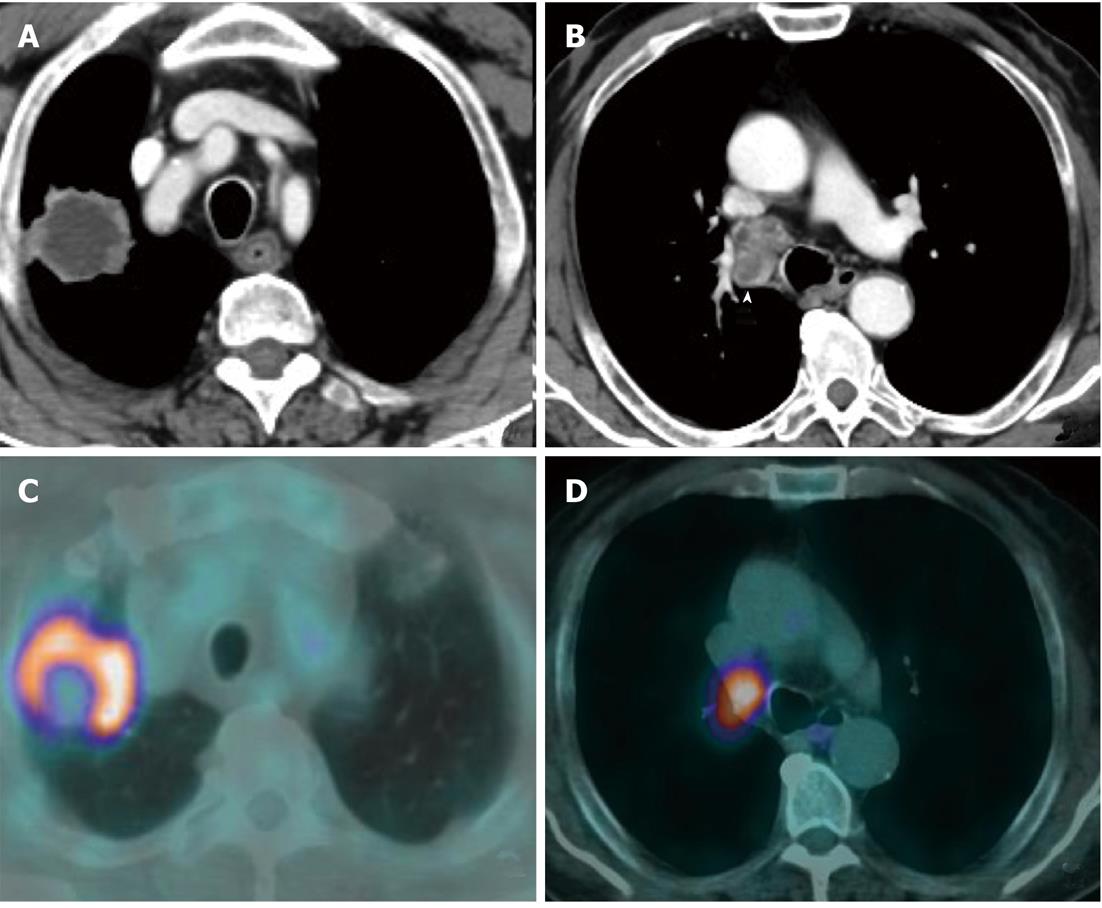
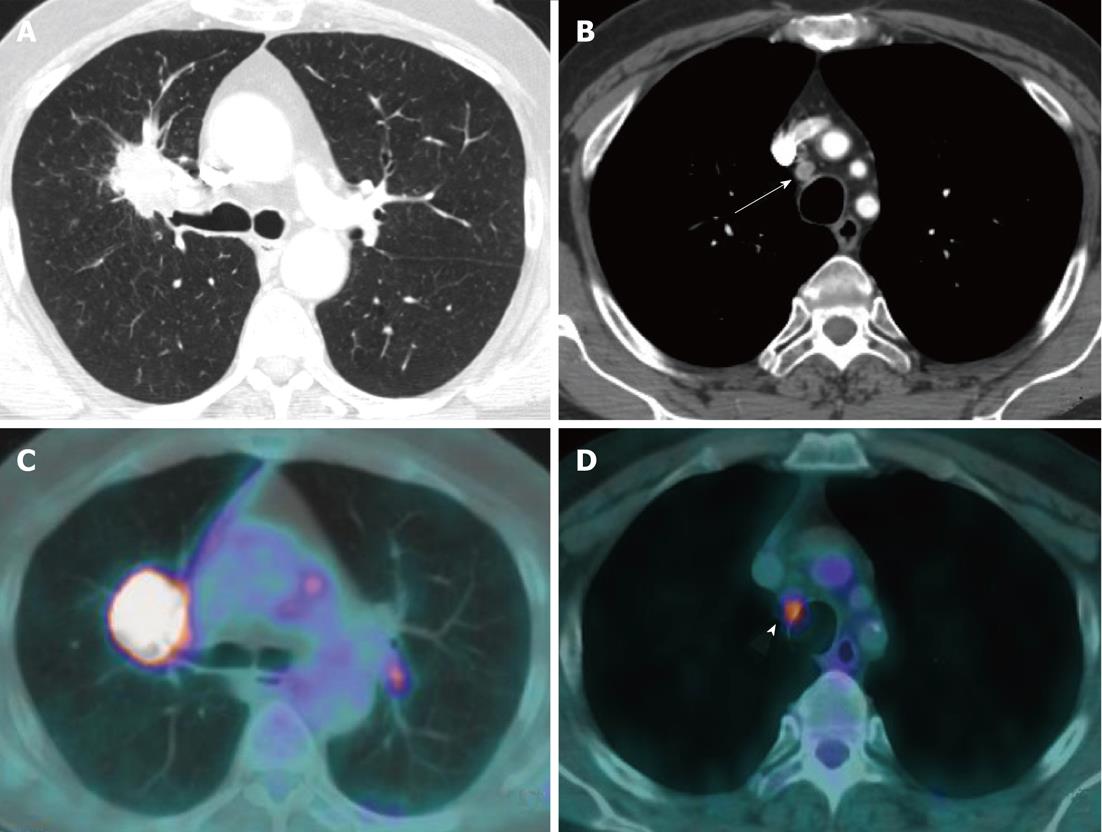
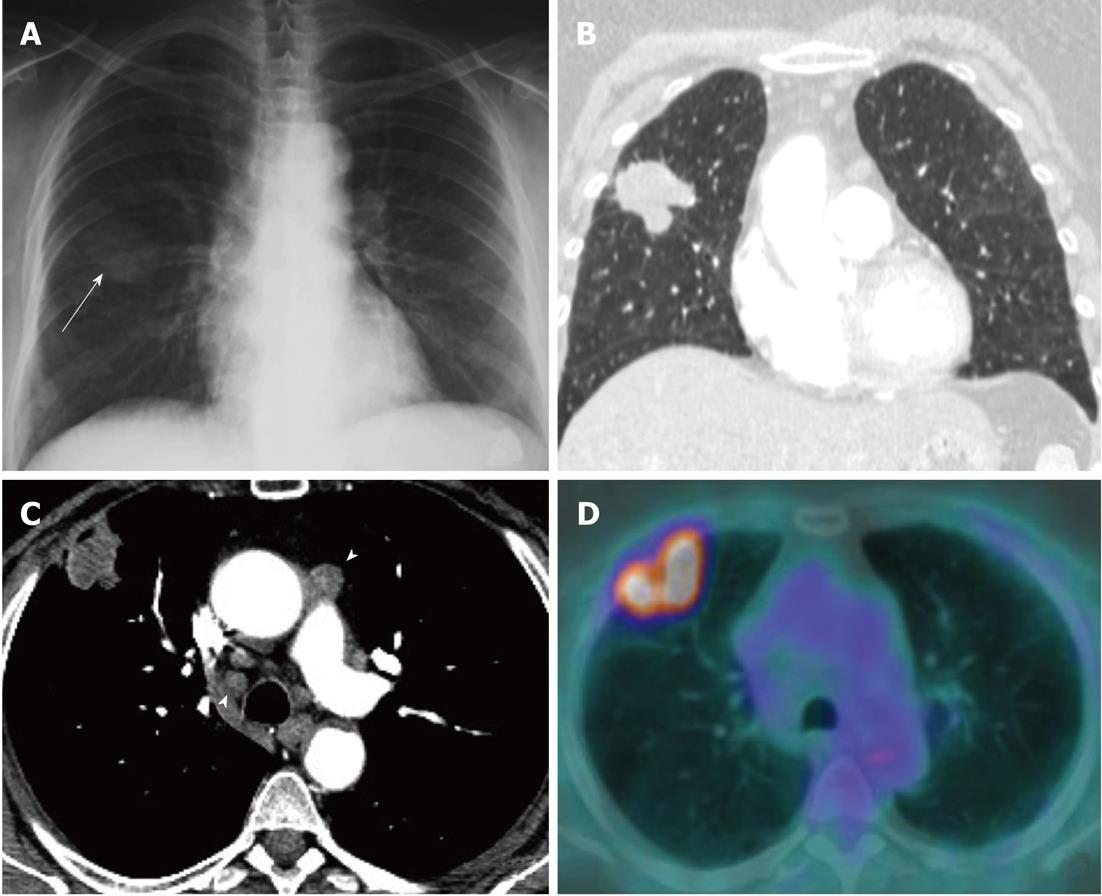
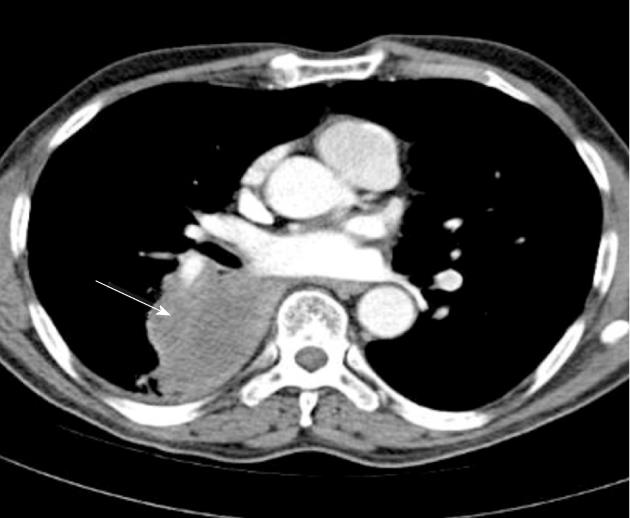
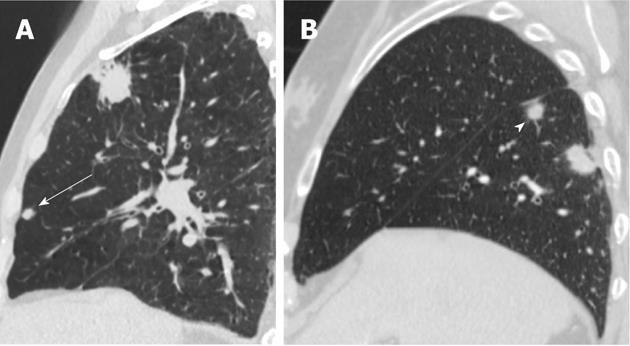
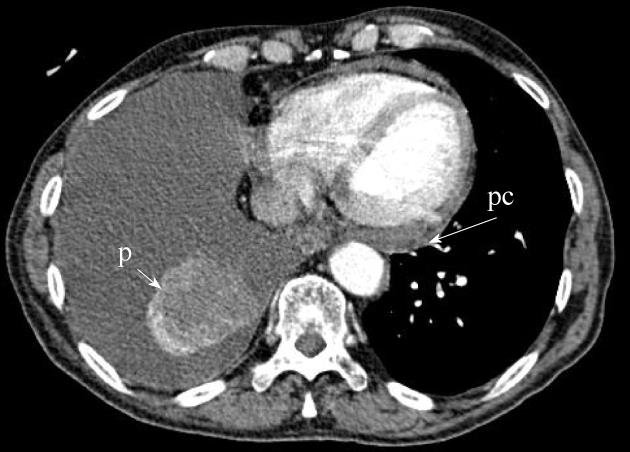
Currently, PET (Figure 1) is routinely used in many centres as an additional diagnostic tool which may change the clinical stage of the disease in a given patient. PET often upstages the disease (in comparison to conventional CT scan) by identifying newer, metabolically active sites of disease (Figure 2). In a minority of cases, it downstages the disease (Figure 3). However, it must be noted that the database analysed for the 7th TNM classification did not include any data from PET studies. Surgical staging refers to pathological staging following surgery or tissue biopsy (e.g., via endoscopical techniques). In addition to T, N and M descriptors, the pathological staging also involves description of resection margins which is indicated as R0 or R1 (R0 is resection margins clear of the malignancy and R1 is involved margins). Following pathological staging, the T, N and M become pT, pN and pM. Obviously, this may result in either upward or downward alteration of clinical staging. The overall level of agreement between clinical and pathological systems is reported to be only 35%-55%[13].
The aim of this overview is to discuss the basis for the changes in the 7th classification of lung cancer and its impact on predicting patients’ prognosis. Potential limitations of the classification and future directions are discussed.
A retrospective international lung cancer database was developed from 46 sources in more than 19 countries with staging and outcome data on 100 869 lung cancer cases managed between 1990 and 2000. After applying exclusion criteria, 81 015 cases remained for analysis. Of these, 67 725 were NSCLC and 13 290 were SCLC. Only the NSCLC cases were included in the analyses of the T, N and M descriptors and the subsequent analysis of TNM subsets and stage groupings[10]. From 67 725 NSCLC cases, 38265 were clinically without metastases, and 28 371 had pathological staging (defined at thoracotomy). Survival was estimated by the Kaplan-Meier method. Prognostic groups were assessed by Cox regression analysis after adjustment for cell type, sex, age and region, using the SAS System for Windows Version 9.0 PHREG procedure[10,14].
The T staging is determined by the size of primary tumour in long axis, or direct extent of the tumour into adjacent structures such as mediastinum or chest wall.
Main changes in staging classification are reflected in the T staging. These changes are largely related to the re-classification of the size and location of the primary tumour and satelite nodules (Table 2).
| Feature | Old - sixth classification 2002-2009 | Current, new - seventh classification - Jan 2010 |
| Tumour < 2 cm | T1 | T1a |
| Tumour > 2 but < 3 cm | T1 | T1b |
| Tumour > 3 cm but < 5 cm | T2 | T2a |
| Tumour > 5 but < 7 cm | T2 | T2b |
| Tumour > 7 cm | T2 | T3 |
| Tumour - same lobe nodules | T4 | T3 |
| Ipsilateral lung nodule - non primary lobe | M1 | T4 |
| Malignant pleural effusion | T4 | M1a |
| Contralateral lung nodule | M1 | M1a |
| Distant metastases | M1 | M1b |
The former staging system divided tumours into two size groups with 3 cm as the cut off point. The new system has 5 size-based categories with cut-off points at 2, 3, 5 and 7 cm. Tumours measuring < 2 cm are classified as T1a, whereas those measuring 2-3 cm are classified as T1b. T2 disease is also subdivided into T2a (> 3 - 5 cm) and T2b (> 5 cm - 7cm). The tumours larger than 7 cm are now classified as T3. The additional cut-off points changes may alter treatment recommendations.
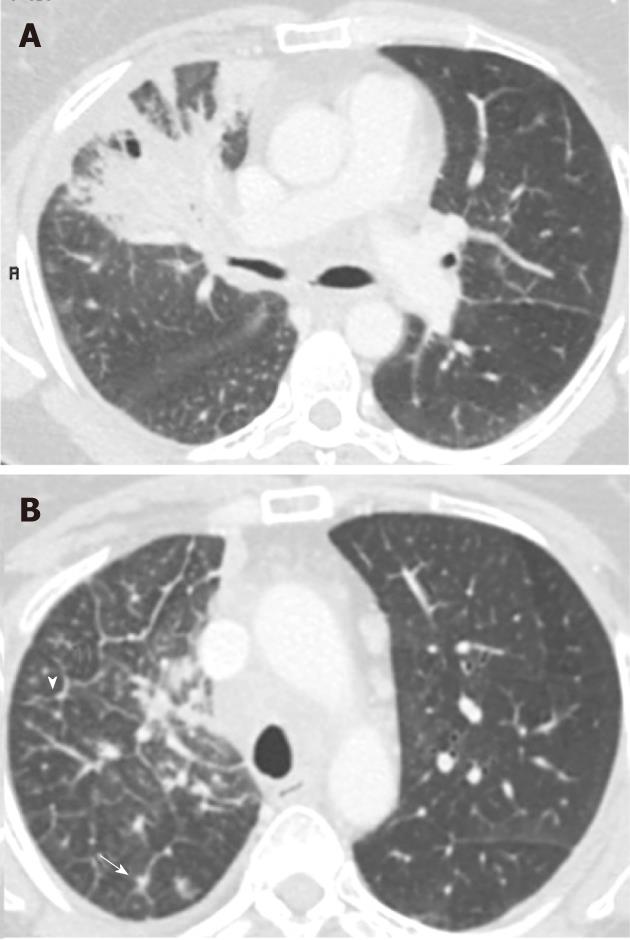
The new calssifcation does not take into account a single tumour which involves two lobes across a fissure. It only considers the tumour size and satellite (non-contagious) nodules in the same and different lobes. Patients previously considered T4 if additional tumour nodules were found in the same lobe are now classified as T3. Additional tumour nodules outside the primary lobe but in the same lung, are now down staged from M1 to T4 and they may be suitable for a pneumonectomy. Classification for a satellite nodule in the contralateral lung has changed from M1 to M1a to indicate intrathoracic spread, which has a slightly favourable prognosis compared to patients with distant metastases (M1b). Figures 4, 5 and 6 show examples of cases in whom the new T classification has changed the staging.
| Old- sixth edition- descriptor | New seventh edition-descriptor | N0 | N1 | N2 | N3 |
| T1 ( ≤ 2 cm) | T1a | I A | II A | III A | III B |
| T1 (> 2-3 cm) | T1b | I A | II A | III A | III B |
| T2 ( ≤ 5 cm) | T2a | I B | II A | III A | III B |
| T2 (> 5-7 cm) | T2b | II A | II B | III A | III B |
| T2 (> 7 cm) | T3 | II B | III A | III A | III B |
| T3 (invasion) | T3 | II B | III A | III A | III B |
| T4 (same lobe nodule) | T3 | II B | III A | III A | III B |
| T4 (extension) | T4 | III A | III A | III B | III B |
| M1 (ipsilateral non primary lobe nodule) | T4 | III A | III A | III B | III B |
| T4 (pleural effusion) | M1a | IV | IV | IV | IV |
| M1 (contralateral lung nodule) | M1a | IV | IV | IV | IV |
| M1 (distant metastases) | M1b | IV | IV | IV | IV |
The N classification descibes the degree of spread to regional lymph nodes. This remained unchanged in the 7th edition as the new data showed no change in node staging related survival.
The regional nodal classification for lung cancer was described by Mountain and Dresler (1997)[15]. Various techniques are used to identify nodal spread. Previous studies showed that the sensitivity and specificity of CT and PET for predicting malignant involvement of mediastinal lymph nodes were 60% and 81%, and 84% and 89%, respectively[16]. Lymph node sampling is regarded as the most accurate predictor of nodal status. Mediastinoscopy has been regarded as the “gold standard” for staging of the mediastinum, but it is invasive and has limitations in accessing to the posterior and inferior mediastinal nodes. Furthermore, the sensitivity for mediastinoscopy is still only 80%-90%, and, in 10%-15% of cases, the technique returns a false-negative diagnosis[17,18].
Endobronchial ultrasound-guided transbronchial needle biopsy (EBUS-TBNA) is reported to have a sensitivity of 85% and a negative predictive value of 90%[19]. Similarly, Rintoul et al[20] reported a sensitivity, specificity and accuracy of 85%, 100% and 89%, respectively for EBUS-TBNA. They also suggested that a combined EBUS and oesophageal endoscopic ultrasound (EUS) allows better access to the mediastinal and hilar lymph nodes than is usually accessible by mediastinoscopy[20]. A further study of 150 consecutive lung cancer patients reported that combination of EUS fine needle aspiration (EUS-FNA) and EBUS-TBNA had higher sensitivity (93%) and higher negative predictive value (97%), when compared to that of each technique[21]. This however needs to be highlighted that not all lymphnode stations are not accessible by EUS techniques.
The M staging defines the presence of metastases beyond regional lymph nodes. In the 7th edition of the lung cancer classification, the pleural or pericardial dissemination (effusions or nodules) are no longer classified T4, but are now upstaged into a new category (M1a). This category also includes additional nodules that are found in the contralateral lung. Distant metastasis is sub-classified as M1b disease.
M1a includes malignant pleural effusion (with median overall survival of 8 mo in 488 patients) and contralateral lung nodules, which had overall survival of 10 mo in 362 patients. M1b refers to extra-thoracic metastases and median overall survival was 6 mo (n = 4343). This contrasts with 13 mo overall median survival in T4M0 any N group (n = 399)[22]. Figure 7 shows an example in which the new TNM classification has changed the T and M staging.
The revised lung cancer staging based on the new TNM classification is shown in Table 3[10].
New staging is based on analyzing survival in large databases based on tumour size and disease proliferation and therefore is expected to assess an individual patient’s prognosis more accurately. Many patients will receive a different staging category based on the 7th edition of the TNM staging system. Those who are down-staged because additional tumours are found in the same lobe as the primary tumour may now be considered candidates for adjunctive chemotherapy along with surgery. Similarly, there may be a greater role for surgery in patients with metastatic nodules in the ipsilateral, non-primary lobe who previously would have been assigned a stage IV diagnosis, but are now stage IIIA.
Those patients who undergo biopsy or surgical resection of the tumour and/or lymph nodes may have their TNM classification revised based on histological findings. The clinical staging of the patient thus changes into pathological staging and is described by adding prefix “p” e.g., T1b N1 M0 may become pT2a pN2 M0 based on pathological measurements and findings, and these would influence treatment strategies and estimated prognosis.
There are glaring deficiencies in the global distribution of the data with no data at all being included from Africa, South America or the Indian subcontinent. Other vast countries such as Russia, China, and Indonesia are not represented or only poorly represented[10]. Moreover, the database used for the 7th edition of lung staging classification (1990-2000) predates the widespread and routine use of PET which has had an enormous impact on clinical staging algorithms[10].
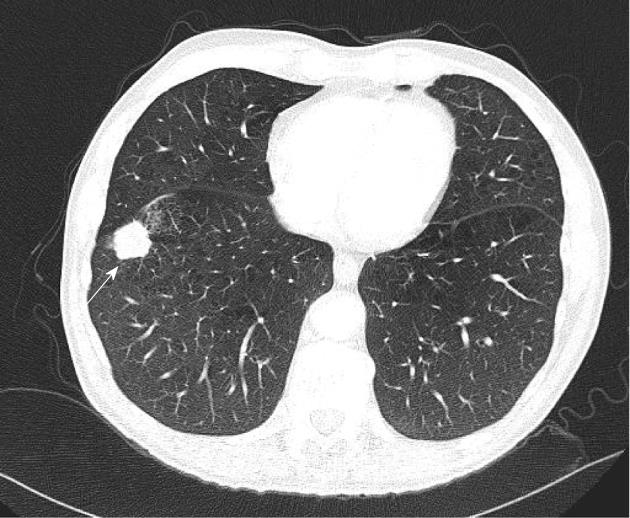
Lympahngitis carcinomatosis (Figure 8) is believed to be associated with worse prognosis in lung cancer patients. However, there is no evidence to support this. The new TNM classification does not specifically take account of lymphangitis.
The process by which TNM classification in lung cancer evolves has been changed irrevocably, and the IASLC has secured a central role in future revisions for the whole of the thoracic oncology community. The IASLC proposes to improve on the above described limitations, especially those relating to PET-CT scanning, in time for the 8th edition of TNM classification. A prospective data set has been agreed, funding has been secured for the 7-year cycle leading up to the 8th edition. A web-based data collection system is being developed and tested to make data submission easier for those who collaborate in this next phase. Data collection has been expanded to incorporate neuro-endocrine tumours and mesothelioma[23].
Peer reviewers: James Chow, PhD, Radiation Physicist, Radiation Medicine Program, Princess Margaret Hospital, 610 University Avenue, Toronto, ON M5G 2M9, Canada; Francesco Lassandro, MD, Department of Radiology, Monaldi Hospital, Via Leonardo Bianchi, 80129 Napoli, Italy
S- Editor Cheng JX L- Editor A E- Editor Xiong L
| 1. | Available from: http://www.ons.gov.uk/ons/rel/vsob1/mortality-statistics--deaths-registered-in-england-and-wales--series-dr-/2009/index.html. [Cited in This Article: ] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [Cited in This Article: ] |
| 3. | Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2000: cancer incidence, mortality and prevalence worldwide. IARC CancerBase No. 5 [CD-ROM]. Version 1.1. Lyon: IARC Press 2001; . [Cited in This Article: ] |
| 4. | Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009;15:4-9. [PubMed] [Cited in This Article: ] |
| 5. | Sobin LH, Wittekind CH. TNM classification of malignant tumours. 6th ed. Hoboken, NJ: John Wiley&Sons 2002; . [Cited in This Article: ] |
| 6. | Denoix PF. Tumor, Node and Metastasis (TNM). Bull Inst. Nat Hyg (Paris). 1944;1:69. [Cited in This Article: ] |
| 7. | Denoix PF. Tumor, Node and Metastasis (TNM). Bull Inst. Nat Hyg (Paris). 1944;2:82. [Cited in This Article: ] |
| 8. | Denoix PF. Tumor, Node and Metastasis (TNM). Bull Inst. Nat Hyg (Paris). 1950;5:81. [Cited in This Article: ] |
| 9. | Denoix PF. Tumor, Node and Metastasis (TNM). Bull Inst. Nat Hyg (Paris). 1952;7:743. [Cited in This Article: ] |
| 10. | Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L, International Association for the Study of Lung Cancer International Staging Committee, Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706-714. [PubMed] [Cited in This Article: ] |
| 11. | Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710-1717. [PubMed] [Cited in This Article: ] |
| 12. | Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z, Goldstraw P, International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol. 2007;2:1067-1077. [PubMed] [Cited in This Article: ] |
| 13. | López-Encuentra A, García-Luján R, Rivas JJ, Rodríguez-Rodríguez J, Torres-Lanza J, Varela-Simo G. Comparison between clinical and pathologic staging in 2,994 cases of lung cancer. Ann Thorac Surg. 2005;79:974-979; discussion 979. [PubMed] [Cited in This Article: ] |
| 14. | Groome PA, Bolejack V, Crowley JJ, Kennedy C, Krasnik M, Sobin LH, Goldstraw P, IASLC International Staging Committee, Cancer Research and Biostatistics; Observers to the Committee, Participating Institutions. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694-705. [PubMed] [Cited in This Article: ] |
| 15. | Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718-1723. [PubMed] [Cited in This Article: ] |
| 16. | Toloza EM, Harpole L, Detterbeck F, McCrory DC. Invasive staging of non-small cell lung cancer: a review of the current evidence. Chest. 2003;123:157S-166S. [PubMed] [Cited in This Article: ] |
| 17. | Luke WP, Pearson FG, Todd TR, Patterson GA, Cooper JD. Prospective evaluation of mediastinoscopy for assessment of carcinoma of the lung. J Thorac Cardiovasc Surg. 1986;91:53-56. [PubMed] [Cited in This Article: ] |
| 18. | Coughlin M, Deslauriers J, Beaulieu M, Fournier B, Piraux M, Rouleau J, Tardif A. Role of mediastinoscopy in pretreatment staging of patients with primary lung cancer. Ann Thorac Surg. 1985;40:556-560. [PubMed] [Cited in This Article: ] |
| 19. | Ømark Petersen H, Eckardt J, Hakami A, Olsen KE, Jørgensen OD. The value of mediastinal staging with endobronchial ultrasound-guided transbronchial needle aspiration in patients with lung cancer. Eur J Cardiothorac Surg. 2009;36:465-468. [PubMed] [Cited in This Article: ] |
| 20. | Rintoul RC, Skwarski KM, Murchison JT, Wallace WA, Walker WS, Penman ID. Endobronchial and endoscopic ultrasound-guided real-time fine-needle aspiration for mediastinal staging. Eur Respir J. 2005;25:416-421. [PubMed] [Cited in This Article: ] |
| 21. | Wallace MB, Pascual JM, Raimondo M, Woodward TA, McComb BL, Crook JE, Johnson MM, Al-Haddad MA, Gross SA, Pungpapong S. Minimally invasive endoscopic staging of suspected lung cancer. JAMA. 2008;299:540-546. [PubMed] [Cited in This Article: ] |
| 22. | Postmus PE, Brambilla E, Chansky K, Crowley J, Goldstraw P, Patz EF, Yokomise H. The IASLC Lung Cancer Staging Project: proposals for revision of the M descriptors in the forthcoming (seventh) edition of the TNM classification of lung cancer. J Thorac Oncol. 2007;2:686-693. [PubMed] [Cited in This Article: ] |
| 23. | Goldstraw P. The 7th Edition of TNM in Lung Cancer: what now? J Thorac Oncol. 2009;4:671-673. [PubMed] [Cited in This Article: ] |