Published online May 26, 2021. doi: 10.4330/wjc.v13.i5.130
Peer-review started: January 9, 2021
First decision: February 28, 2021
Revised: March 4, 2021
Accepted: April 28, 2021
Article in press: April 28, 2021
Published online: May 26, 2021
The established cardiovascular risk factors cannot explain the overall risk of coronary artery disease (CAD), especially in women. Therefore, there is a growing need for the assessment of novel biomarkers to identify women at risk. The receptor for advanced glycation end products (RAGE) and its interaction with the advanced glycation end product (AGE) ligand have been associated with atherogenesis. The soluble fraction of RAGE (sRAGE) antagonizes RAGE signaling and exerts an antiatherogenic effect.
The study aim was to explore the association between plasma levels of sRAGE and CAD in nondiabetic postmenopausal women.
This case-control study included 110 nondiabetic postmenopausal women who were enrolled in two groups. Group I included 55 angiographically proven CAD subjects with > 50% stenosis in at least one of the major coronary arteries and Group II included 55 healthy control women who did not have CAD or had < 50% stenosis of the coronary arteries. Stenosis was confirmed by invasive angiography. Plasma sRAGE was determined by an enzyme-linked immunosorbent assay.
We observed significantly lower plasma sRAGE concentrations in subjects with CAD vs healthy controls (P < 0.05). Univariate and multivariate logistic regression analysis also revealed a significant correlation between plasma sRAGE levels and CAD (P = 0.01). Multivariate odds ratios for CAD revealed that subjects with sRAGE concentrations below 225 pg/mL (lowest quartile) had a 6-fold increase in CAD prevalence independent of other risk factors.
Our findings indicated that low sRAGE levels were independently associated with CAD in nondiabetic postmenopausal women. Risk assessment of CAD in postmenopausal women can be improved by including sRAGE along with other risk factors.
Core Tip: The growing need for the assessment of novel biomarkers led us to identify the risk in women. The receptor for advanced glycation end products (RAGE) and its interaction with the AGE ligand have been shown to play an important role in promoting atherosclerosis. The soluble fraction of RAGE (sRAGE) binds to ligands and antagonizes RAGE signaling, thereby exerting an antiatherogenic effect. This study established that low levels of sRAGE in plasma are independently associated with the presence of coronary artery disease in nondiabetic postmenopausal females.
- Citation: Ghosh S, Kapoor D, Vijayvergiya R, Sangwan S, Wangkheimayum S, Mehta S, Dhawan V. Correlation between soluble receptor for advanced glycation end products levels and coronary artery disease in postmenopausal nondiabetic women. World J Cardiol 2021; 13(5): 130-143
- URL: https://www.wjgnet.com/1949-8462/full/v13/i5/130.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i5.130
Coronary artery disease (CAD) is a leading cause of morbidity and mortality throughout the world. The incidence of CAD is rising rapidly in India, and it is projected that more than 50% of CAD cases in the world will in from India by 2025[1]. Atherosclerosis is the most common cause of CAD. It causes luminal narrowing, which creates an imbalance between supply and demand, thereby impairing the coronary reserve. The etiology of CAD is multifactorial. Traditional risk factors include diabetes, hypertension (HTN), hyperlipidemia, smoking, family history, physical inactivity, obesity, and others. Along with the classical risk factors, oxidative stress and inflammation are now considered as significant risk factors of CAD. Evidence in the literature supports significant positive correlations between various inflammatory mediators including high-sensitivity C-reactive protein (hsCRP), homocysteine, lipoprotein (Lp)-a, matrix metalloproteinases, and tissue inhibitor of matrix metalloproteinase and development of CAD[2]. Postmenopausal status is an important risk factor of CAD, and cardiovascular disease is more common in postmenopausal than in premenopausal women because of decreased endogenous estradiol in that age group[3]. Postmenopausal status is the strongest predictor in women, but it is the least studied factor compared with other traditional risk factors.
Numerous reports in the literature state that CAD occurs in the absence of major risk factors in about one-third of patients in India[4]. Therefore, the overall risk of CAD cannot be explained by traditional and established cardiovascular risk fac
RAGE is a transmembrane receptor of the immunoglobulin superfamily, and it is located in the major histocompatibility complex (gene 6 p21.3)[7]. It can interact with various ligands involved in inflammation, atherosclerosis, and vasoconstriction[8] and is highly expressed at the site of vascular pathology[9]. Activation of the RAGE-dependent pathway is followed by many deleterious effects occur like activation of nuclear factor-B, increased cytokines, and induction of oxidative stress[10,11]. Advanced glycation end products (AGEs) are RAGE ligands for RAGE and their interaction results in coronary atherosclerosis[12]. AGE-RAGE interaction and the subsequent effects are also of great importance in diabetic vasculopathy. Recent studies have shown the key role of this signaling pathway in nondiabetic atheroscle
sRAGE is produced by the cleavage of membrane-bound RAGE. It consists of the extracellular ligand-binding domain only and lacks both cytoplasmic and trans
This case-control prospective observational study was conducted at the Advanced Cardiac Center of the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh. After screening, we enrolled 110 nondiabetic postmenopausal women and assigned them to two groups. Group I included women with CAD and > 50% stenosis in at least one of the major coronary arteries (n = 55). and Group II included healthy women without CAD and with < 50% stenosis in the coronary arteries. Each study subject underwent coronary angiography following the cardiac center protocol. The indications for angiography were suspicion of CAD or preoperative screening for CAD in subjects with valvular disease. Exclusion criteria were patients with diabetes, premenopausal female, surgery or trauma during the month preceding the study, known cardiomyopathy, known malignant diseases, known febrile conditions, subjects using lipid lowering agents, acute or chronic inflammatory disease, connective tissue disorder, renal insufficiency (creatinine > 1.5 mg/dL), abnormal liver function, patients with heart failure or cardiogenic shock, refusal to give informed consent and patient with any neoplasm. Subjects having a concentration of hsCRP > 10 mg/L, a level considered to be indicative of clinically relevant inflammatory conditions, were also excluded from the study[16].
Ethical approval and informed consent: The study was conducted following the ethical standards detailed in the Declaration of Helsinki[17] and was approved by the Institute ethical committee. Informed consent was obtained from all the patients after explaining the protocol prior to their enrolment in the study.
All participants underwent a standard clinical examination. All cardioactive drugs used by the patients were registered, with particular regard to beta-blockers, calcium-antagonists, ACE inhibitors, antiplatelet drugs, statins, and nitrates. Clinical characteristics including height, weight, smoking status, blood pressure (BP), waist circumference, family history of CAD, and body mass index (BMI, kg/m2) were documented. According to the Asian classification, a BMI of < 18.5 kg/m2 is underweight, 18.5-22.99 kg/m2 is normal, 23.0-27.49 kg/m2 is overweight and > 27.5 kg/m2 is obese[18]. A waist circumference cutoff of 80 cm as applied for women[19]. Cigarette smoking was dichotomized into ever vs never, with ever smoking defined as having smoked daily for 1 year or more. Many patients had quit after the onset of CAD, hence the designation as ever smoking rather than current and former. HTN was defined as a systolic BP of > 140 and/or a diastolic BP of > 90 and/or on treatment with antihypertensives, following the eighth Joint National Committee guidelines[20]. American Diabetes Association guidelines, diabetes was taken as either fasting blood sugar (FBS) > 126 mg/dL, 2 h postprandial glucose > 200 mg/dL, glycosylated hemoglobin (HbA1c) > 6.5% or Rutherford backscattering spectroscopy > 200 mg/dL, with symptoms of hyperglycemia in an oral glucose tolerance test[21]. The study protocol excluded subjects with diabetes.
All laboratory determinations were performed in a blinded fashion. Before angio
Lipid profile: All components of the lipid profile were measured in an autoanalyzer. Subjects were classified as dyslipidemic if they had levels of low-density lipoprotein cholesterol (LDL-C) > 100 mg/dL, high-density lipoprotein cholesterol (HDL-C) < 50 mg/dL, triglycerides > 150 mg/dL or total cholesterol > 200 mg/dL[22].
Blood sugar: Plasma FBS and postprandial blood sugar (PPBS) were estimated by the glucose oxidase method. HbA1c was estimated by high-performance liquid chromatography.
hsCRP: hsCRP levels were estimated in samples of plasma containing suspensions of latex particles coated with monoclonal/polyclonal antibodies to human CRP. The plane polarized light passing through the solution was scattered in a manner proportional to the CRP concentration. The intensity of the scattered light was measured against a standard curve prepared using known concentrations of the CRP antigen.
Plasma homocysteine: Plasma homocysteine levels were determined in heparinized blood samples with an enzyme cycling assay.
Renal function tests: For renal function tests, urea levels were measured by Fearon reaction and creatinine levels were measured by Jaffe’s alkaline picrate method in an autoanalyzer.
Lp(a): Lp(a) levels were estimated using a commercially available enzyme-linked immunosorbent assay kit (Assay Max Human Lp(a), Assaypro Catalog no: EL3001-1). This assay employed a quantitative sandwich enzyme immunoassay technique that measured human Lp(a) in less than 4 h. The normal Lp(a) value with this kit was 60-180 μg/mL.
sRAGE: Plasma sRAGE levels were determined using a commercially available enzyme-linked immunosorbent assay kit (BioVendor, Catalog No: RD191116200R) following the manufacturer’s protocol. Briefly, a monoclonal antibody against sRAGE was used to capture sRAGE from plasma. Captured sRAGE was detected with a polyclonal anti-human sRAGE antibody. After washing, plates incubated with streptavidin-horseradish peroxidase, were developed with the appropriate substrate, and the OD450 was determined using an enzyme-linked immunosorbent assay plate reader. Measurements were performed in duplicate and the results were averaged and reported as pg/mL.
Coronary angiography was carried out in all the patients using a standard protocol. The catheter was inserted through femoral or radial artery and moved up to the coronary arteries. Radiocontrast was injected into the coronary arteries under X-ray guidance in order to display the coronary anatomy and possible luminal obstruction. Existing significant CAD was defined as stenosis in the major epicardial coronary arteries that reduced the lumen diameter by 50%.
The sample size of 55 in each group had a power of more than 90% and confidence intervals (CI) of 95%. The statistical analysis was carried out using the Statistical Package for Social Sciences version 20 (IBM Corp.) All quantitative variables were estimated using measures of central location (mean) and measures of dispersion (standard deviation). Normality of data was checked by measures of skewness and Kolmogorov–Smirnov tests of normality. Normally distributed data in groups was expressed as means ± SD. For normally distributed data, differences of the case and control mean values were compared using Student’s t-tests. Qualitative or categorical variables like smoking history, HTN, and family history of CAD were reported as frequencies and percentages. Frequencies were compared using χ2 or Fisher’s exact tests, whichever was applicable. Pearson’s correlation was used to find the associations of variables like serum triglycerides, HDL-C, LDL-C, total cholesterol, Lp(a), and sRAGE. The CAD patients and controls were categorized in quartiles of the plasma sRAGE concentration in the entire study cohort. The interquartile cutoffs of sRAGE concentration were categorized into four categories: I < 225 pg/mL, II = ³225 to < 397.5 pg/mL, III = ³397.5 to < 730 pg/mL, and IV ³730 pg/mL. To evaluate the risk associated with decreasing levels of sRAGE, odds ratio (ORs) for each quartile in the entire study cohort relative to the fourth quartile were calculated. To determine independent predictors of CAD, univariate and multivariate logistic regression were performed for categorical variables like age, HTN, and family history of CAD, and for continuous variables like waist circumference, total cholesterol, triglycerides, LDL-C, HDL-C, Lp(a), hsCRP, and sRAGE. Lastly, Pearson’s correlation was used to find any correlation between sRAGE levels and other biochemical parameters. All baseline variables related to CAD with P < 0.01 and P < 0.05 in simple logistic regression analysis were included in the multivariate model. Crude and multivariate adjusted ORs were reported with their 95%CIs. Values were considered statistically significant at the P < 0.01 and P < 0.05 levels.
The clinical and biochemical characteristics of the study subjects (Group I: Cases; Group II: Controls) are shown in Table 1. All the participants were postmenopausal women and with a similar socioeconomic status. All were never-smokers, 30 patients and 24 control subjects had HTN, and 26 patients and 14 control subjects had family histories of CAD. Student’s t-tests found no significant differences in age, BMI, waist circumference, lipid profile, FBS, PPBS, HbA1c, creatinine, hsCRP, homocysteine, Lp(a), and sRAGE. Chi-square tests found no significant differences in HTN or family history of CAD. No significant differences in age, BMI, waist circumference, HTN, low physical activity, total cholesterol, LDL-C, HDL-C, sugar profile (FBS/ PPBS/ HbA1c), creatinine, and Lp(a) (P > 0.05) were observed among the study groups. Study subjects in Group I had significantly higher levels of triglycerides (TG), hsCRP, and homo
| Variable | Group I (n = 55) | Group II (n = 55) | P value |
| Age (yr) | 57.58 ± 7.75 | 56.29 ± 7.69 | 0.38 |
| BMI (kg/m²) | 25.41 ± 3.05 | 24.81 ± 3.79 | 0.35 |
| WC (cm) | 83.02 ± 5.17 | 81.42 ± 6.11 | 0.14 |
| Serum CHOL (mg/dL) | 175.33 ± 40.68 | 180.12 ± 37.12 | 0.52 |
| Serum TG (mg/dL) | 175.16 ± 48.67 | 150.09 ± 42.67 | 0.05 |
| Serum HDL-C (mg/dL) | 38.57 ± 6.89 | 40.59 ± 8.17 | 0.16 |
| Serum LDL-C (mg/dL) | 123.68 ± 27.73 | 113.17 ± 31.58 | 0.06 |
| FBS (mg/dL) | 92.89 ± 8.81 | 95.71 ± 10.14 | 0.12 |
| PPBS (mg/dL) | 133.33 ± 5.53 | 133.15 ± 3.76 | 0.84 |
| HbA1c (%) | 5.24 ± 0.19 | 5.28 ± 0.14 | 0.14 |
| Serum creatinine (mg/dL) | 0.79 ± 0.16 | 0.85 ± 0.23 | 0.09 |
| Serum hsCRP (μg/mL) | 5.26 ± 1.94 | 1.41 ± 0.44 | 0.00b |
| Plasma HC (μmol/L) | 18.13 ± 5.39 | 12.83 ± 3.99 | 0.00b |
| Lp(a) (μg/mL) | 294.36 ± 202.22 | 251.22 ± 221.42 | 0.28 |
| sRAGE (pg/mL) | 285 (30-2490) | 540 (90-3450) | 0.02a |
| Category 1 (< 225) | 24 | 4 | 0.00b |
| Category 2 (≥ 225-< 397.5) | 8 | 19 | 0.01a |
| Category 3 (≥ 397.5-< 730) | 13 | 15 | 0.66 |
| Category 4 (≥ 730) | 10 | 17 | 0.12 |
| Hypertension | 30 | 24 | 0.25 |
| Family history | 26 | 14 | 0.01a |
Univariate logistic regression analysis: To evaluate the significance of each factor in Table 1, univariate logistic regression analysis was performed. We observed significant differences in family history of CAD, serum cholesterol, LDL-C, homocysteine, Lp(a), hsCRP, and plasma sRAGE levels in the two groups. The results are shown in Table 2.
| Parameter | SE | P value | OR | 95%CI for OR | |
| Lower | Upper | ||||
| Age (yr) | 0.038 | 0.374 | 1.034 | 0.960 | 1.115 |
| HTN | 0.598 | 0.742 | 0.821 | 0.254 | 2.651 |
| BMI (kg/m²) | 0.202 | 0.354 | 1.206 | 0.812 | 1.791 |
| WC (cm) | 0.126 | 0.152 | 0.835 | 0.653 | 1.069 |
| FH CAD | 0.668 | 0.011a | 0.184 | 0.050 | 0.680 |
| S. CHOL (mg/dl) | 0.017 | 0.001b | 1.060 | 1.025 | 1.096 |
| S.LDL-C (mg/dl) | 0.024 | 0.008b | 0.938 | 0.895 | 0.983 |
| S.HDL-C (mg/dl) | 0.041 | 0.963 | 1.002 | 0.924 | 1.087 |
| S.TG (mg/dl) | 0.008 | 0.350 | 0.993 | 0.978 | 1.008 |
| HC (μmol/L) | 0.079 | 0.00b | 0.725 | 0.621 | 0.847 |
| Lp(a) (μg/mL) | 0.002 | 0.041a | 0.996 | 0.993 | 0.999 |
| sRAGE (pg/mL) | 0.001 | 0.010a | 1.002 | 1.001 | 1.003 |
| hsCRP (μg/mL) | 1.037 | 0.00b | 0.023 | 1.001 | 0.178 |
Multivariate logistic regression analysis: After controlling for family history of CAD, serum cholesterol, LDL-C, homocysteine, Lp(a), hsCRP and plasma sRAGE, multi
| Parameter | SE | P value | OR | 95%CI for OR | |
| Lower | Upper | ||||
| FH CAD | 0.639 | 0.014a | 4.827 | 1.379 | 16.904 |
| S. CHOL (mg/dL) | 0.017 | 0.002b | 1.055 | 1.020 | 1.091 |
| LDL-C (mg/dL) | 0.024 | 0.011a | 0.941 | 0.898 | 0.986 |
| HC (mg/dL) | 0.074 | 0.00b | 0.734 | 0.635 | 0.848 |
| sRAGE (pg/mL) | 0.001 | 0.011a | 1.001 | 1.0001 | 1.003 |
| Lp(a) (μg/mL) | 0.001 | 0.052 | 0.997 | 0.994 | 1.000 |
| HTN | 0.571 | 0.755 | 1.195 | 0.390 | 3.662 |
| HDL-C (mg/dL) | 0.040 | 0.853 | 0.993 | 0.918 | 1.073 |
| TG (mg/dL) | 0.008 | 0.265 | 0.991 | 0.976 | 1.007 |
| hsCRP (μg/mL) | 1.037 | 0.00b | 0.023 | 0.003 | 0.178 |
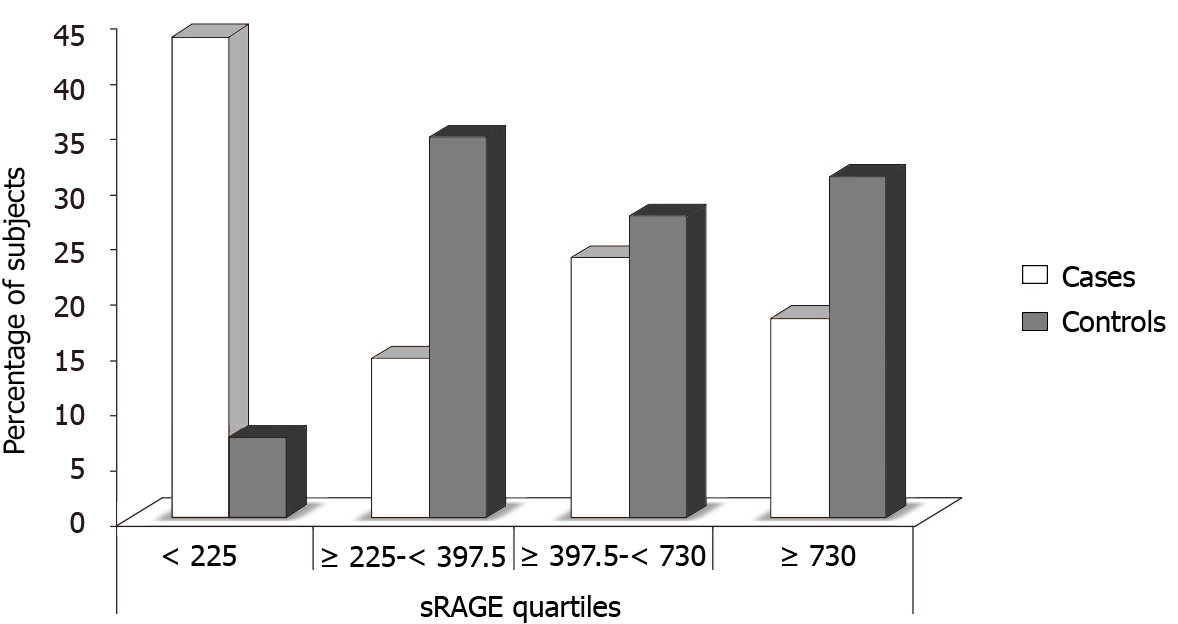
The plasma sRAGE concentration [mean: 474.89 (range: 30-4350) pg/mL] was significantly lower (P < 0.02) in CAD cases (Group I) compared with control subjects (Group II) [mean: 725.73 (range: 90-4800) pg/mL]. The study subjects were further categorized in quartiles of sRAGE concentration, which were found to be significantly different in the multivariate analysis. The interquartile sRAGE concentration cutoffs were Category I: < 225; Category II: ≥ 225 to < 397.5; Category III: ≥ 397.5 to < 730 and Category IV: ≥ 730. The numbers and percentages of subjects in each quartile of plasma sRAGE concentration, are shown in Table 4 and Figure 1. The number of CAD cases was 6-fold higher in the first quartile (plasma sRAGE < 225 pg/mL), than in the control subjects (P = 0.00). In the second (sRAGE ≥ 225-< 397.5), third (sRAGE ≥ 397.5-< 730) and fourth (sRAGE ≥ 730) quartiles, the number of control subjects was 2.38 (P = 0.01), 1.15 (P = 0.66), and 1.7-fold higher (P = 0.12), respectively than the number of CAD patients.
To evaluate the risk associated with decreasing levels of sRAGE, ORs for each quartile in all of the study groups relative to fourth quartile was calculated. As shown in Tables 5 and 6, the ORs of CAD was significantly higher in the first quartile of sRAGE level compared with the fourth quartile (P = 0.001), but no significant differences were observed between second and fourth and third and fourth quartiles.
| Category | P value | OR | 95%CI | |
| Lower | Upper | |||
| Category 1 vs 4 | 0.001 | 0.098 | 0.026 | 0.365 |
| Category 2 vs 4 | 0.564 | 1.397 | 0.448 | 4.355 |
| Category 3 vs 4 | 0.481 | 0.679 | 0.231 | 1.994 |
| Category | P value | OR | 95%CI | |
| Lower | Upper | |||
| Category 1 vs 4 | 0.001 | 0.065 | 0.014 | 0.308 |
| Category 2 vs 4 | 0.533 | 1.555 | 0.387 | 6.243 |
| Category 3 vs 4 | 0.096 | 0.325 | 0.087 | 1.221 |
Pearson’s correlation was used to evaluate the significance of the relationships between sRAGE levels and other biochemical parameters. We found no significant correlations between sRAGE levels and total cholesterol, triglycerides, HDL-C, LDL-C, hsCRP, homocysteine, or Lp(a) levels (Table 7).
| Parameter | CHOL | LDL-C | HDL-C | TG | hsCRP | HC | sRAGE | Lp (a) |
| CHOL | 1 | 0.865b | −0.616b | 0.491b | 0.052 | 0.090 | −0.179 | 0.227 |
| LDL−C | 0.865b | 1 | −0.606b | 0.646b | 0.082 | 0.094 | −0.197 | 0.205 |
| HDL−C | −0.616b | −0.606b | 1 | −0.529b | −0.157 | −0.164 | 0.245 | −0.169 |
| TG | 0.491b | 0.646b | −0.529b | 1 | −0.182 | −0.002 | −0.127 | 0.174 |
| hsCRP | 0.052 | 0.082 | −0.157 | −0.182 | 1 | 0.761b | 0.011 | −0.235 |
| HC | 0.090 | 0.094 | −0.164 | −0.002 | 0.761b | 1 | 0.067 | −0.226 |
| sRAGE | −0.179 | −0.197 | 0.245 | −0.127 | 0.011 | 0.067 | 1 | 0.158 |
| Lp(a) | 0.227 | 0.205 | −0.169 | 0.174 | −0.235 | −0.226 | 0.158 | 1 |
CAD is a major cause of morbidity and mortality worldwide, and a large proportion of the cases of CAD occur in India. CAD mortality is declining in developed nations, but it is increasing in developing countries. In India, CAD prevalence has increased 4-fold over past two decades[23], and postmenopausal women are major contributors. Traditional risk factors like diabetes, HTN, hyperlipidemia and others cannot fully explain the occurrence of CAD in this population. Many studies have been done worldwide to find a correlation between emerging inflammatory risk factors like sRAGE and CAD. Such studies are lacking in India, especially in postmenopausal women. Postmenopausal women are more prone to CAD than premenopausal women because of decreased levels of protective estrogen[24]. Therefore, this study was undertaken in postmenopausal females to understand whether sRAGE, an emerging inflammatory risk factor, was independently correlated with CAD in postmenopausal women. To the best of our knowledge, our study is the first to demonstrate a correlation between plasma sRAGE levels and CAD in nondiabetic postmenopausal women.
Amino groups of proteins bind non-enzymatically to carbonyl groups of reducing sugars to form glycated proteins known as AGEs. The altered proteins lose their normal functions. AGEs increase with ageing, but production is markedly accelerated in diabetes and with oxidative stress[26]. AGEs are associated with atherosclerosis in both diabetic and nondiabetic subjects and increases in the concentration of circulating AGEs are associated with the severity of CAD and adverse clinical outcomes[27]. In addition to extracellular interactions, some AGEs are as specific ligands RAGE, which is a membrane-bound receptor. It is a transmembrane receptor of the immunoglobulin superfamily and is expressed in endothelial, neuronal, smooth muscles, mesangial and mononuclear cells[7]. Bucciarelli et al[13] and Kislinger et al[28] demonstrated that even in the absence of diabetes, the ligand-RAGE axis plays an important role in vascular pathology and the development of atherosclerosis[28]. Again plaque instabi
The study included 110 nondiabetic postmenopausal women, 55 of whom had angiographically proven CAD (Group I) and 55 control subjects (Group II) who were proven by invasive angiography not to have CAD. Blood samples were collected and processed as described in the materials and methods and the results were systematically analyzed. Student t-tests and χ2 tests were performed for continuous variables and categorical variables respectively. When compared with subjects without CAD, those with proven CAD had a significantly higher serum TG, hsCRP, and homocysteine levels and a significant family history of CAD. However, the opposite was found to be true for plasma sRAGE levels, which were found to be significantly lower in subjects with CAD compared with those without CAD (P < 0.05). Univariate and multivariate logistic regression analysis also revealed a significant correlation between plasma sRAGE levels and CAD (P = 0.01). Multivariate ORs for CAD revealed that the study subjects with sRAGE concentrations below 225 pg/mL had a 6-fold increase in CAD prevalence, independent of other risk factors. That finding is consistent with a study carried out by Falcone et al[31] that evaluated the correlation between sRAGE levels and CAD in Italian men, and found that low sRAGE levels (< 776 pg/mL, the first quartile) were associated with a 6.719-fold increase in CAD prevalence, independent of the other risk factors[16]. Similar results were obtained by Mahajan et al[2], who reported in 2009, that subjects with low sRAGE levels (< 607 pg/mL, the first quartile) were associated with a 13.6-fold increase in CAD prevalence, independent of other risk factors[2].
Pearson’s correlation failed to find any significant correlations between sRAGE levels and other biochemical parameters like total cholesterol, triglycerides, HDL-C, LDL-C, hsCRP, homocysteine, or Lp(a) levels. That finding is in contrast to a report by Basta et al[33], who demonstrated a positive correlation between sRAGE and HDL levels in an Italian population[33]. Our finding that no correlation existed between sRAGE levels and HTN contradicts a study by Geroldi et al[34] finding that hypertensive subjects had lower levels of sRAGE compared with normotensive CAD subjects[34]. The cause for this discrepancy is not clear, but it could be explained by differences in the ethnicity and sex of the two studies. Our subjects were all postmenopausal Indian women. We also found that sRAGE levels were lower in women. A 2005 study in nondiabetic Italian men by Falcone et al[31] reported mean sRAGE levels of 966 pg/mL in cases and 1335 pg/mL in controls[16]. In our study population the means were 474.89 in cases and 725.73 pg/mL in controls. The observed differences could have resulted from different study populations and small sample size in our study.
In our study, we found a significant correlation between CAD and some bio
Multivariate analysis revealed that a family history of CAD was an independent risk factor of CAD (P = 0.014). Our findings support Bachmann et al[38] reported in 2012 that a family history of CAD was associated with a persistent increase in CAD and related mortality across a long-term follow-up[38]. Our study also revealed a significant positive correlations of CAD and total cholesterol (P = 0.002) and CAD and LDL-C levels (P = 0.011) It is clear that high hsCRP, homocysteine, Lp(a) levels and decreased sRAGE levels, when considered together, are the best predictor of development and progression of CAD.
Our study has some limitations. First, the study population included only women from northern India. The results might not be obtained in other ethnic groups. Second, we determined only total sRAGE levels and not individual isoforms of sRAGE. Thus, it is possible that the observed reduction in sRAGE levels may reflect a reduction in a distinct circulating sRAGE isoform. Third, follow-up of the study subjects were not done. Therefore, the effects of drugs on the variables under consideration are not known. Last but not the least, the study sample size was small and might thus have been underpowered. Therefore, further studies in a larger number of subjects with CAD are warranted.
In conclusion, CAD in postmenopausal women cannot be fully explained by traditional risk factors. RAGE and its ligand, sRAGE play an important role in the development of atherosclerosis and CAD. Despite the limitations mentioned above, ours is the first study that included only postmenopausal nondiabetic women to demonstrate the correlation of decreased level of sRAGE with CAD. Our findings corroborate existing evidence that the pathogenesis of CAD involves many aspects of inflammation. Risk assessment for CAD in postmenopausal women can be further improved by considering sRAGE with other risk factors. Further studies in a large number of postmenopausal nondiabetic women from different geographical areas are needed to show that its correlation with the development of CAD is applicable to other ethnic groups.
The overall risk assessment for coronary artery disease (CAD) in postmenopausal women cannot rely on traditional risk factors. Thus, assessment of novel markers is needed. Receptor for advanced glycation end products (RAGE) interaction is of great importance in diabetic vasculopathy and plays a key role in atherosclerosis.
The motivation was to add to what is known of the role of soluble fraction of RAGE (sRAGE) in Indian postmenopausal women.
The objective was to investigate the association and correlation between plasma levels of sRAGE and CAD in nondiabetic postmenopausal women.
This case-control study included 55 angiographically proven CAD subjects with > 50% stenosis of at least one of the major coronary arteries and 55 healthy control women. Plasma sRAGE was determined with an enzyme-linked immunosorbent assay.
Plasma sRAGE concentrations were significantly lower in subjects with CAD than it was in healthy controls. A significant correlation between plasma sRAGE levels and CAD was observed using univariate and multivariate analysis.
sRAGE was positively correlated with CAD independent of other traditional risk factors.
sRAGE can be included in the assessment of CAD-risk in postmenopausal women.
We are grateful to all the study subjects who participated in this study and the laboratory staff for their technical support. The authors acknowledge the help of Shruti Chopra, Ruchika Bhatia, and Neha Handa for statistical analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lakusic N S-Editor: Fan JR L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Gupta R, Joshi P, Mohan V, Reddy KS, Yusuf S. Epidemiology and causation of coronary heart disease and stroke in India. Heart. 2008;94:16-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 250] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 2. | Mahajan N, Malik N, Bahl A, Sharma Y, Dhawan V. Correlation among soluble markers and severity of disease in non-diabetic subjects with pre-mature coronary artery disease. Mol Cell Biochem. 2009;330:201-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Güdücü N, Görmüş U, Kutay SS, Kavak ZN, Telatar B. Endogenous sex hormones and their associations with cardiovascular risk factors in post-menopausal women. J Endocrinol Invest. 2013;36:588-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 4. | Kaul U, Dogra B, Manchanda SC, Wasir HS, Rajani M, Bhatia ML. Myocardial infarction in young Indian patients: risk factors and coronary arteriographic profile. Am Heart J. 1986;112:71-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Braunwald E. Shattuck lecture--cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360-1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 967] [Cited by in F6Publishing: 894] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 6. | Niu W, Liu Y, Qi Y, Wu Z, Zhu D, Jin W. Association of interleukin-6 circulating levels with coronary artery disease: a meta-analysis implementing mendelian randomization approach. Int J Cardiol. 2012;157:243-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Laki J, Kiszel P, Vatay A, Blaskó B, Kovács M, Körner A, Madácsy L, Blatniczky L, Almássy Z, Szalai C, Rajczy K, Pozsonyi E, Karádi I, Fazakas A, Hosszúfalusi N, Pánczél P, Arason GJ, Wu YL, Zhou B, Yang Y, Yu CY, Füst G. The HLA 8.1 ancestral haplotype is strongly linked to the C allele of -429T>C promoter polymorphism of receptor of the advanced glycation endproduct (RAGE) gene. Haplotype-independent association of the -429C allele with high hemoglobinA1C levels in diabetic patients. Mol Immunol. 2007;44:648-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Hudson BI, Schmidt AM. RAGE: a novel target for drug intervention in diabetic vascular disease. Pharm Res. 2004;21:1079-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Wendt T, Bucciarelli L, Qu W, Lu Y, Yan SF, Stern DM, Schmidt AM. Receptor for advanced glycation endproducts (RAGE) and vascular inflammation: insights into the pathogenesis of macrovascular complications in diabetes. Curr Atheroscler Rep. 2002;4:228-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res. 1999;84:489-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 542] [Cited by in F6Publishing: 574] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 11. | Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 486] [Cited by in F6Publishing: 496] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 12. | Pollreisz A, Hudson BI, Chang JS, Qu W, Cheng B, Papapanou PN, Schmidt AM, Lalla E. Receptor for advanced glycation endproducts mediates pro-atherogenic responses to periodontal infection in vascular endothelial cells. Atherosclerosis. 2010;212:451-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, Kashyap Y, Stern DM, Schmidt AM. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106:2827-2835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 435] [Cited by in F6Publishing: 434] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 14. | Yu X, Liu J, Zhu H, Xia Y, Gao L, Li Z, Jia N, Shen W, Yang Y, Niu W. An interactive association of advanced glycation end-product receptor gene four common polymorphisms with coronary artery disease in northeastern Han Chinese. PLoS One. 2013;8:e76966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, Yasui K, Takeuchi M, Makita Z, Takasawa S, Okamoto H, Watanabe T, Yamamoto H. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370:1097-1109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 516] [Cited by in F6Publishing: 544] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 16. | Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F; Centers for Disease Control and Prevention; American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4411] [Cited by in F6Publishing: 4437] [Article Influence: 211.3] [Reference Citation Analysis (0)] |
| 17. | World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. Cardiovasc Res. 1997;35:2-3. [PubMed] [Cited in This Article: ] |
| 18. | WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7065] [Cited by in F6Publishing: 7498] [Article Influence: 374.9] [Reference Citation Analysis (0)] |
| 19. | Ness-Abramof R, Apovian CM. Waist circumference measurement in clinical practice. Nutr Clin Pract. 2008;23:397-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5429] [Cited by in F6Publishing: 5256] [Article Influence: 525.6] [Reference Citation Analysis (0)] |
| 21. | American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14-S80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2830] [Cited by in F6Publishing: 2933] [Article Influence: 293.3] [Reference Citation Analysis (0)] |
| 22. | Jellinger PS, Smith DA, Mehta AE, Ganda O, Handelsman Y, Rodbard HW, Shepherd MD, Seibel JA; AACE Task Force for Management of Dyslipidemia and Prevention of Atherosclerosis. American Association of Clinical Endocrinologists' Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis. Endocr Pract. 2012;18 Suppl 1:1-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 296] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 23. | Krishnan MN. Coronary heart disease and risk factors in India - on the brink of an epidemic? Indian Heart J. 2012;64:364-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Walsh BW, Kuller LH, Wild RA, Paul S, Farmer M, Lawrence JB, Shah AS, Anderson PW. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445-1451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 523] [Cited by in F6Publishing: 449] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 25. | Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 930] [Cited by in F6Publishing: 898] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 26. | Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1679] [Cited by in F6Publishing: 1805] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 27. | Kilhovd BK, Juutilainen A, Lehto S, Rönnemaa T, Torjesen PA, Birkeland KI, Berg TJ, Hanssen KF, Laakso M. High serum levels of advanced glycation end products predict increased coronary heart disease mortality in nondiabetic women but not in nondiabetic men: a population-based 18-year follow-up study. Arterioscler Thromb Vasc Biol. 2005;25:815-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Kislinger T, Tanji N, Wendt T, Qu W, Lu Y, Ferran LJ Jr, Taguchi A, Olson K, Bucciarelli L, Goova M, Hofmann MA, Cataldegirmen G, D'Agati V, Pischetsrieder M, Stern DM, Schmidt AM. Receptor for advanced glycation end products mediates inflammation and enhanced expression of tissue factor in vasculature of diabetic apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2001;21:905-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 192] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Lindsey JB, Cipollone F, Abdullah SM, McGuire DK. Receptor for advanced glycation end-products (RAGE) and soluble RAGE (sRAGE): cardiovascular implications. Diab Vasc Dis Res. 2009;6:7-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 856] [Cited by in F6Publishing: 849] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 31. | Falcone C, Emanuele E, D'Angelo A, Buzzi MP, Belvito C, Cuccia M, Geroldi D. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol. 2005;25:1032-1037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 295] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 32. | Kucukhuseyin O, Aydogan HY, Isbir CS, Isbir T. Associations of -374T/A polymorphism of receptor for advanced glycation end products (RAGE) gene in Turkish diabetic and non-diabetic patients with coronary artery disease. In Vivo. 2009;23:949-954. [PubMed] [Cited in This Article: ] |
| 33. | Basta G, Del Turco S, Marchi F, Navarra T, Battaglia D, Mercuri A, Mazzone A, Berti S. Elevated soluble receptor for advanced glycation end product levels in patients with acute coronary syndrome and positive cardiac troponin I. Coron Artery Dis. 2011;22:590-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Geroldi D, Falcone C, Emanuele E, D'Angelo A, Calcagnino M, Buzzi MP, Scioli GA, Fogari R. Decreased plasma levels of soluble receptor for advanced glycation end-products in patients with essential hypertension. J Hypertens. 2005;23:1725-1729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Haidari M, Javadi E, Sadeghi B, Hajilooi M, Ghanbili J. Evaluation of C-reactive protein, a sensitive marker of inflammation, as a risk factor for stable coronary artery disease. Clin Biochem. 2001;34:309-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Liu M, Ding XM. The correlation between serum homocysteine and hsCRP level in acute coronay syndrome patients with type 2 diabetes. Zhongguo Linchuang Baojian Zazhi. 2009;12:268-270. [DOI] [Cited in This Article: ] |
| 37. | Schaffer A, Verdoia M, Cassetti E, Marino P, Suryapranata H, De Luca G; Novara Atherosclerosis Study Group (NAS). Relationship between homocysteine and coronary artery disease. Results from a large prospective cohort study. Thromb Res. 2014;134:288-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 38. | Bachmann JM, Willis BL, Ayers CR, Khera A, Berry JD. Association between family history and coronary heart disease death across long-term follow-up in men: the Cooper Center Longitudinal Study. Circulation. 2012;125:3092-3098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |