Published online Apr 26, 2021. doi: 10.4330/wjc.v13.i4.111
Peer-review started: December 13, 2020
First decision: February 28, 2021
Revised: March 1, 2021
Accepted: April 14, 2021
Article in press: April 14, 2021
Published online: April 26, 2021
Pulmonary artery-to-left atrial fistula is a variant of pulmonary arteriovenous fistula and is a developmental anomaly. Delayed presentation, cyanosis and effort intolerance are some of the important features. The diagnosis is confirmed by computed tomography or pulmonary artery angiography. Catheter-based closure is preferred to surgery.
Left pulmonary artery-to-left atrial fistula is rare. A 40-year-old male presented with effort intolerance, central cyanosis, and recurrent seizures. He had a large and highly tortuous left pulmonary artery-to-left atrial fistula associated with a large aneurysmal sac in the course. Catheter-based closure was performed using a vascular plug.
Left pulmonary artery-to-left atrial fistula is relatively uncommon compared to right pulmonary artery-to-left atrial fistula. Percutaneous closure by either a transeptal technique or guide wire insertion into the pulmonary vein through the pulmonary artery is preferred. The need for an arteriovenous loop depends on the tortuosity of the course of the fistula and the size of the device to be implanted because a larger device needs a larger sheath, necessitating firm guide wire support to facilitate negotiation of the stiff combination of the delivery sheath and dilator.
Core Tip: Pulmonary artery-to-left atrial fistula is a variant of pulmonary arterio
- Citation: Mahapatra R, Mahanta D, Singh J, Acharya D, Barik R. Device closure of fistula from left lower pulmonary artery to left atrium using a vascular plug: A case report. World J Cardiol 2021; 13(4): 111-116
- URL: https://www.wjgnet.com/1949-8462/full/v13/i4/111.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i4.111
Left pulmonary artery-to-left atrial fistula is relatively rare compared to right pulmonary artery-to-left atrial fistula, as previously reported[1]. The first case of pulmonary artery-to-left atrial fistula was reported in 1989 as an unusual cause of cyanosis in a newborn[2]. A significant right-to-left shunt is clinically marked by effort intolerance, cyanosis, and polycythemia, as well as sometimes bleeding issues related to associated hemangioma[3,4]. Pulmonary artery-to-left atrial fistula is a variant of pulmonary arteriovenous malformation. There is significant clinical suspicion of this condition when there is cyanosis without any obvious murmur in the precordium. Echocardiography shows a significant increase in pulmonary venous return to the left atrium, which is an additional clue and can be confirmed by cardiac catheterization or contrast-enhanced computed tomography. This case reports the relevant issues we encountered during the percutaneous device closure of a large and highly tortuous left pulmonary artery-to-left atrial fistula associated with a large aneurysmal sac in its course in an adult.
A 40-year-old male weighing 47 kg presented with effort intolerance, cyanosis, and clubbing.
The patient had a history of recurrent seizures and was on levetiracetam.
The patient was a school teacher in profession and had no other significant comor
The patient had central cyanosis and pandigital clubbing, and his room air oxygen saturation was 87%. There was no murmur on auscultation.
The results of routine blood tests were normal. A genetic study could not be perfor
Chest X-ray examination showed a dilated and enlarged left perihilar region. There were multiple small hemangiomas in several organs, including the brain. Echo
The cardiothoracic surgeon suggested device closure as the first option if possible.
The final diagnosis was a large left lower pulmonary artery-to-left atrial fistula associated with an aneurysmal sac.
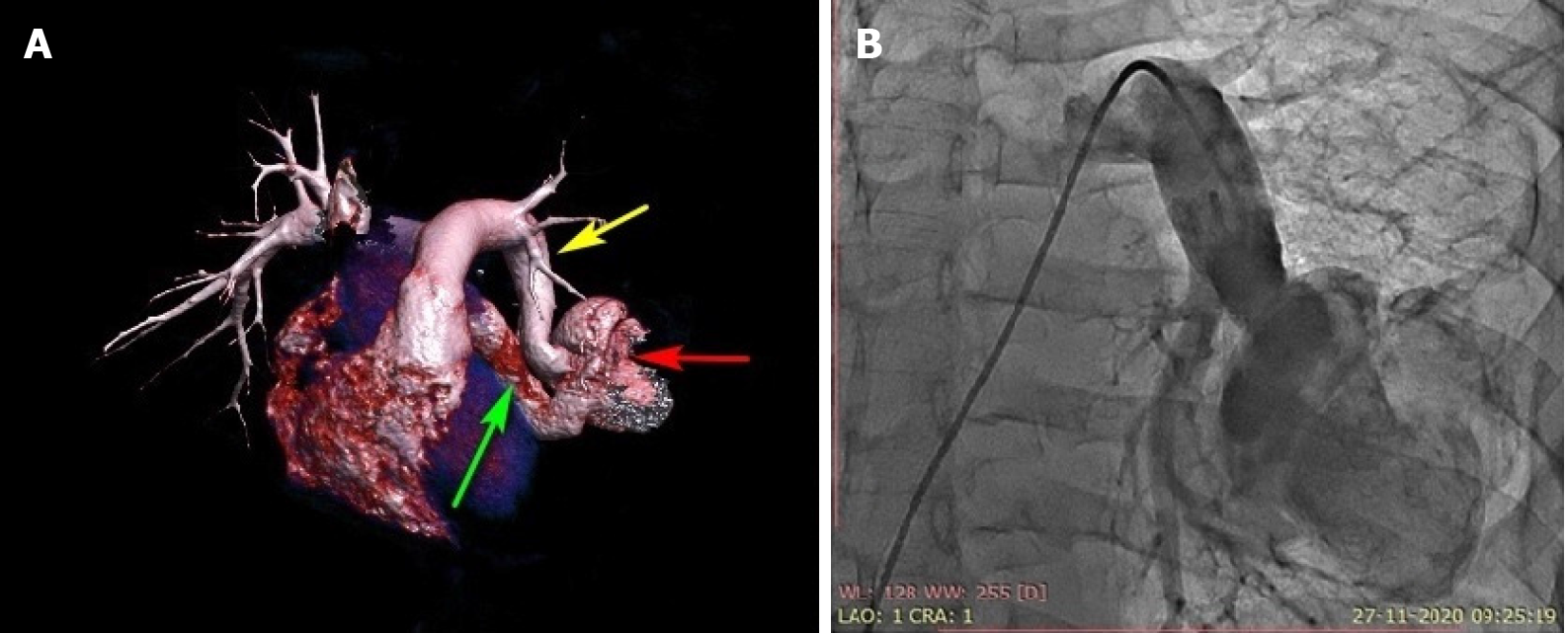
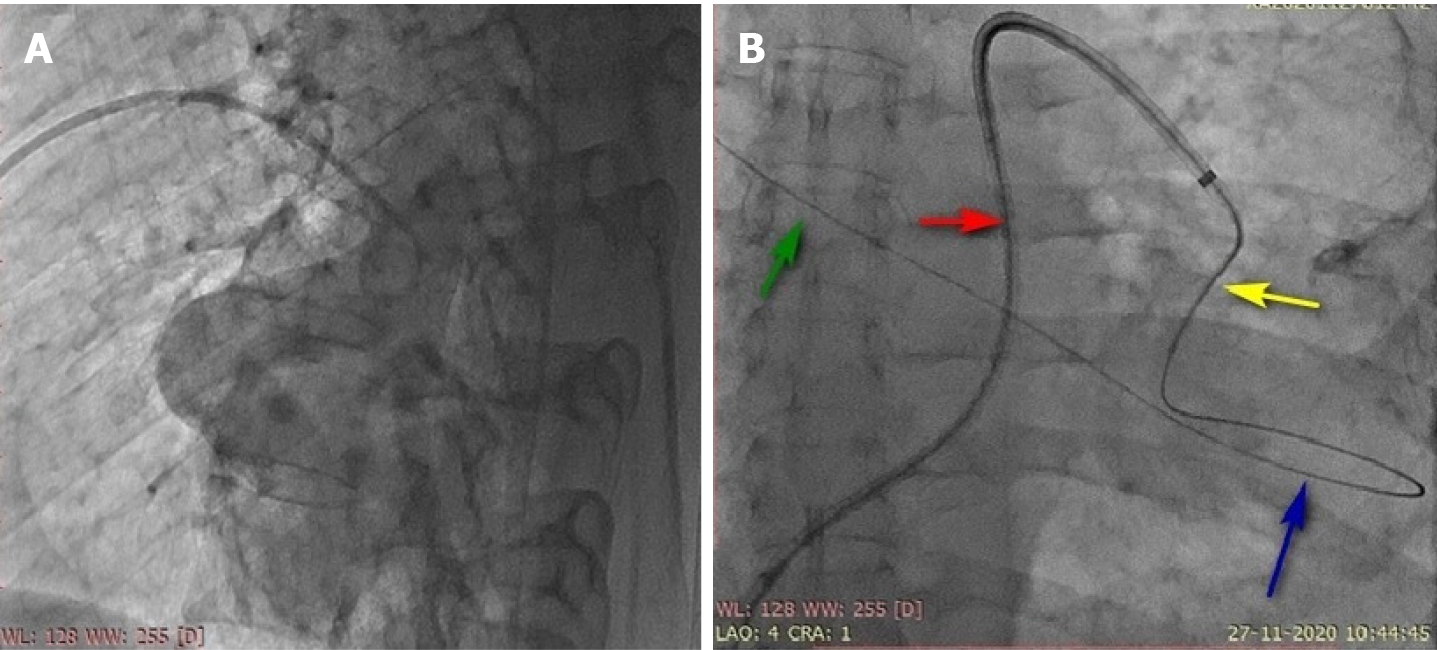
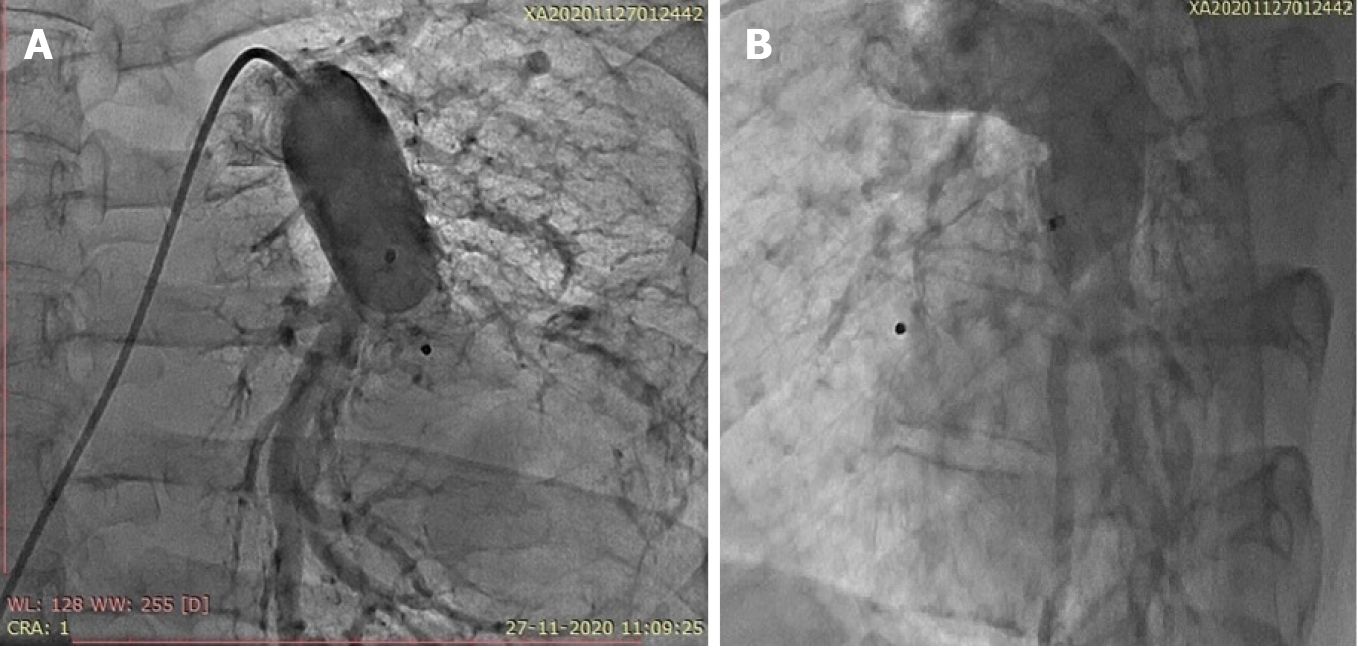
Because the patient had multiple hemangiomas and recurrent seizures related to cerebral hemangioma based on the magnetic resonance angiography findings of the brain, the cardiac team suggested that a catheter-based intervention would be preferred over surgery. Device closure was planned after informed consent was obtained. Under local anesthesia and after infective endocarditis prophylaxis, the patient was taken for percutaneous vascular plugging of the fistula. Right ventricular saturation was 78%, and left ventricular saturation was 92% in room air. The pulmonary artery systolic pressure was 37 mmHg. The angiograms of the frontal and lateral projections showed a significantly tortuous course of the left lower pulmonary artery-to-left atrial fistula with an aneurysmal sac of 6 cm in diameter in its course (Figure 1B). The landing zone diameter was 16 cm, and the landing zone length was 2 cm after the origin of the left lower lobe segmental pulmonary artery branch (Figure 2A). A Terumo wire 0.35 cm × 260 cm in size (Terumo, Tokyo, Japan) was passed across the fistula from the left pulmonary artery through the sac far into the upper right pulmonary vein (Figure 2B). The insertion of a compatible 8-Fr sheath with its dilator was up to the sac was attempted but was not possible because of the tortuous course. The dilator was exchanged with a 5-Fr multipurpose diagnostic catheter, and the sheath was placed just proximal to the neck of the fistula. A 20-mm Amplatzer vascular plug (St. Jude Medical, Minnesota, United States) was deployed without any residual shunting (Figure 3, Video 3 and 4). The arterial oxygen saturation immediately increased to 98%. The patient was discharged on aspirin on day three after the procedure. Oral anticoagulation was avoided in this case because of bleeding issues related to multiple hemangiomas.
At the 2-mo follow-up, contrast-enhanced computed tomography showed the position of the vascular plug in situ; there was no residual shunting, and the patient’s room air saturation was 98%.
Incomplete degeneration of the partition between the arterial and venous plexus of the splanchnic pulmonary vascular bed leads to the formation of thin-walled sacs, resulting in the formation of pulmonary arteriovenous fistulas, which may sometimes be absorbed into the left atrium, causing the pulmonary artery to form a left atrial fistula. The potential right-to-left shunt and aneurysmal dilatation of the pathway can cause thromboembolism and death due to rupture of the sac. The incidence of this kind of fistula is higher in males. Routine frontal chest X-ray examination may show perihilar vascular enlargement. Agitated saline contrast echocardiography could provide additional information when pulmonary artery-to-left atrial fistula is suspected. A close differential diagnosis of pulmonary arteriovenous fistula must always be kept in mind[6]. Although invasive pulmonary angiography is diagnostic, it should be reserved for intervention because computed tomography angiography with three-dimensional reconstruction provides most of the information needed to decide whether a fistula can be percutaneously plugged by a device or requires surgical closure[7]. Various plugging devices, such as vascular plugs, duct occluders and septal defect occluders, can be used depending upon the tortuosity of the course, availability of the landing zone and available delivery sheath and age of the patient. The plug can be deployed with or without an arteriovenous loop depending on the tortuosity of the course and size of the fistula[8] with or without general anesthesia depending upon the age of the patient.
The proximal part of the left lower lobe pulmonary artery was tortuous before its division into anterior and posterior segmental branches. The sac was located along the course of the anterior segmental artery. We faced five major challenges to the catheter-based intervention in this case. (1) Negotiation of the 8-Fr sheath with its stiff default dilator was not possible; we overcame this difficulty by using a 5-Fr multipurpose catheter and substituting the dilator; (2) Although device implantation could have been performed using a transseptal approach with or without a loop, we could manage the antegrade approach from the pulmonary artery because of the good support provided by the guidewire in the upper right pulmonary vein; (3) The proximal end of the landing zone in this case was quite close to the ostium of a large posterior segmental left pulmonary artery branch, but the procedure was completed well because of the proper device selection, which is evident in the provided video clips (Video 3 and 4); (4) The possibility of device embolization in this case was avoided by proper identification of the landing zone and its diameter and the selection of a well-fitting device; and (5) Although there was a fair indication for oral anticoagulation to prevent atheroembolism from the aneurysmal sac after device implantation, we only administered aspirin to avoid bleeding issues related to multiple hemangiomas. Percutaneous closure is preferred to open heart surgery whenever the anatomy allows, as in this case.
Left pulmonary artery-to-left atrial fistula is relatively uncommon compared to right pulmonary artery-to-left atrial fistula. Percutaneous closure by either a transeptal technique or guide wire insertion in the pulmonary vein through the pulmonary artery is preferred. The need for an arteriovenous loop depends upon the tortuosity of the course of the fistula and this size of the device to be implanted because a larger device needs a larger sheath, necessitating firm guide wire support to facilitate negotiation of the stiff combination of the delivery sheath and dilator.
The authors are extremely thankful to Mr. Pramanik S for his kind support during the procedure as our senior catheterization laboratory technician.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: European Society of Cardiology, No. 663523; Cardiology Society of India, No. L-3559; Pediatric Cardiology Society of India, No. L-679; and Indian College of Cardiology, No. L-815.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dai HL S-Editor: Gao CC L-Editor: A P-Editor: Yuan YY
| 1. | Mongé MC, Russell HM, Popescu AR, Robinson JD. Right pulmonary artery to left atrial fistula in a neonate: case report and review of the literature. World J Pediatr Congenit Heart Surg. 2014;5:306-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Jimenez M, Fournier A, Choussat A. Pulmonary artery to the left atrium fistula as an unusual cause of cyanosis in the newborn. Pediatr Cardiol. 1989;10:216-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Sivakumar K, Sean DR. Pulmonary artery to left atrial fistula: haemodynamic changes traced from fetus to infancy until its interventional closure. Cardiol Young. 2018;28:1154-1156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Nikolaou I, Rafailidis V, Kartas A, Kouskouras K, Giannakoulas G. A case of pulmonary arteriovenous malformation in the setting of Rendu Osler Weber syndrome. Radiol Case Rep. 2021;16:483-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Dunphy L, Talwar A, Patel N, Evans A. Hereditary haemorrhagic telangiectasia and pulmonary arteriovenous malformations. BMJ Case Rep. 2021;14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Khanra D, Razi M, Tiwari P, Soni S, Thakur R. Successful occlusion of a large pulmonary arterio-venous fistula with Amplatzer septal occluder in a 16-year-old cyanotic boy. J Cardiol Cases. 2020;21:242-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Singhi AK, Roy RR, De A, Bari EA, Kumar RK. Percutaneous closure of large pulmonary artery to left atrial fistula. J Cardiol Cases. 2020;22:166-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Arya V, Azad S, Radhakrishnan S. Transcatheter closure of right pulmonary artery to left atrium fistula in an infant: technical consideration and possible closure techniques. Cardiol Young. 2019;29:1561-1564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |