Published online Aug 26, 2014. doi: 10.4330/wjc.v6.i8.847
Revised: April 9, 2014
Accepted: June 10, 2014
Published online: August 26, 2014
Pseudoexfoliation (PEX) syndrome is a well-recognized late-onset disease caused by a generalized fibrillopathy. It is linked to a broad spectrum of ocular complications including glaucoma and perioperative problems during cataract surgery. Apart from the long-known intraocular manifestations, PEX deposits have been found in a variety of extraocular locations and they appear to represent a systemic process associated with increased cardiovascular and cerebrovascular morbidity. However, as published results are inconsistent, the clinical significance of the extraocular PEX deposits remains controversial. Identification of PEX deposits in the heart and the vessel wall, epidemiologic studies, as well as, similarities in pathogenetic mechanisms have led to the hypothesis of a possible relation between fibrillar material and cardiovascular disease. Recent studies suggest that PEX syndrome is frequently linked to impaired heart and blood vessels function. Systemic and ocular blood flow changes, altered parasympathetic vascular control and baroreflex sensitivity, increased vascular resistance and decreased blood flow velocity, arterial endothelial dysfunction, high levels of plasma homocysteine and arterial hypertension have all been demonstrated in PEX subjects. Common features in the pathogenesis of both atherosclerosis and PEX, like oxidative stress and inflammation and a possible higher frequency of abdominal aorta aneurysm in PEX patients, could imply that these grey-white deposits and cardiovascular disorders are related or reflect different manifestations of the same process.
Core tip: Although much remains to be clarified concerning causes, pathogenesis and systemic role of pseudoexfoliation aggregations, there is accumulating epidemiologic, clinical and laboratory evidence that this well-described clinical entity may occur as part of a systemic disorder with cardiovascular implications. The present review aims to summarize current knowledge on cardiovascular complications which have been associated with these suspicious whitish-gray deposits.
- Citation: Andrikopoulos GK, Alexopoulos DK, Gartaganis SP. Pseudoexfoliation syndrome and cardiovascular diseases. World J Cardiol 2014; 6(8): 847-854
- URL: https://www.wjgnet.com/1949-8462/full/v6/i8/847.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i8.847
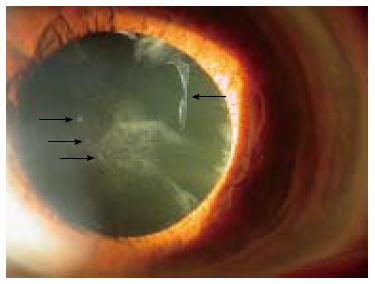
Pseudoexfoliation (PEX) syndrome is an age-related disorder characterized by accumulation and deposition of microfibrillar material on multiple ocular and extraocular structures (Figure 1). The definite clinical diagnosis of the syndrome is based on slit lamp observation of the whitish flake-like deposits on anterior segment structures, particularly on the anterior lens surface and the pupillary border of the iris.
PEX syndrome is the most common identifiable cause of open angle glaucoma, the so-called PEX glaucoma. It is also associated with cataract progression and intraoperative complications like zonular or posterior capsule rupture, poorly dilating pupil, vitreous loss, fibrinoid reaction, as well as, luxation of intraocular lens implants and corneal endothelial decompensation. In addition to the structures of the anterior segment of the eye, similar deposits have been identified in various visceral organs such as lung, heart, brain, vessels, kidney, gallbladder and meninges with unknown clinical significance.
PEX syndrome’s prevalence demonstrates considerable geographic, ethnic and racial variation. Low PEX syndrome rates (< 6% in patients older than 70 years) have been reported in Greenland Eskimos, India, the eastern part of the United States, Germany, Britain, Australia, Japan, Austria, Denmark and Switzerland. In contrast, high PEX syndrome frequencies (> 15%) have been reported in Iceland, Finland, Russia, Tunisia, Saudi Arabia, Sweden, Norway, Turkey and Greece[1-3].
Although specific synthesis and pathogenesis of PEX material are still unknown, the concept of an elastotic process has recently been established. Molecular and biochemical data support the pathogenetic concept of PEX as a type of stress-induced elastic microfibrillopathy. PEX etiopathogenesis involves both genetic and non-genetic factors. Single-nucleotide polymorphisms (SNPs) in the coding region of the lysyl-oxidase-like 1 (LOXL1) gene, which is responsible for cross-linking of elastin, have been identified as strong genetic risk factors for PEX syndrome and PEX glaucoma[4]. Moreover, non-genetic factors including ultraviolet light exposure, dietary factors, infectious agents and trauma, as well as, oxidative stress, hypoxia and inflammation have been suggested to act as co-modulating external factors[5]. Pro-fibrotic cytokines (Interleukin-6), growth factors (GFs) and particularly transforming growth factor-β1 (TGF-β1), impaired cellular protection system with increased cellular and oxidative stress, a change in the local balance between Matrix Metalloproteinases and Tissue Inhibitor of Metalloproteinases appear to be involved in the disorder of the fibrotic matrix with accumulation of extracellular material. Ischemia/hypoxia, cross-linking mechanisms and aggregation of misfolded stressed proteins, as well as, low-grade chronic inflammatory processes have also been implicated[6-9]. PEX material seems to represent a highly cross-linked glycoprotein-proteoglycan complex which is mainly consisted of elastic microfibrillar components, such as fibrillin-1 and latent transforming growth factor binding proteins, as well as, chaperone molecules, such as clusterin, and cross-linking enzymes, such as LOXL1[10].
A variety of epithelial, endothelial and mesenchymal cells may be associated with impaired synthesis of the extracellular fibrillar material in intra- and extraocular sites. Intraocular material seems to be produced mainly in the pre-equatorial lens epithelium, the nonpigmented ciliary epithelium and the iris pigment epithelium, and secondarily in the corneal endothelium, the trabecular endothelium and by almost all cell types of the iris stroma[11].Extraocular PEX material has been detected by electron microscope in connective tissue of visceral organs and in close proximity to fibroblasts, smooth and striated muscle cells, as well as, heart muscle cells[12,13]. These types of cells are probably involved in its production throughout the body. The fibrillar material shows ultrastructural and immunohistochemical similarities in both intra- and extraocular sites.
Although there is no clear-cut evidence that these deposits would cause degeneration of the extraocular tissues, they have been associated with cardiovascular and cerebrovascular morbidity. However, the clinical significance of the PEX-related systemic disorders remains controversial, as published results are inconsistent.
Studies implying a relationship between PEX syndrome and cardiovascular disease are mentioned below, along with others not supporting such a relationship.
In Australia, the Blue Mountains Eye Study proposed that a history of angina, hypertension or a combined history of angina, acute myocardial infarction and stroke are significantly associated with the presence of PEX syndrome after multivariate adjustment including age, sex, glaucoma and vascular risk factors. This was attributed to the effect of elastosis in the vessel wall[14]. Citirik et al[15] found a significantly higher prevalence of PEX in 50 patients with coronary artery disease (CAD) proven by angiography than in healthy controls, and a higher prevalence of CAD in PEX individuals. PEX has been positively associated with presence of CAD among a large cohort of patients scheduled for cataract surgery[16,17]. More recently, French et al[18] reported significant associations of PEX and PEX glaucoma with a variety of cardiovascular disorders, including various stages of ischemic heart disease, cardiomyopathy and aortic aneurysm. Moreover, subclinical myocardial ischemia, by tissue Doppler echocardiography, has been found in PEX patients[19].
The possibility of an association between PEX and asymptomatic myocardial diastolic dysfunction (an important cause of heart failure), as assessed by two-dimensional echocardiography and pulsed Doppler echocardiography, has been suggested[20]. In addition, a higher prevalence of heart failure has been described in PEX individuals[21].
Although there is convincing evidence that PEX syndrome is related to cardiovascular disorders, no significant relationship between PEX and CAD, aortic aneurysm or peripheral artery disease was reported by Emiroglu et al[22].In the same line, arterial hypertension, ischemic heart disease, cerebrovascular disease and prevalence of diabetes mellitus did not differ between patients with or without PEX[23-27]. Of note, a higher prevalence of arrhythmia has been found in PEX individuals[23]. Also, a study by Tarkkanen et al[28] failed to show any significant difference in the frequency of hypertension or ischemic heart disease between patients with primary open-angle glaucoma (POAG) and PEX glaucoma, while the latter had a lower frequency of diabetes mellitus. Moreover, in the Thessaloniki Eye Study, no association was found between PEX and the history of specific or any systemic disease (self-reported history of hypertension, diabetes, cardiovascular disease, migraine, heart attack, coronary artery bypass, vascular surgery)[29]. Avsar et al[30] found no significant differences in time domain heart rate variability parameters (a measure of cardiac autonomic function) between patients with PEX syndrome and control subjects. Furthermore, several studies failed to demonstrate an association between PEX deposits and increased cardiovascular, cerebrovascular or total mortality[31-35].
Major manifestations of cardiovascular diseases such as a decreased blood flow and ischemia have frequently been documented in PEX syndrome. Deposition of PEX material within the vasculature with subsequent increases in vascular resistance and decreases in blood flow, vascular dysregulation and altered parasympathetic vascular control may be implicated in the pathogenesis of cardiovascular disorders in PEX subjects. Moreover, local ischemia and atherosclerosis have been correlated with elastosis in different tissues[36,37].
Increased aortic stiffness in PEX patients, which may be at least partially responsible for the increased incidence of CAD in this patient group has been described[38]. In addition, using the ultrasound wall tracking system Visontai et al[39] reported a lower distensibility and higher rigidity in the common carotid artery, as well as, altered parasympathetic vascular control connected to increased plasma homocysteine level in PEX/PEX glaucoma than the control group. Similar results were drawn by other studies showing lower myocardial peak systolic tissue Doppler imaging velocities and increased carotid intima-media thickness in patients with PEX syndrome when compared to controls. On the contrary, PEX and carotid plaque measurements were weakly correlated[40]. An impairment of parasympathetic cardiovascular regulation, baroreflex sensitivity and pulse wave velocity has also been described in PEX patients[41]. Arterial stiffening is an indicator of increased cardiovascular disease risk and, likewise, decreased baroreflex sensitivity has been described in hypertension, heart failure, myocardial infarction and metabolic syndrome. Lower cutaneous capillary blood flow and altered response to cold and warmth, without any change of plasma endothelin-1 concentration was also demonstrated[42]. Furthermore, Köz et al[43] found high levels of coronary risk markers such as lipoprotein (a), apolipoprotein A, homocysteine, as well as, impaired brachial artery dilation and increased carotid intima-media thickness in PEX patients. In a study by Praveen et al[25] PEX subjects had a significantly lower ankle brachial index as compared to controls, suggestive of PEX as a possible risk factor for peripheral vascular disease.
Dayanir et al[44] concluded that PEX decreases ophthalmic artery blood flow velocities and increases vascular resistance. Similar conclusions were drawn by another study where PEX patients had decreased blood flow velocities in the central retinal and the short posterior cilliary arteries and increased vascular resistance in the ophthalmic and central retinal arteries[45]. Reduced blood flow in choroid, optic nerve head and peripapillary retina of the PEX affected eye has also been found[46,47]. Moreover, Galassi et al[48] using color Doppler imaging found a decrease in ocular perfusion pressure and deterioration of retrobulbar haemodynamics in PEX glaucoma patients as compared to primary open-angle glaucoma patients and healthy controls. Several studies have demonstrated anterior-chamber hypoxia and iris vasculopathy (narrowing, occlusion, neovascularization) in PEX patients[49-52]. PEX as a potential risk factor for central retinal vein occlusion has also been proposed[53,54]. In support of the above, Cursiefen et al[55] found that PEX was significantly more common in eyes enucleated secondary to central retinal vein occlusion as compared to age-matched eyes enucleated for an intraocular tumor; however, morphological evidence of a PEX associated vasculopathy of the central retinal vessels explaining this association was not shown. Endothelin-1, a potent vasoconstrictor which could contribute to the obliterative vasculopathy seems to be increased in the aqueous humor of PEX eyes[56].
A high frequency of PEX syndrome has been reported in patients with transient ischemic attacks[57-59]. A significantly higher prevalence of magnetic resonance images-defined white matter hyperintensities (ischemic changes) in patients with a clinical diagnosis of PEX with or without glaucoma vs control subjects, has also been documented[60]. Studies have indicated that the blood flow velocities of the middle cerebral artery were decreased in patients with PEX and PEX glaucoma[61,62] and there was a decrease in regional brain perfusion in PEX patients[63].
In addition, chronic cerebral diseases such as senile dementia, cerebral atrophy and chronic cerebral ischemia were more common in patients with PEX glaucoma than in those with POAG. The same study showed that patients with PEX glaucoma had higher probability of developing acute cerebrovascular disease than patients with POAG[31]. Alzheimer’s disease has also been correlated to PEX syndrome in several, though not all studies[64-67].
Arterial endothelial dysfunction is an independent predictor of future cardiovascular events. Vascular endothelium has a major role in the control of blood flow by releasing factors which may act either to contract the vascular smooth muscle, such as endothelin-1, or to relax it, such as nitric oxide. Atalar et al[68] found an impaired endothelial function in the brachial artery of patients with PEX syndrome, as assessed by vascular response to reactive hyperemia and sublingual nitroglycerin using high-resolution ultrasound. Endothelial dysfunction was attributed to the pseudoexfoliative fibrillar accumulation in the vessel wall. In the same line, endothelial dysfunction of the brachial artery was described in PEX subjects[69].
A major theory of atherosclerosis is that lesions result from an excessive fibroproliferative response to various forms of insult to the endothelium and smooth muscle of the vascular wall[70]. Endothelial exfoliation has been defined as thin, friable, mobile and translucent tissue, loosely adherent to the vascular wall[71] that may play a functional role in thrombus formation[72].
Nevertheless, other studies failed to demonstrate a correlation between PEX and endothelial damage, as biomarkers levels of endothelial injury (von Willebrand antigen, E-selectin, P-selectin and high sensitivity C-reactive protein) did not differ in blood plasma of patients with PEX vs controls[73,74].
Homocysteine is an independent risk factor for cardiovascular disease. It is associated with vascular injury and, thus, increased risk for stroke, CAD and venous thrombosis. Possible mechanisms of action include endothelial dysfunction, platelet aggregation and perturbation of clotting factors. In addition, alteration of the extracellular matrix of several tissues (mainly vessels), elastolysis and oxidative stress may be implicated.
Hyperhomocysteinemia has been suggested as a possible cause for increased vascular risk because of the potential to trigger the abnormal matrix accumulation in PEX patients. High levels of plasma homocysteine have been found in patients with PEX syndrome and PEX glaucoma[75-84]. Homocysteine concentration has been found to be elevated[83] or unaffected[77] in aqueous humor of patients with PEX glaucoma, while increased in PEX glaucoma patients’ tears[84]. Vitamins B6, B12 and folate, which are involved in homocysteine metabolism and negatively correlated with total plasma homocysteine levels, have been reported to be decreased in PEX glaucoma patients[85], though not differing between PEX and control groups in another study[77]. On the contrary, Turacli et al[86] did not confirm the relationship between plasma homocysteine and PEX syndrome. Hyperhomocysteinemia has also been implicated in the decrease of both LOX activity and expression in vascular endothelial cells[87]. LOX downregulation has been associated with endothelial dysfunction, characteristic of earlier stages of the atherosclerotic process[88]. A possible association between SNPs in the LOXL1 gene (which is linked with PEX syndrome) and spontaneous cervical artery dissection has also been proposed[89].
It is known that hypertension is a major risk factor for stroke, myocardial infarction, heart failure, aneurysms of the arteries (e.g., aortic aneurysm), peripheral arterial disease and chronic kidney disease. At least two studies have demonstrated a higher rate of arterial hypertension in patients with PEX[14,90]. Endothelial damage, impairment of the parasympathetic vascular regulation and elastosis have been implicated. Renal artery stenosis with subsequent arterial hypertension has also been reported[91]. However, reports are conflicting and no clear association has yet been proven, as other studies failed to demonstrate any significant relationship between PEX and arterial hypertension[15,16,17,23-26,61,92], or found arterial hypertension to be less common in PEX subjects[28,93,94].
Impairment in systemic macro- and microcirculation in PEX patients has been suggested. Abdominal aortic aneurysms have been attributed to atherosclerosis, though other factors are involved in their formation. An association between aneurysms of the abdominal aorta and PEX syndrome has been proposed. Histopathological examination of aortic-wall samples from patients with ocular PEX syndrome revealed accumulation of focal PEX deposits in the adventitial and subendothelial connective tissue, pronounced fibrosis, and elastosis of the tunica intima[95]. Abdominal aorta aneurysm was observed with a higher frequency in PEX patients than in control group[91,96], although, other studies failed to demonstrate any significant association[97,98].
Apart from epidemiologic studies and the presence of PEX deposits on vessel wall, a possible relation between PEX material and cardiovascular disease may be supported by similar features in their pathogenesis. In addition to vascular endothelial dysfunction, hyperhomocysteinemia and blood flow changes mentioned above, disorders of the extracellular matrix by growth factors, matrix metalloproteinases, cytokines and altered enzymic action constitute part of atherosclerosis[99] and PEX fibrillopathy process. Altintas et al[100] demonstrated higher serum antiphospholipid antibodies (a risk factor for cardiovascular and cerebrovascular disease) in patients with PEX and PEX glaucoma than in healthy controls and in patients with POAG. In support of the above, serum asymmetric dimethyl arginine and YKL-40 levels (both independent cardiovascular risk factors) have been found higher in PEX patients than those of the control group[101,102].
Atherosclerosis is associated with a number of oxidative events like low density lipoproteins oxidation, production of intracellular reactive oxygen species (ROS) and reactive nitrogen species (RNS), as well as, endothelial dysfunction and plaque disruption[103]. The oxidative-antioxidative balance is disturbed in patients with PEX syndrome as supported by reduced levels of antioxidants such as ascorbic acid, glutathione, trace elements, antioxidative enzymes in aqueous humor and serum and increased levels of oxidants such as hydrogen peroxide or nitric oxide, as well as, oxidative stress markers[104].
Inflammation plays a major role in all phases of atherosclerosis. Inflammatory cells like macrophages and lymphocytes both migrate from the blood and multiply within the atherosclerotic plaques. Activation of these cells leads to lytic enzymes, cytokines, chemokines and growth factors release that induce further damage[105]. Stress-induced, temporally restricted subclinical inflammation in anterior segment tissues is detected during the early stages of the fibrotic PEX process[10]. Moreover, inflammatory markers such as alpha-1 antitrypsin, Interleukin-6, high-sensitivity C-reactive protein and Tumor Necrosis factor alpha have been reported to be increased in PEX subjects[106-108].
Although more data is still required, an increased incidence of cardiovascular disorders in PEX patients and several common features in their pathogenesis suggest that PEX may be an independent risk factor for cardiovascular disease or it may occur as part of a systemic disorder with cardiovascular implications. The pathogenesis of PEX glaucoma and CAD in PEX patients may reflect different manifestations of the same process. Patients with PEX syndrome should be informed and examined frequently as cardiovascular risk may be present throughout.
P- Reviewer: Cumurcu T, Speckauskas M S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Vesti E, Kivelä T. Exfoliation syndrome and exfoliation glaucoma. Prog Retin Eye Res. 2000;19:345-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 116] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 2. | Ringvold A. Epidemiology of the pseudo-exfoliation syndrome. Acta Ophthalmol Scand. 1999;77:371-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Forsius H. Exfoliation syndrome in various ethnic populations. Acta Ophthalmol Suppl. 1988;184:71-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson DF, Stefansson H, Jonsson T, Jonasdottir A, Jonasdottir A, Stefansdottir G. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science. 2007;317:1397-1400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 5. | Schlötzer-Schrehardt U. Pseudoexfoliation syndrome: the puzzle continues. J Ophthalmic Vis Res. 2012;7:187-189. [PubMed] [Cited in This Article: ] |
| 6. | Gartaganis SP, Georgakopoulos CD, Mela EK, Exarchou A, Ziouti N, Assouti M, Vynios DH. Matrix metalloproteinases and their inhibitors in exfoliation syndrome. Ophthalmic Res. 2002;34:165-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Gartaganis SP, Patsoukis NE, Nikolopoulos DK, Georgiou CD. Evidence for oxidative stress in lens epithelial cells in pseudoexfoliation syndrome. Eye (Lond). 2007;21:1406-1411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Schlötzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006;141:921-937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 379] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 9. | Schlötzer-Schrehardt U. Molecular pathology of pseudoexfoliation syndrome/glaucoma--new insights from LOXL1 gene associations. Exp Eye Res. 2009;88:776-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Zenkel M, Lewczuk P, Jünemann A, Kruse FE, Naumann GO, Schlötzer-Schrehardt U. Proinflammatory cytokines are involved in the initiation of the abnormal matrix process in pseudoexfoliation syndrome/glaucoma. Am J Pathol. 2010;176:2868-2879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Ritch R, Schlötzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol. 2001;45:265-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 536] [Cited by in F6Publishing: 572] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 12. | Schlötzer-Schrehardt UM, Koca MR, Naumann GO, Volkholz H. Pseudoexfoliation syndrome. Ocular manifestation of a systemic disorder? Arch Ophthalmol. 1992;110:1752-1756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 276] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Streeten BW, Li ZY, Wallace RN, Eagle RC, Keshgegian AA. Pseudoexfoliative fibrillopathy in visceral organs of a patient with pseudoexfoliation syndrome. Arch Ophthalmol. 1992;110:1757-1762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 267] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Mitchell P, Wang JJ, Smith W. Association of pseudoexfoliation syndrome with increased vascular risk. Am J Ophthalmol. 1997;124:685-687. [PubMed] [Cited in This Article: ] |
| 15. | Citirik M, Acaroglu G, Batman C, Yildiran L, Zilelioglu O. A possible link between the pseudoexfoliation syndrome and coronary artery disease. Eye (Lond). 2007;21:11-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Andrikopoulos GK, Mela EK, Georgakopoulos CD, Papadopoulos GE, Damelou AN, Alexopoulos DK, Gartaganis SP. Pseudoexfoliation syndrome prevalence in Greek patients with cataract and its association to glaucoma and coronary artery disease. Eye (Lond). 2009;23:442-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Sekeroglu MA, Bozkurt B, Irkec M, Ustunel S, Orhan M, Saracbasi O. Systemic associations and prevalence of exfoliation syndrome in patients scheduled for cataract surgery. Eur J Ophthalmol. 2008;18:551-555. [PubMed] [Cited in This Article: ] |
| 18. | French DD, Margo CE, Harman LE. Ocular pseudoexfoliation and cardiovascular disease: a national cross-section comparison study. N Am J Med Sci. 2012;4:468-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Demir N, Ulus T, Yucel OE, Kumral ET, Singar E, Tanboga HI. Assessment of myocardial ischaemia using tissue Doppler imaging in pseudoexfoliation syndrome. Eye (Lond). 2011;25:1177-1180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Bojić L, Ermacora R, Polić S, Ivanisević M, Mandić Z, Rogosić V, Lesin M. Pseudoexfoliation syndrome and asymptomatic myocardial dysfunction. Graefes Arch Clin Exp Ophthalmol. 2005;243:446-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Sainz Gómez C, Moreno-Montañés J, Escudero Berasategui JM, Sádaba Echarri LM, Fernández Hortelano A, García Layana A. [Prevalence and risk factors of pseudoexfoliation syndrome in institutionalized geriatric patients in Navarra]. Arch Soc Esp Oftalmol. 2003;78:383-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Emiroglu MY, Coskun E, Karapinar H, Capkın M, Kaya Z, Kaya H, Akcakoyun M, Kargin R, Simsek Z, Acar G. Is pseudoexfoliation syndrome associated with coronary artery disease? N Am J Med Sci. 2010;2:487-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Brajković J, Kalauz-Surać I, Ercegović A, Miletić-Jurić A, Sušić N, Burić Z. Ocular pseudoexfoliation syndrome and internal systemic diseases. Acta Clin Croat. 2007;46:57-61. [Cited in This Article: ] |
| 24. | Allingham RR, Loftsdottir M, Gottfredsdottir MS, Thorgeirsson E, Jonasson F, Sverisson T, Hodge WG, Damji KF, Stefánsson E. Pseudoexfoliation syndrome in Icelandic families. Br J Ophthalmol. 2001;85:702-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Praveen MR, Shah SK, Vasavada AR, Diwan RP, Shah SM, Zumkhawala BR, Thomas R. Pseudoexfoliation as a risk factor for peripheral vascular disease: a case-control study. Eye (Lond). 2011;25:174-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Spečkauskas M, Tamošiūnas A, Jašinskas V. Association of ocular pseudoexfoliation syndrome with ischaemic heart disease, arterial hypertension and diabetes mellitus. Acta Ophthalmol. 2012;90:e470-e475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Viso E, Rodríguez-Ares MT, Gude F. Prevalence of pseudoexfoliation syndrome among adult Spanish in the Salnés eye Study. Ophthalmic Epidemiol. 2010;17:118-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Tarkkanen A, Reunanen A, Kivelä T. Frequency of systemic vascular diseases in patients with primary open-angle glaucoma and exfoliation glaucoma. Acta Ophthalmol. 2008;86:598-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Anastasopoulos E, Topouzis F, Wilson MR, Harris A, Pappas T, Yu F, Koskosas A, Founti P, Coleman AL. Characteristics of pseudoexfoliation in the Thessaloniki Eye Study. J Glaucoma. 2011;20:160-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Avsar A, Ozturk F, Melek M, Saglam H, Kocogullari CU, Emmiler M, Dursun H, Celik A, Kilit C, Onrat E. Pseudoexfoliation syndrome and cardiac autonomic dysfunction. J Electrocardiol. 2007;40 Suppl 1:S16. [Cited in This Article: ] |
| 31. | Ritland JS, Egge K, Lydersen S, Juul R, Semb SO. Exfoliative glaucoma and primary open-angle glaucoma: associations with death causes and comorbidity. Acta Ophthalmol Scand. 2004;82:401-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Shrum KR, Hattenhauer MG, Hodge D. Cardiovascular and cerebrovascular mortality associated with ocular pseudoexfoliation. Am J Ophthalmol. 2000;129:83-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Ringvold A, Blika S, Sandvik L. Pseudo-exfoliation and mortality. Acta Ophthalmol Scand. 1997;75:255-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Aström S, Stenlund H, Lindén C. Incidence and prevalence of pseudoexfoliations and open-angle glaucoma in northern Sweden: II. Results after 21 years of follow-up. Acta Ophthalmol Scand. 2007;85:832-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Grødum K, Heijl A, Bengtsson B. Glaucoma and mortality. Graefes Arch Clin Exp Ophthalmol. 2004;242:397-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Billis A, Magna LA. Prostate elastosis: a microscopic feature useful for the diagnosis of postatrophic hyperplasia. Arch Pathol Lab Med. 2000;124:1306-1309. [PubMed] [Cited in This Article: ] |
| 37. | Sugai M, Kono R, Kunita Y. A morphologic study on human conduction system of heart considering influences of some disorders of individuals. Acta Pathol Jpn. 1981;31:13-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 38. | Alpaslan M, Karalezli A, Borazan M, Köktekir BE, Müderrisoğlu IH. Decreased aortic root elasticity-as a novel systemic manifestation of the pseudoexfoliation syndrome: an observational study. Anadolu Kardiyol Derg. 2012;12:483-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Visontai Z, Merisch B, Kollai M, Holló G. Increase of carotid artery stiffness and decrease of baroreflex sensitivity in exfoliation syndrome and glaucoma. Br J Ophthalmol. 2006;90:563-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Ulus T, Nadir A, Yaz YA, Ozdemir AO, Mutlu F, Yazici HU, Cavusoglu Y, Yildirim N. Cardiovascular involvement in patients with pseudoexfoliation syndrome. J Cardiovasc Med (Hagerstown). 2013;14:587-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Visontai Z, Horváth T, Kollai M, Holló G. Decreased cardiovagal regulation in exfoliation syndrome. J Glaucoma. 2008;17:133-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Holló G, Lakatos P, Farkas K. Cold pressor test and plasma endothelin-1 concentration in primary open-angle and capsular glaucoma. J Glaucoma. 1998;7:105-110. [PubMed] [Cited in This Article: ] |
| 43. | Köz C, Türkcü F, Gürbüz Köz Ö, Yokuşoǧlu M, Baysan O, Yarangümeli A, Uzun M, Kural G. Endothelial function and novel vascular risk factors in pseudoexfoliation syndrome. Turkiye Klinikleri J Med Sci. 2009;29:1510-1516. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 44. | Dayanir V, Topaloğlu A, Ozsunar Y, Keceli M, Okyay P, Harris A. Orbital blood flow parameters in unilateral pseudoexfoliation syndrome. Int Ophthalmol. 2009;29:27-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Yüksel N, Karabaş VL, Arslan A, Demirci A, Cağlar Y. Ocular hemodynamics in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Ophthalmology. 2001;108:1043-1049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Sibour G, Finazzo C, Boles Carenini A. Monolateral pseudoexfoliatio capsulae: a study of choroidal blood flow. Acta Ophthalmol Scand Suppl. 1997;13-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Ocakoglu O, Koyluoglu N, Kayiran A, Tamcelik N, Ozkan S. Microvascular blood flow of the optic nerve head and peripapillary retina in unilateral exfoliation syndrome. Acta Ophthalmol Scand. 2004;82:49-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Galassi F, Giambene B, Menchini U. Ocular perfusion pressure and retrobulbar haemodynamics in pseudoexfoliative glaucoma. Graefes Arch Clin Exp Ophthalmol. 2008;246:411-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Helbig H, Schlötzer-Schrehardt U, Noske W, Kellner U, Foerster MH, Naumann GO. Anterior-chamber hypoxia and iris vasculopathy in pseudoexfoliation syndrome. Ger J Ophthalmol. 1994;3:148-153. [PubMed] [Cited in This Article: ] |
| 50. | Asano N, Schlötzer-Schrehardt U, Naumann GO. A histopathologic study of iris changes in pseudoexfoliation syndrome. Ophthalmology. 1995;102:1279-1290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 51. | Brooks AM, Gillies WE. The development of microneovascular changes in the iris in pseudoexfoliation of the lens capsule. Ophthalmology. 1987;94:1090-1097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Vannas A. Fluorescein angiography of the vessels of the iris in pseudoexfoliation of the lens capsule, capsular glaucoma and some other forms of glaucoma. Acta Ophthalmol Suppl. 1969;105:1-75. [PubMed] [Cited in This Article: ] |
| 53. | Saatci OA, Ferliel ST, Ferliel M, Kaynak S, Ergin MH. Pseudoexfoliation and glaucoma in eyes with retinal vein occlusion. Int Ophthalmol. 1999;23:75-78. [PubMed] [Cited in This Article: ] |
| 54. | Ritch R, Prata TS, de Moraes CG, Vessani RM, Costa VP, Konstas AG, Liebmann JM, Schlötzer-Schrehardt U. Association of exfoliation syndrome and central retinal vein occlusion: an ultrastructural analysis. Acta Ophthalmol. 2010;88:91-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Cursiefen C, Hammer T, Küchle M, Naumann GO, Schlötzer-Schrehardt U. Pseudoexfoliation syndrome in eyes with ischemic central retinal vein occlusion. A histopathologic and electron microscopic study. Acta Ophthalmol Scand. 2001;79:476-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Koliakos GG, Konstas AG, Schlötzer-Schrehardt U, Hollo G, Mitova D, Kovatchev D, Maloutas S, Georgiadis N. Endothelin-1 concentration is increased in the aqueous humour of patients with exfoliation syndrome. Br J Ophthalmol. 2004;88:523-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Oruç S, Orhan M, Irkeç M. Generalized iris transluminance and pseudoexfoliation syndrome in patients with transient ischemic attack and dark-colored eyes. Ann Ophthalmol. 2001;33:113-115. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Repo LP, Suhonen MT, Teräsvirta ME, Koivisto KJ. Color Doppler imaging of the ophthalmic artery blood flow spectra of patients who have had a transient ischemic attack. Correlations with generalized iris transluminance and pseudoexfoliation syndrome. Ophthalmology. 1995;102:1199-1205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Repo LP, Teräsvirta ME, Koivisto KJ. Generalized transluminance of the iris and the frequency of the pseudoexfoliation syndrome in the eyes of transient ischemic attack patients. Ophthalmology. 1993;100:352-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Yüksel N, Anik Y, Altintaş O, Onur I, Cağlar Y, Demirci A. Magnetic resonance imaging of the brain in patients with pseudoexfoliation syndrome and glaucoma. Ophthalmologica. 2006;220:125-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 61. | Akarsu C, Unal B. Cerebral haemodynamics in patients with pseudoexfoliation glaucoma. Eye (Lond). 2005;19:1297-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Yüksel N, Anik Y, Kiliç A, Karabaş V, Demirci A, Cağlar Y. Cerebrovascular blood flow velocities in pseudoexfoliation. Graefes Arch Clin Exp Ophthalmol. 2006;244:316-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Kaya E, Öztürk F. Evaluation of Regional Brain Perfusion in Patients with Pseudoexfoliation Syndrome. Neuro-Ophthalmol. 2011;35:255-258. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 64. | Linnér E, Popovic V, Gottfries CG, Jonsson M, Sjögren M, Wallin A. The exfoliation syndrome in cognitive impairment of cerebrovascular or Alzheimer’s type. Acta Ophthalmol Scand. 2001;79:283-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Janciauskiene S, Krakau T. Alzheimer’s peptide: a possible link between glaucoma, exfoliation syndrome and Alzheimer’s disease. Acta Ophthalmol Scand. 2001;79:328-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Cumurcu T, Dorak F, Cumurcu BE, Erbay LG, Ozsoy E. Is there any relation between pseudoexfoliation syndrome and Alzheimer’s type dementia? Semin Ophthalmol. 2013;28:224-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Ekström C, Kilander L. Pseudoexfoliation and Alzheimer’s disease: a population-based 30-year follow-up study. Acta Ophthalmol. 2014;92:355-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 68. | Atalar PT, Atalar E, Kilic H, Abbasoglu OE, Ozer N, Aksöyek S, Ovünç K, Ozmen F, Gürsel E. Impaired systemic endothelial function in patients with pseudoexfoliation syndrome. Int Heart J. 2006;47:77-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 69. | Naji M, Naji F, Suran D, Gracner T, Kanic V, Pahor D. [Systemic endothelial dysfunction in patients with pseudoexfoliation syndrome]. Klin Monbl Augenheilkd. 2008;225:963-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7939] [Cited by in F6Publishing: 7639] [Article Influence: 246.4] [Reference Citation Analysis (0)] |
| 71. | Itoh A, Miyazaki S, Nonogi H, Daikoku S, Haze K. Angioscopic prediction of successful dilatation and of restenosis in percutaneous transluminal coronary angioplasty. Significance of yellow plaque. Circulation. 1995;91:1389-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Rössig L, Dimmeler S, Zeiher AM. Apoptosis in the vascular wall and atherosclerosis. Basic Res Cardiol. 2001;96:11-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 73. | Stafiej J, Malukiewicz G, Lesiewska-Junk H, Rość D, Kaźmierczak K. Endothelial cell markers in patients with pseudoexfoliation syndrome. ScientificWorldJournal. 2012;2012:863949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 74. | Yüksel N, Pirhan D, Altintaş O, Cağlar Y. Systemic high-sensitivity C-reactive protein level in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. J Glaucoma. 2010;19:373-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 75. | Leibovitch I, Kurtz S, Shemesh G, Goldstein M, Sela BA, Lazar M, Loewenstein A. Hyperhomocystinemia in pseudoexfoliation glaucoma. J Glaucoma. 2003;12:36-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 76. | Vessani RM, Ritch R, Liebmann JM, Jofe M. Plasma homocysteine is elevated in patients with exfoliation syndrome. Am J Ophthalmol. 2003;136:41-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 77. | Puustjärvi T, Blomster H, Kontkanen M, Punnonen K, Teräsvirta M. Plasma and aqueous humour levels of homocysteine in exfoliation syndrome. Graefes Arch Clin Exp Ophthalmol. 2004;242:749-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 78. | Altintaş O, Maral H, Yüksel N, Karabaş VL, Dillioğlugil MO, Cağlar Y. Homocysteine and nitric oxide levels in plasma of patients with pseudoexfoliation syndrome, pseudoexfoliation glaucoma, and primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2005;243:677-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 79. | Clement CI, Goldberg I, Healey PR, Graham SL. Plasma homocysteine, MTHFR gene mutation, and open-angle glaucoma. J Glaucoma. 2009;18:73-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Cumurcu T, Sahin S, Aydin E. Serum homocysteine, vitamin B 12 and folic acid levels in different types of glaucoma. BMC Ophthalmol. 2006;6:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 81. | Tranchina L, Centofanti M, Oddone F, Tanga L, Roberti G, Liberatoscioli L, Cortese C, Manni G. Levels of plasma homocysteine in pseudoexfoliation glaucoma. Graefes Arch Clin Exp Ophthalmol. 2011;249:443-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 82. | Turgut B, Kaya M, Arslan S, Demir T, Güler M, Kaya MK. Levels of circulating homocysteine, vitamin B6, vitamin B12, and folate in different types of open-angle glaucoma. Clin Interv Aging. 2010;5:133-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 83. | Bleich S, Roedl J, Von Ahsen N, Schlötzer-Schrehardt U, Reulbach U, Beck G, Kruse FE, Naumann GO, Kornhuber J, Jünemann AG. Elevated homocysteine levels in aqueous humor of patients with pseudoexfoliation glaucoma. Am J Ophthalmol. 2004;138:162-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Roedl JB, Bleich S, Reulbach U, Rejdak R, Kornhuber J, Kruse FE, Schlötzer-Schrehardt U, Jünemann AG. Homocysteine in tear fluid of patients with pseudoexfoliation glaucoma. J Glaucoma. 2007;16:234-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Roedl JB, Bleich S, Reulbach U, Rejdak R, Naumann GO, Kruse FE, Schlötzer-Schrehardt U, Kornhuber J, Jünemann AG. Vitamin deficiency and hyperhomocysteinemia in pseudoexfoliation glaucoma. J Neural Transm. 2007;114:571-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 86. | Turaçli ME, Tekeli O, Ozdemir F, Akar N. Methylenetetrahydrofolate reductase 677 C-T and homocysteine levels in Turkish patients with pseudoexfoliation. Clin Experiment Ophthalmol. 2005;33:505-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | Raposo B, Rodríguez C, Martínez-González J, Badimon L. High levels of homocysteine inhibit lysyl oxidase (LOX) and downregulate LOX expression in vascular endothelial cells. Atherosclerosis. 2004;177:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 88. | Rodríguez C, Martínez-González J, Raposo B, Alcudia JF, Guadall A, Badimon L. Regulation of lysyl oxidase in vascular cells: lysyl oxidase as a new player in cardiovascular diseases. Cardiovasc Res. 2008;79:7-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 89. | Kuhlenbäumer G, Friedrichs F, Kis B, Berlit P, Maintz D, Nassenstein I, Nabavi D, Dittrich R, Stoll M, Ringelstein B. Association between single nucleotide polymorphisms in the lysyl oxidase-like 1 gene and spontaneous cervical artery dissection. Cerebrovasc Dis. 2007;24:343-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 90. | Miyazaki M, Kubota T, Kubo M, Kiyohara Y, Iida M, Nose Y, Ishibashi T. The prevalence of pseudoexfoliation syndrome in a Japanese population: the Hisayama study. J Glaucoma. 2005;14:482-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 91. | Gonen KA, Gonen T, Gumus B. Renal artery stenosis and abdominal aorta aneurysm in patients with pseudoexfoliation syndrome. Eye (Lond). 2013;27:735-741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 92. | Shazly TA, Farrag AN, Kamel A, Al-Hussaini AK. Prevalence of pseudoexfoliation syndrome and pseudoexfoliation glaucoma in Upper Egypt. BMC Ophthalmol. 2011;11:18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 93. | Jonas JB, Gründler AE. Prevalence of diabetes mellitus and arterial hypertension in primary and secondary open-angle glaucomas. Graefes Arch Clin Exp Ophthalmol. 1998;236:202-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 94. | Shingleton BJ, Heltzer J, O’Donoghue MW. Outcomes of phacoemulsification in patients with and without pseudoexfoliation syndrome. J Cataract Refract Surg. 2003;29:1080-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 95. | Schumacher S, Schlötzer-Schrehardt U, Martus P, Lang W, Naumann GO. Pseudoexfoliation syndrome and aneurysms of the abdominal aorta. Lancet. 2001;357:359-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 140] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 96. | Djordjevic-Jocic J, Jovanovic P, Bozic M, Tasic A, Rancic Z. Prevalence and early detection of abdominal aortic aneurysm in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Curr Eye Res. 2012;37:617-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 97. | Hietanen J, Soisalon-Soininen S, Kivelä T, Tarkkanen A. Evaluation of the clinical association between exfoliation syndrome and abdominal aortic aneurysm. Acta Ophthalmol Scand. 2002;80:617-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 98. | Pierre Filho PTP, de Araújo LC, Costa VP, de Medeiros CAF, Lucas GC. Analysis of correlation between pseudoexfoliation syndrome and aneurysm of the abdominal aorta. Arq Bras Oftalmol. 2004;67:407-410. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 99. | Rajavashisth TB, Liao JK, Galis ZS, Tripathi S, Laufs U, Tripathi J, Chai NN, Xu XP, Jovinge S, Shah PK. Inflammatory cytokines and oxidized low density lipoproteins increase endothelial cell expression of membrane type 1-matrix metalloproteinase. J Biol Chem. 1999;274:11924-11929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 147] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 100. | Altintas O, Yuksel N, Sonmez GT, Ozkan B, Altintas L, Caliskan Ş, Caglar Y. Serum antiphospholipid antibody levels in pseudoexfoliation. J Glaucoma. 2012;21:326-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 101. | Tosun M, Erdurmus M, Bugdayci G, Celebi S, Alcelik A. Aqueous humour and serum concentration of asymmetric dimethyl arginine in pseudoexfoliation syndrome. Br J Ophthalmol. 2012;96:1137-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Türkyılmaz K, Öner V, Kırbas A, Sevim MS, Sekeryapan B, Özgür G, Durmus M. Serum YKL-40 levels as a novel marker of inflammation and endothelial dysfunction in patients with pseudoexfoliation syndrome. Eye (Lond). 2013;27:854-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 103. | Stocker R, Keaney JF. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381-1478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1746] [Cited by in F6Publishing: 1719] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 104. | Schlötzer-Schrehardt U. [Oxidative stress and pseudoexfoliation glaucoma]. Klin Monbl Augenheilkd. 2010;227:108-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 105. | Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15621] [Cited by in F6Publishing: 15151] [Article Influence: 606.0] [Reference Citation Analysis (0)] |
| 106. | Cumurcu T, Ozyurt H, Demir HD, Yardim H. Serum alpha-1-antitriypsin levels in patients with pseudoexfolative syndrome. Curr Eye Res. 2008;33:159-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 107. | Yildirim Z, Yildirim F, Uçgun NI, Sepici-Dinçel A. The role of the cytokines in the pathogenesis of pseudoexfoliation syndrome. Int J Ophthalmol. 2013;6:50-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 15] [Reference Citation Analysis (0)] |
| 108. | Sorkhabi R, Ghorbanihaghjo A, Ahoor M, Nahaei M, Rashtchizadeh N. High-sensitivity C-reactive Protein and Tumor Necrosis Factor Alpha in Pseudoexfoliation Syndrome. Oman Med J. 2013;28:16-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |