Published online Nov 26, 2015. doi: 10.4330/wjc.v7.i11.776
Peer-review started: June 7, 2015
First decision: June 18, 2015
Revised: July 21, 2015
Accepted: September 16, 2015
Article in press: September 18, 2015
Published online: November 26, 2015
Chronic inflammation of the native vessel wall with infiltration of lipid-laden foamy macrophages through impaired endothelium results in atherosclerosis. Percutaneous coronary intervention, including metallic stent implantation, is now widely utilized for the treatment of atherosclerotic lesions of the coronary artery. Bare-metal stents and the subsequently developed drug-eluting stents seal the atherosclerosis and resolve lumen stenosis or obstruction of the epicardial coronary artery and myocardial ischemia. After stent implantation, neointima proliferates within the stented segment. Chronic inflammation caused by a foreign body reaction to the implanted stent and subsequent neovascularization, which is characterized by the continuous recruitment of macrophages into the vessel, result in the transformation of the usual neointima into an atheromatous neointima. Neointima with an atherosclerotic appearance, such as that caused by thin-cap fibroatheromas, is now recognized as neoatherosclerosis, which can sometimes cause in-stent restenosis and acute thrombotic occlusion originating from the stent segment following disruption of the atheroma. Neoatherosclerosis is emerging as a new coronary stent-associated problem that has not yet been resolved. In this review article, we will discuss possible mechanisms, clinical challenges, and the future outlook of neoatherosclerosis.
Core tip: Percutaneous coronary intervention, including metallic stent implantation, causes chronic inflammation of the coronary artery and neovascularization, which involves the continuous recruitment of macrophages into the vessel. The phenomenon of stent neointima transformation from normal neointima to atherosclerotic lesions is now recognized as neoatherosclerosis, which causes in-stent restenosis and acute thrombotic occlusion. Neoatherosclerosis is now emerging as a new atherosclerosis-related problem that has not yet been solved. In this review, we will discuss possible mechanisms, clinical challenges, and the future outlook of neoatherosclerosis.
- Citation: Komiyama H, Takano M, Hata N, Seino Y, Shimizu W, Mizuno K. Neoatherosclerosis: Coronary stents seal atherosclerotic lesions but result in making a new problem of atherosclerosis. World J Cardiol 2015; 7(11): 776-783
- URL: https://www.wjgnet.com/1949-8462/full/v7/i11/776.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i11.776
Atherosclerosis is caused by chronic inflammation at the site of damaged vascular endothelium and lipid-laden foamy macrophages derived from infiltration of monocytes into the arterial wall, and it results in coronary stenosis and thrombotic obstruction after atherosclerotic plaque disruption[1]. Percutaneous coronary intervention (PCI) is now widely accepted worldwide for the treatment of coronary artery disease due to atherosclerosis. In 1977, PCI by plain old balloon angioplasty (POBA) was performed for the first time by Gruntzig[2] to treat angina pectoris. In 1986, Sigwart et al[3] implanted a self-expandable stainless-steel stent to prevent acute occlusion and chronic restenosis caused by intimal dissection after balloon dilatation and elastic recoil of the coronary artery, respectively. In 1994, randomized clinical trials showed that bare-metal stent (BMS) implantation was superior to POBA with regard to short-term procedural success and long-term arterial patency[4,5]. However, in-stent restenosis (ISR) occurred in approximately 20%-30% of cases, causing the long-term failure of PCI that was bestowed the title of the “Achilles’ heel” of PCI. According to pathological investigations, the primary pathogenesis of ISR is neointimal hyperplasia due to migration and proliferation of vascular smooth muscle cells (VSMCs) from the media. In the 2000s, the drug-eluting stent (DES) was introduced to prevent inhibition of neointimal hyperplasia and ISR of the BMS. Application of the DES to coronary artery disease has dramatically reduced the incidence of ISR in the clinical setting[6,7]. The so-called “first-generation DESs” were composed of a stainless steel stent platform and was coated with durable polymer-releasing anti-proliferative drugs. Although the first-generation DES, the sirolimus-eluting stent (SES) and paclitaxel-eluting stent (PES), decreased ISR, they are associated with a steady increase in very late stent thrombosis (VLST; > 1 year post-stent implantation) due to delayed re-endothelialization or a hypersensitivity reaction to the stent polymer[8]. Therefore, the next-generation DES were developed with new technology; specifically, the main feature of these DES was the inclusion of a biocompatible or biodegradable polymer to reduce vessel inflammation and a thin stent strut for normalization of rheological flow around the strut to diminish thrombogenicity. The second-generation DES, namely, zotarolimus-eluting stents, everolimus-eluting stents, and biodegradable polymer-coated biolimus-eluting stents, showed reduced incidences of VLST[9-11]. Nevertheless, the placement of second-generation DES was found to cause acute coronary syndrome originating from the stent segment[12].
Although metallic coronary stents, BMS, and DES resolve the problem of coronary lumen stenosis or occlusion in the acute phase after their implantation, they potentially cause new problems in the chronic phase, such as late ISR and VLST. It is now understood that some of these phenomena arise from the new pathogenic concept of “neoatherosclerosis”, which is defined as the phenomenon of the transformation of stent neointima from normal neointima to an atherosclerotic lesion. We will review basic and clinical studies concerning topical problems of neoatherosclerosis that are associated with coronary stenting.
Mechanical injury of the vessel wall cannot be avoided by PCI, such as balloon dilatation and stent implantation. PCI procedures cause denudation of the coronary artery endothelium, resulting in exposure of the myointima, fissures in the atheromatous plaque, and overstretching of the circumferential vessel layers[13]. The endothelium regulates vascular tone, controls inflammation, maintains lipid and tissue-fluid homeostasis, and possesses antithrombotic properties[14]. The vascular endothelium protects against thrombus formation and blood coagulation through its production of nitric oxide, prostacyclin, tissue plasminogen activator, heparin-like molecules, tissue-factor pathway inhibitor, thrombomodulin, and other molecules[14]. Perturbation of the normal endothelium function by PCI is related to the pathogenesis of atherosclerosis and results in accelerated formation of atheromatous lesions[13]. Incomplete re-endothelialization in the coronary vascular wall induces thrombotic events after stent implantation in the early, late (> 1 mo, ≤ 1 year post-stenting), or very late phase[15]. Denudation of the endothelium after PCI causes VSMCs to be exposed to blood flow directly, which modulates the proliferation and viability of the VSMCs[16,17]. Although BMS implantation is superior to POBA with respect to procedural success and long-term target lesion patency[4,5], dysfunction of the regenerated endothelium is more pronounced after stent implantation than after ballooning[18]. Any interventional procedure, even POBA, causes denudation of the endothelium and is associated with the same risk of very late thrombosis as BMS[19], and the regenerated endothelial cells are not structurally and functionally normal[20]. Stent implantation into the vessel leads to perturbations in blood flow, and flow reversal and disturbed shear stress around the stent strut promote vascular inflammation and injury[21,22]. The thickness of the stent struts determines the size of blood flow recirculation, which is associated with thrombogenicity within the stent segment[23]. Compared with the BMS, the first-generation DES, namely, SES and PES, which incorporated anti-proliferative drugs and durable polymers, were associated with dramatic reductions in the proliferation of the neointimal hyperplasia and ISR[6,7]. However, an increased risk of VLST was observed for these first-generation DES compared with BMS[24,25]. Autopsy studies showed that a lack of re-endothelialization with > 30% of the stent strut uncovered per cross-section was a strong predictor of late stent thrombosis (LST) and VLST[26]. Moreover, the polymer-induced type IV hypersensitivity reaction is one of the mechanisms of LST or VLST associated with SES. In contrast, excessive fibrin deposition and consequent stent mal-apposition (detachment of the stent struts from the coronary arterial wall) are associated with thromboses in the case of PES[26,27]. The new stent technology of second-generation DES involved minimization of vessel injury and normalization of micro-rheology around the stent strut, thinner struts, and the use of a biocompatible or biodegradable polymer[28]. The pathophysiology of LST and VLST is multifactorial, as mentioned above. However, other mechanisms are possibly linked to stent thrombosis. LST or VLST after placement of BMS and DES is an unresolved problem, and the new pathological concept of neoatherosclerosis is another mechanism of stent failure. It is understood that the pathophysiology and development of neoatherosclerosis differ between BMS and DES.
Neointimal hyperplasia associated with BMS was considered to be stable, with peaks at 6 mo and 1 year after stenting during a 3-year follow-up[29]. However, extended follow-up of BMS showed that late luminal re-narrowing beyond 4 years was common[30]. Moreover, one-third of patients implanted with BMS who had restenosis presented with acute myocardial infarction or unstable angina 5 years after the index procedure that was not clinically benign[31]. Some reports have documented the occurrence of ACS due to the disruption of neoatherosclerosis after BMS implantation[32].
The findings of a histopathological study suggested the mechanism of the catastrophic late events after BMS implantation[33]. This study, which assessed nineteen stented coronary arteries obtained from 19 patients autopsied after non-cardiac death 2-7 years post-BMS implantation, showed that after more than 4 years of stenting, there was prominent infiltration of lipid-laden macrophages with strong collagen-degrading matrix metalloproteinase expressing ruptured and vulnerable plaque accompanied by thrombi around the struts evoked by remarkable foreign-body inflammation[33]. Regenerated endothelium after PCI forms poor endothelial cell junctions and expresses reduced numbers of antithrombotic molecules and nitric oxide, which contributes to neoatherosclerosis[15,18,34]. Neoatherosclerosis is now recognized as chronic inflammation in the vessel wall caused by the stent itself and subsequent neo-vessel formation, which causes continuous recruitment of macrophages and forms unstable lesions called thin-cap fibroatheroma (TCFA) that contribute to disruption of neointima and thrombus formation, leading to VLST[15].
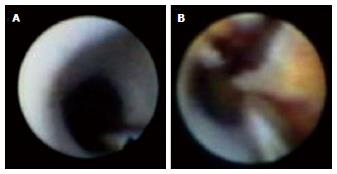
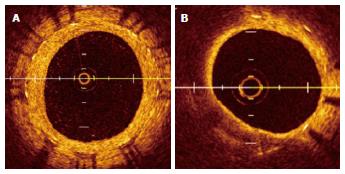
Serial angioscopic observation at baseline, 6 to 12 mo, and ≥ 4 years after BMS implantation revealed changes in the smooth white intima characterized by atheromatous yellow plaque with vulnerable features, such as surface disruption and thrombus formation, during the study period (Figure 1)[35]. In addition, the atheromatous transformation was correlated with ISR[35]. Optical coherence tomography (OCT) is a near-infrared light-based imaging modality with high-resolution that can accurately characterize tissue components in vivo[36]. Although there are no data regarding the angioscopic findings and histopathologic correlation in intimal tissue, an OCT study showed that the angioscopic yellow neointima likely corresponds to foamy macrophages infiltrating into the fibrous cap and underlying lipid accumulation, as well as that the intensity of yellow likely signifies the thickness of the fibrous cap and amount of necrotic core[37]. OCT observation of BMS segments was performed in the early phase (< 6 mo) and late phase (≥ 5 years) after BMS implantation[38]. The normal neointima proliferated homogeneously, and the lipid-laden intima was not observed in the early phase. In the late phase, the lipid-laden intima was found in 67% of cases (Figure 2)[38]. Additionally, pathological characteristics, such as intimal disruption and thrombus formation, appeared (38% and 52% of cases, respectively). There was a similar incidence of peri-stent neovascularization in the 2 phases. However, the location of neovascularization was different between the two phases. Intra-intima neovascularization was more prevalent in the late phase than the early phase (62% and 0%, respectively; P < 0.01) and in segments with lipid-laden intima compared with non-lipidic segments (79% and 29%, respectively; P = 0.026)[38]. There are few reports showing that neoatherosclerosis of BMS increases ACS, clinically diagnosed as VLST. Therefore, further careful follow-up of neoatherosclerosis after BMS implantation is needed.
Chronic inflammation and insufficient functional endothelialization induce neoatherosclerosis inside both BMS and DES, causing ISR and thrombosis in the late phase[39]. In intravascular ultrasound (IVUS) analyses of VLST, neointimal rupture was observed within the stent segment in 43.5% of the DES and all of the BMS[40]. OCT also indicated that ruptured atherosclerosis and thrombosis in BMS and DES was the most common mechanism of definite VLST presenting as myocardial infarction with ST-segment elevation[41].
Pathological analysis of human coronary arteries with stented segments showed that unstable lesions, such as TCFA or intimal rupture, were associated with shorter implant durations for first-generation DES (1.5 ± 0.4 years) compared with BMS (6.1 ± 1.5 years). These results indicate that neoatherosclerosis in first-generation DES is more frequent and occurs earlier than that in BMS[39]. Pathology of second-generation everolimus-eluting cobalt chromium stents implanted < 3 years showed less uncovered strut area and milder inflammation compared with first-generation DES. However, neoatherosclerotic changes were confirmed even in second-generation DES, and there was no significant difference in neoatherosclerosis between first-generation DES and second-generation DES[42]. Neoatherosclerosis occurs more rapidly in DES than BMS, possibly because the eluted drug prevents endothelial cell proliferation, viability, and migration, which allows infiltration of lipid-laden foamy macrophage into the vessel, thereby accelerating atherosclerotic changes[43-46].
In first-generation DES, angioscopic follow-up of SES at baseline, 6 mo, and 2 years after implantation showed that neointimal growth inside the SES progressed heterogeneously, uncovered struts persisted in 20% of the patients for up to 2 years, and subclinical thrombus formation was not a rare phenomenon[47]. Although uncovered stent struts on angioscopic images do not correspond to incomplete re-endothelialization, uncovered struts may play a role in promoting atherosclerosis. An angioscopic follow-up study demonstrated that the neointima at baseline changed into a lipid-rich atherosclerotic and yellow neointima at 10 mo, with intramural thrombi being more frequently detected on newly formed yellow neointima[48]. Serial angioscopic findings up to 2 years after SES implantation showed that neointimal coverage was completed by 3 to 6 mo in BMS, whereas SES demonstrated the presence of thrombi and yellow plaques as long as 2 years after implantation[49]. The long-term vascular response was evaluated by serial angioscopic follow-up at 2 and 5 years after SES implantation, and incomplete neointimal stent coverage and the prevalence of latent thrombus within the SES segments did not decrease from 2 to 5 years[50].
In-stent neoatherosclerosis was recognized as an important mechanism of DES failure, especially late after implantation, regardless of its generation[51]. OCT was performed on a total of 50 lesions with angiographic in-stent restenosis (30 stable and 20 unstable angina patients, median follow-up time of 32 mo). Patients with unstable angina had a thinner fibrous cap and a higher incidence of TCFA, including intimal rupture and thrombi, than those with stable angina[51]. A direct comparison of the characteristics of neointimal hyperplasia and its time course between BMS and DES using OCT showed that lipid-rich neoatherosclerosis develops within stent segments earlier (< 9 mo) in DES than in BMS (≥ 48 mo), and the majority of ISR lesions developed lipid-laden neointima in both groups by 48 mo[52]. Morphological analysis of first-generation DES-ISR by OCT revealed that early (< 1 year) ISR showed a speckled pattern; in contrast, very late ISR (> 3 years) exhibited a pattern more similar to that of TCFA[53]. Angiographic and integrated backscatter IVUS analysis of ISR lesions after SES and BMS implantation showed that focal angiographic restenosis was predominantly present in the SES group, whereas diffuse restenosis was more common in the BMS group. The neointimal tissue in SES-related ISR lesions consisted of a significantly larger percentage of lipid tissue and a smaller percentage of fibrous tissue compared with that in BMS-related ISR lesions[54]. Characterization of neointimal tissue approximately 9 mo after DES implantation by OCT revealed that heterogeneous lesion type can be helpful in predicting outcomes regardless of DES generation[55]. Second-generation 40 zotarolimus, 36 everolimus, and 35 biolimus stents were not more protective against neoatherosclerosis compared with the first-generation 65 SES and 36 PES[12]. Table 1 summarizes the characteristics of each type of stent with regard to neoatherosclerosis. Taken together, continuous follow-up is required to clarify clinical events after DES regardless of its generation.
| Stent type | BMS | First-generation DES | Second-generation DES | BRS |
| Strut thickness | Thick | Thick | Thin | Thick |
| Incorporated drug | None | Rapamycin derivatives/paclitaxel | Rapamycin derivatives | None/rapamaycin derivatives |
| Polymer | None | Durable | Durable/biodegradable | None/biodegradable |
| Inflammation | Not available Foreign-body inflammatory reaction[33] | Strong | Slightly | Slightly[65] |
| Onset of neoathero- sclerosis | After 4 yr[39] | SES 70 d[42] PES 120 d[42] | CoCr EES 270 d[42] | Not available |
There are drugs and mechanical interventions available to treat neoatherosclerosis. In the clinical setting, univariate analysis revealed that smoking and angiotensin-converting enzyme inhibitor or angiotensin II inhibitor usage were associated with the presence of neoatherosclerosis[56]. Chronic kidney disease and > 70 mg/dL of low-density cholesterol at OCT follow-up were independent predictors of neoatherosclerosis[12]. Whether interventions addressing these risk factors and aggressive lipid-lowering therapy can improve neoatherosclerosis should be assessed in prospective trials.
Regarding PCI, OCT observation at 9 mo following treatment for DES-ISR using a paclitaxel-coated balloon to avoid repeated stenting showed a heterogeneous pattern in the neointima with speckled structures consistent with macrophage infiltration and a lipid pool consistent with neoatherosclerosis, indicating insufficient treatment of DES-ISR[57]. New stent technologies that accelerate endothelial healing through the use of a thinner stent strut, biodegradable polymer with contraluminal drug coating (Synergy™; Boston Scientific, Ultimaster™; Terumo), or luminal surface coating with CD34 antibody (COMBO™; Orbusneich Medical Technologies) to capture endothelial precursor cells, rendering the stents free from neoatherosclerosis, are expected in future clinical trials[58].
Complete bio-absorption of the vascular scaffold [bio-resorbable scaffolds (BRS)] a few years after implantation, which potentially reduces late adverse events such as VLST provoked by neoatherosclerosis in the stent caused by the permanent presence of a polymer and metallic artificial implant[59,60], can restore endothelial function[61,62]. IVUS analysis of ABSORB BVS revealed a significant plaque media reduction without a significant change in the vessel wall area (plaque regression)[61]. Nevertheless, unresolved problems remain regarding BRS. If overstretched, BRS can lose their radial strength, leading to stent fracture[63]. BRS demonstrated a higher probability of procedural side branch occlusion in small side branches compared with everolimus-eluting metallic stents[64]. Moreover, most of the data on BRS use are derived from relatively small and non-randomized studies with short or mid-term follow-up, and further studies are warranted to determine the real-world efficacy and safety of BRS[59]. The features of each stent are summarized in Table 1.
Cardiologists have been combatting coronary atherosclerosis through stent implantation and preventive medicine. Neoatherosclerosis is now emerging as a new problem that has not yet been solved. Although coronary stenting resolves the problem of atherosclerotic lesion-induced myocardial ischemia, it results in a new problem of neoatherosclerosis. New stent technology or drugs may solve this problem in the future.
P- Reviewer: Farand P, Prosser HC S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
| 1. | Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045-2051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1404] [Cited by in F6Publishing: 1507] [Article Influence: 125.6] [Reference Citation Analysis (0)] |
| 2. | Gruntzig A. Transluminal dilatation of coronary-artery stenosis. Lancet. 1978;1:263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 969] [Cited by in F6Publishing: 792] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 3. | Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987;316:701-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1377] [Cited by in F6Publishing: 1151] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 4. | Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, Detre K, Veltri L, Ricci D, Nobuyoshi M. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331:496-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3153] [Cited by in F6Publishing: 2951] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 5. | Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, Emanuelsson H, Marco J, Legrand V, Materne P. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994;331:489-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3312] [Cited by in F6Publishing: 3086] [Article Influence: 102.9] [Reference Citation Analysis (0)] |
| 6. | Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773-1780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3050] [Cited by in F6Publishing: 2861] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 7. | Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2134] [Cited by in F6Publishing: 2035] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 8. | Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Jüni P, Sianos G, Hellige G. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369:667-678. [PubMed] [Cited in This Article: ] |
| 9. | Kedhi E, Joesoef KS, McFadden E, Wassing J, van Mieghem C, Goedhart D, Smits PC. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010;375:201-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 546] [Cited by in F6Publishing: 561] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 10. | Leon MB, Nikolsky E, Cutlip DE, Mauri L, Liberman H, Wilson H, Patterson J, Moses J, Kandzari DE. Improved late clinical safety with zotarolimus-eluting stents compared with paclitaxel-eluting stents in patients with de novo coronary lesions: 3-year follow-up from the ENDEAVOR IV (Randomized Comparison of Zotarolimus- and Paclitaxel-Eluting Stents in Patients With Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2010;3:1043-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Jensen LO, Thayssen P, Hansen HS, Christiansen EH, Tilsted HH, Krusell LR, Villadsen AB, Junker A, Hansen KN, Kaltoft A. Randomized comparison of everolimus-eluting and sirolimus-eluting stents in patients treated with percutaneous coronary intervention: the Scandinavian Organization for Randomized Trials with Clinical Outcome IV (SORT OUT IV). Circulation. 2012;125:1246-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 12. | Lee SY, Hur SH, Lee SG, Kim SW, Shin DH, Kim JS, Kim BK, Ko YG, Choi D, Jang Y. Optical coherence tomographic observation of in-stent neoatherosclerosis in lesions with more than 50% neointimal area stenosis after second-generation drug-eluting stent implantation. Circ Cardiovasc Interv. 2015;8:e001878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Ip JH, Fuster V, Badimon L, Badimon J, Taubman MB, Chesebro JH. Syndromes of accelerated atherosclerosis: role of vascular injury and smooth muscle cell proliferation. J Am Coll Cardiol. 1990;15:1667-1687. [PubMed] [Cited in This Article: ] |
| 14. | Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527-3561. [PubMed] [Cited in This Article: ] |
| 15. | Otsuka F, Finn AV, Yazdani SK, Nakano M, Kolodgie FD, Virmani R. The importance of the endothelium in atherothrombosis and coronary stenting. Nat Rev Cardiol. 2012;9:439-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 263] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 16. | Asada H, Paszkowiak J, Teso D, Alvi K, Thorisson A, Frattini JC, Kudo FA, Sumpio BE, Dardik A. Sustained orbital shear stress stimulates smooth muscle cell proliferation via the extracellular signal-regulated protein kinase 1/2 pathway. J Vasc Surg. 2005;42:772-780. [PubMed] [Cited in This Article: ] |
| 17. | Ekstrand J, Razuvaev A, Folkersen L, Roy J, Hedin U. Tissue factor pathway inhibitor-2 is induced by fluid shear stress in vascular smooth muscle cells and affects cell proliferation and survival. J Vasc Surg. 2010;52:167-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | van Beusekom HM, Whelan DM, Hofma SH, Krabbendam SC, van Hinsbergh VW, Verdouw PD, van der Giessen WJ. Long-term endothelial dysfunction is more pronounced after stenting than after balloon angioplasty in porcine coronary arteries. J Am Coll Cardiol. 1998;32:1109-1117. [PubMed] [Cited in This Article: ] |
| 19. | Yamaji K, Kimura T, Morimoto T, Nakagawa Y, Inoue K, Kuramitsu S, Soga Y, Arita T, Shirai S, Ando K. Very long-term (15 to 23 years) outcomes of successful balloon angioplasty compared with bare metal coronary stenting. J Am Heart Assoc. 2012;1:e004085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Weidinger FF, McLenachan JM, Cybulsky MI, Gordon JB, Rennke HG, Hollenberg NK, Fallon JT, Ganz P, Cooke JP. Persistent dysfunction of regenerated endothelium after balloon angioplasty of rabbit iliac artery. Circulation. 1990;81:1667-1679. [PubMed] [Cited in This Article: ] |
| 21. | Duraiswamy N, Jayachandran B, Byrne J, Moore JE, Schoephoerster RT. Spatial distribution of platelet deposition in stented arterial models under physiologic flow. Ann Biomed Eng. 2005;33:1767-1777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Van der Heiden K, Gijsen FJ, Narracott A, Hsiao S, Halliday I, Gunn J, Wentzel JJ, Evans PC. The effects of stenting on shear stress: relevance to endothelial injury and repair. Cardiovasc Res. 2013;99:269-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich KL, Giddings VL, Coleman L, Wong GK, Edelman ER. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123:1400-1409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 573] [Cited by in F6Publishing: 607] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 24. | Bavry AA, Kumbhani DJ, Helton TJ, Bhatt DL. What is the risk of stent thrombosis associated with the use of paclitaxel-eluting stents for percutaneous coronary intervention?: a meta-analysis. J Am Coll Cardiol. 2005;45:941-946. [PubMed] [Cited in This Article: ] |
| 25. | Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med. 2006;119:1056-1061. [PubMed] [Cited in This Article: ] |
| 26. | Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435-2441. [PubMed] [Cited in This Article: ] |
| 27. | Nakazawa G, Finn AV, Vorpahl M, Ladich ER, Kolodgie FD, Virmani R. Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. J Am Coll Cardiol. 2011;57:390-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 28. | Cerrato E, Echavarría-Pinto M, Tandjung K, Macaya C, Escaned J. Optimizing vessel healing following drug eluting stent implantation with biodegradable polymer DES. Minerva Cardioangiol. 2014;62:407-420. [PubMed] [Cited in This Article: ] |
| 29. | Kimura T, Yokoi H, Nakagawa Y, Tamura T, Kaburagi S, Sawada Y, Sato Y, Yokoi H, Hamasaki N, Nosaka H. Three-year follow-up after implantation of metallic coronary-artery stents. N Engl J Med. 1996;334:561-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 423] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 30. | Kimura T, Abe K, Shizuta S, Odashiro K, Yoshida Y, Sakai K, Kaitani K, Inoue K, Nakagawa Y, Yokoi H. Long-term clinical and angiographic follow-up after coronary stent placement in native coronary arteries. Circulation. 2002;105:2986-2991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Doyle B, Rihal CS, O’Sullivan CJ, Lennon RJ, Wiste HJ, Bell M, Bresnahan J, Holmes DR. Outcomes of stent thrombosis and restenosis during extended follow-up of patients treated with bare-metal coronary stents. Circulation. 2007;116:2391-2398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 32. | Takano M, Yamamoto M, Mizuno K. Two cases of coronary stent thrombosis very late after bare-metal stenting. JACC Cardiovasc Interv. 2009;2:1286-1287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Inoue K, Abe K, Ando K, Shirai S, Nishiyama K, Nakanishi M, Yamada T, Sakai K, Nakagawa Y, Hamasaki N. Pathological analyses of long-term intracoronary Palmaz-Schatz stenting; Is its efficacy permanent? Cardiovasc Pathol. 2004;13:109-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Farb A, Shroff S, John M, Sweet W, Virmani R. Late arterial responses (6 and 12 months) after (32)P beta-emitting stent placement: sustained intimal suppression with incomplete healing. Circulation. 2001;103:1912-1919. [PubMed] [Cited in This Article: ] |
| 35. | Yokoyama S, Takano M, Yamamoto M, Inami S, Sakai S, Okamatsu K, Okuni S, Seimiya K, Murakami D, Ohba T. Extended follow-up by serial angioscopic observation for bare-metal stents in native coronary arteries: from healing response to atherosclerotic transformation of neointima. Circ Cardiovasc Interv. 2009;2:205-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1221] [Cited by in F6Publishing: 1288] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 37. | Takano M, Jang IK, Inami S, Yamamoto M, Murakami D, Okamatsu K, Seimiya K, Ohba T, Mizuno K. In vivo comparison of optical coherence tomography and angioscopy for the evaluation of coronary plaque characteristics. Am J Cardiol. 2008;101:471-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Takano M, Yamamoto M, Inami S, Murakami D, Ohba T, Seino Y, Mizuno K. Appearance of lipid-laden intima and neovascularization after implantation of bare-metal stents extended late-phase observation by intracoronary optical coherence tomography. J Am Coll Cardiol. 2009;55:26-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 39. | Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, Kolodgie FD, Finn AV, Virmani R. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011;57:1314-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 689] [Cited by in F6Publishing: 691] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 40. | Lee CW, Kang SJ, Park DW, Lee SH, Kim YH, Kim JJ, Park SW, Mintz GS, Park SJ. Intravascular ultrasound findings in patients with very late stent thrombosis after either drug-eluting or bare-metal stent implantation. J Am Coll Cardiol. 2010;55:1936-1942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 41. | Kang SJ, Lee CW, Song H, Ahn JM, Kim WJ, Lee JY, Park DW, Lee SW, Kim YH, Mintz GS. OCT analysis in patients with very late stent thrombosis. JACC Cardiovasc Imaging. 2013;6:695-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Otsuka F, Vorpahl M, Nakano M, Foerst J, Newell JB, Sakakura K, Kutys R, Ladich E, Finn AV, Kolodgie FD. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation. 2014;129:211-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 381] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 43. | Barilli A, Visigalli R, Sala R, Gazzola GC, Parolari A, Tremoli E, Bonomini S, Simon A, Closs EI, Dall’Asta V. In human endothelial cells rapamycin causes mTORC2 inhibition and impairs cell viability and function. Cardiovasc Res. 2008;78:563-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | Jiang P, Lan Y, Luo J, Ren YL, Liu DG, Pang JX, Liu J, Li J, Wang C, Cai JP. Rapamycin promoted thrombosis and platelet adhesion to endothelial cells by inducing membrane remodeling. BMC Cell Biol. 2014;15:7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Moss SC, Lightell DJ, Marx SO, Marks AR, Woods TC. Rapamycin regulates endothelial cell migration through regulation of the cyclin-dependent kinase inhibitor p27Kip1. J Biol Chem. 2010;285:11991-11997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Habib A, Karmali V, Polavarapu R, Akahori H, Cheng Q, Pachura K, Kolodgie FD, Finn AV. Sirolimus-FKBP12.6 impairs endothelial barrier function through protein kinase C-α activation and disruption of the p120-vascular endothelial cadherin interaction. Arterioscler Thromb Vasc Biol. 2013;33:2425-2431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Takano M, Yamamoto M, Xie Y, Murakami D, Inami S, Okamatsu K, Seimiya K, Ohba T, Seino Y, Mizuno K. Serial long-term evaluation of neointimal stent coverage and thrombus after sirolimus-eluting stent implantation by use of coronary angioscopy. Heart. 2007;93:1533-1536. [PubMed] [Cited in This Article: ] |
| 48. | Higo T, Ueda Y, Oyabu J, Okada K, Nishio M, Hirata A, Kashiwase K, Ogasawara N, Hirotani S, Kodama K. Atherosclerotic and thrombogenic neointima formed over sirolimus drug-eluting stent: an angioscopic study. JACC Cardiovasc Imaging. 2009;2:616-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 49. | Awata M, Kotani J, Uematsu M, Morozumi T, Watanabe T, Onishi T, Iida O, Sera F, Nanto S, Hori M. Serial angioscopic evidence of incomplete neointimal coverage after sirolimus-eluting stent implantation: comparison with bare-metal stents. Circulation. 2007;116:910-916. [PubMed] [Cited in This Article: ] |
| 50. | Yamamoto M, Takano M, Murakami D, Inami T, Kobayashi N, Inami S, Okamatsu K, Ohba T, Ibuki C, Hata N. The possibility of delayed arterial healing 5 years after implantation of sirolimus-eluting stents: serial observations by coronary angioscopy. Am Heart J. 2011;161:1200-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Kang SJ, Mintz GS, Akasaka T, Park DW, Lee JY, Kim WJ, Lee SW, Kim YH, Whan Lee C, Park SW. Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug-eluting stent implantation. Circulation. 2011;123:2954-2963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 269] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 52. | Yonetsu T, Kim JS, Kato K, Kim SJ, Xing L, Yeh RW, Sakhuja R, McNulty I, Lee H, Zhang S. Comparison of incidence and time course of neoatherosclerosis between bare metal stents and drug-eluting stents using optical coherence tomography. Am J Cardiol. 2012;110:933-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Habara M, Terashima M, Nasu K, Kaneda H, Yokota D, Ito T, Kurita T, Teramoto T, Kimura M, Kinoshita Y. Morphological differences of tissue characteristics between early, late, and very late restenosis lesions after first generation drug-eluting stent implantation: an optical coherence tomography study. Eur Heart J Cardiovasc Imaging. 2013;14:276-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Ando H, Amano T, Takashima H, Harada K, Kitagawa K, Suzuki A, Kunimura A, Shimbo Y, Harada K, Yoshida T. Differences in tissue characterization of restenotic neointima between sirolimus-eluting stent and bare-metal stent: integrated backscatter intravascular ultrasound analysis for in-stent restenosis. Eur Heart J Cardiovasc Imaging. 2013;14:996-1001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Kim JS, Lee JH, Shin DH, Kim BK, Ko YG, Choi D, Jang Y, Hong MK. Long-term outcomes of neointimal hyperplasia without neoatherosclerosis after drug-eluting stent implantation. JACC Cardiovasc Imaging. 2014;7:788-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 56. | Vergallo R, Yonetsu T, Uemura S, Park SJ, Lee S, Kato K, Jia H, Abtahian F, Tian J, Hu S. Correlation between degree of neointimal hyperplasia and incidence and characteristics of neoatherosclerosis as assessed by optical coherence tomography. Am J Cardiol. 2013;112:1315-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Alfonso F, Jimenez-Quevedo P, Gonzalo N, Medina M, Bañuelos C. Neoatherosclerosis after paclitaxel-coated balloon angioplasty for in-stent restenosis. Circulation. 2014;129:923-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Granada JF, Inami S, Aboodi MS, Tellez A, Milewski K, Wallace-Bradley D, Parker S, Rowland S, Nakazawa G, Vorpahl M. Development of a novel prohealing stent designed to deliver sirolimus from a biodegradable abluminal matrix. Circ Cardiovasc Interv. 2010;3:257-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 59. | Iqbal J, Onuma Y, Ormiston J, Abizaid A, Waksman R, Serruys P. Bioresorbable scaffolds: rationale, current status, challenges, and future. Eur Heart J. 2014;35:765-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 60. | Ormiston JA, Serruys PW, Onuma Y, van Geuns RJ, de Bruyne B, Dudek D, Thuesen L, Smits PC, Chevalier B, McClean D. First serial assessment at 6 months and 2 years of the second generation of absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study. Circ Cardiovasc Interv. 2012;5:620-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 61. | Serruys PW, Garcia-Garcia HM, Onuma Y. From metallic cages to transient bioresorbable scaffolds: change in paradigm of coronary revascularization in the upcoming decade? Eur Heart J. 2012;33:16-25b. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 245] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 62. | Onuma Y, Serruys PW, Perkins LE, Okamura T, Gonzalo N, García-García HM, Regar E, Kamberi M, Powers JC, Rapoza R. Intracoronary optical coherence tomography and histology at 1 month and 2, 3, and 4 years after implantation of everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model: an attempt to decipher the human optical coherence tomography images in the ABSORB trial. Circulation. 2010;122:2288-2300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 268] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 63. | Ormiston JA, De Vroey F, Serruys PW, Webster MW. Bioresorbable polymeric vascular scaffolds: a cautionary tale. Circ Cardiovasc Interv. 2011;4:535-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 64. | Muramatsu T, Onuma Y, García-García HM, Farooq V, Bourantas CV, Morel MA, Li X, Veldhof S, Bartorelli A, Whitbourn R. Incidence and short-term clinical outcomes of small side branch occlusion after implantation of an everolimus-eluting bioresorbable vascular scaffold: an interim report of 435 patients in the ABSORB-EXTEND single-arm trial in comparison with an everolimus-eluting metallic stent in the SPIRIT first and II trials. JACC Cardiovasc Interv. 2013;6:247-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 65. | Otsuka F, Pacheco E, Perkins LE, Lane JP, Wang Q, Kamberi M, Frie M, Wang J, Sakakura K, Yahagi K. Long-term safety of an everolimus-eluting bioresorbable vascular scaffold and the cobalt-chromium XIENCE V stent in a porcine coronary artery model. Circ Cardiovasc Interv. 2014;7:330-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |