Published online Sep 28, 2019. doi: 10.4329/wjr.v11.i9.116
Peer-review started: March 4, 2019
First decision: August 2, 2019
Revised: August 10, 2019
Accepted: August 21, 2019
Article in press: Auguet 21, 2019
Published online: September 28, 2019
Primary appendiceal cancers are rare, and they generally present with liver and/or peritoneal metastases. Currently there are no guidelines to treat metastatic appendiceal cancer, and hence they are treated as metastatic colorectal cancer. Combining Yttrium 90 (Y-90) radioembolization (RE) with systemic chemotherapy early in the treatment of right sided colon cancers has been shown to improve survival. Based on this data, a combination of systemic chemotherapy and Y-90 RE was used to treat a case of metastatic appendiceal cancer.
A 76-year-old male presented to the emergency room with progressive right lower quadrant pain. A Computed Tomography of the abdomen and pelvis was performed which showed acute appendicitis and contained perforation. Urgent laparoscopic appendectomy was then followed by histological analysis, which was significant for appendiceal adenocarcinoma. After complete workup he underwent right hemicolectomy and lymph node dissection. He received adjuvant chemotherapy as the local lymph nodes were positive. Follow-up imaging was significant for liver metastasis. Due to rapid growth of the liver lesions and new peritoneal nodules, the patient was treated with a combination of Y-90 RE and folinic acid, fluorouracil, and irinotecan with bevacizumab and not microwave ablation as previously planned. Follow up imaging demonstrated complete response of the liver lesions. At 12-mo follow-up, the patient continued to enjoy good quality of life with no recurrent disease.
Utilization of Y-90 RE concomitantly with systemic chemotherapy early in the treatment of appendiceal cancer may provide improved control of this otherwise aggressive cancer.
Core tip: Primary appendiceal cancers are rare and there are no current guidelines for treatment of metastatic appendiceal cancer. We describe an unusual case of appendiceal cancer metastatic to the liver and peritoneum that was successfully treated with a combination of systemic chemotherapy and Yttrium 90 radioembolization. This complete and sustained response may support a role for combination therapy in the more aggressive right sided colon cancers.
- Citation: Bhat AP, Schuchardt PA, Bhat R, Davis RM, Singh S. Metastatic appendiceal cancer treated with Yttrium 90 radioembolization and systemic chemotherapy: A case report. World J Radiol 2019; 11(9): 116-125
- URL: https://www.wjgnet.com/1949-8470/full/v11/i9/116.htm
- DOI: https://dx.doi.org/10.4329/wjr.v11.i9.116
Primary cancers of the appendix are rare and often aggressive. There are no evidence based guidelines to deal with patients who present with aggressive metastatic appendiceal cancers. Surgical cytoreduction and hyperthermic intraoperative chemotherapy (HIPEC) have shown survival benefit in some patients[1]. However, it is a rather extensive and major operation and not suitable for all patients. In patients who are not suitable candidates, there are very limited options and hence, they have a dismal prognosis. In the past decade, the role of Yttrium 90 (Y-90) radioembolization (RE) in chemorefractory metastatic colorectal cancer (CRC) has been extensively studied and has produced promising results[2-4]. Due to its proven synergistic role with systemic chemotherapy, a combination of Y-90 RE and chemotherapy was used to successfully treat a patient with metastatic appendiceal cancer.
A 76-year-old male initially presented to the emergency room with progressive pain in the right lower quadrant.
The pain was localized to the right lower quadrant without any radiation. The pain was associated with anorexia, without nausea or vomiting. Low grade fever was present.
Significant for hypertension controlled with medications and benign prostatic hyperplasia.
No history of alcohol, or drug abuse.
Vital signs: Heart rate 108/min, respiratory rate 23/min, BP 130/82 mmHg. Temperature: 37.1 °C.
Cardiovascular: Tachycardia with normal heart sounds.
Respiratory: Normal breath sounds.
Abdomen: Moderate tenderness in the right lower quadrant. No abdominal distension. Normal bowel sounds. Neurological: Alert, oriented, without neurological deficits.
The blood work showed leukocytosis, with white blood cell: 12 × 109 L. The rest of the labs were normal.
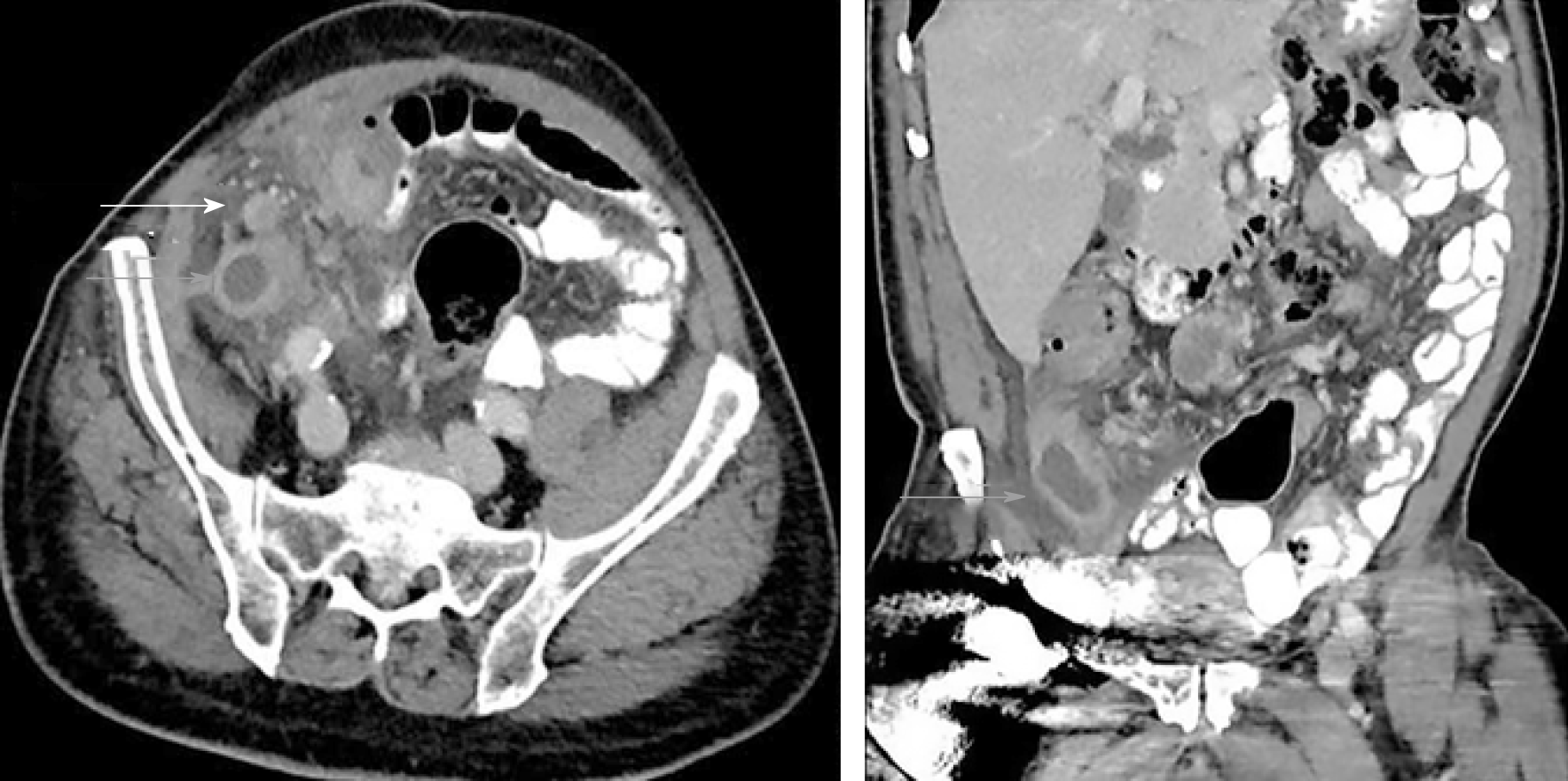
Contrast-enhanced computed tomography (CT) of the abdomen and pelvis demonstrated a markedly dilated appendix with surrounding inflammatory changes (Figure 1) and regional lymphadenopathy. This was originally interpreted as acute appendicitis with contained perforation.
The patient then underwent urgent laparoscopic appendectomy and drainage of the intra-abdominal abscess. Pathology of the surgical specimen revealed a moderately differentiated appendiceal adenocarcinoma, measuring 1.7 cm, with tumor invading the mesoappendix. Extensive transmural inflammatory changes were noted, though the base of the appendix was not involved. The patient underwent colonoscopy to excluded synchronous lesions. As the colonoscopy was negative for additional lesions, the patient subsequently underwent laparoscopic right hemicolectomy with primary anastomosis and lymph node dissection. During the operation the rest of the abdomen was thoroughly inspected for metastatic disease. After performing laparoscopic right hemicolectomy with primary anastomosis and lymph node dissection, histopathology demonstrated 6/18 lymph nodes positive for metastatic adenocarcinoma with extra-nodal extension present in the largest lymph node. The distal ileum and cecum were free of malignancy. The staging was completed by obtaining a CT of the chest (as it had not been obtained in the initial presentation), and it showed no evidence of metastatic disease.
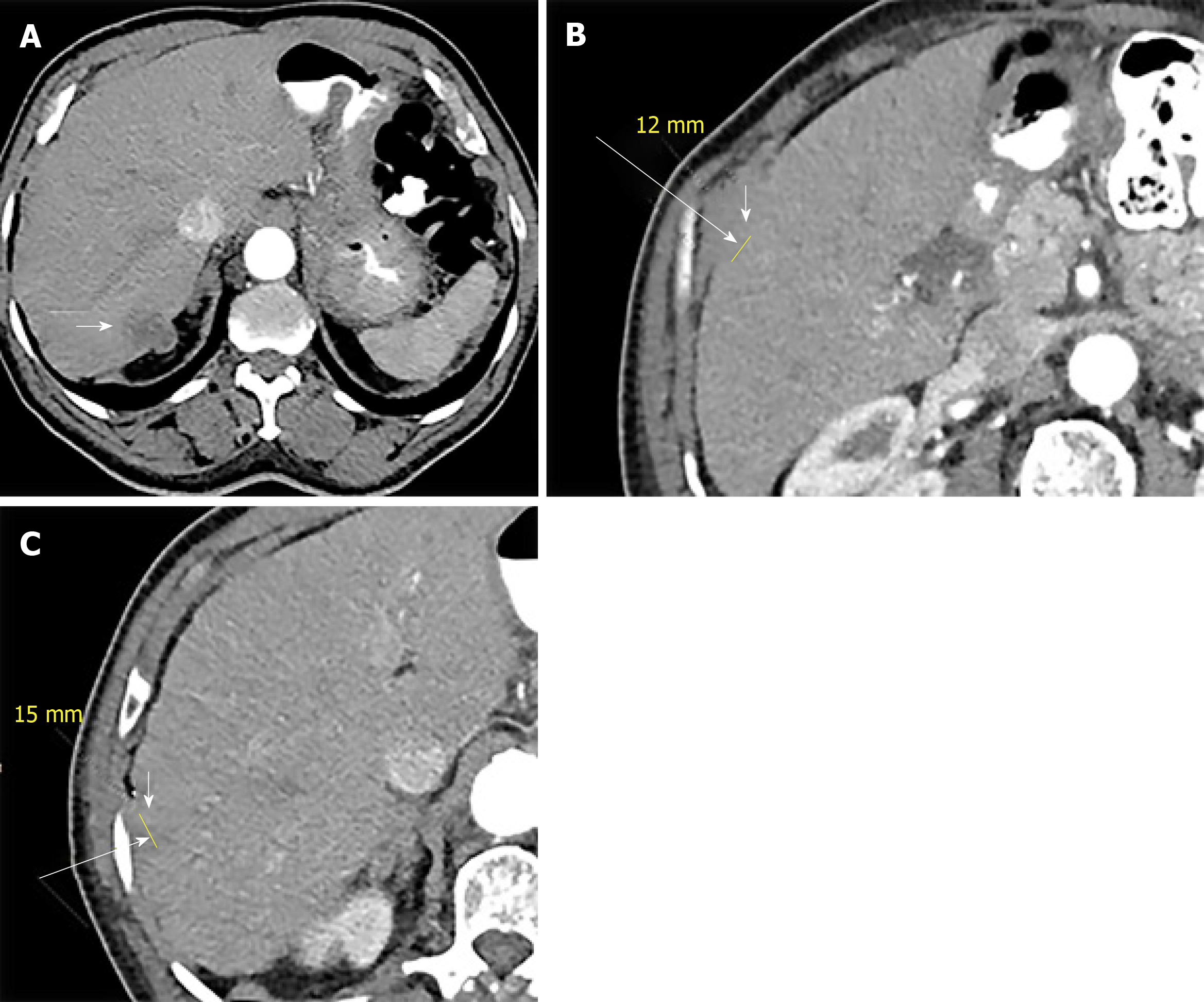
The patient then received 12 cycles of adjuvant folinic acid, fluorouracil, and oxaliplatin (FOLFOX) chemotherapy for a duration of 7 mo from the initial diagnosis. Follow-up CT 10 mo after the last cycle of chemotherapy demonstrated three hypodense hypovascular lesions in the right lobe of the liver (two in segment 7 and one in segment 2) (Figure 2) with concurrent rise in serum carcinoembryonic antigen level. Ultrasound guided biopsy of the liver lesions revealed metastatic adenocarcinoma, consistent with an appendiceal primary.
Final diagnosis was stage IIIC (T3, N2, Mx) moderately differentiated appendiceal adenocarcinoma.
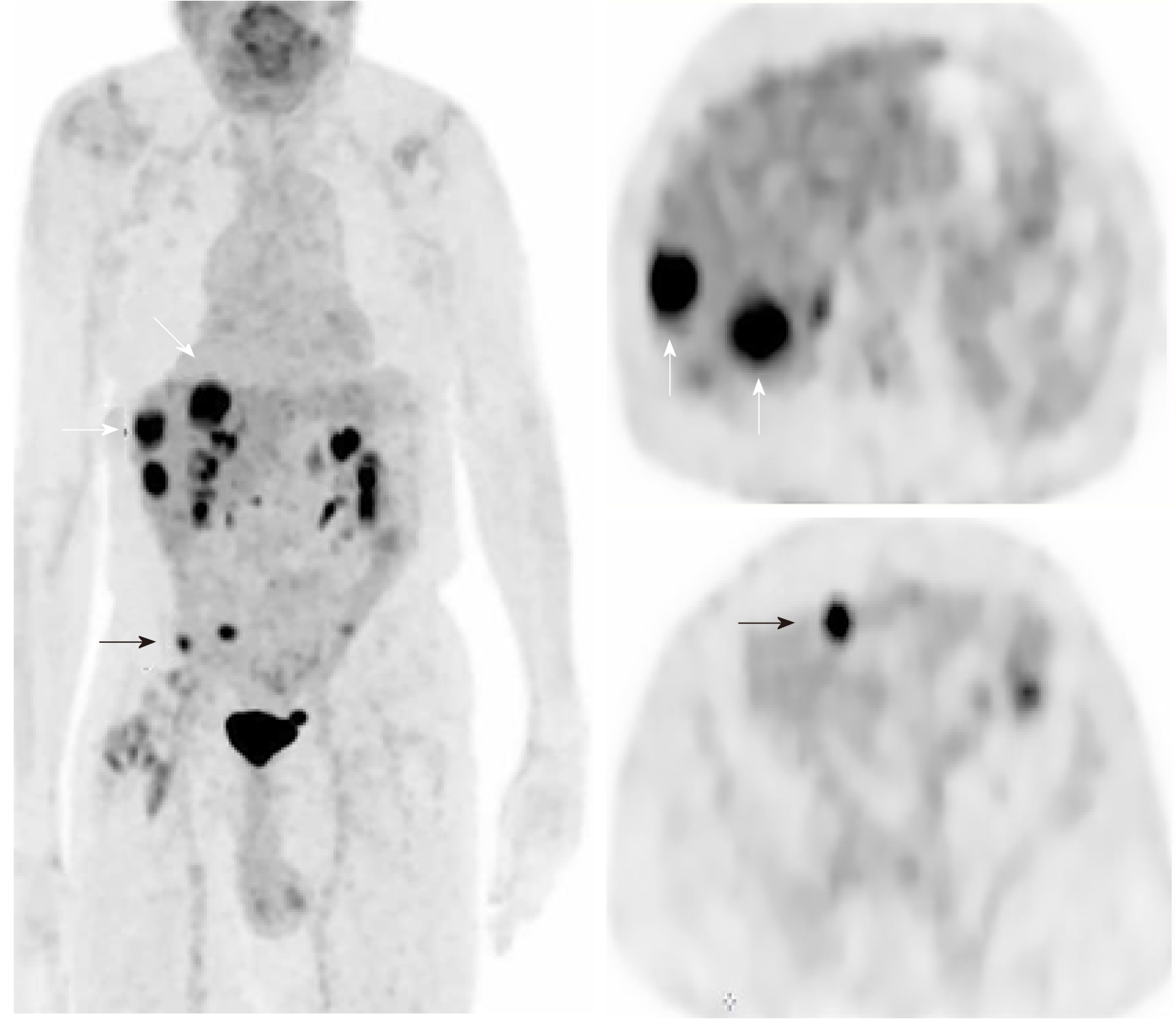
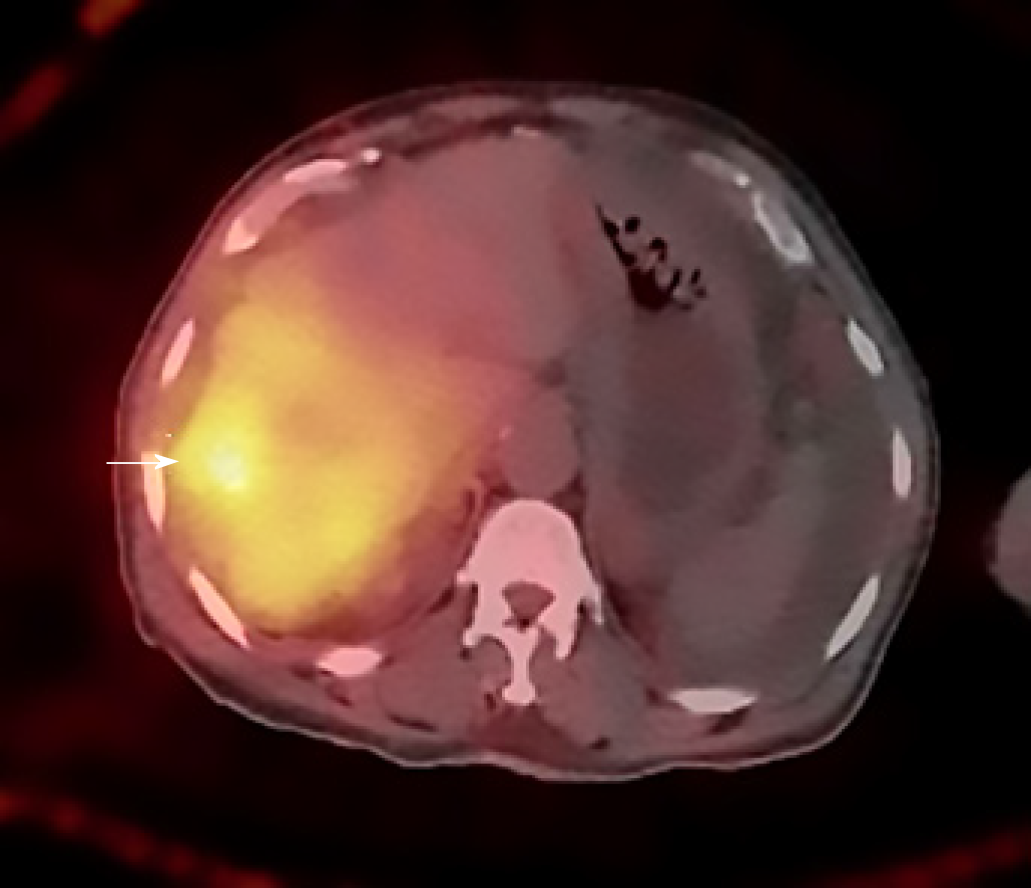
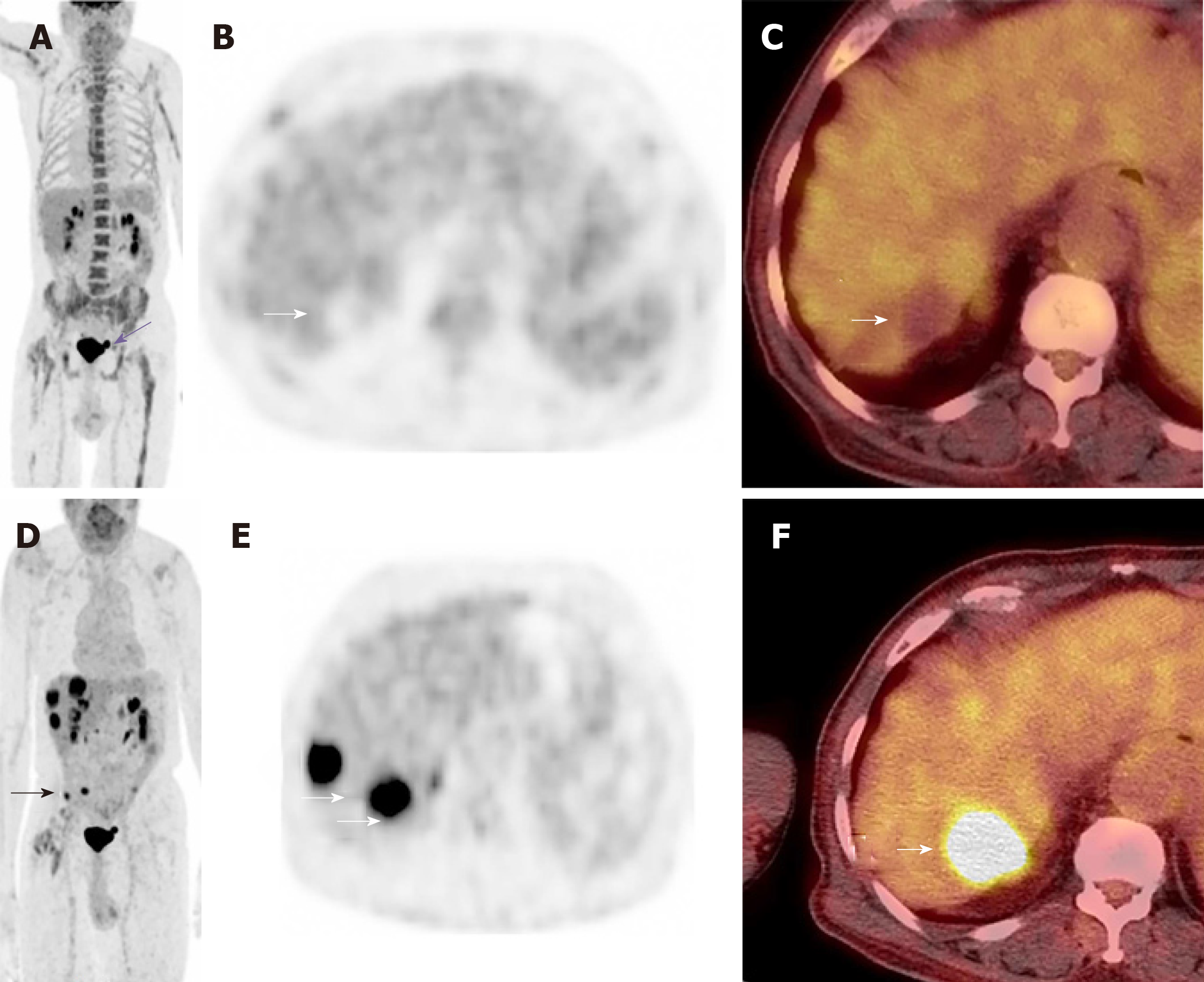
Due to the complexity of the case. It was presented at the multidisciplinary tumor board and a decision was made to proceed with microwave ablation of the liver lesions. Fluorodeoxyglucose (FDG) positron emission tomography (PET) CT obtained prior to microwave ablation demonstrated an increase in the size of the liver lesions (FDG avid) and new sub-centimeter FDG avid lesions in the peritoneum, suggestive of metastatic implants (Figure 3). Two of the three lesions, now measured more than 3 cm, prompting us to hold off on the microwave ablation. The patient was again presented to the multidisciplinary tumor board and was not considered a surgical candidate for hepatic metastasectomy or HIPEC due to general deconditioning, including severe weight loss. In light of the progressive hepatic and limited extrahepatic disease burden, the patient was offered selective internal radiation treatment or RE with Y90 and initiation of folinic acid, fluorouracil, and irinotecan (FOLFIRI) with bevacizumab, as a palliative measure. FOLFIRI was initiated prior to the Y-90 RE procedure; however, the bevaxizumab was not started until after the RE procedure.
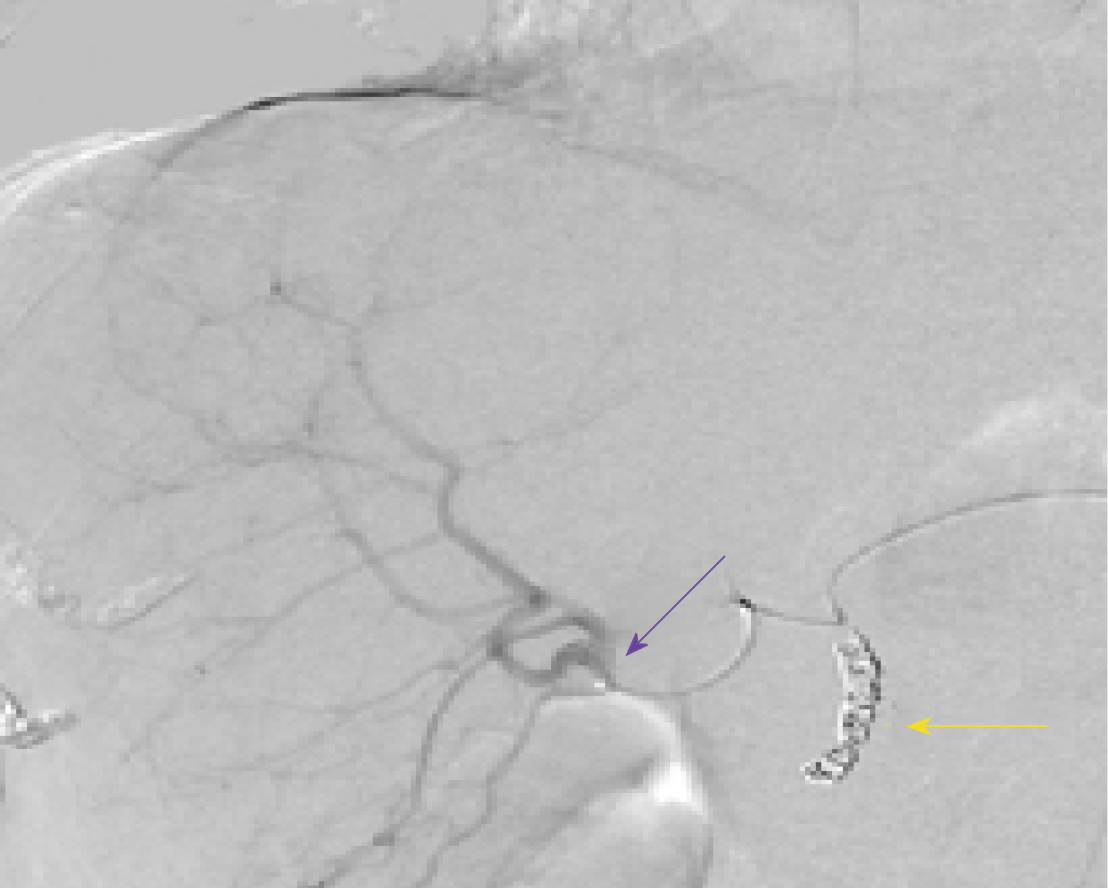
A mapping angiogram was performed one week prior to the Y-90 RE treatment to evaluate the vascular anatomy and estimate the lung shunt fraction (LSF). As a part of the mapping angiogram, the gastroduodenal artery was embolized using 4 and 5 mm 0.018” Azur® CX coils (Terumo Interventional Systems). Based on the planar images obtained after injection of 99mtechnetium-labeled macroaggregated albumin (99mTcMAA) into the right hepatic artery, the shunt fraction toward the lung was calculated at approximately 2.5%.
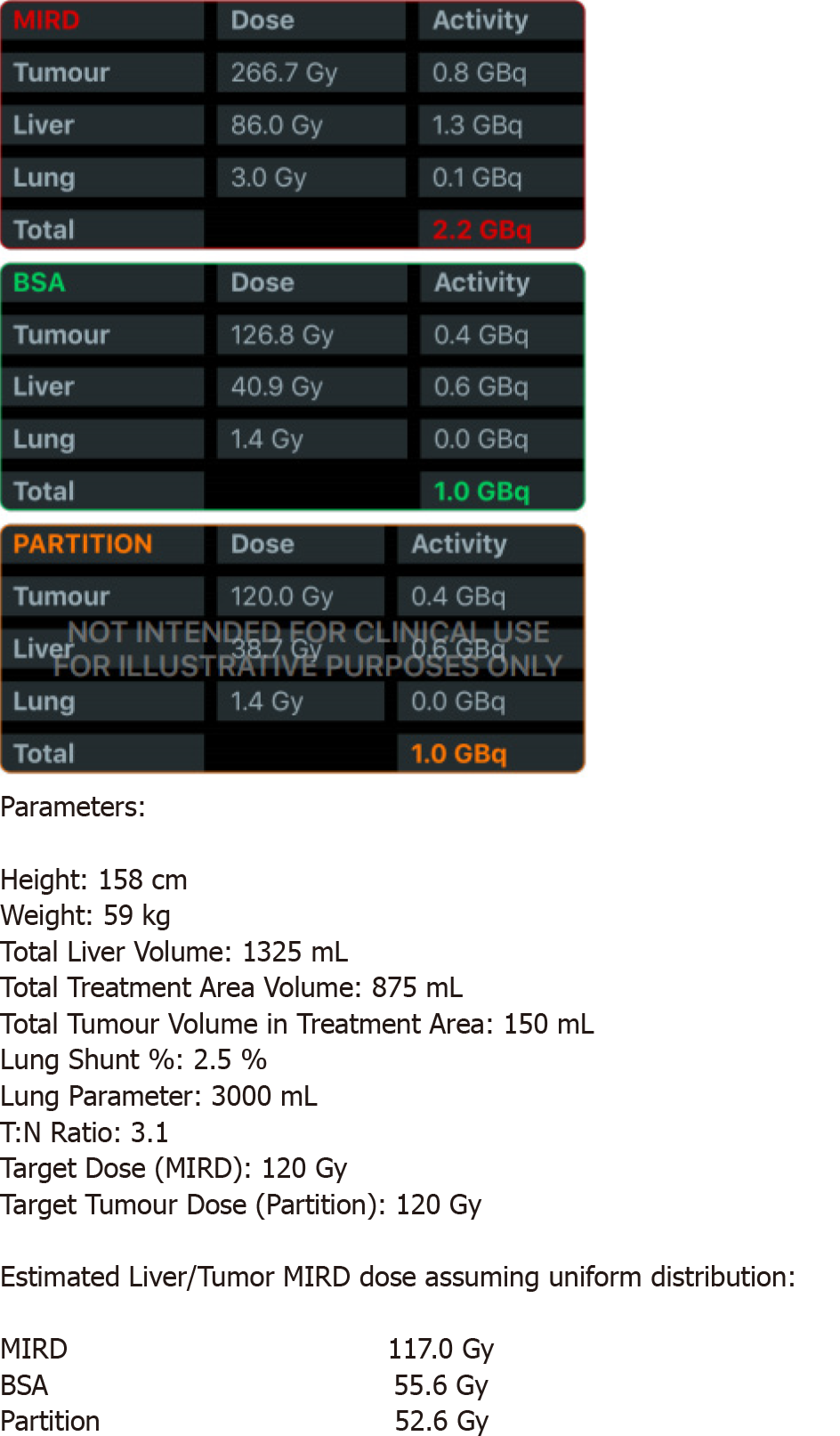
Calculations of liver and tumor volumes were performed on a 3D segmentation application (TeraRecon Inc Foster City, CA, United States). The whole liver volume was 1325 mL. The right lobe measured 875 mL, the tumor volume was 150 mL, and the tumor to normal liver ratio (T:N) based off the 99mTcMAA injection during the mapping angiogram was 3:1. The prescribed activity was calculated using the DAVYR application (Dose and activity visualizer for Y-90 RE) (Figure 4) and the SIR sphere microsphere activity calculator (SMAC) chart provided by the manufacturer and a 1.0 Gigabecquerel (Gbq) activity of Y90 was prescribed for treatment of the right lobe metastatic lesions. Based on the body surface area (BSA) method recommended by the manufacturer, the dose to the tumor was estimated as 126 Gray (Gy) and dose to the healthy liver was estimated at 40.9 Gray (Gy) (Figure 4). The prescribed activity was successfully administered by positioning a Progreat 2.8 F 130 cm catheter (Terumo Interventional Systems) in the right hepatic artery distal to the origin of the cystic artery (Figure 5). Post procedure Bremsstrahlung planar and single photon emission computed tomography (SPECT) demonstrated appropriate distribution of Y90 spheres in the hepatic lobe without evidence of non-target embolization (Figure 6).
Post RE FDG PET/CT performed two months after treatment (Figure 7) showed complete response, with no residual FDG-avid lesions in the liver or peritoneum. No new lesions were identified. At this point, based on the good response to treatment, the oncologist switched him to a combination of oral capecitabine and bevacizumab. The patient has been followed for 12 mo after the Y-90 treatment and continues to demonstrate good quality of life with no recurrent disease. There were no significant changes to his liver functions after treatment, except slight elevation of his alkaline phosphatase. The only toxicity was hyperpigmentation of the fingers and oral ulcers (hand foot mouth disease) which started 10 mo into the treatment and resolved with reduction of the capecitabine dose.
The incidence of appendiceal cancer is 1.2 per 100000 people per year in the United States. Potentially due in part to this rarity, there are no accepted American Joint Commission on Cancer staging system or National Comprehensive Cancer Network evidence-based guidelines recommended for appendiceal cancer[5]. Colon cancer workup, staging, and treatment is commonly applied to appendiceal carcinoma.
Appendiceal cancers can either present with or progress to peritoneal dissemination and the optimal therapy is complete surgical cytoreduction in combination with HIPEC[6]. For patients not eligible for complete cytoreduction and HIPEC, as in this case, systemic chemotherapy with regimens utilized for metastatic CRC are generally considered the mainstay therapy. We embarked on a literature review to find potential treatment options for our patient.
A systematic review of the literature was performed using Pubmed for articles from inception to January 2019 using the key words “Y90”, “metastatic Appendix cancer” and “systemic chemotherapy”. We did not find any studies in which Y-90 RE was used to treat liver metastasis form appendix cancer. However we found several studies using Y 90 in the salvage setting following failure of chemotherapy with improved overall survival numbers[2-4]. Data was extracted by two extractors.
Interventional oncology treatments for liver dominant CRC metastasis include, RE, drug eluting beads irinotecan (DEBIRI) transarterial chemoemboization (TACE) and thermal ablation.
RE with Y-90 microspheres is based on the same principle as that of TACE, in that the hepatic artery provides the primary vascular supply to the tumor, while the portal vein provides the majority of blood flow to normal hepatic parenchyma[7]. Furthermore, the microvascular density of hepatic tumors is much higher than the hepatic parenchyma[8], resulting in a proportionately higher exposure to microspheres. CRC cells are highly radiosensitive and even chemo-refractive lesions do not generally demonstrate any cross-resistance to radiation. Additionally, radiation works synergistically when used with radiation-sensitizing chemotherapeutic drugs.
The two commercial manufacturers of Y-90 microspheres are TheraSpheres (BTG International, ON, Canada) and SIR-spheres (Sirtex Medical Inc., Sydney, Australia). These microspheres, that have been specifically developed to deliver Y-90, measure 20-60 μm in size. Y-90 primarily emits beta radiation, with a half-life of 64.1 h and an average energy of 0.94 MeV. The beta particles have a range of 1.1 cm (average 2.5 mm in vivo) from the source, with 94% of the dose being deposited within the first 11 d of administration[9]. Hence, RE works from “inside out” and inflicts minimal damage to the normal liver. This is in contrast to external beam radiation which exposes the normal liver parenchyma to excessive amounts of radiation, in the process of killing multifocal tumors, thus increasing collateral damage. One of the most critical components of the RE procedure is the dosimetry. There is one activity calculation method for Y-90 glass microspheres[10], however three alternatives exist for resin microspheres[11-13].
Both liver and tumor volumes, calculated based on CT or magnetic resonance imaging are used to determine resin microsphere activity. The empiric model recommends administration of 2.0 GBq for < 25% involvement, 2.5 GBq for 25%-50% involvement, and 3.0 GBq for > 50% involvement[13]. This method has low safety margins[13,14] and is generally not recommended. The empiric method adjusted for patient BSA has been more widely utilized to calculate the activity needed to treat liver tumors[12]. This model assumes that the patient’s BSA is proportional to liver volume, thus leading to a method of dose adjustment. Activity in GBq = (BSA - 0.2) + [VTumor / (VTumor + VLiver)]. Where VTumor is the tumor volume and VLiver is liver volume[12].
Lastly, the partition model is the only calculation method predicated on Medical Internal Radiation Dose principles (MIRD)[15-17]. The partition model identifies the lungs, tumor, and uninvolved liver parenchyma as three distinct vascular compartments that can be partitioned during RE[15] and the dose to the individual components can be estimated for optimization and safety of the procedure. A slightly simplified MIRD based equation calculates the dose to the liver as follows: D liver (Gy) = Injected activity (GBq) × 50 × fractional uptake liver × [m liver (kg)] – 1.
With fractional uptake (FU) of the liver defined as: FU liver = (1-LSF) [m liver/TLR × m tumor+ m liver], where m liver is the liver mass, TLR is tumor to liver ratio and LSF is LSF.
This model usually works well with discrete tumor that can be separated from the surrounding parenchyma (generally hepatocellular cancer). The BSA activity planning is suited for small lesions with diffuse or infiltrative margins, which cannot be separated from the uninvolved liver parenchyma (generally metastasis).
A tumoricidal dose for SIR spheres is considered to be 120 Gy, so little benefit is gained when exceeding this dose, while parenchymal damage continues to increase with larger radiation doses[18,19]. The dose for uninvolved, normal parenchyma should preferably be at 50 Gy and should never exceed 70 Gy.
For TheraSphere® noncompartmental MIRD macrodosimetry proposed for by Salem et al[11] is used. It uses the following equation for computation of the activity and assumes Y 90 distributes uniformly within the liver and decays completely in situ. Activity required (GBq) = [desired dose (Gy)] × [target liver mass (kg)]/50.
Of note, Therasphere dosimetry is independent of the tumor volume and depends solely on the infused tissue mass. For Therasphere, the recommended mean absorbed is between 80 and 150 Gy per lobe[11]. The recommended tumoricidal doses is 120 Gy for HCC (due to the underlying cirrhosis) and 150 Gy for metastases[11]. Maximum exposure to the lungs should be less than 30 Gy per session and 50 Gy cumulatively[17]. Both manufacturers recommend prescribed activity reductions for patients with lung shunts higher than 10%[10,13].
Several investigators have studied the application of Y-90 RE in the salvage setting for liver dominant metastatic CRC[2,3]. Van Hazel et al[4] reported results of combined Y-90 RE with systemic irinotecan therapy in patients who failed initial 5-FU chemotherapy. In a group of 25 patients, 11 (48%) had partial response (PR) and 9 (39%) had stable disease. Median survival was 12.2 mo.
DEBIRI TACE has been proposed by some as a possible alternative to RE, however evidence within the literature is currently limited. In our institute we consider it in cases where Y-90 RE is contraindicated or in the setting of failed RE therapy. TACE with DEBIRI involves two separate treatment sessions for each lobe, with each session occurring 4 wk apart[20]. Based on this, patients with bilobar disease, may need four procedures. During each treatment session, as much as one vial of 100- to 300-μm LC Beads (Biocompatibles, Oxford, CT) loaded with 100-mg irinotecan is selectively delivered to treat the hepatic lesions. Furthermore, post-embolization syndrome is more severe after DEBERI as compared to Y-90, with moderate to severe abdominal pain observed in up to 30% of treatment sessions[20,21].
Thermal ablation has been successfully used in treatment of non rescectable CRC liver metastasis, with best outcomes for isolated liver lesions less than 3 cm without systemic spread[22]. Although our patient initially appeared to be a good candidate for thermal ablation, his staging PET showed increased in size of the hepatic lesions and new lesions in the peritoneum, making him a less suitable candidate for liver ablation alone and prompted us to use systemic therapy and more aggressive liver directed therapy in the form of Y-90 RE to the right lobe.
Several studies in the past 2 years have reported that patients have worse survival outcomes with right sided colon cancer, and they may benefit less from standard therapies[23,24]. The subset of patients with right sided tumors in the SIRFLOX, FOXFIRE and SIRFLOX global trials had a better overall survival from addition of RE in combination with first line chemotherapy[25]. Gene expression analyses have identified 4 consensus molecular subtypes (CMSs) which are different with right and left-sided CRCs, with greater proportions of the “microsatellite unstable/immune” CMS1 and the “metabolic” CMS3 subtypes found in right-sided colon cancers[26], which may explain their different behavior and natural history.
To the best of our knowledge, this is the first report of Y-90 RE used with systemic chemotherapy to treat hepatic metastatic disease from appendiceal cancer. The aggressive disease progression demonstrated in this case appears to mirror the aggressiveness one might see from typical right sided colonic malignancies. Most studies using Y90 RE in the salvage setting report PR of liver lesions. The patient in this case report had a complete response of both liver and peritoneal metastases presumably form a combination of Y-90 RE and systemic chemotherapy. The relative contribution of the two individual treatments or any possible synergy between the treatments is unknown, however initiating Y-90 RE early in the treatment may have contributed to the excellent control of what was otherwise a rapidly progressive cancer. More studies are needed to assess the benefit of adding Y-90 RE to chemotherapy treatments early in the course of appendiceal/right sided colon cancers.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bazeed MF, Gavriilidis P S-Editor: Ma RY L-Editor: A E-Editor: Liu MY
| 1. | Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737-3743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1396] [Cited by in F6Publishing: 1415] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 2. | Cosimelli M, Golfieri R, Cagol PP, Carpanese L, Sciuto R, Maini CL, Mancini R, Sperduti I, Pizzi G, Diodoro MG, Perrone M, Giampalma E, Angelelli B, Fiore F, Lastoria S, Bacchetti S, Gasperini D, Geatti O, Izzo F; Italian Society of Locoregional Therapies in Oncology (SITILO). Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103:324-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 3. | Bester L, Meteling B, Pocock N, Pavlakis N, Chua TC, Saxena A, Morris DL. Radioembolization versus standard care of hepatic metastases: comparative retrospective cohort study of survival outcomes and adverse events in salvage patients. J Vasc Interv Radiol. 2012;23:96-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | van Hazel GA, Pavlakis N, Goldstein D, Olver IN, Tapner MJ, Price D, Bower GD, Briggs GM, Rossleigh MA, Taylor DJ, George J. Treatment of fluorouracil-refractory patients with liver metastases from colorectal cancer by using yttrium-90 resin microspheres plus concomitant systemic irinotecan chemotherapy. J Clin Oncol. 2009;27:4089-4095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | McCusker ME, Coté TR, Clegg LX, Sobin LH. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973-1998. Cancer. 2002;94:3307-3312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 367] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 6. | Sugarbaker PH, Zhu BW, Sese GB, Shmookler B. Peritoneal carcinomatosis from appendiceal cancer: results in 69 patients treated by cytoreductive surgery and intraperitoneal chemotherapy. Dis Colon Rectum. 1993;36:323-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 92] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969-977. [PubMed] [Cited in This Article: ] |
| 8. | Lien WM, Ackerman NB. The blood supply of experimental liver metastases. II. A microcirculatory study of the normal and tumor vessels of the liver with the use of perfused silicone rubber. Surgery. 1970;68:334-340. [PubMed] [Cited in This Article: ] |
| 9. | Kalva SP, Thabet A, Wicky S. Recent advances in transarterial therapy of primary and secondary liver malignancies. Radiographics. 2008;28:101-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Package Insert-TheraSphere® Yttrium-90 Glass Microspheres. Available from: http://www.therasphere.com. [Cited in This Article: ] |
| 11. | Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251-1278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 491] [Cited by in F6Publishing: 481] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 12. | Lau WY, Kennedy AS, Kim YH, Lai HK, Lee RC, Leung TW, Liu CS, Salem R, Sangro B, Shuter B, Wang SC. Patient selection and activity planning guide for selective internal radiotherapy with yttrium-90 resin microspheres. Int J Radiat Oncol Biol Phys. 2012;82:401-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, Benson A, Espat J, Bilbao JI, Sharma RA, Thomas JP, Coldwell D. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68:13-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 506] [Cited by in F6Publishing: 498] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 14. | Kennedy AS, McNeillie P, Dezarn WA, Nutting C, Sangro B, Wertman D, Garafalo M, Liu D, Coldwell D, Savin M, Jakobs T, Rose S, Warner R, Carter D, Sapareto S, Nag S, Gulec S, Calkins A, Gates VL, Salem R. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int J Radiat Oncol Biol Phys. 2009;74:1494-1500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 15. | Ho S, Lau WY, Leung TW, Chan M, Ngar YK, Johnson PJ, Li AK. Partition model for estimating radiation doses from yttrium-90 microspheres in treating hepatic tumours. Eur J Nucl Med. 1996;23:947-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 217] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Gulec SA, Mesoloras G, Stabin M. Dosimetric techniques in 90Y-microsphere therapy of liver cancer: The MIRD equations for dose calculations. J Nucl Med. 2006;47:1209-1211. [PubMed] [Cited in This Article: ] |
| 17. | Ho S, Lau WY, Leung TW, Chan M, Johnson PJ, Li AK. Clinical evaluation of the partition model for estimating radiation doses from yttrium-90 microspheres in the treatment of hepatic cancer. Eur J Nucl Med. 1997;24:293-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Lau WY, Leung WT, Ho S, Leung NW, Chan M, Lin J, Metreweli C, Johnson P, Li AK. Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: a phase I and II study. Br J Cancer. 1994;70:994-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 163] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Campbell AM, Bailey IH, Burton MA. Tumour dosimetry in human liver following hepatic yttrium-90 microsphere therapy. Phys Med Biol. 2001;46:487-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Martin RC, Howard J, Tomalty D, Robbins K, Padr R, Bosnjakovic PM, Tatum C. Toxicity of irinotecan-eluting beads in the treatment of hepatic malignancies: results of a multi-institutional registry. Cardiovasc Intervent Radiol. 2010;33:960-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Fiorentini G, Aliberti C, Tilli M, Mulazzani L, Graziano F, Giordani P, Mambrini A, Montagnani F, Alessandroni P, Catalano V, Coschiera P. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer Res. 2012;32:1387-1395. [PubMed] [Cited in This Article: ] |
| 22. | Tanis E, Nordlinger B, Mauer M, Sorbye H, van Coevorden F, Gruenberger T, Schlag PM, Punt CJ, Ledermann J, Ruers TJ. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983. Eur J Cancer. 2014;50:912-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 23. | Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, Passalacqua R, Sgroi G, Barni S. Prognostic Survival Associated With Left-Sided vs Right-Sided Colon Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2017;3:211-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 467] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 24. | Venook AP, Niedzwiecki D, Innocenti F. Impact of Primary (1°) Tumor Location on Overall Survival (OS) and Progression-Free Survival (PFS) in Patients (Pts) with Metastatic Colorectal Cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2016;34. [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 209] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 25. | Wasan HS, Gibbs P, Sharma NK, Taieb J, Heinemann V, Ricke J, Peeters M, Findlay M, Weaver A, Mills J, Wilson C, Adams R, Francis A, Moschandreas J, Virdee PS, Dutton P, Love S, Gebski V, Gray A; FOXFIRE trial investigators; SIRFLOX trial investigators; FOXFIRE-Global trial investigators, van Hazel G, Sharma RA. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017;18:1159-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 213] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 26. | Lee MS, Menter DG, Kopetz S. Right Versus Left Colon Cancer Biology: Integrating the Consensus Molecular Subtypes. J Natl Compr Canc Netw. 2017;15:411-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 213] [Article Influence: 30.4] [Reference Citation Analysis (0)] |