Published online Jul 28, 2018. doi: 10.4329/wjr.v10.i7.65
Peer-review started: March 13, 2018
First decision: April 23, 2018
Revised: April 29, 2018
Accepted: May 23, 2018
Article in press: May 23, 2018
Published online: July 28, 2018
To evaluate radiological imaging findings of patients who had been found to have pineal cyst (PC) in brain magnetic resonance imaging (MRI).
A total of 9546 patients who had brain MRI examination in March 2010-January 2018 period were studied. Fifty-six patients (44 female and 12 male) found to have PC were evaluated. Eighteen of the patients had had follow-up examinations of 2-94 mo (mean 30.50 ± 28.83). PC dimensions and volume, radiological imaging features (signal intensities, contours, internal septation-loculation and contrast-enhancement features) and natural history in cases who had been followed-up were evaluated by two radiologists.
Of 9546 patients, 5555 were female (58.2%) and 3991 male (41.8%). Age range was 1-99 (mean 43.18 ± 20.94). PC frequency was calculated to be 0.58%. Forty-four of the 56 patients (78.57%) with PC were female and 12 male (21.43%), and their age range was 5-61 (mean 31.26 ± 12.73). Thirty-five of the PCs were typical (62.50%) and 21 (37.50%) were atypical. No significant difference was found between initial and final imaging sizes of PCs which were monitored by follow-up examinations (P > 0.05).
PCs are cysts which do not show clear size and natural changes and are more frequently observed in females and in adult ages. Most of them are isointense with cerebrospinal fluid on T1 and T2A weighted images, hyperintense compared to cerebrospinal fluid on fluid-attenuated inversion recovery; sequence and smoothly contoured. Their typical forms have peripheral rim and multilocular ones may have septal contrast-enhancement.
Core tip: In this retrospective study, brain magnetic resonance images of 9546 patients were studied to detect incidence, size, contour, septation and contrast-enhancement features of pineal cysts (PCs). In addition, size and natural changes in follow-up examinations were also investigated. Classification of PCs based on routine magnetic resonance imaging examinations could change when examination was performed using high resolution sequences due to detection of septations within them. The present study revealed that no significant size or natural change was observed in follow-up examinations of PCs.
- Citation: Gokce E, Beyhan M. Evaluation of pineal cysts with magnetic resonance imaging. World J Radiol 2018; 10(7): 65-77
- URL: https://www.wjgnet.com/1949-8470/full/v10/i7/65.htm
- DOI: https://dx.doi.org/10.4329/wjr.v10.i7.65
Pineal cysts (PC) are frequently asymptomatic, small sized, unilocular, benign pineal lesions which do not show size change[1]. They are generally made of three layers: fibrocollagen layer at the outside, pineal parenchymal layer which may have calcium deposits at the middle and hypocellular glial tissue layer which may have hemosiderin inside[1,2]. PCs may develop as secondary to focal degeneration of pineal gland or distension of pineal diverticulum remnant[3]. Increasing resolution and more frequent use of cranial imaging techniques along with use of contrast agent have led to an increase in incidental diagnosis of PCs[1]. Unilocular, smooth edged, round or ovoid shaped cysts which have homogenous interior signal feature and rim-shaped contrast-enhancement with less than 2 mm wall thickness on MRI are referred as typical PC[4,5]. Although there is an excess amount of reports in literature about typical PC stating that they are indeed incidental findings of radiological imaging and that they are stable over time, more complex and atypical pineal cystic lesions constitute a problem for radiologists[4-7]. In addition, it has been reported that pineal neoplasia could have imaging features similar to those of PC[5,8]. Some studies mentioned some cystic lesions in pineal region with complex imaging features, which are probably normal variants[5,9,10]. In the present study, radiological imaging findings of PCs determined in patients who had undergone brain magnetic resonance imaging (MRI) due to various reasons in a past eight-year period were evaluated.
After the approval of local ethic committee (No. 18-KAEK-015), 9546 patients who had brain MRI examinations in March 2010-January 2018 period in Radiology Department of Gaziosmanpaşa University Medical School were studied retrospectively. A total of 11695 consecutive brain MRI examinations were scanned. Scans were reviewed by experienced neuroradiologists (Gokce E and Beyhan M) to confirm radiologic diagnoses of PC. Patients with solid or semisolid masses in pineal gland location or other cystic lesions (arachnoid cyst, etc.) developing from neighboring structures and patients with operation history were excluded. There were no exclusion criteria related to pineal cyst size. Fifty-six patients (44 female and 12 male) with PCs were evaluated. Among the reasons for MRI requests were headache, cerebrovascular disease, epilepsy, vertigo, hand tremors and encephalitis. MRI examinations were carried out using an 8-channel 1.5 T MRI machine (GE Signa Excite HD; GE Healthcare, Milwaukee, WI, United States, 2005) until 2017, and a 16-channel 1.5 T MRI machine (GE Signa Explorer SV 25; GE Healthcare, Milwaukee, WI, United States, 2016) after 2017. MRI examination parameters before and after 2017 are given in Tables 1 and 2. Eighteen patients had 2-94 mo (mean 30.50 ± 28.83) of follow-ups. Contrast-enhanced MRI was performed in 21 of 56 patients.
| Parameter | T1 (SPGR) | T2 (SE) | FLAIR | DWI | Ax SPGR + C | Sag T1 SE + C | Ax T1 FS + C |
| TR (repetition time) (ms) | 6.36 | 5700 | 8002 | 8000 | 6.36 | 440 | 620 |
| TE (echo time) (ms) | 2.23 | 134 | 80.9 | 104 | 2.23 | 12 | 20 |
| Field of view (mm) | 260 | 250 | 220 | 250 | 260 | 250 | 250 |
| Slice thickness (mm) | 4 | 5 | 5.5 | 5 | 4 | 5.5 | 5.5 |
| Slice gap (mm) | 2 | 6.5 | 6.5 | 6.5 | 2 | 6.5 | 7 |
| NEX (No. of excitations) | 1 | 1.5 | 1 | 2 | 1 | 1 | 1 |
| TI (time inversion) ms | 450 | 2000 | 450 | ||||
| Machine | 1.5 T GE Signa Excite HD (8 channel head coil) | ||||||
| Parameter | T1 (BRAVO) | T2 (SE) | FLAIR FS | DWI | T1 (BRAVO) +C | Cor T1 (SE) + C | Ax T1 FS + C |
| TR (repetition time) (ms) | 9.25 | 6282 | 10000 | 6992 | 9.25 | 3509 | 2744 |
| TE (echo time) (ms) | 3.58 | 124 | 91.7 | 84.4 | 3.58 | 17.8 | 17.5 |
| Field of view (mm) | 256 | 220 | 240 | 270 | 256 | 240 | 256 |
| Slice thickness (mm) | 1 | 5.5 | 5.5 | 5 | 1 | 5.5 | 5.5 |
| Slice gap (mm) | 0 | 7 | 7 | 5.5 | 0 | 7 | 7 |
| NEX (No. of excitations) | 1 | 2 | 1 | 2 | 1 | 1 | 1 |
| TI (time inversion) (ms) | 420 | 2688 | 420 | 1146 | 922 | ||
| Machine | 1.5 T GE Signa Explorer SV 25 (16 channel neurovascular-head coil) | ||||||
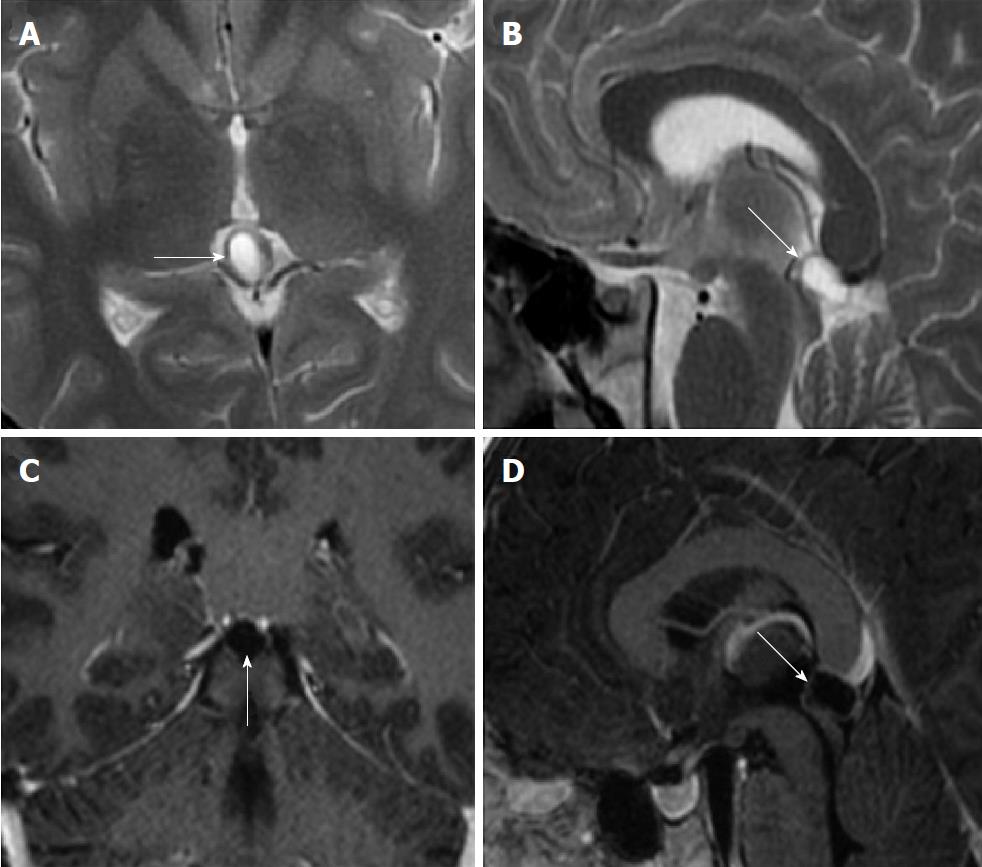
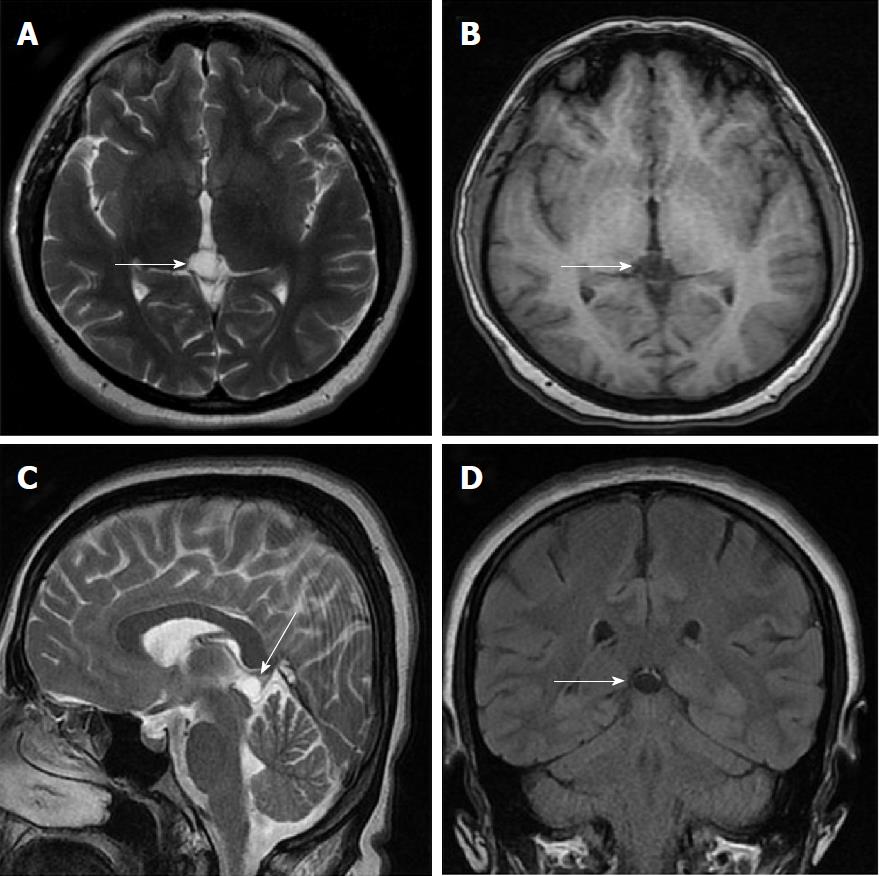
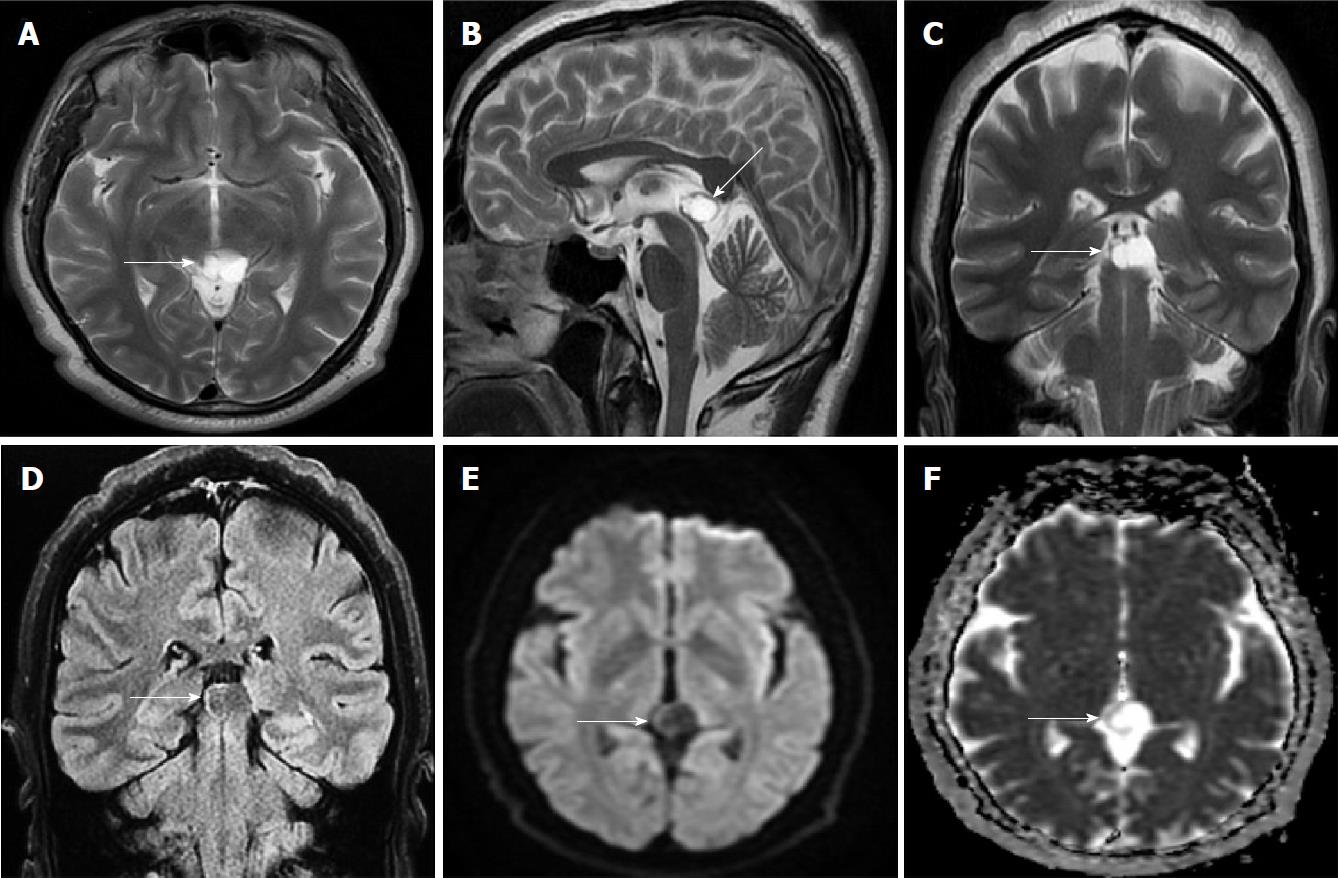
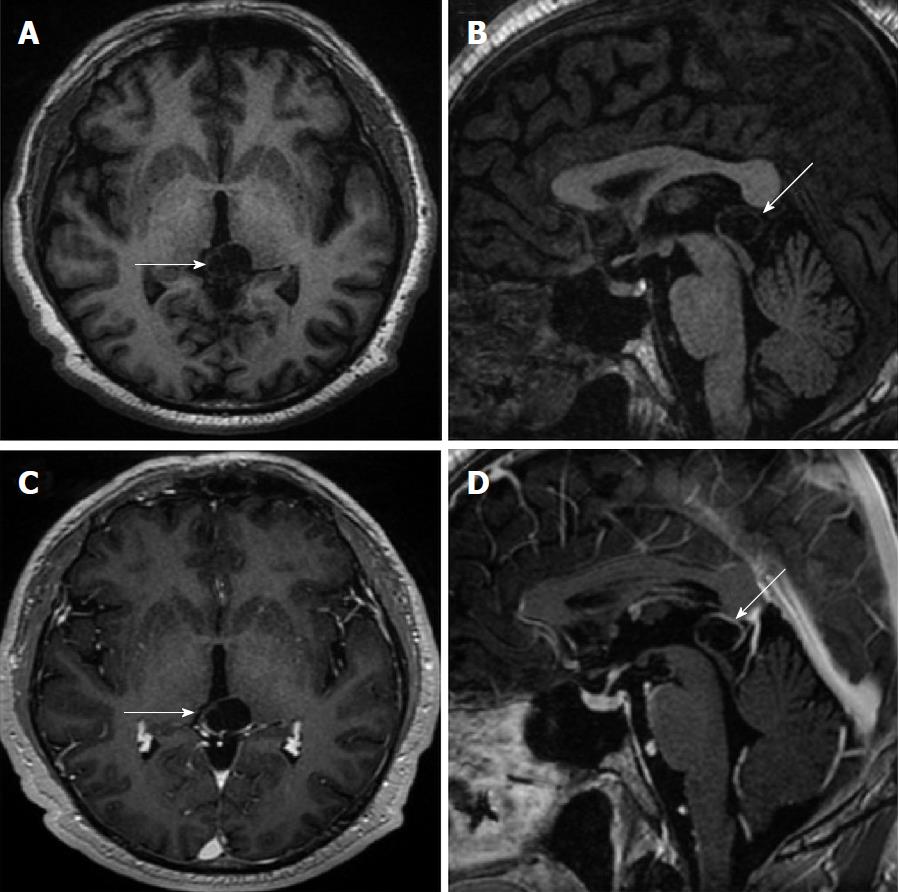
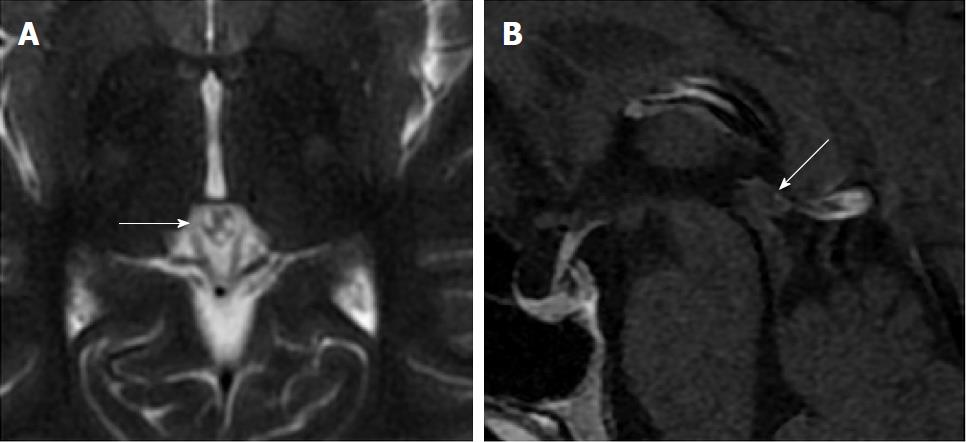
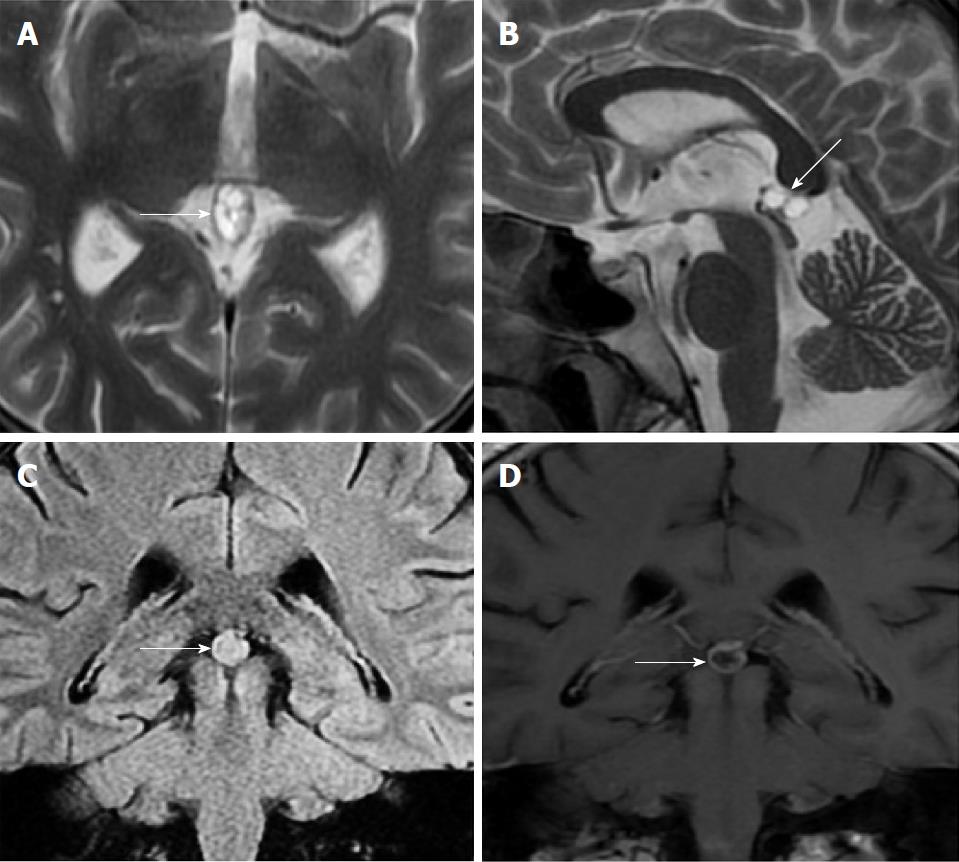
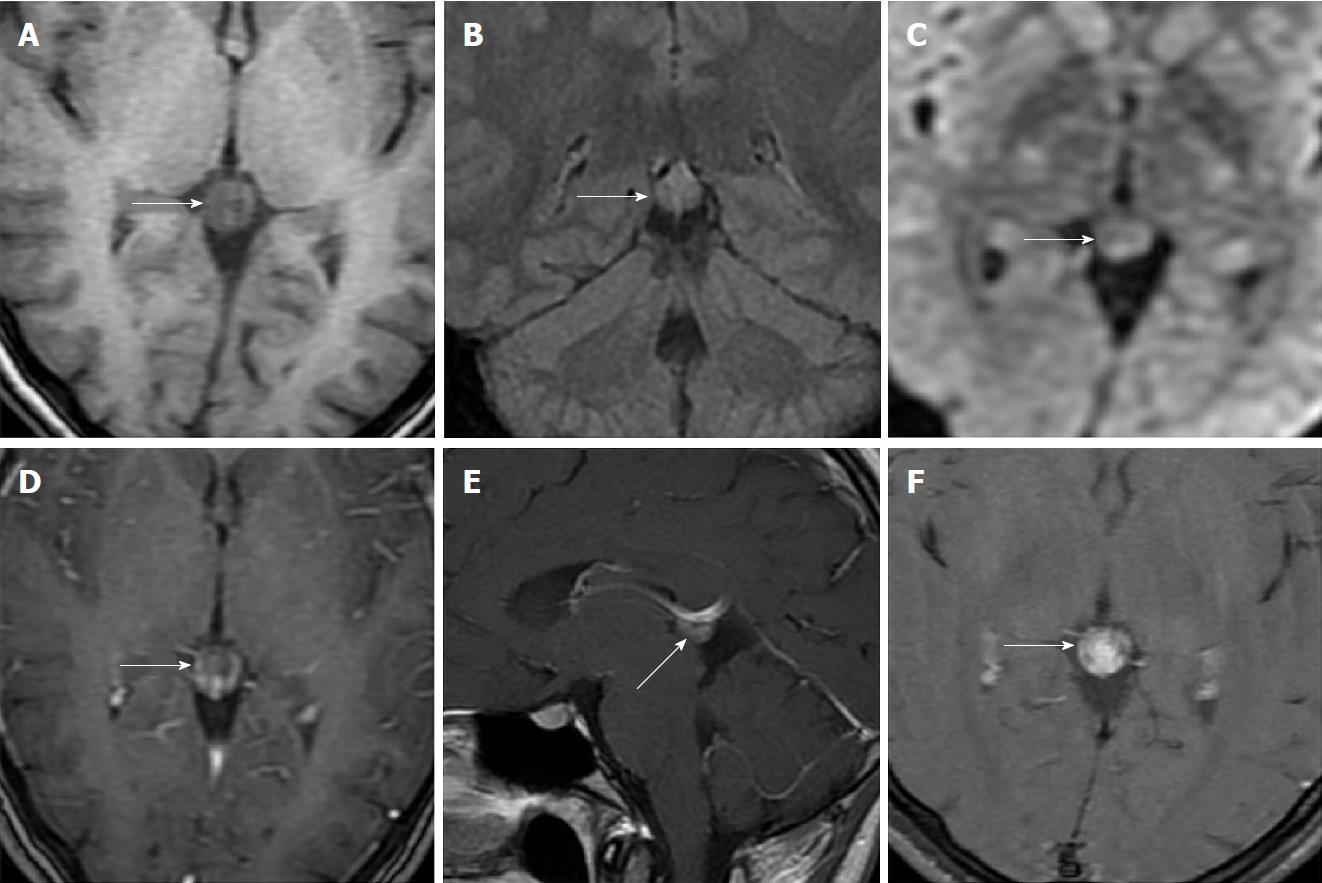
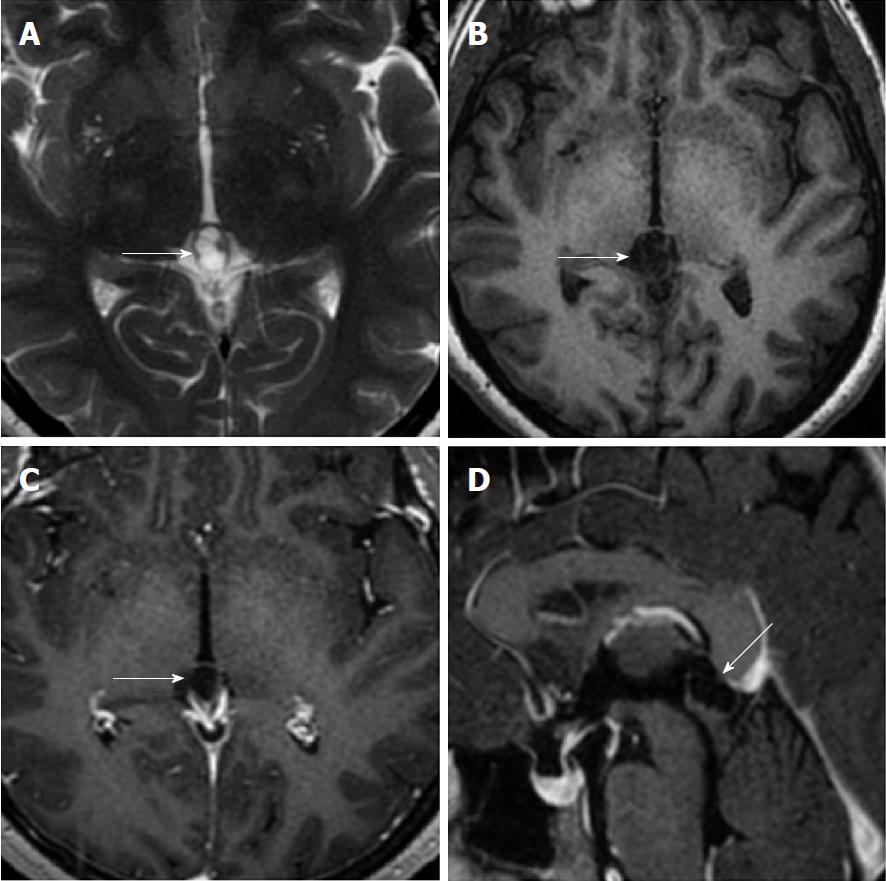
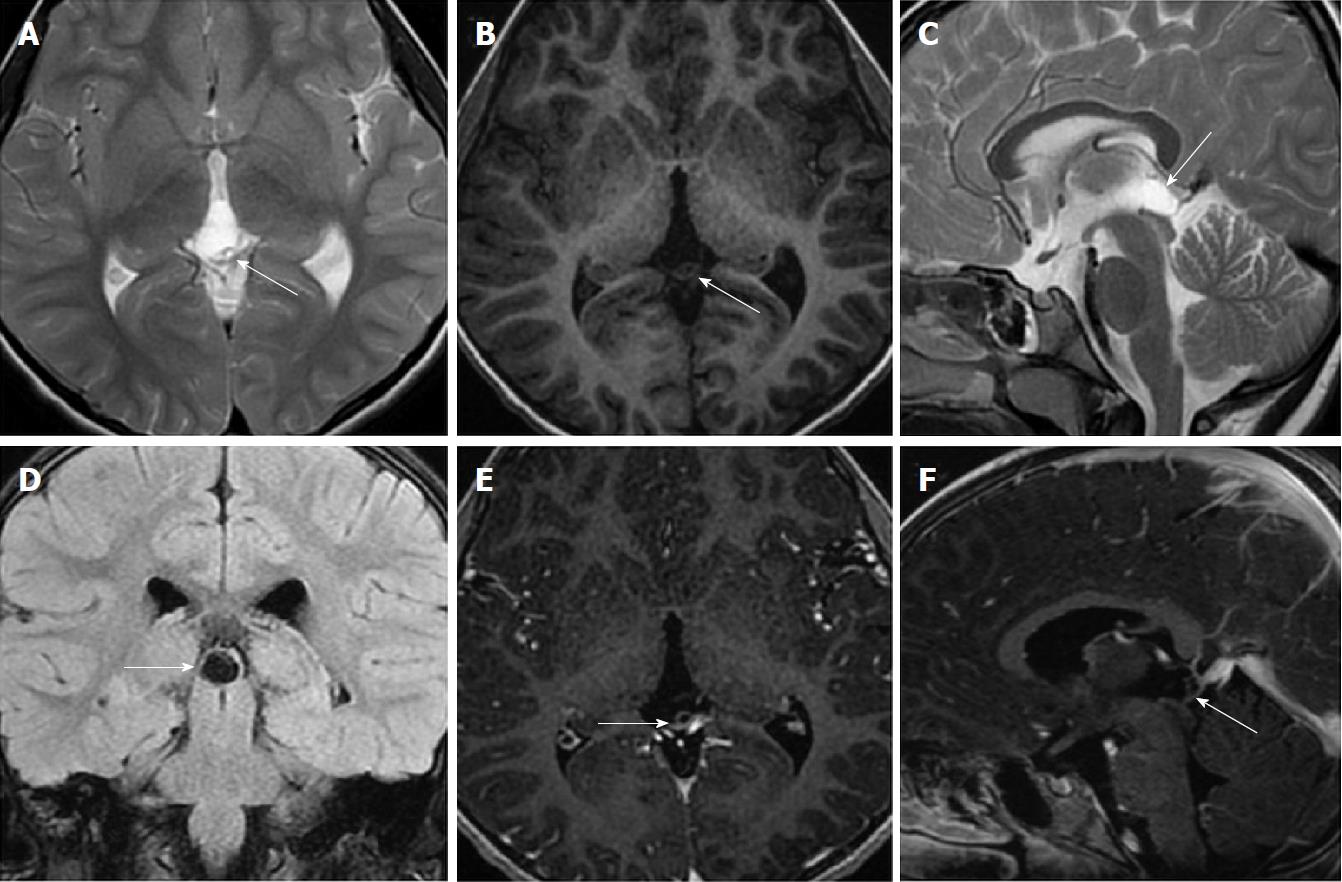
Size and volume, radiological imaging features (signal intensities, contours, internal septation-loculation and contrast-enhancement properties) and size and natural changes of PCs (in patients who had follow-ups) were evaluated by two radiologists. PC dimensions were measured from outer wall to outer wall in sagittal, axial and coronal planes. Unilocular, smooth edged, ovoid PCs with homogenous interior structure and less than 2 mm wall thickness were considered typical PC (Figures 1-3), while multilocular PCs with walls thicker than 2 mm, septation or lobulated contours were considered atypical (Figures 4-10). In patients who had had follow-up examinations, size and natural differences were compared in initial and follow-up examinations.
Descriptive statistics were expressed as mean ± SD. Data regarding categorical variables were given as n (%). Categorical variables were compared using χ2 tests. Independent-samples t-test and Mann-Whitney U test were used to compare the means of two groups. The results of PC dimension measurements were compared using paired-samples t-test. A P-value of < 0.05 was considered significant. Statistical analyses were performed using the Statistical Package for Social Sciences 18.0 software (IBM Corp.; Armonk, NY, United States).
Studied brain MRI examinations belonged to 5555 women (58.2%) and 3991 men (41.8%), and the age range was 1-99 (mean 43.18 ± 20.94). Fifty-six patients were found to have had PC. Forty-four of them (78.57%) were female and 12 were men (21.43%). Calculated frequency of PC was 0.58%. Age range of patients with PC was 5-61 (mean: 31.26 ± 12.73). When the groups with and without PC were compared, a significant difference was found between the two groups for average age (P < 0.001). The difference between the genders for frequency of PC incidence was also significant (P = 0.002). The most common reason for MRI examination request was headache (42 patients). Demographic and clinical characteristics of patients with PC are given in Table 3.
| Demographic and clinical characteristics | n (%) |
| No. of patients | 56 |
| Pineal cyst prevalence | 0.58% |
| Median age (mean ± SD, range) (yr) | 31.26 ± 12.73 (5–61) |
| Sex | |
| Female | 44 (78.57) |
| Male | 12 (21.43) |
| MRI request reasons | |
| Headache | 42 (75) |
| Epilepsy | 7 (12.50) |
| Cerebrovascular disease | 4 (7.14) |
| Vertigo | 1 (1.78) |
| Encephalitis | 1 (1.78) |
| Hand tremor | 1 (1.78) |
One PC was slightly hyperintense on T2 weighed series compared to cerebrospinal fluid (CSF), but others were isointense (Figures 1-3). On T1 weighted series, three PCs were slightly hyperintense compared to CSF and other PCs were isointense. On fluid-attenuated inversion recovery (FLAIR) series, on the other hand, only six PCs were isointense with CSF, and others were hyperintense (Figure 2). No diffusion limitation was observed on diffusion MRI series in any PC (Figure 4). Contrast-enhancement was detected in PCs of all 21 patients who had undergone contrast-enhanced MRI examination. Vast majority of PC (71.42%) showed peripheral rim-like contrast-enhancement (Figure 1). However, peripheral rim-like contrast-enhancement and septal contrast-enhancement or partial rim style enhancement were less frequent (14.28% and 9.52%, respectively) (Figures 5, 7 and 10). One PC (4.76%) had intense heterogeneous contrast-enhancement, and complicated PC-pineocytoma differentiation could not be done radiologically (Figure 8). Most of the PCs were concluded to be typical (62.50%), while a smaller proportion was atypical (37.50%). Eighteen of the atypical PCs (32.4%) had two or more septation-loculation, and 11 (19.64%) had peripheral lobulation. Septation-loculation was observed in 9 (81.81%) of lobule-contoured PCs (Figure 9). MRI features of PCs are given in Table 4. Minimum and maximum PC diameters varied from 5.0 to 19.7 mm. Average PC sizes were 11.18 ± 3.03 mm in anteroposterior (AP), 10.41 ± 2.72 mm in mediolateral (ML) and 8.63 ± 2.47 mm in craniocaudal (CC) directions. Average PC volume was 0.62 ± 0.54 cm3. A total of 18 PCs had follow-up examinations, and 10 of them were typical and 8 atypical. Natural change was not observed in any PCs with follow-ups. Amount of average dimension changes in PCs with follow-ups were (-0.08 ± 0.53) mm, (-0.22 ± 0.87) mm, and (0.16 ± 0.56) mm in AP, ML and CC dimensions, respectively. Thus, AP, ML and CC dimensions decreased in 6, 8 and 5 PCs, and increased in 5, 5 and 7 PCs, respectively. No significant difference was found between initial and final imaging sizes of PCs which were monitored by follow-up examinations (P > 0.05). Initial dimensions and volumes of PCs and observed changes during the follow-up period are given in Table 5.
| MRI features of pineal cysts | n (%) |
| Cyst type | |
| Typical | 35 (62.50) |
| Atypical | 21 (37.50) |
| Two or more septation-loculation | 18 (32.14) |
| Contour lobularity | 11 (19.64) |
| Cyst contrast-enhancement | 21 |
| Peripheral rim enhancement | 15 (71.42) |
| Peripheral rim and septal enhancement | 3 (14.28) |
| Partial rim enhancement | 2 (9.52) |
| Dense heterogeneous enhancement | 1 (4.76) |
| Cyst intensity relative to CSF on T2 weighted images | |
| Isointense | 55 (98.21) |
| Hyperintense | 1 (1.79) |
| Cyst intensity relative to CSF on T1 weighted images | |
| Isointense | 53 (94.64) |
| Hyperintense | 3 (5.36) |
| Cyst intensity relative to CSF on FLAIR images | |
| Isointense | 6 (10.71) |
| Hyperintense | 50 (89.29) |
| Diffusion restriction | 0 |
| Pineal cyst dimensions and volume | Value (range) |
| Minimal-maximal linear dimension | 5.0-19.70 mm |
| All plane mean dimension (AP, ML, CC) | 10.07 ± 2.93 mm |
| Cyst volume (mean ± SD, range) | 0.62 ± 0.54 (0.11-3.35) cm³ |
| Cyst dimension (mean ± SD, range) (mm) | |
| Anteroposterior (AP) | 11.18 ± 3.03 (6.50-19.00) |
| Mediolateral (ML) | 10.41 ± 2.72 (5.80-19.70) |
| Craniocaudal (CC) | 8.63 ± 2.47 (5.0-18.30) |
| Changes in dimensions during follow-up period (mean ± SD, range) (mm) | |
| Anteroposterior (AP) | -0.08 ± 0.53 (-1.60-0.7) |
| Mediolateral (ML) | -0.22 ± 0.87 (-3-0.8) |
| Craniocaudal (CC) | 0.16 ± 0.56 (-0.70-1.40) |
PC are observed in 0.6-10.8% of all random or consecutive brain MRI studies[5,11-14] and in 23% of healthy volunteers[15]. Al-Holou et al[16] found an incidence rate of 1.9% in an MRI study on a child population comprising 10821 children under 18 years of age. Lacroix-Boudhrioua et al[13] found a PC incidence rate of 11% in a high-resolution MRI study on a child patient group without a neurological indication. Considerable differences in reported PC incidence in MRI literature could be due to technical parameters or methodology (slice thickness, sequence type, strength of the magnetic field, size threshold of cysts included, etc.) as well as due to population differences (age, gender and race)[17]. In cadaver autopsies, pineal cyst incidence of up to 40% have been reported[5,18]. Higher incidence in autopsy series could be explained by the fact that small sized cysts of 2-5 mm can be detected only in cadaveric studies[13]. In the present study, only six of the 1327 patients (0.4%) who were 17 years of age and under were found to have PC. Frequency of PC in all ages combined was 0.58%, which was lower than the incidence rates reported in literature. Lower incidence rate in the present study could be due to population difference.
Al-Holou et al[12] found a prevalence of 2.0% in an adult population in the range of 19-30 years of age. Sawamura et al[19] reported a decrease in PC incidence after the age of 40. Al-Holou et al[12], on the other hand, mentioned that PC prevalence peaked at late childhood period and then started to decrease in adult age range. Most studies in literature reported lower PC incidence rates in infants and in old ages (older adults)[4,6,12,16,20-22]. In the present study, no PC was observed in infantile period, and the incidence of PC tended to increase towards the end of the second decade and peaked at the fourth decade. A slight decrease in prevalence was observed at the fifth decade, but the decrease in older ages was more pronounced.
There are many studies in literature reporting higher incidence of PCs in women[12,14,16,19]. Studying a population of children and young adults, Al-Holou et al[16] mentioned PC frequency of 2.4% in women and 1.5% in men. In a retrospective study by Al-Holou et al[12] carried out on 48417 patients who had brain MRI, frequency of pineal cyst was reported to be 1.1% in women and 0.8% in men. Similarly, Sawamura et al[19] found PC incidence rates of 1.6% for women and 0.96% for men. In the present study, on the other hand, relatively lower incidence rates of PC were observed, women having higher incidence rates (0.8%) than men (0.3%) similar to literature.
Most PCs are small sized[1,2]. Barboriak et al[4] reported that average PC diameter was 11.2 mm and volume was 1.42 cm3. They also reported that 47% of PCs had 10 mm or smaller maximum linear dimension. Nevins et al[14] evaluated 281 PC, and found that median size of PCs at diagnosis was 10 mm. Al-Holou et al[12] found that starting dimensions of PCs were 9.7 ± 3.8 mm in sagittal anteroposterior, 6.8 ± 2.9 mm in sagittal craniocaudal and 7.0 ± 2.8 mm in axial width dimensions, and that 50% of PCs had less than 10 mm of maximum size. The authors also mentioned that sizes of PC in women and men were not significantly different, and volume of PCs was not significantly associated with age. Average PC dimension in the present study was 10.07 ± 2.93 mm in all planes (AP, ML and CC dimensions). Maximum dimension was less than 10 mm in 37.5% of PCs (n = 21), which was somewhat lower than what was reported in literature. This may be due to different measurement techniques used to determine PC dimensions. PC volumes in the present study were not significantly associated with gender or age of the patients (P = 0.74 and P = 0.81, respectively).
PCs typically have a benign prognosis, but some studies reported rare size changes of PCs over time[4,12,23]. Tamaki et al[7] and Golzarian et al[6] reported that size of PCs did not change in follow-up examinations. Al-Holou et al[12] found that only 2.6% of PCs which were monitored for periods varying from 6 mo to 3 years had an average maximum diameter increase of 3.5 mm, while size decreased in 15% and remained stable in 82% of them. In 32 patients monitored for periods ranging from six months to nine years, Barboriak et al[4] observed that maximal size did not change in 75.0% of PCs, 2-4 mm size decreases were observed in 9.37%, and 2-3 mm size increases were observed in 6.25% of the PCs. On the other hand, they also found that two cysts resolved completely, and a new cyst developed and grew to 12 mm. Nevins et al[14] reported that only 11 of the 181 PC that they followed for periods varying from 1 to 68 mo had dimensional changes. Seven of them had a 2 mm median diameter increase and the other four had 2.5 mm median diameter decrease[14]. Of the 18 PCs which were monitored by follow-up examinations in the present study, three (16.66%) had no size change, while five (27.77%) had size increases in all dimensions, four (22.22%) had size decreases and six (33.33%) had both increase and decreases in at least one dimension. Average increase in maximum diameter was 0.64 ± 0.37 mm (range: 0.1-1.4 mm), and average decrease in maximum diameter was 0.62 ± 0.45 mm (range: 0.1-1.6 mm). Size changes in PCs were much lower than those in literature. Barboriak et al[4] reported that no significant difference was observed for average volumes and maximum linear dimensions of PCs in initial and final MRI screenings. In parallel with Barboriak et al[4], changes between initial and final sizes of PCs were not statistically significant in the present study (P > 0.05). Barboriak et al[4] mentioned that MRI monitoring of incidentally determined asymptomatic cysts are not practical and suggested that cysts with atypical imaging features should be monitored. Nevins et al[14] recommended a single follow-up MRI scan with gadolinium at 12 mo after diagnosis and discharge if the pineal cyst has not increased in size. It has been indicated that follow-up imaging and even tissue sampling could be necessary for a lesion which does not meet MRI criteria of a typical pineal cyst or which manifests itself with clinical symptoms[10]. Nevertheless, many benign PC were reported to have irregular nodular enhancement on MR images[9]. Fleege et al[9] reported that 14 of 19 pineal lesions confirmed through histological examinations had been preoperatively concluded to be pineal neoplasms. The authors noted that PC had the appearance of complex cysts and cysts with fluid levels, calcification, hemorrhage, and enhancement[9]. Similarly, Fain et al[24] found abnormal rim enhancement on intracranial imaging in 50% of benign cysts confirmed by histological examination. It has been proposed that this abnormal peripheral rim enhancement could be associated with surrounding venous structures or displaced pineal gland[1,6,16]. Therefore, it was concluded that presence of a solid contrast-enhancement component in PC should be considered as a worrying appearance[5].
Radiological appearance of PCs changes by imaging modalities and parameters used. PCs are smooth-edged ovoid lesions which could generally be better visualized on sagittal plane in MRI. There are different reports in literature about signal properties of PCs obtained by different sequence parameters[6,19,21,25]. Osborn[26] indicated that almost all PCs appeared isointense or slightly intense with CSF on T2 weighted MR images, but on T1 weighted images, 50%-60% of them appeared slightly hyperintense compared to CSF, about 40% appeared isointense and 1%-2% with intracystic hemorrhage, on the other hand, appeared hyperintense. The author also reported that signal of most PCs was not completely suppressed on FLAIR images and appeared moderately hyperintense compared to brain parenchyma. Nevertheless, signal properties of PCs were found to vary depending upon its content, presence of hemorrhage and calcification[26]. More than 60%-90% of PCs were shown to have contrast-enhancement on contrast-enhanced series[1,2,26]. On diffusion MRI content of cyst typically does not have diffusion limitation[27]. Almost all PCs in the present study (98.21%) were isointense with CSF, and only one (1.79%) was slightly hyperintense on T2 weighted images. On T1 weighed series, 94.64% were isointense with CSF and only 5.36% was slightly hyperintense. On FLAIR series, 89.29% were hyperintense with CSF and 10.71% were isointense. Contrast-enhancement was observed in PCs of all patients who had contrast-enhanced examination. Barboriak et al[4] reported that only one cyst showed signal change on proton density weighted sequence during follow-up examinations. In the present study, no change was observed in MRI signal feature of any cyst in any sequence.
PCs were reported to have unilocular appearance in literature dealing with routine brain MRI studies[4,25]. Al-Holou et al[12], on the other hand, found that 11% of PCs had multicystic appearance or had atypical features due to abnormal contrasting. Jinkins et al[25] mentioned that most PCs were unilocular but PCs in two patients had septation. Using FIESTA (fast imaging employing steady-state acquisition) sequence, Pastel et al[28] detected that six of the 10 PCs (60%) had internal septation or multiloculation. In their high-resolution MRI studies, Lacroix-Boudhrioua et al[13] found that 74% of PCs had septation. In addition, pathological studies reported multiple septations as common findings in PCs[12,18]. This fact means that majority of PC septations could not be detected in routine MRI series. In the present study, septation was observed in 18 PCs (32.14%). Three PCs which had been described as typical based on MRI exams before our MRI machine was upgraded (before 2017) were classified atypical in follow-up MRI exams carried out in 2017 and later due to observed internal septations. It was concluded that especially BRAVO sequence (high resolution three-dimensional T1-weighted gradient images) of isotropic 1 mm3, both contrast-enhanced and without contrast-enhanced, improved the detection of internal septations. Studies in literature mentioned that growth and changing patterns of septated cysts are not significantly different from those of unilocular ones[5,12,28]. Similar to the studies in literature, no significant difference was observed between growth patterns of atypical and typical PCs (P > 0.05).
PCs are localized in pineal gland and may partly or completely occupy it. A typical pineal cyst shows a contrasting wall feature in a thin peripheral rim style of less than 2 millimeter[4]. Since there is no blood-brain barrier around pineal gland, contrast-enhancement is observed in cyst walls[11]. In the center of cyst, contrast-enhancement is not normally observed in images taken right after contrast matter administering. Nevertheless, in images taken 60-90 min later, cyst may have contrast-enhancement in a uniform and solid appearance[1]. In atypical PCs, findings such as internal septation or loculation, irregular nodular contrast-enhancement, edge lobulation and hemorrhage can be observed[1,4,11,16]. However, these atypical findings are not necessarily related to malignity or cyst enlargement[1]. In fact, high resolution MRI studies showed that internal septations and loculations can be detected in great majority of PCs[13,28]. Follow-up examinations in many studies, including the present one, showed that atypical PCs are not different from the typical ones in terms of size and natural change[4,12]. This finding suggests that internal septation-loculations or lobulations are in fact inherent in PCs and that typical-atypical classification based on these criteria should be reconsidered. Nevertheless, despite the advances in high resolution MRI, there are not definite radiological methods to distinguish benign PC from pineal region malignancies containing cystic components such as pineocytomas, pineoblastomas, germinomas or mature teratomas[29]. In addition, similar to pineal area tumors such as pineoblastomas, teratomas or pilocytic astrocytoma which look like large cysts, benign PCs which lead to intracystic hemorrhage and hydrocephaly and have a complicated appearance may mimic malignant tumors[9,30]. Since malignancy possibility is higher in PCs which grow and have high contrast-enhancement and hemorrhage, more frequent follow-ups or neurosurgical intervention may be necessary with these PCs[29].
PC could enlarge in time due to both intracystic fluid increase and hemorrhage and become symptomatic. Because of their mass effect on midbrain next to them, PCs could lead to Parinaud syndrome (paralysis of upward gaze, retraction in eyelid and abnormal pupil reactions)[31,32]. Sudden death events have been reported because of intra-cystic hemorrhage, also called pineal apoplexy, and acute hydrocephaly[33,34]. PCs with diameters smaller than 10 mm typically do not exert compression on adjacent structures such as cerebral aqueduct, vein of Galen, and the quadrigeminal plate, and they are frequently asymptomatic[1,20]. However, PCs with diameters larger than 15 mm could make local mass effect on adjacent structures and lead to neurological symptoms as a result of hydrocephaly due to compression of cerebral aqueduct[1]. Although there were seven PCs with maximum diameter of more than 15 mm in the present study, gross local mass effect or cerebral aqueduct compression was not observed in any patient. Patients may have a large scale of symptoms due to PC, headache being the most common. Other frequently observed symptoms in PC patients are seizures, dizziness, blurred vision, hemiparesis and vomiting[29]. Previously, headache in these patients were thought to be due to increased intracranial pressure. However, recent studies indicated a hormonal imbalance indicating melatonin as culprit[29,35]. In addition, a recent study reported that MR biomarkers (tectum-splenium-cyst ratio and thalamic and periventricular edema) could be associated with central venous hypertension and severity of symptoms in non-hydrocephalic, symptomatic PC patients[36]. Although PCs did not lead to clear compression findings in the present study, the most common symptom experienced by the patients was headache (75%).
Asymptomatic cysts could be accompanied by tectal deformities of different intensities[4]. Although higher deformity levels are observed in larger cysts as expected, Barboriak et al[4] reported that they could not obtain any finding indicating that cysts with higher level of deformities could further enlarge in follow-up examinations. Some studies reported hydrocephaly in patients with PCs larger than 20 mm[37]. On the other hand, Barboriak et al[4] found that only a moderate enlargement in ventricle was observed in two patients with PCs of that size. No patients had cysts with maximum diameter of more than 20 mm diameter, and hydrocephaly due to the mass effect of PC was not observed in any patient in the present study.
Because of the uncertainties about the natural history of PC, especially about asymptomatic ones, there is no consensus in the literature about what is the most appropriate treatment approach for PC[29]. Management options for asymptomatic cysts vary from total ignoring even without any follow-ups to surgical intervention. Surgical intervention is commonly refrained in asymptomatic patients[29]. Some clinicians suggest yearly follow-ups using clinical examination and imaging, but others do not recommend routine imaging for known PC[12,29]. Similarly, while some studies highly recommended routine clinical examinations and imaging in children[16,23,29,31], others considered PCs as common incidental findings and suggested no follow-ups or contrast-enhanced examinations for children without any neurological indications[13]. In symptomatic patients, especially in ones with hydrocephaly, surgical interventions such as shunt placement, cyst excision, endoscopic or stereotactic aspiration, and endoscopic third ventriculostomy could be preferred[29,38]. In their review paper, Májovský et al[39] reported positive feedbacks for elimination of symptoms after PC surgery in most symptomatic patients and even in patients with non-specific symptoms. Although the authors considered microsurgical resection of PCs, using supracerebellar-infratentorial approach, as a viable option for symptomatic patients, they noted that this suggestion was based on limited number of reports[39].
The present study has some limitations. First, relatively fewer PC were evaluated retrospectively in the present study. Second, only 21 of the patients (37.5%) had contrast-enhanced examination. Third, the number of cases with follow-ups was few and follow-up periods were not standard for patients who had them. Fourth, none of the patients had histopathological examinations. Finally, quite small size increase or decrease was observed in a small number of PC. Although we are confident that the change in size is accurate, there is a slight possibility that some of the changes may reflect measurement error.
In conclusion, PCs are cysts which do not have marked dimensional and natural changes. Their frequency is higher in women and adults, and their sizes are not associated with gender or age. Great majority of them are isointense with CSF on T1 and T2A series. On FLAIR sequence, they are hyperintense compared to CSF, and they may be smoothly contoured, unilocular or multilocular. Typical ones may have contrast-enhancement in peripheral rim style, while multilocular ones may have septal contrast-enhancement.
Pineal cysts (PC) are cysts which are frequently detected incidentally in brain magnetic resonance imaging (MRI). No clear consensus has been reached yet over the classification and follow-up procedures in routine clinical practice. PCs were classified based on MRI findings in the present study. Unilocular, smooth edged, ovoid PCs with homogenous interior structure and less than 2 mm wall thickness were considered typical PC, while multilocular PCs with walls thicker than 2 mm, septation or contour lobulation were considered atypical. In addition, size and natural changes in follow-up examinations were also investigated.
Lack of a consensus over radiological classification and follow-up of PC, and their radiological findings that resemble other lesions of pineal area create difficulties. Presentation of radiological studies dealing with PCs in different population including follow-up images taken at different series could help to resolve this uncertainty.
In the present study, PCs detected using brain MRI examinations in our population were classified based on radiological imaging features. In addition, whether PCs had significant size or nature changes were also evaluated.
A total of 9546 patients who had brain MRI examination in March 2010-January 2018 period were studied retrospectively for the presence of PCs. Sizes in three dimensions, volumes, contours, signal intensities, internal septation or loculation features and contrasting patterns of PCs were evaluated. Size and natural changes of PCs were investigated in patients during follow-up examinations with durations varying from 2 to 96 mo. Associations between PC frequency and gender, between PC volumes and gender and age, and amount of changes between initial and final sizes of PCs were statistically analyzed.
Fifty-six patients (44 female and 12 male) were found to have had PC. Age range of patients with PC was 5-61 (mean: 31.26 ± 12.73). Frequency of PC was 0.58%. PC incidence rates were significantly different in men and women. In terms of classification, 62.50% of the PCs were typical and 37.50% were atypical. Average PC sizes were 11.18 ± 3.03 mm in AP, 10.41 ± 2.72 mm in ML and 8.63 ± 2.47 mm in CC directions. Natural change was not observed in any PC with follow-ups. Average dimension changes in PCs with follow-ups were (-0.08 ± 0.53) mm, (-0.22 ± 0.87) mm, and (0.16 ± 0.56) mm in AP, ML and CC dimension, respectively. No significant difference was found between initial and final sizes of PCs which were monitored by follow-up examinations.
It was revealed in the present study that classification of PCs concluded to be unilocular (i.e., typical) based on routine MRI sequences could change to atypical when high resolution sequences indicated internal septations. No significant size or natural change was observed in follow-up examinations of PCs. Therefore, it could be suggested that asymptomatic PCs which do not show any size or natural changes during one- or two-year follow-ups should be removed from routine follow-up.
The present study showed that PCs are cysts frequently observed as incidental lesions in brain MRI series, that they have an average diameter of 10 mm and that they have signal features similar to CSF in T1 and T2 weighed series while giving higher signal intensities than CSF in FLAIR sequence. In addition, it was revealed that typical or atypical classification of PCs could change based on resolution of sequence used in identification of PCs. Elimination of frequent follow-ups of asymptomatic PCs could lower cost and labor burden on health care system.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Kumar J, Xiao E S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
| 1. | Choy W, Kim W, Spasic M, Voth B, Yew A, Yang I. Pineal cyst: a review of clinical and radiological features. Neurosurg Clin N Am. 2011;22:341-351, vii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Osborn AG, Preece MT. Intracranial cysts: radiologic-pathologic correlation and imaging approach. Radiology. 2006;239:650-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 437] [Cited by in F6Publishing: 376] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 3. | Kahilogullari G, Massimi L, Di Rocco C. Pineal cysts in children: case-based update. Childs Nerv Syst. 2013;29:753-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Barboriak DP, Lee L, Provenzale JM. Serial MR imaging of pineal cysts: implications for natural history and follow-up. AJR Am J Roentgenol. 2001;176:737-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Cauley KA, Linnell GJ, Braff SP, Filippi CG. Serial follow-up MRI of indeterminate cystic lesions of the pineal region: experience at a rural tertiary care referral center. AJR Am J Roentgenol. 2009;193:533-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Golzarian J, Balériaux D, Bank WO, Matos C, Flament-Durand J. Pineal cyst: normal or pathological? Neuroradiology. 1993;35:251-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Tamaki N, Shirataki K, Lin TK, Masumura M, Katayama S, Matsumoto S. Cysts of the pineal gland. A new clinical entity to be distinguished from tumors of the pineal region. Childs Nerv Syst. 1989;5:172-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Hayashida Y, Hirai T, Korogi Y, Kochi M, Maruyama N, Yamura M, Yamashita Y. Pineal cystic germinoma with syncytiotrophoblastic giant cells mimicking MR imaging findings of a pineal cyst. AJNR Am J Neuroradiol. 2004;25:1538-1540. [PubMed] [Cited in This Article: ] |
| 9. | Fleege MA, Miller GM, Fletcher GP, Fain JS, Scheithauer BW. Benign glial cysts of the pineal gland: unusual imaging characteristics with histologic correlation. AJNR Am J Neuroradiol. 1994;15:161-166. [PubMed] [Cited in This Article: ] |
| 10. | Fakhran S, Escott EJ. Pineocytoma mimicking a pineal cyst on imaging: true diagnostic dilemma or a case of incomplete imaging? AJNR Am J Neuroradiol. 2008;29:159-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Mamourian AC, Towfighi J. Pineal cysts: MR imaging. AJNR Am J Neuroradiol. 1986;7:1081-1086. [PubMed] [Cited in This Article: ] |
| 12. | Al-Holou WN, Terman SW, Kilburg C, Garton HJ, Muraszko KM, Chandler WF, Ibrahim M, Maher CO. Prevalence and natural history of pineal cysts in adults. J Neurosurg. 2011;115:1106-1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Lacroix-Boudhrioua V, Linglart A, Ancel PY, Falip C, Bougnères PF, Adamsbaum C. Pineal cysts in children. Insights Imaging. 2011;2:671-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Nevins EJ, Das K, Bhojak M, Pinto RS, Hoque MN, Jenkinson MD, Chavredakis E. Incidental Pineal Cysts: Is Surveillance Necessary? World Neurosurg. 2016;90:96-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Pu Y, Mahankali S, Hou J, Li J, Lancaster JL, Gao JH, Appelbaum DE, Fox PT. High prevalence of pineal cysts in healthy adults demonstrated by high-resolution, noncontrast brain MR imaging. AJNR Am J Neuroradiol. 2007;28:1706-1709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Al-Holou WN, Garton HJ, Muraszko KM, Ibrahim M, Maher CO. Prevalence of pineal cysts in children and young adults. Clinical article. J Neurosurg Pediatr. 2009;4:230-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Jussila MP, Olsén P, Salokorpi N, Suo-Palosaari M. Follow-up of pineal cysts in children: is it necessary? Neuroradiology. 2017;59:1265-1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Hasegawa A, Ohtsubo K, Mori W. Pineal gland in old age; quantitative and qualitative morphological study of 168 human autopsy cases. Brain Res. 1987;409:343-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 123] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Sawamura Y, Ikeda J, Ozawa M, Minoshima Y, Saito H, Abe H. Magnetic resonance images reveal a high incidence of asymptomatic pineal cysts in young women. Neurosurgery. 1995;37:11-15; discussion 15-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 80] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Sener RN. The pineal gland: a comparative MR imaging study in children and adults with respect to normal anatomical variations and pineal cysts. Pediatr Radiol. 1995;25:245-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Lee DH, Norman D, Newton TH. MR imaging of pineal cysts. J Comput Assist Tomogr. 1987;11:586-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Lum GB, Williams JP, Machen BC, Akkaraju V. Benign cystic pineal lesions by magnetic resonance imaging. J Comput Tomogr. 1987;11:228-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Mandera M, Marcol W, Bierzyńska-Macyszyn G, Kluczewska E. Pineal cysts in childhood. Childs Nerv Syst. 2003;19:750-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Fain JS, Tomlinson FH, Scheithauer BW, Parisi JE, Fletcher GP, Kelly PJ, Miller GM. Symptomatic glial cysts of the pineal gland. J Neurosurg. 1994;80:454-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 88] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Jinkins JR, Xiong L, Reiter RJ. The midline pineal “eye”: MR and CT characteristics of the pineal gland with and without benign cyst formation. J Pineal Res. 1995;19:64-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Osborn AG. Osborn’s Brain: Imaging, Pathology, and Anatomy. 1st ed. Canada: Amirsys 2013; 788-791. [Cited in This Article: ] |
| 27. | Fang AS, Meyers SP. Magnetic resonance imaging of pineal region tumours. Insights Imaging. 2013;4:369-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Pastel DA, Mamourian AC, Duhaime AC. Internal structure in pineal cysts on high-resolution magnetic resonance imaging: not a sign of malignancy. J Neurosurg Pediatr. 2009;4:81-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Starke RM, Cappuzzo JM, Erickson NJ, Sherman JH. Pineal cysts and other pineal region malignancies: determining factors predictive of hydrocephalus and malignancy. J Neurosurg. 2017;127:249-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Sarikaya-Seiwert S, Turowski B, Hänggi D, Janssen G, Steiger HJ, Stummer W. Symptomatic intracystic hemorrhage in pineal cysts. Report of 3 cases. J Neurosurg Pediatr. 2009;4:130-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Wisoff JH, Epstein F. Surgical management of symptomatic pineal cysts. J Neurosurg. 1992;77:896-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Ramji S, Touska P, Rich P, MacKinnon AD. Normal neuroanatomical variants that may be misinterpreted as disease entities. Clin Radiol. 2017;72:810-825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Milroy CM, Smith CL. Sudden death due to a glial cyst of the pineal gland. J Clin Pathol. 1996;49:267-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Richardson JK, Hirsch CS. Sudden, unexpected death due to “pineal apoplexy”. Am J Forensic Med Pathol. 1986;7:64-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Peres MF. Melatonin, the pineal gland and their implications for headache disorders. Cephalalgia. 2005;25:403-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Eide PK, Pripp AH, Ringstad GA. Magnetic resonance imaging biomarkers indicate a central venous hypertension syndrome in patients with symptomatic pineal cysts. J Neurol Sci. 2016;363:207-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Fetell MR, Bruce JN, Burke AM, Cross DT, Torres RA, Powers JM, Stein BM. Non-neoplastic pineal cysts. Neurology. 1991;41:1034-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Berhouma M, Ni H, Delabar V, Tahhan N, Memou Salem S, Mottolese C, Vallee B. Update on the management of pineal cysts: Case series and a review of the literature. Neurochirurgie. 2015;61:201-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Májovský M, Netuka D, Beneš V. Is surgery for pineal cysts safe and effective? Short review. Neurosurg Rev. 2018;41:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |