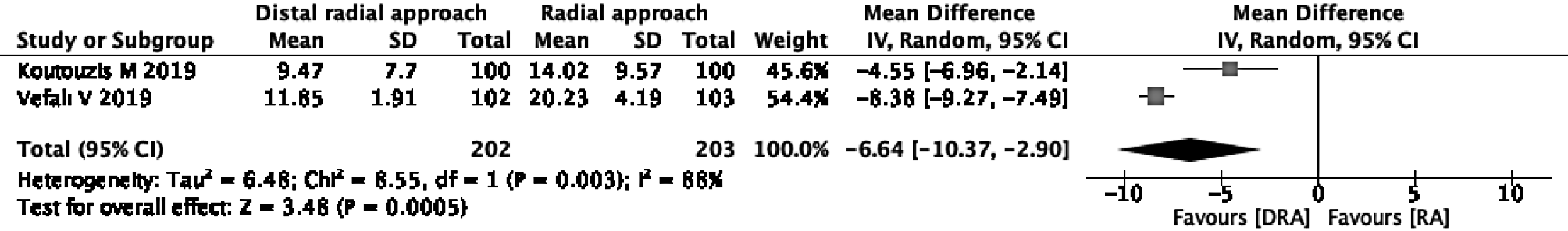Published online May 26, 2021. doi: 10.4330/wjc.v13.i5.144
Peer-review started: January 11, 2021
First decision: February 28, 2021
Revised: March 9, 2021
Accepted: April 26, 2021
Article in press: April 26, 2021
Published online: May 26, 2021
The traditional radial approach (RA) is recommended as the standard method for coronary angiography (CAG), while a distal RA (DRA) has been recently used for CAG.
To assess the efficacy and safety of the DRA vs RA during CAG.
The following databases were searched through December 2020: MEDLINE, the Cochrane Central Register of Controlled Trials, EMBASE, the World Health Organization International Clinical Trials Platform Search Portal, and ClinicalTrials.gov. Individual randomized-controlled trials for adult patients undergoing cardiac catheterization were included. The primary outcomes were the successful cannulation rate and the incidence of radial artery spasm (RAS) and radial artery occlusion (RAO). Study selection, data abstraction and quality assessment were independently performed using the Grading of Recommendations, Assessment, Development, and Evaluation approach.
Three randomized control trials and 13 registered trials were identified. The two approaches showed similar successful cannulation rates [risk ratio (RR) 0.90, 95% confidence interval (CI): 0.72-1.13]. The DRA did not decrease RAS (RR 0.43, 95%CI: 0.08-2.49) and RAO (RR 0.48, 95%CI: 0.18-1.29). Patients with the DRA had a shorter hemostasis time in comparison to those with the RA (mean difference -6.64, 95%CI: -10.37 to -2.90). The evidence of certainty was low.
For CAG, the DRA would be safer than the RA with comparable cannulation rates. Given the limited data, additional research, including studies with standard protocols, is necessary.
Core Tip: No consensus is available in the literature about which technique for coronary angiography—distal radial approach (DRA) or radial approach (RA)—is more beneficial to patients. This is the first systematic review and meta-analysis to compare clinical data on the DRA and RA. We investigated the successful cannulation rate, the incidence of radial artery spasm and radial artery occlusion, the mean number of punctures, and the mean time for hemostasis with the two approaches. The present study indicated the DRA to be safer than the RA, with comparable procedure rates. Further research, including studies with standard protocols, is required to establish clinical practice using the DRA.
- Citation: Izumida T, Watanabe J, Yoshida R, Kotani K. Efficacy and safety of distal radial approach for cardiac catheterization: A systematic review and meta-analysis. World J Cardiol 2021; 13(5): 144-154
- URL: https://www.wjgnet.com/1949-8462/full/v13/i5/144.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i5.144
Coronary angiography (CAG) is an invasive but essential part of the diagnosis and treatment for coronary artery disease (CAD). Annually, it is estimated that 1016000 inpatient diagnostic CAG and 480000 inpatient percutaneous coronary intervention (PCI) procedures are performed in the United States[1]. In European countries, it is estimated that 4500 diagnostic coronary angiograms per million people and 2000 PCI procedures per million people are performed each year[2]. Interventional cardiologists gain access via a peripheral artery, and the latest guidelines from the European Society of Cardiology, National Institute for Health and Care Excellence, and American College of Cardiology/American Heart Association recommended the radial approach (RA) over the transfemoral, transbrachial, and transulnar approaches, because it is associated with a reduced risk of cardiac death, all-cause mortality, bleeding, and access site complications[3-5].
The distal RA (DRA) was recently introduced, as this approach may have some potential advantages in comparison to the RA[6,7]. Previous observational studies showed that the two approaches were associated with similar successful cannulation rates[8], while the rates of vascular complications in the DRA, including radial artery occlusion (RAO) and radial artery spasm (RAS), were less frequent than the RA[9-16]. The DRA is assumed to be an alternative approach to the RA, but the efficacy of the two approaches has never been systematically reviewed and analyzed.
Therefore, the present study aimed to evaluate the efficacy and safety of the DRA in comparison to the RA. To achieve this aim, a systematic review and meta-analysis of only randomized-controlled trials (RCTs) were conducted to produce high-quality evidence that would inform clinical practice decisions for guidance of the cardiac catheterization procedures concerning these two approaches.
Our review protocol was registered in protocol.io (dx.doi.org/10.17504/protocols.io. bramm2c6). Our study was performed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) Statement[17].
Individual RCTs were included to evaluate the efficacy and safety of the RA vs DRA for cardiac catheterization. All papers, including published and unpublished articles, abstracts of conferences, and letters, were included, regardless of language, country restrictions, or publication year. Non-RCTs were excluded. The inclusion criteria were adult patients (≥ 18 years of age) undergoing diagnostic CAG and PCI for CAD. Patients for whom a > 7-Fr sheath was used were excluded (available on a commercial basis)[18]. The DRA is a method of puncturing distal radial arteries at the proximal part of the anatomical snuffbox or the first intermetacarpal space. After successful artery puncture, a guidewire is smoothly passed through the needle and used to guide the sheath through the artery. After introduction of the sheath, interventional cardiologists perform diagnostic CA and PCI with the coronary catheters through the sheath[19]. The RA is a method of puncturing radial artery at the forearm, a few centimeters above the wrist joint[20]. The primary outcomes were the successful cannulation rate and the incidence of RAS and RAO. The successful cannulation rate was defined as completion of the procedure without cross-over to another access site or as defined by practitioners. RAS was diagnosed by angiographic evaluation of the radial artery. RAO was diagnosed based on the absence of flow on color Doppler ultrasound. The secondary outcomes were the mean number of punctures per patient and the mean time for hemostasis. The success of hemostasis was defined as no bleeding or hematoma formation after release. The total time was defined as the time from when the sheath was removed to when successful hemostasis was confirmed. All outcomes included the definitions of the authors of original studies.
The following databases were searched through December 2020: MEDLINE, the Cochrane Central Register of Controlled Trials, and EMBASE (Supplementary material, Appendix 1). The World Health Organization International Clinical Trials Platform Search Portal and ClinicalTrials.gov databases were also searched for ongoing or unpublished trials (Supplementary material, Appendix 2). The original authors were asked for unpublished or additional data if necessary. The reference lists of studies, including international guidelines published by the European Society of Cardiology, National Institute for Health and Care Excellence, and American College of Cardiology/American Heart Association[3-5], as well as the reference lists of eligible studies and articles citing eligible studies, were checked.
Two independent reviewers (Izumida T and Yoshida R) screened the titles and abstracts, then assessed the eligibility based on the full text. We contacted the original authors when relevant data were missing. Disagreements between the two reviewers were resolved by discussion, and when this failed, a third reviewer acted as an arbiter (Watanabe J).
Two reviewers (Izumida T and Yoshida R) performed independent data extraction of the included studies using a standardized data collection form. The form included information on the study design, study population, interventions, and outcomes. Any disagreements were resolved by discussion, and when this failed, a third reviewer acted as an arbiter (Watanabe J).
Two reviewers (Izumida T and Yoshida R) evaluated the risk of bias independently using the Risk of Bias 2[21]. Disagreements between the two reviewers were resolved by discussion, and when this failed, a third reviewer acted as an arbiter (Watanabe J).
We pooled the relative risk ratios (RRs) and 95% confidence intervals (CIs) for the following binary variables: Cannulation success, RAS, and RAO. We pooled the mean differences and the 95%CIs for the following continuous variables: Mean time for hemostasis. An intention-to-treat analysis was performed for all dichotomous data (to the extent that was possible). For continuous data, missing data were not imputed based on the recommendation of the Cochrane handbook[22]. A meta-analysis was performed using the available data in the original study. The Review Manager software program (RevMan 5.4.1) was used to perform the meta-analysis. A random-effects model was used. The statistical heterogeneity was evaluated by a visual inspection of forest plots and calculation of the I2statistic (I2 values of 0%-40%: Might not be important; 30%-60%: May represent moderate heterogeneity; 50%-90%: May represent substantial heterogeneity; 75%-100%: May represent considerable heterogeneity)[22]. When there was substantial heterogeneity (I2> 50%), the reason for heterogeneity was assessed. The Cochrane chi-squared test (Q-test) was performed for the I2 statistic, and P values of < 0.10 were considered statistically significant. A funnel plot was not created and the Egger test was not performed because < 10 trials were included in our analysis[22]. The following subgroup analyses of the primary out
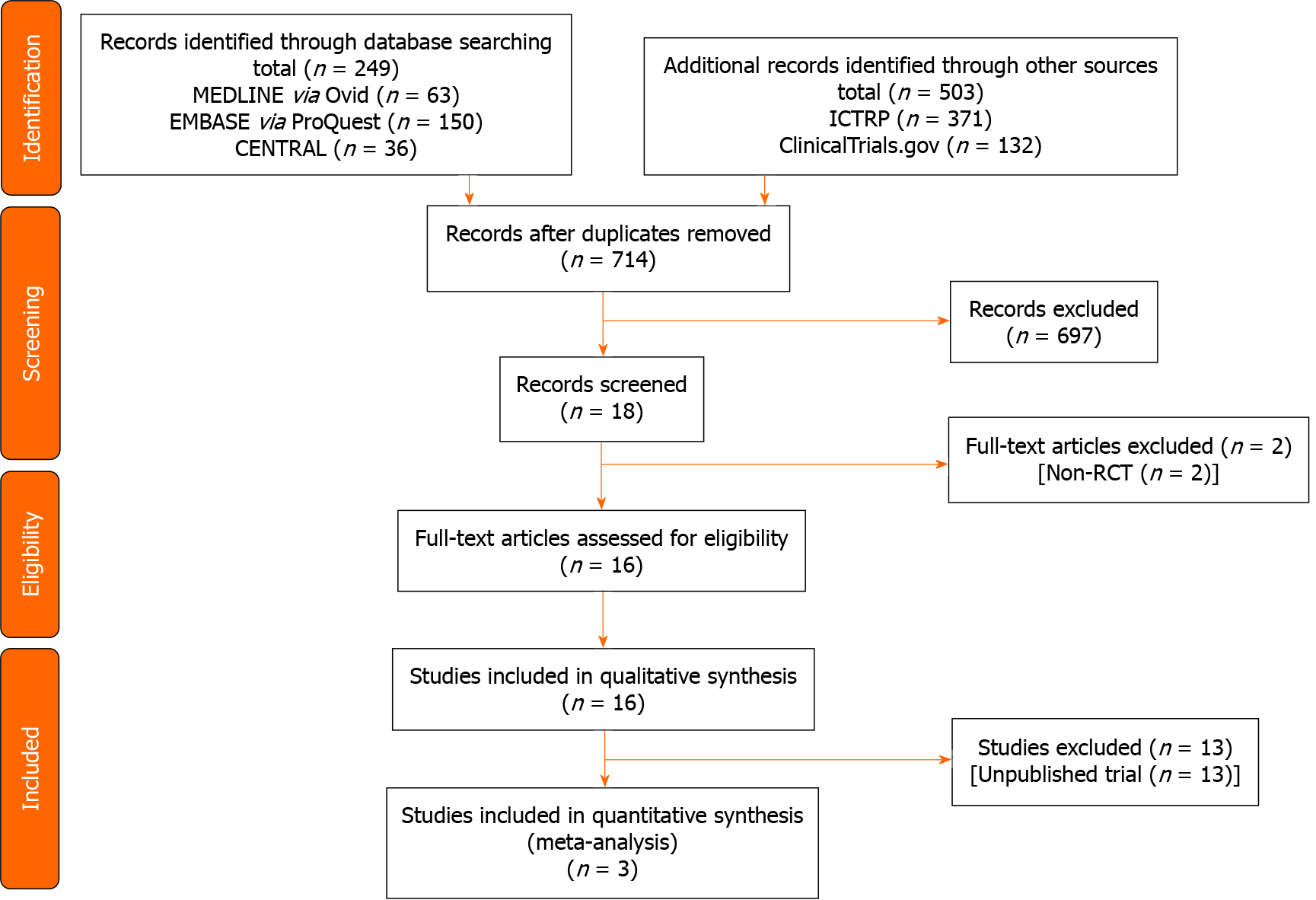
Figure 1 shows the flow of the study selection of studies comparing the DRA vs RA for cardiac catheterization. We identified a total 752 records (MEDLINE 63 records, EMBASE 150 records, CENTRAL 36 records, ClinicalTrials.gov 132 records, and ICTRP 371 records) published prior to December 7, 2020. After the initial screening, 16 trials met the inclusion criteria. Among these trials, we identified eight ongoing trials (NCT03611725, NCT03986151, NCT04171570, NCT04194606, NCT04211584, NCT04232488, NCT04318990, KCT0004537), five protocols without results (NCT03373565, NCT04001764, NCT04023838, NCT04125992, NCT04238026), and three clinical trials.
Table 1 summarizes the characteristics of eligible studies. Three studies included 519 participants[16,25,26]. Table 2 and Supplementary Tables 1-4 show the risk of bias in each study. The overall risk of bias for the successful cannulation rate was similar in the three studies.
| Ref. | Country | Subject No. | Mean age in yr | Male, % | Right arm/left arm, n | CAG/PCI (n) | 5-Fr sheath/6-Fr sheath, n | Operators | Medications to prevent radial artery spasm | Approach to hemostasis | Timing of assessment of radial artery occlusion |
| Mokbel et al[26], 2018 | Romania | 200 | 63.4 | NS | NS | NS | NS | NS | Nitrate | NS | At discharge |
| Koutouzis et al[25], 2019 | Greece | 205 | 63.3 | 75.5 | 152/48 | 200/0 | 0/200 | Specialists | Verapamil | Manual compression | At discharge |
| Vefalı et al[16], 2020 | Turkey | 114 | 60.4 | 69.3 | 33/172 | 156/49 | 205/0 | NS | NS | Manual compression | NS |
| Ref. | Risk of bias 2 tool assessment | |||||
| Bias arising from the randomization process | Bias due to deviations from intended interventions | Bias due to missing outcome data | Bias in measurement of the outcome | Bias in selection of the reported results | Overall risk of bias | |
| Mokbel et al[26] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Koutouzis et al[25] | Low | Low | Low | Low | Some concerns | Some concerns |
| Vefalı et al[16] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
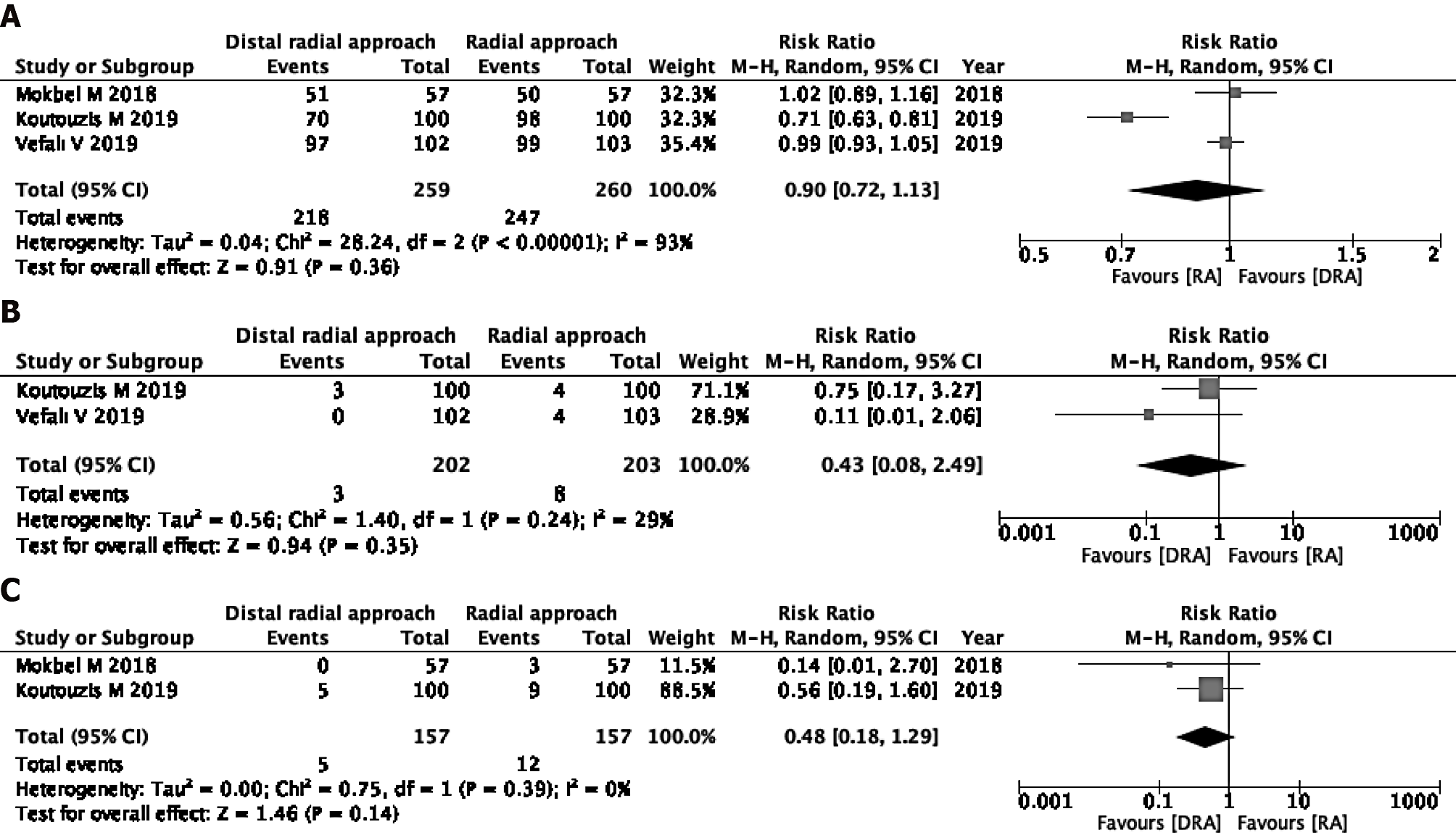
Successful cannulation rate: Three studies were eligible for the evaluation of the successful cannulation rate[16,25,26]. In one study, the operators were specialists, and in the other two studies, the operators’ skills were unknown. The DRA resulted in little to no difference in the successful cannulation rate in comparison to the RA (RR 0.90, 95%CI: 0.72-1.13; I2 = 93%) (Figure 2A).
Incidence of RAS: The incidence of RAS was measured in two of three studies[16,25]. The two studies used verapamil and nitrate, respectively[16,25]. The DRA did not reduce the incidence of RAS (RR 0.43, 95%CI: 0.08-2.49; I2 = 29%) (Figure 2B).
Incidence rate of RAO: Two of the three studies were eligible for the evaluation of incidence of RAO[25,26]. The DRA did not reduce the incidence of RAO (RR 0.48, 95%CI: 0.18-1.29; I2 = 0%) (Figure 2C).
We could not perform a pre-specified subgroup analysis or sensitivity analyses for the successful cannulation rate, the incidence of RAS, or the incidence of RAO.
Mean number of punctures: We included one RCT for the evaluation of mean number of punctures[25]. In the study, the mean number of punctures per patient was 2.4 with the DRA and 1.6 with the RA.
Mean time for hemostasis: Two of the three studies were eligible for the evaluation of the mean time for hemostasis[16,26]. In one study, hemostasis was performed only by manual compression without using a device[16], and in the other study, it was unclear whether a device was used[26]. The DRA reduced the mean time for hemostasis in comparison to the RA (mean difference -6.64, 95%CI: -10.37 to -2.90; I2 = 88%) (Figure 3).
The certainty of the evidence was low for the successful cannulation rate because of inconsistency due to substantial heterogeneity and imprecision due to the small sample size. The certainty of evidence was low for RAS, RAO, and the mean number of punctures because of imprecision due to small sample size and the small number of participants. The certainty of the evidence was very low for the mean time for hemostasis because of substantial heterogeneity, imprecision, and a high risk of bias (Table 3).
| Patient or population: Adults; Setting: Diagnostic coronary angiography and percutaneous coronary intervention; Intervention: Radial approach (RA); Comparison: Distal radial approach (DRA) | ||||||
| Outcomes | Anticipated absolute effects1 (95%CI) | Relative effect (95%CI) | Patient number (studies) | Certainty of the evidence, GRADE | Comments | |
| Risk with RA | Risk with DRA | |||||
| Successful cannulation rates | 950 per 1000 | 798 per 1000 (532-1000) | RR 0.90 [0.72-1.13] | 519 (3 RCTs) | Low2,3 | DRA resulted in little to no difference in successful cannulation rates |
| Radial artery spasm | 39 per 1000 | 16 per 1000 (4-56) | RR 0.43 [0.08-2.49] | 405 (2 RCTs) | Low3 | DRA may reduce incidence of radial artery spasm |
| Radial artery occlusion | 32 per 1000 | 14 per 1000 (5-41) | RR 0.48 [0.18-1.29] | 314 (2 RCTs) | Low3 | DRA may reduce the incidence of radial artery occlusion |
| Mean number of punctures per patient | The mean number of punctures per patient were 2.4 in DRA in comparison to 1.6 in RA | 200 (1 RCT) | Low3 | DRA may reduce the mean number of punctures per patient | ||
| Mean time for hemostasis | - | MD 6.64 min lower (10.37 lower to 2.9 lower) | - | 405 (2 RCTs) | Very low2,3,4 | DRA reduced mean time for hemostasis |
In the present review, the rate of cannulation failure with the DRA was suggested to be similar to that with the RA. Furthermore, the DRA might reduce the incidence of RAS and RAO in comparison to the RA. Additionally, the DRA had a shorter hemostasis time. These findings indicate the safe clinical practice analyses of the DRA to guide cardiac catheterization procedures.
The puncture of the distal radial artery has some caveats because of anatomical features such as the superficial position of the artery and the bone basement. The puncture site in the DRA is either the distal radial artery of the anatomic snuffbox or the more distal radial artery, which is located on the vertex of the angle between the tendon of the extensor pollicis longus and the second metacarpal bone[7]. Some studies showed that the diameter of distal radial artery was smaller and might have the increased tortuosity and angulations in comparison to forearm radial arte
RAS is one of the most frequent complications in cardiac catheterization[29,30] and can be caused by mechanical stimulation by guide wires or catheters and increasing catecholamine levels, which are induced by pain and discomfort[28]. In previous systematic reviews, additional drugs, such as local anesthetics and vasodilatory medications, reduced RAS[31,32]. In the present review, the DRA arm was likely to reduce the incidence of RAS, despite the use of additional medications. Although the detailed mechanism remains unknown, a previous study reported that the DRA might be associated with more advantages in terms of patient satisfaction and the analgesic effect[16,33].
RAO is relatively common, with an incidence ranging from 0.6% to 2.2%; it occurs through the inflammation and endothelial dysfunction of the radial artery[34,35]. Regarding possible explanations for the lower incidence of RAO in the DRA arm, the first possibility seemed to be the anatomical features of the distal radial artery. The antegrade flow through the superficial palmar arch can be maintained during compression of the distal radial artery, resulting in a low risk of retrograde thrombus formation[6]. The second possibility was the shorter duration of hemostasis with the DRA[7], which appeared to be related to the structure of the anatomic snuffbox with a bony basement surrounded by tendons.
The mean number of punctures in the DRA could be mostly comparable to that in the RA. The operators were mainly specialists in the study setting; however, in the clinical setting, the DRA is associated with a learning curve because it involves the puncture of small and weak arteries[36]. Ultrasound is useful for increasing the rate of successful puncture and for reducing adverse events. The measurement of the diameter of the distal radial artery helps to select a suitable sheath, leading to reduced damage of the endothelium and reduced development of RAS and RAO[28]. The use of ultrasound may alter the results of similar studies in the future. Research is needed to evaluate the usefulness of ultrasound in the DRA.
The shorter time of hemostasis in the DRA, as found in the present review, is a useful aspect of this approach for the prevention of vascular damage. Due to the anatomical features of the distal radial artery, the DRA can reduce the hemostasis time. A new compression hemostasis device for the puncture site of the distal radial artery was also developed, and the safety and efficacy of the device were valida
The present review had some limitations. First, our review included a relatively small number of studies. Second, various definitions may have been applied for RAS, RAO, and hemostasis, because the protocols were not described. To improve the quality of evidence and draw convincing conclusions, it will be necessary to perform large cohort studies with standard protocols.
This first systematic review and meta-analysis to compare clinical data using the DRA and RA indicated that the DRA would be safer than the RA, with comparable procedure rates. Given the limited data, accumulating more knowledge by further research, including studies with standard protocols, is required to establish clinical practice using the DRA.
While the traditional radial approach (RA) is the gold standard method for cardiac catheterization, a distal RA (DRA) has been recently introduced.
The DRA may have some advantages compared to RA; however, it is not fully understood as to which technique for coronary angiography—DRA or RA—is more beneficial to the patients.
Via the systematic review and meta-analysis, we compared clinical data using the DRA and RA.
The databases MEDLINE, the Cochrane Central Register of Controlled Trials, EMBASE, the World Health Organization International Clinical Trials Platform Search Portal and ClinicalTrials.gov were searched. All randomized-controlled trials for adult patients undergoing cardiac catheterization until December 2020 were included. The primary outcomes were the successful cannulation rate and the incidence of radial artery spasm (RAS) and radial artery occlusion (RAO). The statistical analysis was performed on a random-effect model to pool the relative risk ratios (RRs) and 95% confidence intervals (CIs) for the binary variables, such as cannulation success, RAS, and RAO.
Three randomized-control trials including 519 participants and 13 registered trials were identified. The two approaches showed similar successful cannulation rates (RR 0.90, 95%CI: 0.72-1.13). The DRA did not decrease RAS (RR 0.43, 95%CI: 0.08-2.49) and RAO (RR 0.48, 95%CI: 0.18-1.29). The evidence of certainty was low.
The present study indicated the DRA to be safer than the RA, with comparable procedure rates. Importantly, there are limitations, including the limited study numbers and no studies with standard protocols, that prevent definitive conclusions.
Further research, including studies with standard protocols, is required to establish clinical practice using the DRA.
The authors would like to thank Dr. Babunashvili A, Dr. Lee JW, and Dr. Park JC for providing us with unpublished details of studies for the review. We thank Ms. Fujiwara C for providing us with International Clinical Trials Registry Platform search data.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Darbari DA, Santomauro M S-Editor: Gao CC L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139-e596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3254] [Cited by in F6Publishing: 4602] [Article Influence: 1150.5] [Reference Citation Analysis (1)] |
| 2. | Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, Mossialos EA, Maggioni AP, Kazakiewicz D, May HT, De Smedt D, Flather M, Zuhlke L, Beltrame JF, Huculeci R, Tavazzi L, Hindricks G, Bax J, Casadei B, Achenbach S, Wright L, Vardas P; European Society of Cardiology. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur Heart J. 2020;41:12-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 569] [Article Influence: 142.3] [Reference Citation Analysis (1)] |
| 3. | Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2791] [Cited by in F6Publishing: 3481] [Article Influence: 870.3] [Reference Citation Analysis (0)] |
| 4. | National Institute for Health and Care Excellence. Acute Coronary Syndrome. NICE guidelines [NG185]. [cited 19 November 2020]. In: National Institute for Health and Care Excellence [Internet]. Available from: https://www.nice.org.uk/guidance/ng185. [Cited in This Article: ] |
| 5. | Mason PJ, Shah B, Tamis-Holland JE, Bittl JA, Cohen MG, Safirstein J, Drachman DE, Valle JA, Rhodes D, Gilchrist IC; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. An Update on Radial Artery Access and Best Practices for Transradial Coronary Angiography and Intervention in Acute Coronary Syndrome: A Scientific Statement From the American Heart Association. Circ Cardiovasc Interv. 2018;11:e000035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 299] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 6. | Kiemeneij F. Left distal transradial access in the anatomical snuffbox for coronary angiography (ldTRA) and interventions (ldTRI). EuroIntervention. 2017;13:851-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 7. | Cai G, Huang H, Li F, Shi G, Yu X, Yu L. Distal transradial access: a review of the feasibility and safety in cardiovascular angiography and intervention. BMC Cardiovasc Disord. 2020;20:356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Pua U, Sim JZT, Quek LHH, Kwan J, Lim GHT, Huang IKH. Feasibility Study of “Snuffbox” Radial Access for Visceral Interventions. J Vasc Interv Radiol. 2018;29:1276-1280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Babunashvili A. Novel distal transradial approach for coronary and peripheral interventions. J Am Coll Cardiol. 2018;72:B323. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Uddin MJ, Hashem S, Momen A, Sarker S, Rahman AU, Hasan M, Saha BP, Mozumder MR, Shahriar MS. Right distal radial artery access for coronary intervention initial experience in Bangladesh. J Am Coll Cardiol. 2019;73:S72. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Soydan E, Akın M. Coronary angiography using the left distal radial approach - An alternative site to conventional radial coronary angiography. Anatol J Cardiol. 2018;19:243-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Valsecchi O, Vassileva A, Cereda AF, Canova P, Satogami K, Fiocca L, Guagliumi G. Early Clinical Experience With Right and Left Distal Transradial Access in the Anatomical Snuffbox in 52 Consecutive Patients. J Invasive Cardiol. 2018;30:218-223. [PubMed] [Cited in This Article: ] |
| 13. | Kim Y, Ahn Y, Kim I, Lee DH, Kim MC, Sim DS, Hong YJ, Kim JH, Jeong MH. Feasibility of Coronary Angiography and Percutaneous Coronary Intervention via Left Snuffbox Approach. Korean Circ J. 2018;48:1120-1130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 14. | Yu W, Hu P, Wang S, Yao L, Wang H, Dou L, Lu M, Bo G, Yu X, Chen J, Chen C, Luo Y, Yang M, Dong Z, Huang S. Distal radial artery access in the anatomical snuffbox for coronary angiography and intervention: A single center experience. Medicine (Baltimore). 2020;99:e18330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Aoi S, Htun WW, Freeo S, Lee S, Kyaw H, Alfaro V, Coppola J, Pancholy S, Kwan T. Distal transradial artery access in the anatomical snuffbox for coronary angiography as an alternative access site for faster hemostasis. Catheter Cardiovasc Interv. 2019;94:651-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 16. | Vefalı V, Sarıçam E. The Comparison of Traditional Radial Access and Novel Distal Radial Access for Cardiac Catheterization. Cardiovasc Revasc Med. 2020;21:496-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9970] [Cited by in F6Publishing: 10260] [Article Influence: 684.0] [Reference Citation Analysis (0)] |
| 18. | Gasparini GL, Garbo R, Gagnor A, Oreglia J, Mazzarotto P. First prospective multicentre experience with left distal transradial approach for coronary chronic total occlusion interventions using a 7 Fr Glidesheath Slender. EuroIntervention. 2019;15:126-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 19. | Al-Azizi KM, Lotfi AS. The distal left radial artery access for coronary angiography and intervention: A new era. Cardiovasc Revasc Med. 2018;19:35-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Campeau L. Percutaneous radial artery approach for coronary angiography. Cathet Cardiovasc Diagn. 1989;16:3-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 582] [Cited by in F6Publishing: 538] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 21. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6581] [Cited by in F6Publishing: 9590] [Article Influence: 1918.0] [Reference Citation Analysis (0)] |
| 22. | Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V. Cochrane Handbook for Systematic Reviews of Interventions version 6.0. Cochrane, 2019. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5433] [Cited by in F6Publishing: 5846] [Article Influence: 1169.2] [Reference Citation Analysis (0)] |
| 23. | De Rosa S, Torella D, Caiazzo G, Giampà S, Indolfi C. Left radial access for percutaneous coronary procedures: from neglected to performer? Int J Cardiol. 2014;171:66-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Shah RM, Patel D, Abbate A, Cowley MJ, Jovin IS. Comparison of transradial coronary procedures via right radial vs left radial artery approach: A meta-analysis. Catheter Cardiovasc Interv. 2016;88:1027-1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Koutouzis M, Kontopodis E, Tassopoulos A, Tsiafoutis I, Katsanou K, Rigatou A, Didagelos M, Andreou K, Lazaris E, Oikonomidis N, Maniotis C, Ziakas A. Distal Versus Traditional Radial Approach for Coronary Angiography. Cardiovasc Revasc Med. 2019;20:678-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 26. | Mokbel M, Sinescu C, Florescu N. Snuff-box vs distal forearm for trans-radial access: performance and radial patency. Eur Heart J. 2018;39:P4398. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Naito T, Sawaoka T, Sasaki K, Iida K, Sakuraba S, Yokohama K, Sato H, Soma M, Okamura E, Harada T, Yoshimachi F. Evaluation of the diameter of the distal radial artery at the anatomical snuff box using ultrasound in Japanese patients. Cardiovasc Interv Ther. 2019;34:312-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Norimatsu K, Kusumoto T, Yoshimoto K, Tsukamoto M, Kuwano T, Nishikawa H, Matsumura T, Miura SI. Importance of measurement of the diameter of the distal radial artery in a distal radial approach from the anatomical snuffbox before coronary catheterization. Heart Vessels. 2019;34:1615-1620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Coghill EM, Johnson T, Morris RE, Megson IL, Leslie SJ. Radial artery access site complications during cardiac procedures, clinical implications and potential solutions: The role of nitric oxide. World J Cardiol. 2020;12:26-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Ruiz-Salmerón RJ, Mora R, Vélez-Gimón M, Ortiz J, Fernández C, Vidal B, Masotti M, Betriu A. [Radial artery spasm in transradial cardiac catheterization. Assessment of factors related to its occurrence, and of its consequences during follow-up]. Rev Esp Cardiol. 2005;58:504-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Curtis E, Fernandez R, Lee A. The effect of topical medications on radial artery spasm in patients undergoing transradial coronary procedures: a systematic review. JBI Database System Rev Implement Rep. 2018;16:738-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Curtis E, Fernandez R, Lee A. The effect of vasodilatory medications on radial artery spasm in patients undergoing transradial coronary artery procedures: a systematic review. JBI Database System Rev Implement Rep. 2017;15:1952-1967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Liao JM, Lin CF, Ting H, Chang CC, Lin YJ, Lin TB. Electroacupuncture at Hoku elicits dual effect on autonomic nervous system in anesthetized rats. Neurosci Res. 2002;42:15-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Babunashvili A. Novel distal transradial approach for coronary and peripheral interventions. J Am Coll Cardiol. 2018;72:B323. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Kaledin A, Kochanov IN, Podmetin PS, Seletsky SS, Ardeev VN. Distal radial artery in endovascular interventions, 2017. [DOI] [Cited in This Article: ] |
| 36. | Hammami R, Zouari F, Ben Abdessalem MA, Sassi A, Ellouze T, Bahloul A, Mallek S, Triki F, Mahdhaoui A, Jeridi G, Abid L, Charfeddine S, Kammoun S, Jdidi J. Distal radial approach vs conventional radial approach: a comparative study of feasibility and safety. Libyan J Med. 2021;16:1830600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Kawamura Y, Yoshimachi F, Nakamura N, Yamamoto Y, Kudo T, Ikari Y. Impact of dedicated hemostasis device for distal radial arterial access with an adequate hemostasis protocol on radial arterial observation by ultrasound. Cardiovasc Interv Ther. 2021;36:104-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Petroglou D, Didagelos M, Chalikias G, Tziakas D, Tsigkas G, Hahalis G, Koutouzis M, Ntatsios A, Tsiafoutis I, Hamilos M, Kouparanis A, Konstantinidis N, Sofidis G, Pancholy SB, Karvounis H, Bertrand OF, Ziakas A. Manual Versus Mechanical Compression of the Radial Artery After Transradial Coronary Angiography: The MEMORY Multicenter Randomized Trial. JACC Cardiovasc Interv. 2018;11:1050-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |