Published online May 26, 2021. doi: 10.4330/wjc.v13.i5.117
Peer-review started: December 28, 2020
First decision: February 28, 2021
Revised: March 11, 2021
Accepted: April 21, 2021
Article in press: April 21, 2021
Published online: May 26, 2021
Right ventricular outflow tract (RVOT) obstruction is present in a variety of congenital heart disease states including tetralogy of Fallot, pulmonary atresia/stenosis and other conotruncal abnormalities etc. After surgical repair, these patients develop RVOT residual abnormalities of pulmonic stenosis and/or insufficiency of their native outflow tract or right ventricle to pulmonary artery conduit. There are also sequelae of other surgeries like the Ross operation for aortic valve disease that lead to right ventricle to pulmonary artery conduit dysfunction. Surgical pulmonic valve replacement (SPVR) has been the mainstay for these patients and is considered standard of care. Transcatheter pulmonic valve implantation (TPVI) was first reported in 2000 and has made strides as a comparable alternative to SPVR, being approved in the United States in 2010. We provide a comprehensive review in this space–indications for TPVI, detailed procedural facets and up-to-date review of the literature regarding outcomes of TPVI. TPVI has been shown to have favorable medium-term outcomes free of re-interventions especially after the adoption of the practice of pre-stenting the RVOT. Procedural mortality and complications are uncommon. With more experience, recognition of risk of dreaded outcomes like coronary compression has improved. Also, conduit rupture is increasingly being managed with transcatheter tools. Questions over endocarditis risk still prevail in the TPVI population. Head-to-head comparisons to SPVR are still limited but available data suggests equivalence. We also discuss newer valve technologies that have limited data currently and may have more applicability for treatment of native dysfunc
Core Tip: As patients with congenital heart defects continue to survive well into adult life, right ventricular function preservation and management of the right ventricular outflow tract has become increasingly important and sophisticated. As an alternative to surgical pulmonary valve replacement (PVR), the advent of transcatheter pulmonic valve therapy has offered a less invasive approach for many patients. Improved techniques and technologies have rapidly expanded its use in increasingly complex anatomy. In this review we summarize the indications for PVR, transcatheter pulmonic valve implantation techniques, clinical outcomes and also future directions of this treatment.
- Citation: Law MA, Chatterjee A. Transcatheter pulmonic valve implantation: Techniques, current roles, and future implications . World J Cardiol 2021; 13(5): 117-129
- URL: https://www.wjgnet.com/1949-8462/full/v13/i5/117.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i5.117
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart disease occurring in approximately 3 per 10000 live births[1]. This defect is comprised of the following four components: Ventricular septal defect (VSD), aortic override, right ventricular hypertrophy, and right ventricular outflow tract (OT) obstruction in the form of infundibular stenosis/pulmonary valve stenosis/pulmonary artery stenosis. Correction of this heart defect involves patch closure of the VSD and relief of the right ventricular outflow tract (RVOT) obstruction. The end result uniformly leaves the patient with residual OT dysfunction with some form of pulmonic stenosis (PS)[2] and/or pulmonary valve insufficiency (PI) leading to deleterious effects over the long-term[3,4]. The chronic effects of pressure and volume loading on the right ventricular (RV) ultimately lead to symptomatic ventricular dysfunction and arrhythmia[2,3].
While TOF is the stereotypic diagnosis for the management of residual RVOT disease, other congenital heart defects are managed similarly. Conotruncal defects such as truncus arteriosus, d-transposition of the great arteries with a VSD and pulmonary stenosis, double outlet right ventricle can be repaired with a VSD closure to the aortic valve and placement of a RV to pulmonary artery conduit. Another common example is the Ross operation for aortic valve stenosis which leaves the patient with a reconstructed RV to pulmonary artery connection. These surgically reconstructed outflows all universally fail over time leading to the same long-term deleterious effect on the RV.
Until recently, surgical pulmonic valve replacement (SPVR) was utilized universally for pulmonary valve replacement (PVR) and relief of RVOT dysfunction. In 2000, Bonhoeffer et al[5] implanted the first transcatheter valve into a human[5]. This led to the development of Melody Transcatheter Pulmonary Valve (Medtronic Inc, Minneapolis, MN), receiving Humanitarian Device Exemption (HDE) Food and Drug approval (FDA) for commercial use in dysfunctional RV to pulmonary conduits in the United States in 2010. Subsequent studies allowed for approval of the Sapien XT valve (Edwards Lifesciences LLC, Irvine, California) as transcatheter PVR in failed RV to pulmonary conduits[6].
In the following review we will review the indications for transcatheter pulmonic valve implantation (TPVI), discuss procedural considerations, techniques, outcomes as well as future considerations.
The indications for PVR, especially via transcatheter means are gradually evolving. Most clinical societies agree with need for PVR in symptomatic patients in the TOF spectrum with moderate to severe PS or PI (Table 1). However, the question of asymptomatic PI persists. The deleterious effects of progressive RV dilation with respect to increased mortality are well known, however it has not been convincingly proven that SPVR improves mortality or the incidence of ventricular arrhythmias[7]. There have been multiple studies to investigate what threshold of RV dilation should be used to pursue PVR in an asymptomatic patient which allows a reasonable chance of reversal of structural abnormalities[8]. However, surgical PVR for asymptomatic patients with lesser degrees of RV dilation has also been shown to increase chances of heart failure, atrial arrhythmias or ventricular tachycardia[9]. Thus, the need to balance the deleterious effects of progressive RV dilation with those of repeated operations and cardiopulmonary bypass need to be weighed together. With the increase in use of TPVI, the latter can be circumvented and with solutions for native RVOT also on horizon, it is likely that more asymptomatic patients may benefit from the procedure. Currently, generally accepted indications for TPVI in asymptomatic patients are the following[3]: (1) RV indexed end diastolic volume > 160 mL/m2 or RV indexed end systolic volume > 80 mL/m2; (2) RV end diastolic volume > 2 times of left ventricular end diastolic volume; (3) Significant RV dysfunction (RV ejection fraction < 45%); (4) Progressive tricuspid regurgitation in the setting of a dilated RV; and (5) Ventricular arrhythmias with a dilated RV.
| Indication | Recommendation | Level of evidence | ||
| ACC/AHA 2018 | ESC 2010 | ACC/AHA 2018 | ESC 2010 | |
| Symptomatic with moderate/severe PR | I | I | B | C |
| Asymptomatic with moderate/severe PR and RV dilation | IIa | IIa | B | C |
| Asymptomatic with moderate/severe PR and ventricular arrhythmias | IIb | IIa | C | C |
Generally multidisciplinary discussion with different cardiology subspecialties (almost always with adult congenital specialists), congenital cardiac surgery and cardiac anesthesia is recommended to determine best strategy for each individual patient. Multi-modality imaging review and good understanding of prior operations is essential. It is of vital importance to understand the nominal size of RV to pulmonary artery conduits and bioprosthetic pulmonary valves as well as any known coronary artery anomalies.
General anesthesia is usually preferred[10]. Biplane fluoroscopy is most often employed to understand the RVOT and pulmonary artery anatomy in two views and requires patient positioning with the arms elevated in a non-stretched position to avoid brachial plexus injury[11]. Most patients have had multiple catheteriza
A baseline right and left heart catheterization hemodynamic assessment is performed as well as RVOT, pulmonary artery, and aorta/coronary angiography. From this data, a plan for conduit rehabilitation and final balloon sizing is developed as well as ascertaining risk for conduit rupture and coronary artery compression. It also identifies other pulmonary artery stenosis that might need to be intervened upon during the procedure. After baseline data is obtained a stiff wire [Lunderquist Extra Stiff Guidewire (Cook Medical, Bloomington, IN), Amplatz Superstiff Guidewire (Boston Scientific, Boston, MA)] is positioned into the distal lower lobe of right or left pulmonary artery.
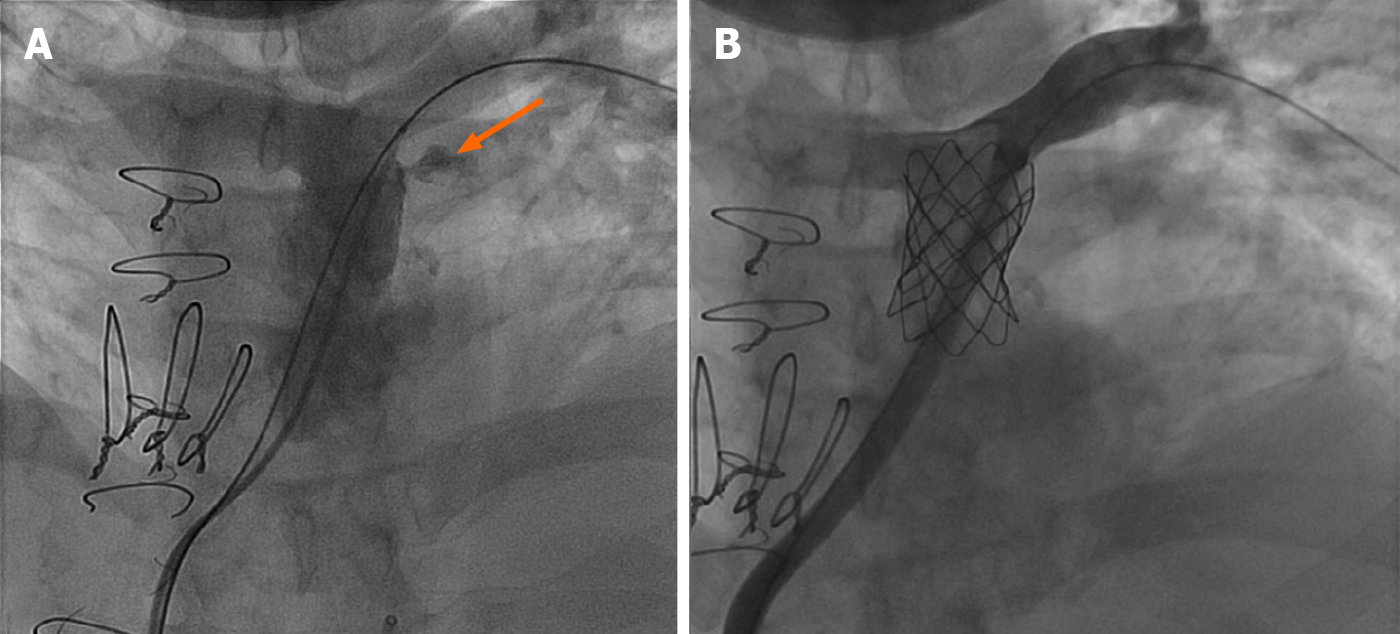
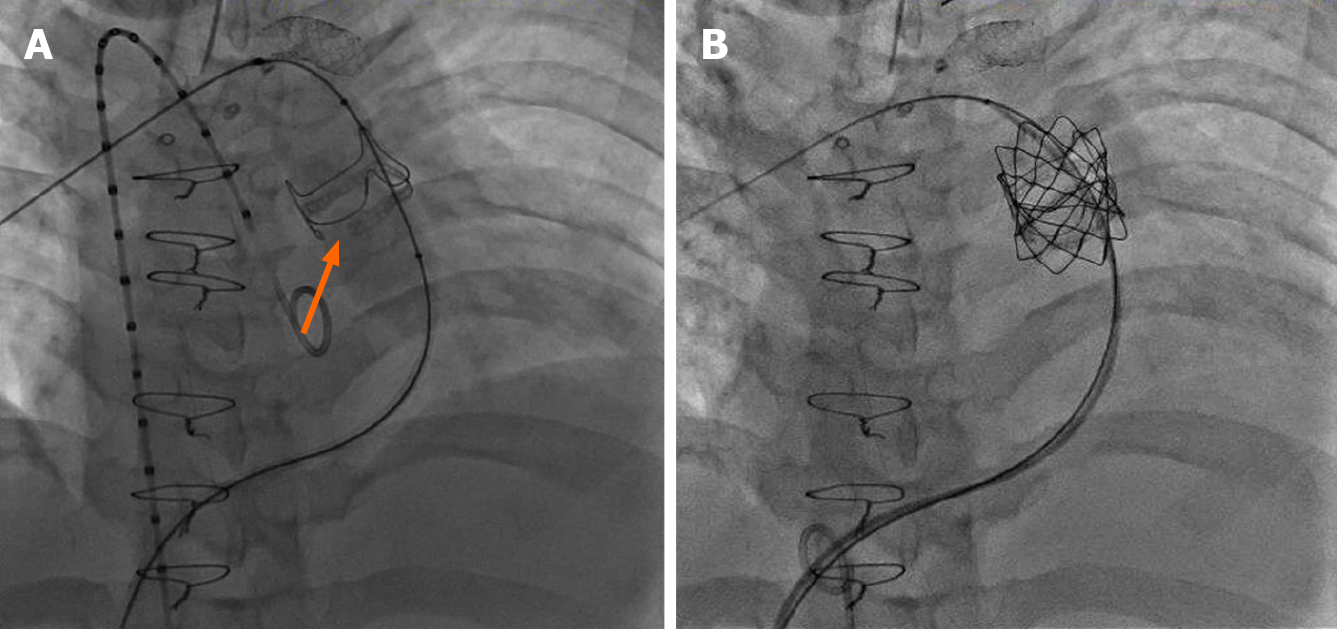
Many dysfunctional RVOTs involve significant conduit stenosis. This requires conduit rehabilitation and stenting to achieve acceptable post procedure RV pressure. Serial balloon dilation with high pressure balloon angioplasty is often necessary. This places the conduit at risk for rupture necessitating covered stent angioplasty with a Cheatham-platinum covered stent (Numed, Hopkinton, New York, United States) (Figure 1)[13]. Once assured that an adequate final diameter can be achieved, bare metal stent angioplasty with large bore hand mounted stainless-steel stents [Palmaz XL (Cordis, Milpitas, CA, United States), InstraStent Max LD (EV3, Plymouth, MN, United States)] are implanted. Because of stent fracture risk of the Melody valve, multiple stents might be necessary to avoid recoil and limit metal fatigue[14]. Ideally, for an adult, the final diameter of the conduit should be at least 20-22 mm, otherwise, a residual RVOT gradient will persist.
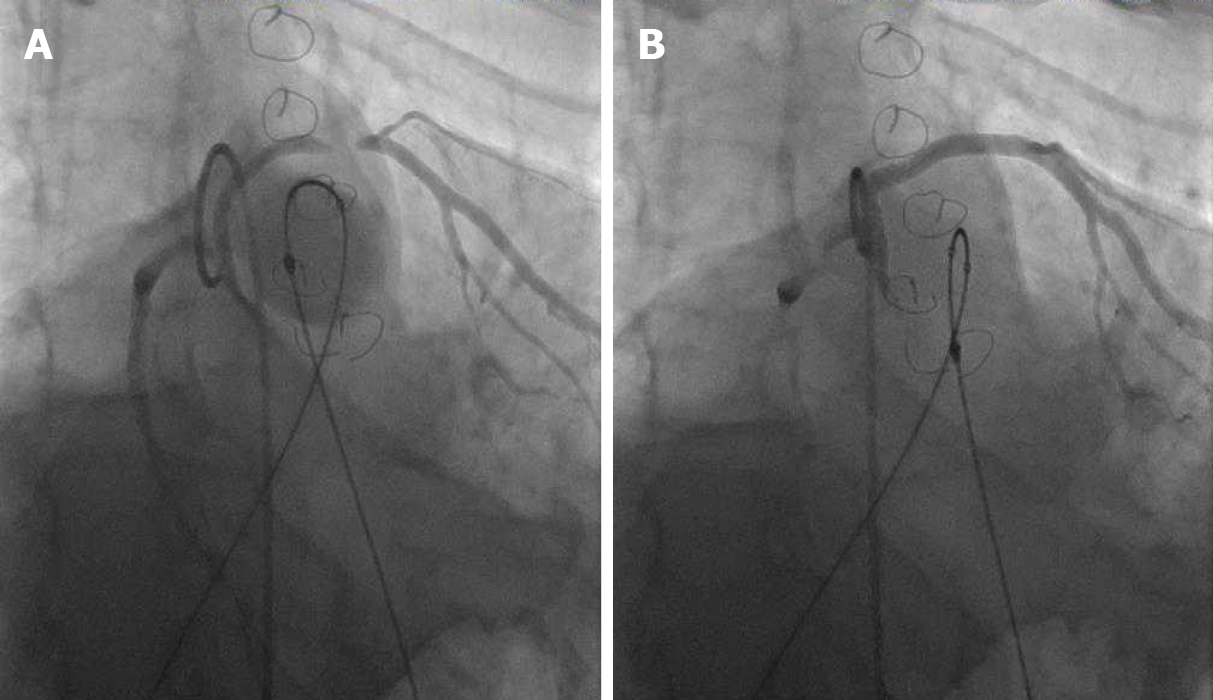
Prior to initial stenting of the conduit, assessment for risk of coronary artery obstruction is necessary. A variety of coronary artery anomalies can be at risk including a left anterior descending from the right coronary artery, ostial stenosis, abnormal location secondary to reimplantation. Coronary artery testing is performed with either aortic root angiography and/or selective coronary angiography. This is performed with the Anteroposterior tube angled caudal to perform and “down the barrel” assessment (Figure 2). If the coronary artery flow is compromised during balloon testing, this is considered a contraindication to proceeding with stent or PVI. While less commonly a problem, aortic root distortion causing aortic valve insufficiency has also been demonstrated[15].
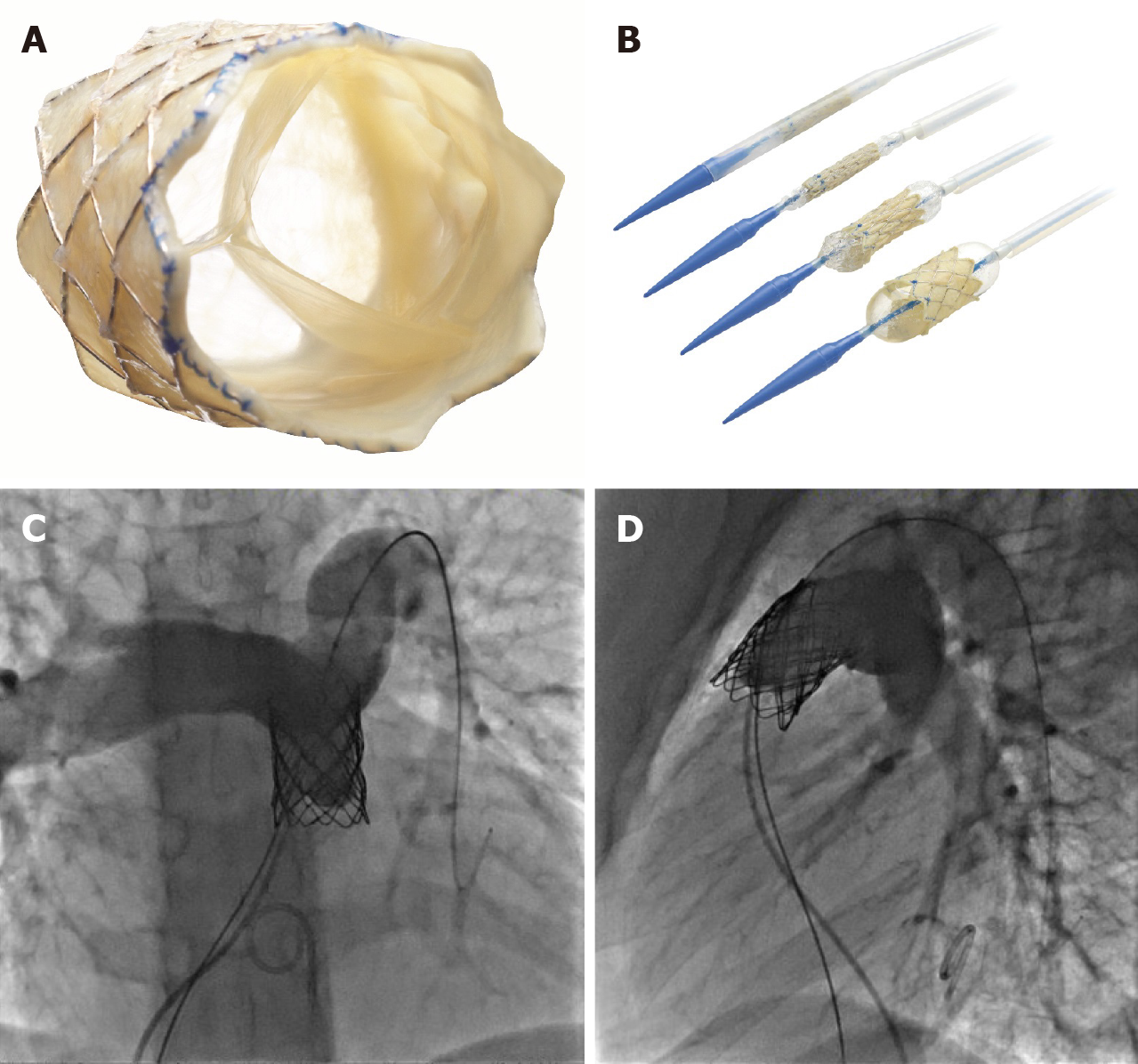
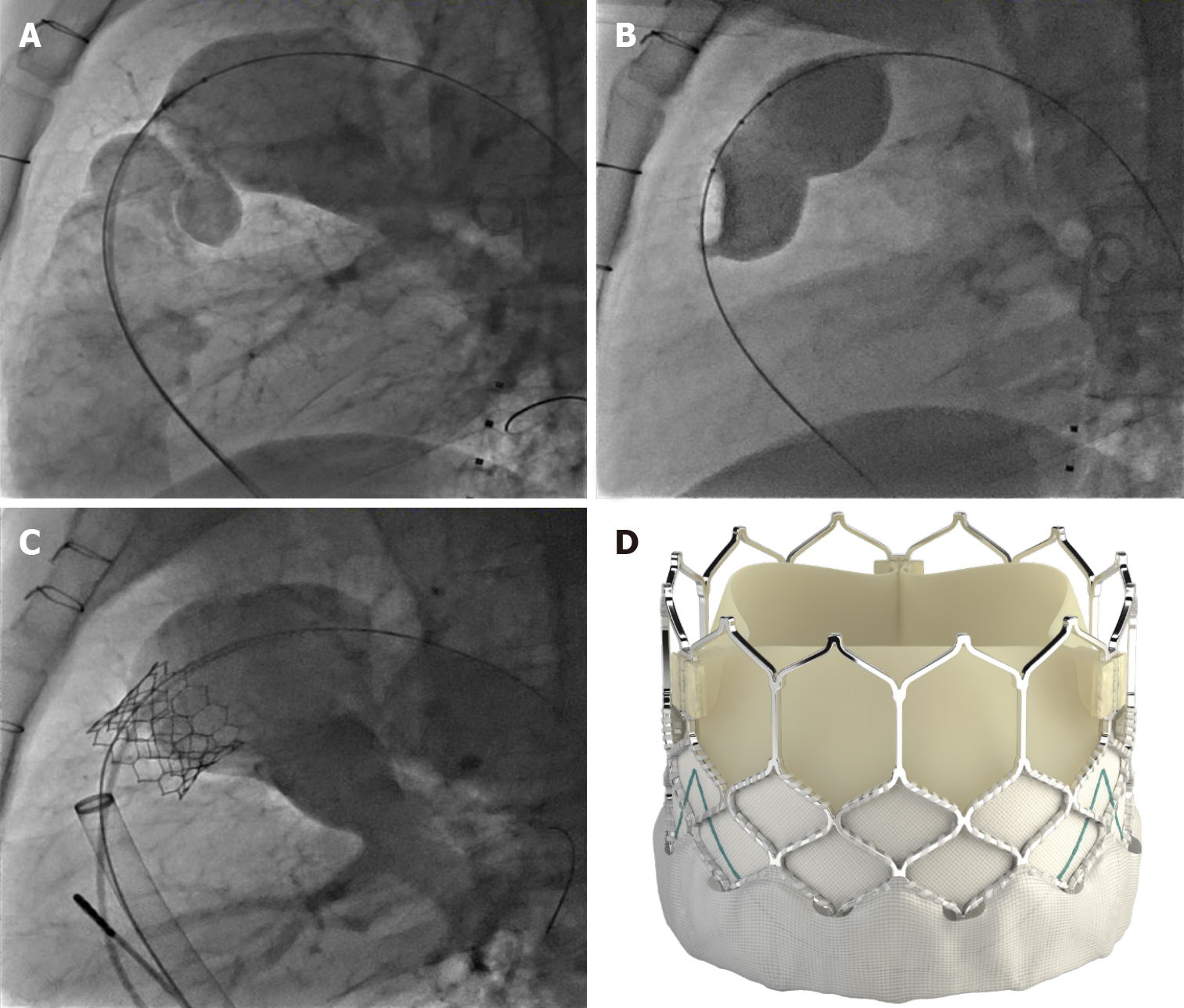
Once the conduit is adequately prepared-the Melody valve is hand crimped onto the balloon Ensemble system (Medtronic, Minneapolis, MN, United States) (Figure 3). Balloons range from 18-22 mm and there are descriptions of maintained valve competency even when mounted on a 24 mm balloon[16]. The outer diameter of the valve is approximately 2 mm larger than the balloon diameter. The Edwards XT valve is crimped onto the Novaflex delivery system using the proprietary process (Figure 4).
After delivery system preparation, the delivery system is advanced into the appropriate position. Numerous techniques can be used to aid in delivery system advancement such as balloon assisted or a buddy wire methods[17]. Secondary to the stiffness of the delivery system, Edwards Sapien XT valve is more difficult to pass into the OT. Use of the Sapien S3 and the smaller delivery system is more amenable to traversing the RVOT. Use of the Edwards XT or Sapien S3 through a long (65 cm) large bore Gore sheath (Flagstaff, AZ) into position has mitigated some of the difficulty[18]. While the valve implant is typically stable, rapid pacing at 140-160 beats per minute can be performed to aid in valve positioning. Following valve implant, post implant hemodynamic data are obtained, and an angiogram is performed to evaluate for perivalvar leak and valve competence. Some have advocated for intracardiac echocardiogram imaging post implant to evaluate the implant and have a reference for the future[19]. Typically for hemostasis post procedure a figure of 8 stitch is sufficient[20].
Valve in bioprosthetic valve implantation tends to be a less complex procedure. The risk of coronary compression is less as the valve cannot be over-expanded at low pressure, and generally there is no need to pre-stent unless there is significant conduit stenosis separate from the valve. The type and size of bioprosthetic valve is of utmost importance as the inner diameter dictates the size of transcatheter valve. Many descriptions of valve fracture exist, expanding the valve to the size or just larger of the outer diameter with a high pressure True balloon (Atlas, Atlas Gold, or True Balloon, Bard Peripheral Vascular, Inc, Tempe, AZ) (Figure 5)[21]. This avoids future deve
The OT after transannular patch repair for TOF can be quite complex. Many times, it lacks a tubular landing zone and can have a funnel shape from the RV out to the pulmonary arteries. However, some native OTs have anatomy suitable for currently available valve to be used off-label. Balloon sizing with a large diameter (> 35 mm) compliant balloon can identify a suitable landing zone for a balloon expandable stent. In appropriate anatomic arrangements, this is reported with good short- and long-term success (Figure 6)[22]. Valve implant into one or both of the proximal branch pulmonary arteries has also been shown to be effective in reducing the degree of PI[23].
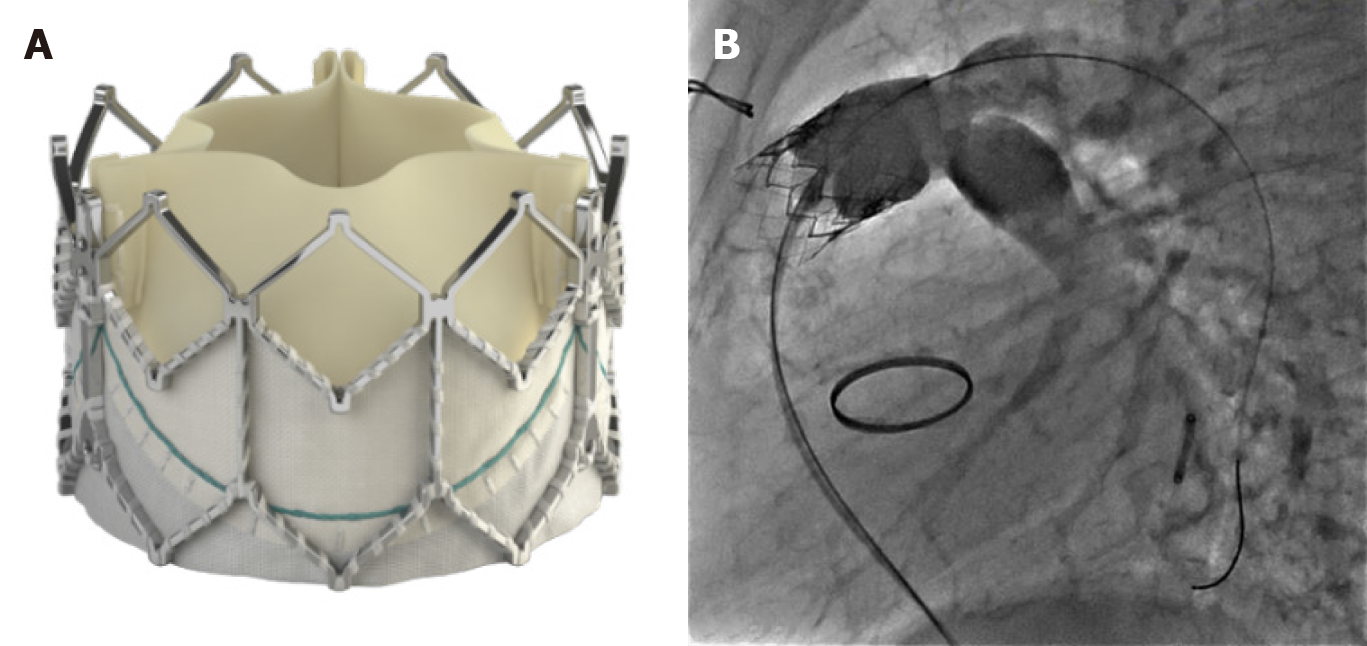
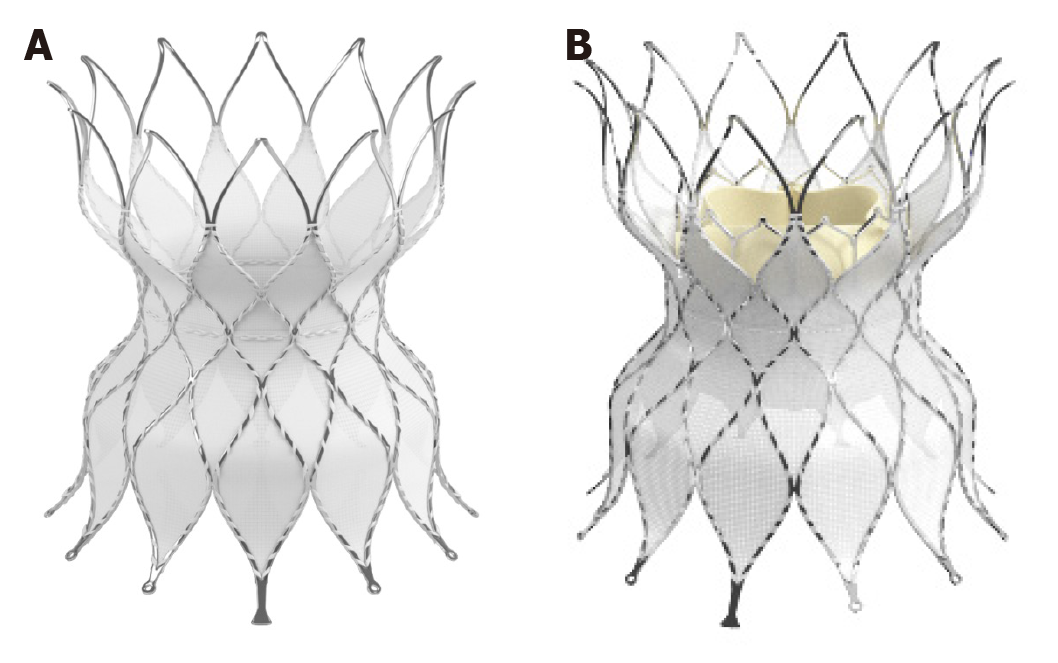
The Medtronic Harmony Valve (Minneapolis, MN, United States) (Figure 7) is a self-expanding valve that can be used in native OT after three-dimensional sizing data has been obtained. Similarly, the Alterra pre-stent (Edwards Lifesciences LLC, Irvine, California) (Figure 8) allows for creation of a landing zone to allow for implantation of a 29 Sapien S3 valve. Both are currently under investigation at the time of writing this review[24,25].
The Melody valve (Medtronic Inc, Minneapolis, MN, United States) traces its origins to the implantation of an 18 mm bovine jugular vein mounted on a platinum stent in a 12 year old patient with conduit failure by Bonhoeffer et at[5]. In its current iteration, the Melody valve is a bovine jugular vein sutured inside of a Platinum Iridium stent, available in two sizes – 20 and 22 mm. The two sizes use 16 and 18 mm vein segments intended to be expanded up to 20 and 22 mm respectively. The valve is delivered via the Medtronic Ensemble delivery system which utilizes a “balloon-in-balloon” (NuMED Inc, Hopkinton, NY, United States). The delivery system is 22F and is available in 18, 20, and 22 mm sizes.
Experience from European centers with the Melody valve predates use in the United States–Lurz et al[26] reported their series of 155 implants with an excellent success rate of 96.7%, freedom from reoperation rates of 70% after 70 mo and lower re-operation rates with increasing familiarity/expertise with the valve system[26]. The United States Investigational Device Exemption trial reported its medium to long term results in 2015 enrolling a total of 171 patients with 148 successful implants and a re-intervention free rate of 76% at 5 years[27]. The Melody valve received Conformitè Europëenne Mark approval in 2006; the FDA granted HDE approval in 2010 and full post market approval in 2015. The United States post market approval study had 100 implants with 98% success rate and a 98% rate of freedom from catheter/surgical re-intervention[28].
The Edwards Sapien XT (Edwards Lifesciences, Irvine, CA, United States) transcatheter heart valve (THV) was approved by the FDA for use in the pulmonic position in 2016 (Figure 4). The valve is made of bovine pericardial tissue and mounted on a cobalt chromium stent with a fabric skirt on the outside lower part of the stent to minimize perivalvular regurgitation. The valve sizes are 23, 26 and 29 mm and delivered via the Edwards Novaflex delivery system. The COMPASSION clinical trial enrolled 81 patients and implanted the Sapien XT THV in 69 with a 93.7% freedom from re-intervention at 3 years[6]. There are ongoing clinical trials, for post market approval surveillance of the use of this valve and also for the evaluation of the Edwards Sapien 3 THV (Figure 4). The Sapien 3 expands the valve sizes with a 20 mm option–the Commander valve delivery system is also believed to be better suited for delivery into the RVOT. Data from a series of 56 patients from Germany suggests excellent procedural success and no reinterventions were needed up to 2 years of follow up[29].
The venus P valve (Venus Medtech, Hangzhou, China) is a newer generation valve using porcine pericardium leaflets mounted inside the middle segment of a self-expanding nitinol stent. The middle segment measures 22-36 mm in diameter and 20-35 mm in length. The proximal and distal ends are flared to provide an hourglass shape that helps with anchoring in the RVOT and main pulmonary artery. The delivery system consists of a 19-24F capsule which houses the crimped stent valve and a 15F shaft. This system is designed keeping in mind the unmet need of treating PI in native RVOT which in most circumstances sizes out of approved valve systems’ range in adults. Multiple small series report favorable outcomes with the venus P valve–in a 38-patient retrospective multi center report, 37 implantations were successful; 1 patient subsequently required surgical repositioning. With a median follow up of 25 mo, no significant valve dysfunction was reported. Stent fractures were reported in 27% patients but the significance of those with this valve system is not yet known[30].
Another valve system with a similar concept is the Pulsta valve (TaeWoong Medical Co, Gyeonggi-do, South Korea). It also has a knitted double strand Nitinol wire frame which is self-expanding with treated porcine pericardial tissue leaflets sutured on. The valve is available in 18-28 mm sizes (2 mm increments). The length of the stent frame is 28-38 mm depending on the valve size and the ends are flared 4 mm wider than the outer diameter for anchoring. The diameter of the “valve loading zone” is 18F while the shaft of the delivery system is 12F in caliber. There is a “hook block” to minimize chances of abrupt delivery and migration. In a Korean feasibility study, ten TOF patients with PR were treated with the Pulsta valve–five with 26 mm and other five with 28 mm valve. There were no major complications with all 10 implants showing good valve function and significant reduction in RV volumes[31].
With growing experience, procedural success has improved remarkably–in a prior meta-analysis, we reported a success rate of 96.2% using observational reports, which is consistent with the 98% success rate reported in the Melody United States post market approval surveillance study[28,32]. The most dreaded complications for TPVI are conduit rupture and coronary compression. Conduit rupture is estimated in about 4.1% patients–more so in homografts[32,33]. In recent years, the practice of performing TPVI off label in conduits sized < 16 mm has increased, and the rate of conduit rupture is much more in this population. Varied estimates from 16% to 50% have been reported-however most of these ruptures are limited and can usually be managed with a covered stent implant[34,35].
Coronary compression was reported more frequently in the earlier TPVI experience and led to procedural mortality. In our pooled analysis of 1044 attempted TPVI, there were a total of five coronary complications. There were only two deaths in elective cases, and both were attributed to coronary compression[32]. However, with more experience and careful testing prior to stent/valve implant, these have become extremely rare. In the United States post market approval study, only 1 out of 100 implants had coronary compression was reported which required conversion to surgery. Five other patients out of the originally considered 120 patients had evidence of coronary compression on balloon testing[28]. It should especially be noted that anomalous origin of coronaries especially left coronary artery arising from the right cusp is a strong predictor of coronary compression[36].
The issue of needing frequent re-interventions or re-operations has largely been deemed secondary to stent fractures of the Melody valve and the rates for same have vastly improved since the adoption of pre-stenting. The United States Investigational Device Exemption trial for example had only 10% pre-stenting in the first tertile of the study and reported a freedom from re-intervention rate of 76% after 5 years. The other factors leading to re-interventions were found to be implant in a homograft, a higher post implant gradient (> 25 mmHg) and pre-existing significant tricuspid regurgi
Endocarditis/blood stream infection has been described as a late complication after TPVI. There have been variable reports of endocarditis after Melody and Sapien valve implants, more for the former. McElhinney et al[38] used three patient cohorts in North America and Europe with a cumulative sample of 309 patients and a follow up of 5.1 years–Endocarditis was diagnosed in 46 patients with TPV related endocarditis occurring in 35 patients[38]. Median time to endocarditis was 3.1 years. At 5 years, 89% patients were free of endocarditis. However widely variable reports of endo
It is also interesting to note that there are differences in endocarditis rates amongst surgical PVR recipients–rates are much higher in patients receiving bovine jugular vein conduits as compared to homografts–20.4% vs 2.4%[41].
Very few head-to-head comparisons of SPVR and TPVI exist. Caughron et al[42] reported a study of 66 patients (SPVR 36; TPVI 30) and found no differences in mortality, cardiovascular re-admissions or repeat interventions in the two groups[42]. A recent meta-analysis of 1132 TPVI and 4939 SPVR patients also did not show any difference in mortality or repeat interventions. However, there were fewer procedure related complications and higher rate of endocarditis with TPVI[43]. A study using the Nationwide Inpatient Sample database also suggested the same–comparing an estimated 8449 SPVR and 555 TPVI admissions, it showed a higher in-hospital mortality with SPVR along with other iatrogenic cardiac complications. Also, TPVI predictably had less hospital length of stay decreasing the impact on societal loss of wages for patients/care givers[44].
TPVI has dramatically evolved over that past decade and has become a common procedure to manage many patients with dysfunctional RVOT. The short and midterm results are overall quite favorable with a low and acceptable morbidity and mortality. Ideally in the future, smaller and more flexible delivery systems will be developed. With native OTs, intervention becomes quite appealing if a new valve can be placed inside indefinitely thus avoiding surgery except for at the time of initial repair. As all the existing valves have been implanted for a short time with limited follow up, future data is necessary to understand their overall longevity and types of valve failure. Furthermore, as many patients have smaller and stenotic conduits even in a growing child, adoption of new surgical techniques to allow for further expansion of small and stenotic conduits could be of significant clinical benefit.
The Authors would like to personally thank both Edwards Lifesciences, LLC, Irvine, CA, United States and Medtronic, Inc, Minneapolis, MN, United States for granting permission of their product images for use in this manuscript.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kim GB S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY
| 1. | Perry LW, Neill CA, Ferencz C, Rubin JD, Loffredo CA. Infants with congenital heart disease: the cases. Perspectives in pediatric cardiology Vol 4: Epidemiology of congenital heart disease, the Baltimore-Washington Infant Study 1981-1989. Ferencz C, Loffredo CA, Rubin JD, Magee Ca, editors. Mount Kisco, NY: Futura Publishing Company; 1993: 33-62. [Cited in This Article: ] |
| 2. | Sabate Rotes A, Connolly HM, Warnes CA, Ammash NM, Phillips SD, Dearani JA, Schaff HV, Burkhart HM, Hodge DO, Asirvatham SJ, McLeod CJ. Ventricular arrhythmia risk stratification in patients with tetralogy of Fallot at the time of pulmonary valve replacement. Circ Arrhythm Electrophysiol. 2015;8:110-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:1494-1563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 306] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 4. | Kirklin JK, Kirklin JW, Blackstone EH, Milano A, Pacifico AD. Effect of transannular patching on outcome after repair of tetralogy of Fallot. Ann Thorac Surg. 1989;48:783-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 114] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D, Acar P, Le Bidois J, Sidi D, Kachaner J. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403-1405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 754] [Cited by in F6Publishing: 676] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 6. | Kenny D, Rhodes JF, Fleming GA, Kar S, Zahn EM, Vincent J, Shirali GS, Gorelick J, Fogel MA, Fahey JT, Kim DW, Babaliaros VC, Armstrong AK, Hijazi ZM. 3-Year Outcomes of the Edwards SAPIEN Transcatheter Heart Valve for Conduit Failure in the Pulmonary Position From the COMPASSION Multicenter Clinical Trial. JACC Cardiovasc Interv. 2018;11:1920-1929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 7. | de Torres-Alba F, Kaleschke G, Baumgartner H. Impact of Percutaneous Pulmonary Valve Implantation on the Timing of Reintervention for Right Ventricular Outflow Tract Dysfunction. Rev Esp Cardiol (Engl Ed). 2018;71:838-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Therrien J, Siu SC, McLaughlin PR, Liu PP, Williams WG, Webb GD. Pulmonary valve replacement in adults late after repair of tetralogy of fallot: are we operating too late? J Am Coll Cardiol. 2000;36:1670-1675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 356] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Bokma JP, Geva T, Sleeper LA, Babu Narayan SV, Wald R, Hickey K, Jansen K, Wassall R, Lu M, Gatzoulis MA, Mulder BJ, Valente AM. A propensity score-adjusted analysis of clinical outcomes after pulmonary valve replacement in tetralogy of Fallot. Heart. 2018;104:738-744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Arboleda Salazar R, Heggie J, Wolski P, Horlick E, Osten M, Meineri M. Anesthesia for Percutaneous Pulmonary Valve Implantation: A Case Series. Anesth Analg. 2018;127:39-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Souza Neto EP, Durand PG, Sassolas F, Vial C, Lehot JJ. Brachial plexus injury during cardiac catheterisation in children. Report of two cases. Acta Anaesthesiol Scand. 1998;42:876-879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Zampi JD, Berman DP, Bocks ML, Yu S, Zahn EM, Lu JC, Shaya JA, Armstrong AK. Factors associated with the internal jugular venous approach for Melody™ Transcatheter Pulmonary Valve implantation. Cardiol Young. 2016;26:948-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Delaney JW, Goldstein BH, Bishnoi RN, Bisselou KSM, McEnaney K, Minahan M, Ringel RE; PARCS Investigators. Covered CP Stent for Treatment of Right Ventricular Conduit Injury During Melody Transcatheter Pulmonary Valve Replacement. Circ Cardiovasc Interv. 2018;11:e006598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Cardoso R, Ansari M, Garcia D, Sandhu S, Brinster D, Piazza N. Prestenting for prevention of melody valve stent fractures: A systematic review and meta-analysis. Catheter Cardiovasc Interv. 2016;87:534-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Torres AJ, McElhinney DB, Anderson BR, Turner ME, Crystal MA, Timchak DM, Vincent JA. Aortic Root Distortion and Aortic Insufficiency During Balloon Angioplasty of the Right Ventricular Outflow Tract Prior to Transcatheter Pulmonary Valve Replacement. J Interv Cardiol. 2016;29:197-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Cheatham SL, Holzer RJ, Chisolm JL, Cheatham JP. The Medtronic Melody® transcatheter pulmonary valve implanted at 24-mm diameter--it works. Catheter Cardiovasc Interv. 2013;82:816-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Shah RR, Poommipanit P, Law MA, Amin Z. Anchor balloon, buddy wire, and wire and sheath techniques to deploy percutaneous pulmonary valves in tetralogy of fallot patients. Catheter Cardiovasc Interv. 2018;92:915-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Kenny D, Morgan GJ, Murphy M, AlAlwi K, Giugno L, Zablah J, Carminati M, Walsh K. Use of 65 cm large caliber Dryseal sheaths to facilitate delivery of the Edwards SAPIEN valve to dysfunctional right ventricular outflow tracts. Catheter Cardiovasc Interv. 2019;94:409-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Whiteside W, Pasquali SK, Yu S, Bocks ML, Zampi JD, Armstrong AK. The Utility of Intracardiac Echocardiography Following Melody™ Transcatheter Pulmonary Valve Implantation. Pediatr Cardiol. 2015;36:1754-1760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Morgan GJ, Waragai T, Eastaugh L, Chaturvedi RC, Lee KJ, Benson L. The fellows stitch: large caliber venous hemostasis in pediatric practice. Catheter Cardiovasc Interv. 2012;80:79-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Shahanavaz S, Asnes JD, Grohmann J, Qureshi AM, Rome JJ, Tanase D, Crystal MA, Latson LA, Morray BH, Hellenbrand W, Balzer DT, Gewillig M, Love JC, Berdjis F, Gillespie MJ, McElhinney DB. Intentional Fracture of Bioprosthetic Valve Frames in Patients Undergoing Valve-in-Valve Transcatheter Pulmonary Valve Replacement. Circ Cardiovasc Interv. 2018;11:e006453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Georgiev S, Tanase D, Ewert P, Meierhofer C, Hager A, von Ohain JP, Eicken A. Percutaneous pulmonary valve implantation in patients with dysfunction of a "native" right ventricular outflow tract - Mid-term results. Int J Cardiol. 2018;258:31-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Qureshi AM, Bansal N, McElhinney DB, Boudjemline Y, Forbes TJ, Maschietto N, Shahanavaz S, Cheatham JP, Krasuski R, Lamers L, Chessa M, Morray BH, Goldstein BH, Noel CV, Wang Y, Gillespie MJ. Branch Pulmonary Artery Valve Implantation Reduces Pulmonary Regurgitation and Improves Right Ventricular Size/Function in Patients With Large Right Ventricular Outflow Tracts. JACC Cardiovasc Interv. 2018;11:541-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Zahn EM, Chang JC, Armer D, Garg R. First human implant of the Alterra Adaptive PrestentTM : A new self-expanding device designed to remodel the right ventricular outflow tract. Catheter Cardiovasc Interv. 2018;91:1125-1129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | Bergersen L, Benson LN, Gillespie MJ, Cheatham SL, Crean AM, Hor KN, Horlick EM, Lung TH, McHenry BT, Osten MD, Powell AJ, Cheatham JP. Harmony Feasibility Trial: Acute and Short-Term Outcomes With a Self-Expanding Transcatheter Pulmonary Valve. JACC Cardiovasc Interv. 2017;10:1763-1773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Lurz P, Coats L, Khambadkone S, Nordmeyer J, Boudjemline Y, Schievano S, Muthurangu V, Lee TY, Parenzan G, Derrick G, Cullen S, Walker F, Tsang V, Deanfield J, Taylor AM, Bonhoeffer P. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117:1964-1972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 366] [Cited by in F6Publishing: 381] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 27. | Cheatham JP, Hellenbrand WE, Zahn EM, Jones TK, Berman DP, Vincent JA, McElhinney DB. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131:1960-1970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 28. | Armstrong AK, Balzer DT, Cabalka AK, Gray RG, Javois AJ, Moore JW, Rome JJ, Turner DR, Zellers TM, Kreutzer J. One-year follow-up of the Melody transcatheter pulmonary valve multicenter post-approval study. JACC Cardiovasc Interv. 2014;7:1254-1262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Lehner A, Dashkalova T, Ulrich S, Fernandez Rodriguez S, Mandilaras G, Jakob A, Dalla-Pozza R, Fischer M, Schneider H, Tarusinov G, Kampmann C, Hofbeck M, Dähnert I, Kanaan M, Haas NA. Intermediate outcomes of transcatheter pulmonary valve replacement with the Edwards Sapien 3 valve - German experience. Expert Rev Med Devices. 2019;16:829-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Morgan G, Prachasilchai P, Promphan W, Rosenthal E, Sivakumar K, Kappanayil M, Sakidjan I, Walsh KP, Kenny D, Thomson J, Koneti NR, Awasthy N, Thanopoulos B, Roymanee S, Qureshi S. Medium-term results of percutaneous pulmonary valve implantation using the Venus P-valve: international experience. EuroIntervention. 2019;14:1363-1370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Kim GB, Song MK, Bae EJ, Park EA, Lee W, Lim HG, Kim YJ. Successful Feasibility Human Trial of a New Self-Expandable Percutaneous Pulmonary Valve (Pulsta Valve) Implantation Using Knitted Nitinol Wire Backbone and Trileaflet α-Gal-Free Porcine Pericardial Valve in the Native Right Ventricular Outflow Tract. Circ Cardiovasc Interv. 2018;11:e006494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Chatterjee A, Bajaj NS, McMahon WS, Cribbs MG, White JS, Mukherjee A, Law MA. Transcatheter Pulmonary Valve Implantation: A Comprehensive Systematic Review and Meta-Analyses of Observational Studies. J Am Heart Assoc. 2017;6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 33. | Boudjemline Y, Malekzadeh-Milani S, Patel M, Thambo JB, Bonnet D, Iserin L, Fraisse A. Predictors and outcomes of right ventricular outflow tract conduit rupture during percutaneous pulmonary valve implantation: a multicentre study. EuroIntervention. 2016;11:1053-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Hascoet S, Martins JD, Baho H, Kadirova S, Pinto F, Paoli F, Bitar F, Haweleh AA, Uebing A, Acar P, Ghez O, Fraisse A. Percutaneous pulmonary valve implantation in small conduits: A multicenter experience. Int J Cardiol. 2018;254:64-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Shahanavaz S, Qureshi AM, Levi DS, Boudjemline Y, Peng LF, Martin MH, Bauser-Heaton H, Keeshan B, Asnes JD, Jones TK, Justino H, Aboulhosn JA, Gray RG, Nguyen H, Balzer DT, McElhinney DB. Transcatheter Pulmonary Valve Replacement With the Melody Valve in Small Diameter Expandable Right Ventricular Outflow Tract Conduits. JACC Cardiovasc Interv. 2018;11:554-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Morray BH, McElhinney DB, Cheatham JP, Zahn EM, Berman DP, Sullivan PM, Lock JE, Jones TK. Risk of coronary artery compression among patients referred for transcatheter pulmonary valve implantation: a multicenter experience. Circ Cardiovasc Interv. 2013;6:535-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 37. | Fraisse A, Aldebert P, Malekzadeh-Milani S, Thambo JB, Piéchaud JF, Aucoururier P, Chatelier G, Bonnet D, Iserin L, Bonello B, Assaidi A, Kammache I, Boudjemline Y. Melody ® transcatheter pulmonary valve implantation: results from a French registry. Arch Cardiovasc Dis. 2014;107:607-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | McElhinney DB, Sondergaard L, Armstrong AK, Bergersen L, Padera RF, Balzer DT, Lung TH, Berger F, Zahn EM, Gray RG, Hellenbrand WE, Kreutzer J, Eicken A, Jones TK, Ewert P. Endocarditis After Transcatheter Pulmonary Valve Replacement. J Am Coll Cardiol. 2018;72:2717-2728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 39. | Abdelghani M, Nassif M, Blom NA, Van Mourik MS, Straver B, Koolbergen DR, Kluin J, Tijssen JG, Mulder BJM, Bouma BJ, de Winter RJ. Infective Endocarditis After Melody Valve Implantation in the Pulmonary Position: A Systematic Review. J Am Heart Assoc. 2018;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | Hascoet S, Mauri L, Claude C, Fournier E, Lourtet J, Riou JY, Brenot P, Petit J. Infective Endocarditis Risk After Percutaneous Pulmonary Valve Implantation With the Melody and Sapien Valves. JACC Cardiovasc Interv. 2017;10:510-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 41. | Van Dijck I, Budts W, Cools B, Eyskens B, Boshoff DE, Heying R, Frerich S, Vanagt WY, Troost E, Gewillig M. Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart. 2015;101:788-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 42. | Caughron H, Kim D, Kamioka N, Lerakis S, Yousef A, Maini A, Reginauld S, Sahu A, Shashidharan S, Jokhadar M, Rodriguez FH 3rd, Book WM, McConnell M, Block PC, Babaliaros V. Repeat Pulmonary Valve Replacement: Similar Intermediate-Term Outcomes With Surgical and Transcatheter Procedures. JACC Cardiovasc Interv. 2018;11:2495-2503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Ribeiro JM, Teixeira R, Lopes J, Costa M, Pires A, Gonçalves L. Transcatheter Versus Surgical Pulmonary Valve Replacement: A Systemic Review and Meta-Analysis. Ann Thorac Surg. 2020;110:1751-1761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Chatterjee A, Bhatia N, Torres MG, Cribbs MG, Mauchley DC, Law MA. Comparison of Transcatheter Pulmonic Valve Implantation With Surgical Pulmonic Valve Replacement in Adults (from the National Inpatient Survey Dataset). Am J Cardiol. 2020;125:135-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |