Published online Apr 26, 2021. doi: 10.4330/wjc.v13.i4.68
Peer-review started: February 7, 2021
First decision: February 28, 2021
Revised: March 1, 2021
Accepted: March 29, 2021
Article in press: March 29, 2021
Published online: April 26, 2021
Drug-induced gingival overgrowth (DIGO) is a pathological growth of gingival tissue, primarily associated with calcium channel blockers and immunosuppre
Core Tip: Drug-induced gingival overgrowth is a side-effect of the drugs such as calcium channel blockers and immunosuppressants, commonly used in cardiovascular and transplanted patients. The condition is multifactorial and mainly depends on the potential of the used drug to cause gingival changes and the state of oral hygiene. Patients who develop drug-induced gingival overgrowth may experience severe discomfort and pain in addition to troubles with mastication, speech, and maintaining oral hygiene. Since it significantly reduces the quality of life, preventive and curative measures should be implemented as part of a care plan for patients at risk.
- Citation: Bajkovec L, Mrzljak A, Likic R, Alajbeg I. Drug-induced gingival overgrowth in cardiovascular patients. World J Cardiol 2021; 13(4): 68-75
- URL: https://www.wjgnet.com/1949-8462/full/v13/i4/68.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i4.68
Drug-induced gingival overgrowth (DIGO) is a pathological growth of the gingiva characterized by the accumulation of connective tissue that primarily affects the anterior regions of the maxilla and mandibula[1-3]. The first large DIGO case series was described in 1939, showing DIGO in 68 out of 119 patients treated by antiepileptic drug phenytoin[4].
Since then, various medications showed to be associated with this side-effect[5]. Although more than 20 different drugs are now known to cause DIGO, it most frequently results from calcium channel blockers (CCBs) and immunosuppressants[6,7]. Antiepic drugs (e.g., phenytoin, valproic acid, phenobarbital, vigabatrin) are recognized as a prominent group of medications causing DIGO, although in recent years, cases of DIGO resulting from these drugs were less frequently reported[8].
Cardiovascular and transplanted patients are at particular risk due to the extensive use of CCBs alone or in combination with immunosuppressants[9,10]. Significant variability among patients medicated with the same drugs is observed[1], indicating the importance of additional risk factors involved in the pathogenesis. Genetic factors, male gender, bacterial plaque, and gingival inflammation are associated with increased DIGO risk[11]. Aside from the cosmetic effect, which is the most apparent feature, patients who develop DIGO experience difficulty maintaining oral hygiene, pronunciation, and mastication. Simultaneously, the extensive disease can cause pain and loss of the teeth. As a result, quality of life is reduced significantly[12,13]. Since this side-effect is not rare in a group of cardiovascular patients, oral health needs to be emphasized and included as part of a care plan for patients treated with the drugs mentioned above.
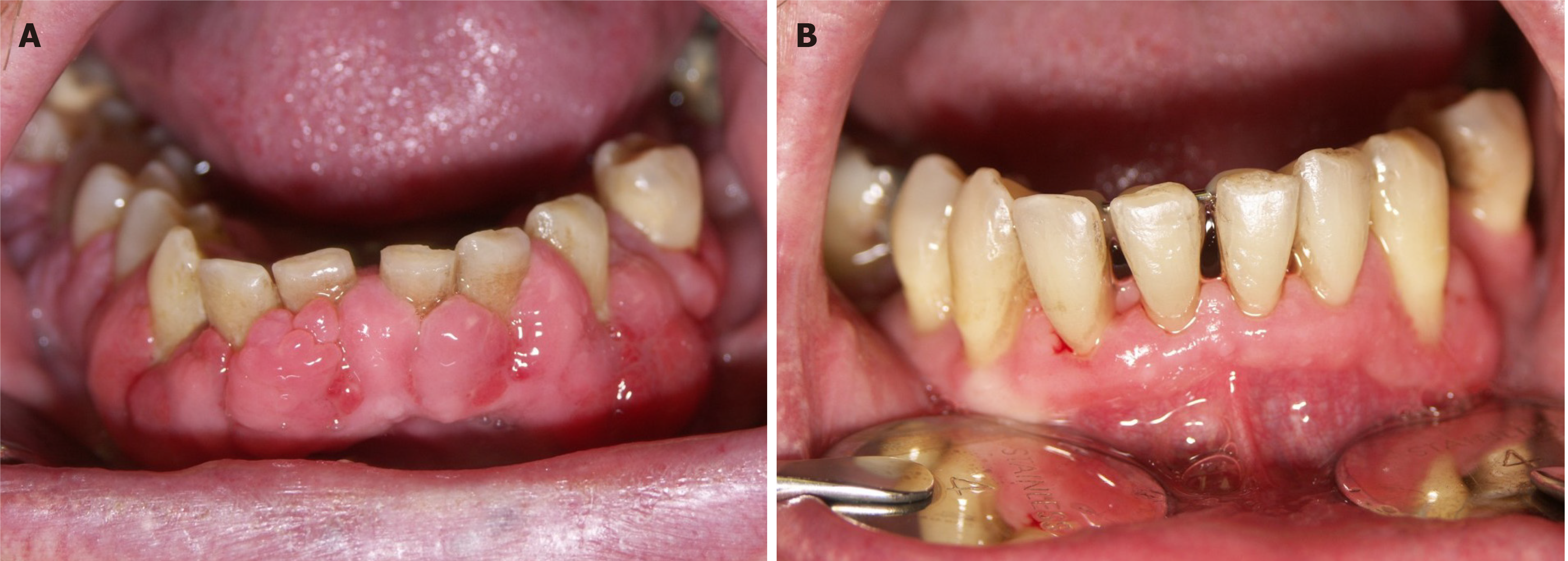
DIGO usually starts as painless enlargement of interdental papillae and progresses towards facial and lingual margins, covering the teeth crowns. Fully developed, it forms generalized changes throughout the mouth, affecting the anterior gingiva the most[5], although it can also occur as a localized lesion[14]. A possible explanation for the predominance of lesions in anterior regions could be higher exposure of anterior gingiva to the irritation resulting from plaque[15]. DIGO initially appears as pink, lobulated, and thickened gingival tissue without concomitant inflammation, with no tendency to bleed[5,16]. In its course it becomes inflamed with red or bluish-red discolorations and frequent bleeding[5]. As it progresses, it spreads both vertically and horizontally and affects mastication and speech[14].
In advanced forms, enlarged gingiva may even interfere with the occlusion[5]. Patients with DIGO have problems maintaining oral hygiene, which leads to susceptibility to infections and periodontal disease and may result in the loss of the teeth[14]. A weakened immune system predisposes to more severe periodontal disease and puts patients on immunosuppressants at additional risk[17]. Furthermore, periodontitis may potentially carry a risk for cardiovascular diseases, including myocardial infarction, peripheral artery disease, stroke, and heart failure. In theory, possible mechanisms behind this association could be dissemination of oral pathogens into the bloodstream and invasion of cardiovascular organs and tissues to induce inflammatory response on a local and systemic level[18]. Additionally, periodontitis is associated with endothelial dysfunction, an important factor in atherosclerosis development[18,19]. Aside from the functional impairment and cardiovascular risk, gingival changes also represent a significant esthetic problem for the patients[14].
Etiopathology of DIGO is multifactorial and not fully understood[2,20]. Genetic factors (cytochrome P450, HLA, and MDR1 gene polymorphisms) influence the interindividual difference in gingival response to DIGO-inducing drugs and could have a role in identifying patients at risk[2,21,22]. The main histological finding in DIGO is the accumulation of collagen in the gingiva's extracellular matrix, along with the infiltration of inflammatory cells[1]. Most of the DIGO-inducing drugs act as inhibitors of calcium ion influx[23]. An inhibited influx of cations into fibroblasts causes a decrease in cation-dependent folic acid uptake. Folic acid is necessary for the proper function of matrix metalloproteinases, which activate collagenase. With no collagenase, there is no collagen breakdown, and it accumulates in connective tissue[1]. Furthermore, studies demonstrated a drug-induced increase in glycosaminoglycans[1] and collagen[24] production along with the proliferation of gingival fibroblasts[25]. These changes are mainly mediated by inflammatory cytokines that are a part of an inflammatory response to drugs[23]. Inflammatory infiltrates found in the gingiva mainly consist of plasma cells[26]. Periodontal status is a significant predictor of DIGO, as bacterial plaque induces inflammation. A significant correlation was found between bacterial plaque and a higher risk for DIGO in patients treated with CCBs or cyclosporin[1,15,27]. In gingival tissue of patients with CCBs-associated DIGO, higher expression of androgen receptors accompanied with higher levels of type I collagen were detected, implying androgens' role in its pathogenesis[28].
The first CCB-DIGO cases date from the early 80-ties, primarily associated with nifedipine[29] and later with the use of other CCBs such as verapamil, diltiazem, amlodipine, and felodipine[30]. Early studies demonstrated the highest prevalence in patients on nifedipine (6.3%), which remains to date the leading cause of DIGO in this drug group. Other CCBs, such as amlodipine (1.7%) and diltiazem (2.2%), have a lower potential to cause DIGO[31]. The various prevalence of DIGO among different CCBs may be a consequence of pharmacokinetic characteristics, as nifedipine is more lipophilic, so it passes through cell membranes more quickly and has a half-life which allows it to reach peak levels in the plasma needed for the initiation of gingival changes[31]. However, the prevalence of DIGO caused by CCBs varies in various studies, as for CCBs in general, it ranges between 10%-20%[32]. It amounts from 15% to 85%[33,34], for nifedipine, meaning that there are additional influencing factors. Male gender, drug dosage, smoking, periodontal status, previous myocardial infarction, and concomitant use of diuretics or antiepileptic drugs increase the risk of developing DIGO in CCBs users[12,31,35]. However, drug dosage depends mostly on pharmacokin
Cyclosporin and tacrolimus, from the group of calcineurin inhibitors, are the leading cause of DIGO among immunosuppressive drugs[38]. Cyclosporin was the primary immunosuppressive drug in heart-transplant patients since 1980[39]. Many heart transplant recipients (8.3%-67%) develop gingival enlargement, most of whom are treated with cyclosporin[40]. The role of cyclosporin as an inducing drug in DIGO development is well known and recognized[41]. Gingival changes in patients treated with cyclosporine show symmetry between mandibula and maxilla and within the jaw, while incisors and canines are affected the most[15]. According to the research of Hatahira et al[13], 70% of gingival overgrowth is attributed to cyclosporin. On the other hand, tacrolimus is another immunosuppressant often used as an alternative to cyclosporin in primary or rescue therapy[27]. It is 100 times more potent and tends to cause some side-effects common to other immunosuppressants[27,42]. However, it less often causes gingival overgrowth, and changes are less severe than those caused by cyclosporin[27]. The prevalence of DIGO among tacrolimus treated patients is around 14%[27,43]. DIGO can be detected as soon as 1-3 mo after immunosuppressive therapy initiation, and the plateau phase is reached at 9-12 mo[44]. However, gingival changes resulting from tacrolimus use appear later compared to cyclosporin, as in various studies, no changes were observed before 90 d of treatment[38,45]. Interestingly, immunosuppressants differ from other DIGO-inducing drugs since high inflammation levels and low fibrosis mostly mediate the changes[46]. Some of the predisposing factors among cyclosporin users are male gender, gingivitis, bacterial plaque, and higher cyclosporine concentrations[15,22]. Furthermore, younger patients are more frequently affected by DIGO[15], and high rates have been reported among the group of pediatric heart-transplant patients treated with cyclosporin[39,47,48]. Younger age was also a risk factor for more severe changes in patients on tacrolimus[27]. Different therapeutic patterns and higher potential of fibroblasts to proliferate and produce collagen in a group of younger patients could be a possible explanation[15,49]. Additionally, a higher risk for DIGO in tacrolimus users was observed in patients with the worse periodontal state, those previously medicated with cyclosporin[27], and a with longer duration of tacrolimus therapy[50]. The occurrence and severity of changes also depend on the concomitant use of other medications. Simultaneous use of cyclosporin and CCBs doubles the risk for DIGO, compared to using cyclosporin alone (51.9% vs 25%)[22]. Furthermore, the use of CCBs in tacrolimus-treated patients results in higher severity of gingival changes[27]. These findings indicate the synergistic effect of CCBs and calcineurin inhibitors. On the contrary, azathioprine has a protective effect in patients on cyclosporin or tacrolimus and lowers the risk for DIGO[27,51]. Although tacrolimus provides a better safety profile regarding DIGO and could be a treatment option in patients with cyclosporin-induced gingival overgrowth, it is important to point out that in some cases, changes persist even after the switching of therapy, especially with concomitant use of CCBs[27].
Management of DIGO can be conservative or surgical, with the aim to provide a satisfactory cosmetic outcome and minimize discomfort and pain[1]. Non-surgical methods are the treatment of choice, including proper oral hygiene and mechanical removal of dental plaque, together with the mandatory exclusion of the offending drug[14]. Periodontal treatment reduces inflammation and prevents the need for surgical treatment in cyclosporin-treated patients[52]. A rigorous oral hygiene regime has been recommended for patients with DIGO resulting from CCBs use[32]. Since a worse periodontal state has been associated with a higher risk for DIGO[15,27], preventive measures targeting oral health could be valuable. Reduction of drug dose or switching to that of a lower potential for side-effects should always be considered, if possible. In that case, complete improvement can be expected in 1-8 wk[53] (Figure 1).
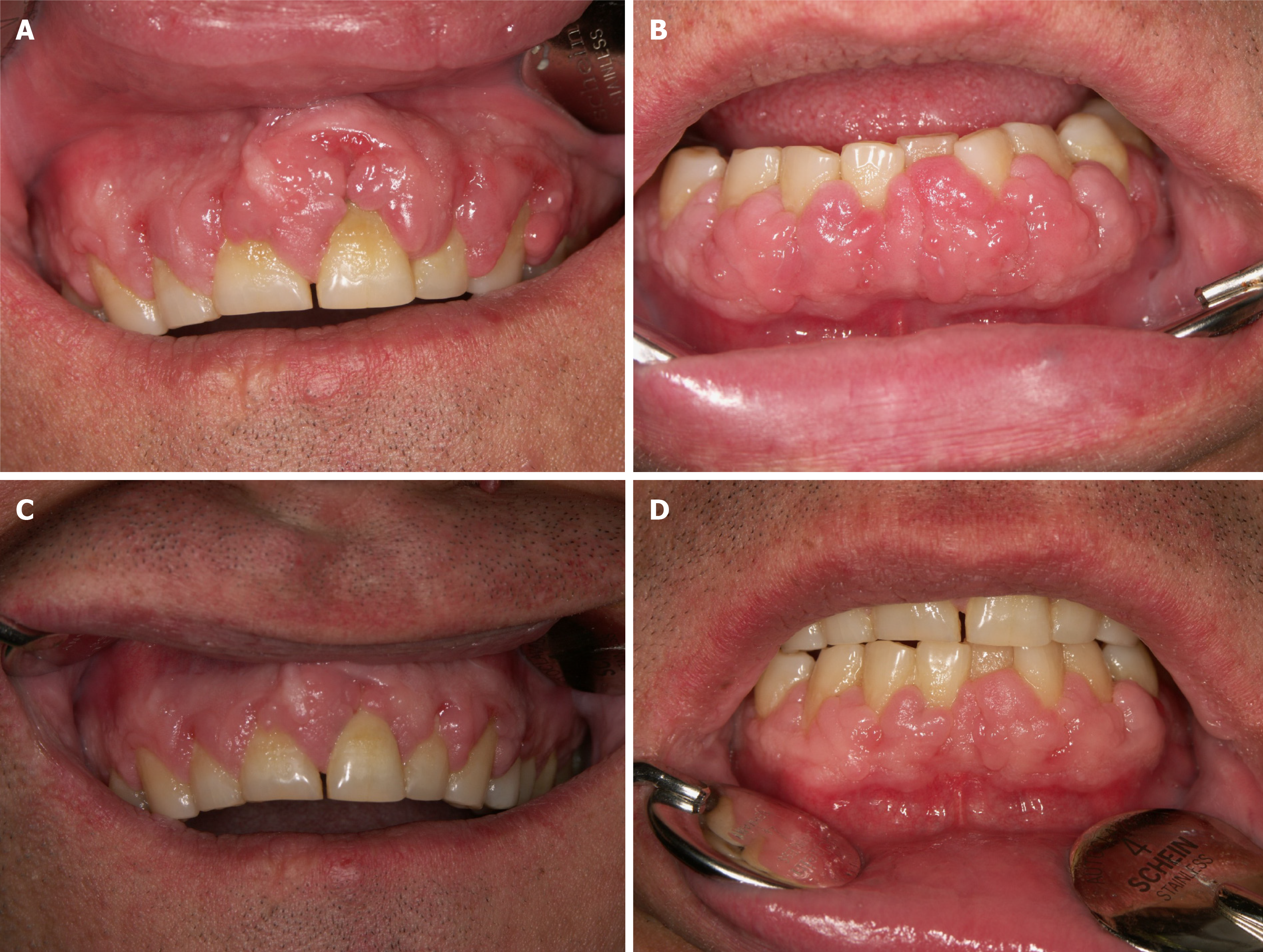
Stopping the use of CCBs or switching to non-CCB antihypertensives provides satisfactory results, but it is not always possible, as some patients may have problems controlling their hypertension[54]. In an attempt to treat gingival overgrowth caused by cyclosporin, replacing this medication with tacrolimus or everolimus remains an option[55,56] (Figure 2).
Flutamide, an androgen receptor antagonist, inhibits gingival cells' response to nifedipine and could be used to prevent or treat nifedipine associated DIGO[28]. Non-surgical methods are often not sufficient if the drug cannot be withdrawn for other reasons[54]. Persistent DIGO requires surgical treatment, which could either involve gingivectomy or periodontal flap[57]. The use of carbon dioxide lasers is a solid choice that provides adequate postoperative hemostasis[23]. Recurrence of DIGO after surgical treatment was reported in about 40% of the patients still treated with the offending drug[58]. In conclusion, the prognosis of DIGO is good, as it can be successfully managed and resolved with discontinuation of the inducing drugs[1].
Since CCBs and immunosuppressants are widely used medications in patients with hypertension or after heart transplantation[9,10], health professionals should be aware of gingival overgrowth as an unpleasant side-effect that can result from the use of these drugs[23,55]. Although it might not be life-threatening, it poses a significant problem for the patients, not only because of the cosmetic effect but also due to the impairment of speech, eating, and maintaining oral hygiene[12,13]. Furthermore, infections resulting from the lack of proper oral hygiene could enhance the risk for cardiovascular diseases[59]. Recognizing the importance of DIGO and its effect on the patients' health is crucial for providing better health outcomes and satisfactory quality of life. If possible, treatment of choice should be changing of a drug and conservative periodontal treatment, whereas surgical treatment is reasonable only in resistant cases. Since it is multifactorial and reoccurs if the drug must be continued, efforts need to be made to find each patient's optimal treatment. An interdisciplinary approach and cooperation of medical and dental professionals are necessary to reach this goal.
Manuscript source: Invited manuscript
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciccone MM, Spartalis M S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Tungare S, Paranjpe AG. Drug Induced Gingival Overgrowth. 2020 Oct 5. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. [PubMed] [Cited in This Article: ] |
| 2. | Seymour RA, Thomason JM, Ellis JS. The pathogenesis of drug-induced gingival overgrowth. J Clin Periodontol. 1996;23:165-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 267] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Ustaoğlu G, Erdal E, Karaş Z. Influence of different anti-hypertensive drugs on gingival overgrowth: A cross-sectional study in a Turkish population. Oral Dis. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Kimball OP. The treatment of epilepsy with sodium diphenyl hydantoinate. JAMA. 1939;112:1244-1245. [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 180] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Kanade K. Drug Induced Gingival Enlargement. Munich: GRIN Verlag, 2018: 2-10. [Cited in This Article: ] |
| 6. | Rees TD, Levine RA. Systematic drugs as a risk factor for periodontal disease initiation and progression. Compendium. 1995;16:20, 22, 26 passim; quiz 42. [PubMed] [Cited in This Article: ] |
| 7. | Marshall RI, Bartold PM. Medication induced gingival overgrowth. Oral Dis. 1998;4:130-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Lin K, Guilhoto LMFF, Yacubian EMT. Drug-induced gingival enlargement - Part II. Antiepileptic drugs: not only phenytoin is involved. J Epilepsy Clin Neurophysiol. 2007;13:83-88. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Asif SM, Shaik N, Barthunia B, Kaleem SM, Zakirulla M, Kota MZ, Baig FAH. Nifedipine induced gingival enlargement in an edentulous patient: a case report with one year follow up. BMC Oral Health. 2018;18:227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Kobashigawa J, Luu M. Immunosuppression Strategies in Heart Transplantation. In: Kobashigawa J. Clinical Guide to Heart Transplantation. 1st ed. Cham: Springer, 2017: 109-135. [Cited in This Article: ] |
| 11. | Seymour RA, Ellis JS, Thomason JM. Risk factors for drug-induced gingival overgrowth. J Clin Periodontol. 2000;27:217-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 178] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Kaur G, Verhamme KM, Dieleman JP, Vanrolleghem A, van Soest EM, Stricker BH, Sturkenboom MC. Association between calcium channel blockers and gingival hyperplasia. J Clin Periodontol. 2010;37:625-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Hatahira H, Abe J, Hane Y, Matsui T, Sasaoka S, Motooka Y, Hasegawa S, Fukuda A, Naganuma M, Ohmori T, Kinosada Y, Nakamura M. Drug-induced gingival hyperplasia: a retrospective study using spontaneous reporting system databases. J Pharm Health Care Sci. 2017;3:19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Livada R, Shiloah J. Calcium channel blocker-induced gingival enlargement. J Hum Hypertens. 2014;28:10-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (3)] |
| 15. | Somacarrera ML, Hernández G, Acero J, Moskow BS. Factors related to the incidence and severity of cyclosporin-induced gingival overgrowth in transplant patients. A longitudinal study. J Periodontol. 1994;65:671-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 105] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Sabarudin MA, Taib H. Drug-influenced Gingival Enlargement: Overview of the Clinical Features and Assessment Methods. J Dentists. 2019;7:1-7. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Hasturk H, Kantarci A. Activation and resolution of periodontal inflammation and its systemic impact. Periodontol 2000. 2015;69:255-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 18. | Liccardo D, Cannavo A, Spagnuolo G, Ferrara N, Cittadini A, Rengo C, Rengo G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 188] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 19. | Radomski MW, Palmer RM, Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987;92:181-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 611] [Cited by in F6Publishing: 660] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 20. | Rapone B, Ferrara E, Santacroce L, Cesarano F, Arazzi M, Liberato LD, Scacco S, Grassi R, Grassi FR, Gnoni A, Nardi GM. Periodontal Microbiological Status Influences the Occurrence of Cyclosporine-A and Tacrolimus-Induced Gingival Overgrowth. Antibiotics (Basel). 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Meisel P, Giebel J, Kunert-Keil C, Dazert P, Kroemer HK, Kocher T. MDR1 gene polymorphisms and risk of gingival hyperplasia induced by calcium antagonists. Clin Pharmacol Ther. 2006;79:62-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Thomason JM, Seymour RA, Ellis JS, Kelly PJ, Parry G, Dark J, Wilkinson R, Ilde JR. Determinants of gingival overgrowth severity in organ transplant patients. An examination of the rôle of HLA phenotype. J Clin Periodontol. 1996;23:628-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Dongari-Bagtzoglou A; Research, Science and Therapy Committee; American Academy of Periodontology. Drug-associated gingival enlargement. J Periodontol. 2004;75:1424-1431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Duncan MR, Berman B. Stimulation of collagen and glycosaminoglycan production in cultured human adult dermal fibroblasts by recombinant human interleukin 6. J Invest Dermatol. 1991;97:686-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 247] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Goriuc A, Foia LG, Minea B, Luchian AI, Surdu AE, Toma V, Costuleanu M, Mârţu I. Drug-induced gingival hyperplasia - experimental model. Rom J Morphol Embryol. 2017;58:1371-1376. [PubMed] [Cited in This Article: ] |
| 26. | Deliliers GL, Santoro F, Polli N, Bruno E, Fumagalli L, Risciotti E. Light and electron microscopic study of cyclosporin A-induced gingival hyperplasia. J Periodontol. 1986;57:771-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Ellis JS, Seymour RA, Taylor JJ, Thomason JM. Prevalence of gingival overgrowth in transplant patients immunosuppressed with tacrolimus. J Clin Periodontol. 2004;31:126-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Lu HK, Tseng CC, Lee YH, Li CL, Wang LF. Flutamide inhibits nifedipine- and interleukin-1 beta-induced collagen overproduction in gingival fibroblasts. J Periodontal Res. 2010;45:451-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Lederman D, Lumerman H, Reuben S, Freedman PD. Gingival hyperplasia associated with nifedipine therapy. Report of a case. Oral Surg Oral Med Oral Pathol. 1984;57:620-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 135] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Marshall RI, Bartold PM. A clinical review of drug-induced gingival overgrowths. Aust Dent J. 1999;44:219-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Ellis JS, Seymour RA, Steele JG, Robertson P, Butler TJ, Thomason JM. Prevalence of gingival overgrowth induced by calcium channel blockers: a community-based study. J Periodontol. 1999;70:63-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 167] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Meisel P, Schwahn C, John U, Kroemer HK, Kocher T. Calcium antagonists and deep gingival pockets in the population-based SHIP study. Br J Clin Pharmacol. 2005;60:552-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Barak S, Engelberg IS, Hiss J. Gingival hyperplasia caused by nifedipine. Histopathologic findings. J Periodontol. 1987;58:639-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 106] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Fattore L, Stablein M, Bredfeldt G, Semla T, Moran M, Doherty-Greenberg JM. Gingival hyperplasia: a side effect of nifedipine and diltiazem. Spec Care Dentist. 1991;11:107-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Nery EB, Edson RG, Lee KK, Pruthi VK, Watson J. Prevalence of nifedipine-induced gingival hyperplasia. J Periodontol. 1995;66:572-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Andrew W, Evelyn W, Francis M, Mark J, Mark C. Pattern of Gingival Overgrowth among Patients on Antihypertensive Pharmacotherapy at a Nairobi Hospital in Kenya. Open J Stomato. 2014;4:169-173. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Pradhan S, Mishra P. Gingival enlargement in antihypertensive medication. JNMA J Nepal Med Assoc. 2009;48:149-152. [PubMed] [Cited in This Article: ] |
| 38. | Sekiguchi RT, Paixão CG, Saraiva L, Romito GA, Pannuti CM, Lotufo RF. Incidence of tacrolimus-induced gingival overgrowth in the absence of calcium channel blockers: a short-term study. J Clin Periodontol. 2007;34:545-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Minami K, von Knyphausen E, Niino T, Blanz U, Tenderich G, Wlost S, Meyer H, Körfer R. Long-term results of pediatric heart transplantation. Ann Thorac Cardiovasc Surg. 2005;11:386-390. [PubMed] [Cited in This Article: ] |
| 40. | Gruter MO, Brand HS. Oral health complications after a heart transplant: a review. Br Dent J. 2020;228:177-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Montebugnoli L, Bernardi F, Magelli C. Cyclosporin-A-induced gingival overgrowth in heart transplant patients. A cross-sectional study. J Clin Periodontol. 1996;23:868-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Jacobson P, Uberti J, Davis W, Ratanatharathorn V. Tacrolimus: a new agent for the prevention of graft-versus-host disease in hematopoietic stem cell transplantation. Bone Marrow Transplant. 1998;22:217-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Greenberg KV, Armitage GC, Shiboski CH. Gingival enlargement among renal transplant recipients in the era of new-generation immunosuppressants. J Periodontol. 2008;79:453-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Lauritano D, Moreo G, Limongelli L, Palmieri A, Carinci F. Drug-Induced Gingival Overgrowth: The Effect of Cyclosporin A and Mycophenolate Mophetil on Human Gingival Fibroblasts. Biomedicines. 2020;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Paixão CG, Sekiguchi RT, Saraiva L, Pannuti CM, Silva HT, Medina-Pestana J, Romito GA. Gingival overgrowth among patients medicated with cyclosporin A and tacrolimus undergoing renal transplantation: a prospective study. J Periodontol. 2011;82:251-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Trackman PC, Kantarci A. Molecular and clinical aspects of drug-induced gingival overgrowth. J Dent Res. 2015;94:540-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 47. | Lowry LY, Welbury RR, Seymour RA, Waterhouse PJ, Hamilton JR. Gingival overgrowth in paediatric cardiac transplant patients: a study of 19 patients aged between 2 and 16 years. Int J Paediatr Dent. 1995;5:217-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Ansari F, Ferring V, Schulz-Weidner N, Wetzel WE. Concomitant oral findings in children after cardiac transplant. Pediatr Transplant. 2006;10:215-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Daley TD, Wysocki GP, Day C. Clinical and pharmacologic correlations in cyclosporine-induced gingival hyperplasia. Oral Surg Oral Med Oral Pathol. 1986;62:417-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 155] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Pamuk F, Cetinkaya BO, Gulbahar MY, Gacar A, Keles GC, Erisgin Z, Arik N. Effects of tacrolimus and nifedipine, alone or in combination, on gingival tissues. J Periodontol. 2013;84:1673-1682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Wilson RF, Morel A, Smith D, Koffman CG, Ogg CS, Rigden SP, Ashley FP. Contribution of individual drugs to gingival overgrowth in adult and juvenile renal transplant patients treated with multiple therapy. J Clin Periodontol. 1998;25:457-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Aimetti M, Romano F, Debernardi C. Effectiveness of periodontal therapy on the severity of cyclosporin A-induced gingival overgrowth. J Clin Periodontol. 2005;32:846-850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Wynn RL. Calcium channel blockers and gingival hyperplasia. Gen Dent. 1991;39:240-243. [PubMed] [Cited in This Article: ] |
| 54. | Fardal Ø, Lygre H. Management of periodontal disease in patients using calcium channel blockers - gingival overgrowth, prescribed medications, treatment responses and added treatment costs. J Clin Periodontol. 2015;42:640-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Penninga L, Møller CH, Gustafsson F, Steinbrüchel DA, Gluud C. Tacrolimus vs cyclosporine as primary immunosuppression after heart transplantation: systematic review with meta-analyses and trial sequential analyses of randomised trials. Eur J Clin Pharmacol. 2010;66:1177-1187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 56. | Stypmann J, Engelen MA, Eckernkemper S, Amler S, Gunia S, Sindermann JR, Rothenburger M, Rukosujew A, Drees G, Welp HA. Calcineurin inhibitor-free immunosuppression using everolimus (Certican) after heart transplantation: 2 years' follow-up from the University Hospital Münster. Transplant Proc. 2011;43:1847-1852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Camargo PM, Melnick PR, Pirih FQ, Lagos R, Takei HH. Treatment of drug-induced gingival enlargement: aesthetic and functional considerations. Periodontol 2000. 2001;27:131-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Ilgenli T, Atilla G, Baylas H. Effectiveness of periodontal therapy in patients with drug-induced gingival overgrowth. Long-term results. J Periodontol. 1999;70:967-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review. Ann Periodontol. 2003;8:38-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 308] [Article Influence: 15.4] [Reference Citation Analysis (0)] |