Revised: December 22, 2009
Accepted: December 28, 2009
Published online: December 31, 2009
Coronary heart disease is the single most common cause of illness and death in the developed world. Coronary atherosclerosis is by far the most frequent cause of ischemic heart disease, and plaque disruption with superimposed thrombosis is the main cause of the acute coronary syndromes of unstable angina, myocardial infarction, and sudden death. Atherosclerosis is the result of a complex interaction between blood elements, disturbed flow, and vessel wall abnormality, involving several pathological processes: inflammation, with increased endothelial permeability, endothelial activation, and monocyte recruitment; growth, with smooth muscle cell proliferation, migration, and matrix synthesis; degeneration, with lipid accumulation; necrosis, possibly related to the cytotoxic effect of oxidized lipid; calcification/ossification, which may represent an active rather than a dystrophic process; and thrombosis, with platelet recruitment and fibrin formation. In this review we discuss these processes and the possible pathological effects of Chlamydia infection and the ensuing phlogosis.
- Citation: Fazio G, Giovino M, Gullotti A, Bacarella D, Novo G, Novo S. Atherosclerosis, inflammation and Chlamydia pneumoniae. World J Cardiol 2009; 1(1): 31-40
- URL: https://www.wjgnet.com/1949-8462/full/v1/i1/31.htm
- DOI: https://dx.doi.org/10.4330/wjc.v1.i1.31
Approximately one third of patients with coronary artery disease (CAD) do not have traditional risk factors. New evidence shows that systemic markers of inflammation are a strong predictor of cardiovascular events, adding independently to traditional risk factors. Inflammation systemically or locally within an atherosclerotic plaque is believed to play a major role in the initiation and progression of CAD and the precipitation of acute coronary events. Cardiovascular events may most commonly arise from sites of “nonsignificant” stenosis, suggesting that plaque instability rather than the degree of stenosis is the key risk factor. This plaque instability is believed related to inflammation within the plaque, with activated macrophages releasing inflammatory mediators, activating matrix metalloproteinases (MMP), and breaking down the protective fibrous cap. Sources of this inflammation may include non-infectious triggers [e.g. oxidized low-density lipoprotein (LDL), oxidation products of smoking, endothelial injury, genetics, etc.] or a number of proposed infectious triggers[1-9].
Currently, it is known that local and systemic inflammatory processes play an important role in the genesis and development of atherosclerotic lesions and in the pathophysiology of acute coronary syndromes. This hypothesis is supported by findings of elevated parameters of the inflammatory reaction in the blood of atherosclerotic patients as well as findings of histopathological characteristics of unstable plaques (thin fibrous cap, large necrotic core, less smooth muscle cells and abundant foamy cells and lymphocytes). Furthermore, several studies have demonstrated that inflammation has a determining role in the rupture of the coronary plaque, and investigations have been carried out to identify the etiopathogenetic basis of the inflammation itself, trying to correlate coronary atherosclerosis and its development with some infectious agents[7-12].
Potentially, acute or chronic infections could initiate and promote CAD in the absence of traditional risk factors. More likely, infections act to augment CAD risk in the presence of other risk factors. A number of mechanisms have been proposed which could link infection to atherosclerosis[10].
Understanding the pathogenesis of atherosclerosis and the role of inflammation first requires some knowledge of the structure and biology of the normal artery and its indigenous cell types.
Normal arteries have a well-developed trilaminar structure. The innermost layer, the tunica intima, is a monolayer of endothelial cells abutting directly on a basal lamina and constitutes the crucial contact surface with blood. Arterial endothelial cells possess many highly regulated mechanisms of capital importance for vascular homeostasis that often go awry during the pathogenesis of arterial diseases. The internal elastic membrane serves as the border between the intimal layer and the underlying tunica media[8-12]. The media of elastic arteries such as the aorta have well-developed concentric layers of smooth muscle cells, interleaved with layers of elastin-rich extracellular matrix. This structure appears well adapted for the storage of the kinetic energy of left ventricular systole by the walls of great arteries. The lamellar structure also doubtless contributes to the structural integrity of the arterial trunks. In the media of smaller muscular arteries there are usually smooth muscle cells residing within the surrounding matrix in a more continuous manner than in lamellar array. In the normal artery the smooth muscle cells are generally quiescent from the standpoint of growth control and there is a state of homeostasis of extracellular matrix. The external elastic lamina forms a border with the adventitial layer. The adventitia contains collagen fibrils and a cellular population such as fibroblasts and mast cells. Vasa vasorum and nerve endings localize in this outermost layer of the arterial wall[12-15].
The pathogenesis of atherogenesis and of its development remain largely conjectural. One of the first ultrastructural alterations is an accumulation of small lipoprotein particles (LDL) in the intima, where binding of lipoproteins to proteoglycan occurs which tends to coalesce into aggregates. This process is supported by permeability of the endothelial monolayer. Lipoprotein particles bound to proteoglycan appear to exhibit increased susceptibility to oxidative or other chemical modifications such as enzymatic processing and glycation which can modify LDL in the intima. The second morphologically definable event in the initiation of atheroma is leukocyte recruitment and accumulation; these adhere to the endothelium by means of adhesion molecules, and diapedese between endothelial cell junctions to enter the intima, where they begin to accumulate lipids and transform into foam cells. In addition to the monocytes, T lymphocytes also tend to accumulate in early atherosclerotic lesions. The current concept of directed migration of leukocytes involves the action of protein molecules known as chemoattractant cytokines, or chemokines, produced by the endothelium and smooth muscle in response to oxidized lipoprotein and other stimuli[16-19].
Until now the natural history of the atherosclerotic process has not been totally understood. Some researchers have invoked a multicentric origin hypothesis of atherogenesis, positing that atheromas arise as benign leiomyomas of the artery wall. However, the location of sites of lesion predilection at proximal portions of arteries after branch points or bifurcations at flow dividers suggests a hydrodynamic basis for early lesion development. Locally disturbed flow could induce alterations that promote the steps of early atherogenesis; alternatively, the laminar flow may elicit antiatherogenic homeostatic mechanisms (atheroprotective functions). This hypothesis is supported by in vitro data suggesting that laminar shear stress can augment the expression of genes that may protect against atherosclerosis, including forms of the enzymes superoxide dismutase (which reduces oxidative stress by catabolizing the reactive and injurious superoxide anion) or nitric oxide synthase (which produces nitric oxide, an endogenous vasodilator and anti-inflammatory agent)[19,20].
Whereas the early events in atheroma initiation involve primarily altered endothelial function and recruitment and accumulation of leukocytes, the subsequent evolution of atheroma into more complex plaques additionally involves smooth muscle cells. Some smooth muscle cells likely migrate from the underlying media into the intima, attracted by molecules such as platelet-derived growth factor, secreted by activated macrophages and overexpressed in atherosclerosis. These smooth muscle cells begin to replication themselves; furthermore, death of these cells may also participate in complications of the atherosclerotic plaque. The vascular smooth muscle cell produces extracellular matrix molecules which make up much of the volume of an advanced atherosclerotic plaque. This matrix is catalyzed in part by enzymes known as MMP. This dissolution also likely plays a role in the arterial remodeling that accompanies lesion growth. During the first part of the life history of an atheromatous lesion, growth of the plaque is outward, in an abluminal direction, rather than inward. The smooth muscle cell is not alone in its proliferation and migration within the evolving atherosclerotic plaque. Endothelial cell migration and replication also occur as plaques develop in microcirculation, characterized by plexuses of newly formed vessels. The microvascularization of plaques may also allow growth of the plaque, overcoming diffusion limitations on oxygen and nutrient supply. Finally, the plaque microvessels may be friable and prone to rupture. Hemorrhage and thrombosis in situ could promote a local cycle of smooth muscle cell proliferation and matrix accumulation in the area immediately adjacent to the microvascular disruption. Plaques often develop areas of calcification as they evolve[21-23].
The process of initiation and evolution of the atherosclerotic plaque generally takes place over many years, during which the affected person often has no symptoms. After the plaque burden exceeds the capacity of the artery to remodel outward, encroachment on the arterial lumen begins. Eventually the stenosis may progress to a degree that impedes blood flow through the artery. The development of chronic stable angina pectoris or intermittent claudication on increased demand is a common presentation of this type of atherosclerotic disease. However, several kinds of clinical observation suggest that most myocardial infarctions result not from critical blockages but from lesions that produce stenoses which do not limit flow. Instead of progressive growth of the intimal lesion to a critical stenosis, we now recognize that thrombosis, complicating a not necessarily occlusive plaque, most often causes episodes of unstable angina or acute myocardial infarction. Thrombosis is the consequence of a fracture of the plaque’s fibrous cap, or of a superficial erosion of the intima[24-28].
The “risk factor” is a characteristic or feature of an individual or population that is present early in life which is associated with an increased risk of developing future cardiovascular disease. The risk factor of interest may be a behavior (e.g. smoking), an inherited trait (e.g. family history), or a laboratory measurement (e.g. cholesterol). Features can be classified as conventional atherosclerotic risk factors such as hyperlipidemia, smoking, hypertension, insulin resistance and diabetes, physical activity, obesity, and hormone status; or novel atherosclerotic risk factors, including levels of homocysteine, fibrinogen, lipoprotein(a) [Lp(a)], as well as infective agents, markers of inflammation [e.g. high-sensitivity C-reactive protein (CRP)], indices of fibrinolytic function [e.g. tissue-type plasminogen activator (t-PA) and plasminogen activator inhibitor 1 (PAI-1)][10,14,25].
Dyslipidemia encompasses disorders that include high average total plasma cholesterol levels, particularly LDL, but low high-density lipoproteins (HDL). Several studies point out the relationship between LDL and possibly very LDL and CAD. The role of HDL as a protective fraction has also emerged. Cholesterol (C) plays an important role in the atherosclerotic process, as it actually is a main constituent of the plaque. Furthermore, the presence of small, dense LDL particles may be related to features of the “metabolic syndrome”, characterized by the presence of abdominal obesity, peripheral insulin resistance, high blood pressure, and a dyslipoproteinemia with elevated plasma triglycerides and reduced HDL-C levels[21-26].
Cigarette consumption constitutes the single most important modifiable risk factor for CAD. Smoking affects atherothrombosis via several mechanisms. In addition to accelerating atherosclerotic progression, long-term smoking may enhance oxidation of LDL-C and reduce levels of HDL-C. Smoking also impairs endothelium-dependent coronary artery vasodilation and has multiple adverse hemostatic effects; it actually increases inflammatory markers such as CRP, soluble intercellular adhesion molecule (ICAM-1), and fibrinogen, causes spontaneous platelet aggregation and increases monocyte adhesion to endothelial cells[26].
Hypertension is often a silent cardiovascular risk factor. The risk increases in the presence of other cardiovascular risk factors such as insulin resistance and obesity. This cluster of metabolic and cardiovascular risk factors is named “Metabolic Syndrome” and also includes dyslipidemia, prothrombotic state and inflammatory state[27].
Diabetic patients have a greater atherosclerotic burden both in the major arteries and in the microvascular circulation. Insulin resistance also produces a prothrombotic state due to increased levels of PAI-1 and fibrinogen. In addition to these systemic metabolic abnormalities, hyperglycemia causes accumulation of advanced glycation end products inculpated in vascular damage. Furthermore, diabetic patients have markedly impaired endothelial and smooth muscle function and appear to have increased leukocyte adhesion to vascular endothelium, a critical early step in atherogenesis[28].
Regular physical exercise reduces myocardial oxygen demand and increases exercise capacity, both of which are associated with lower levels of coronary risk. The mechanisms by which exercise lowers cardiovascular risk remain uncertain but likely include favorable effects on blood pressure, weight control, lipid profiles, and improved glucose tolerance. Exercise also improves endothelial function, enhances fibrinolysis, reduces platelet reactivity, and reduces propensity for in situ thrombosis.
Controversy remains as to whether obesity itself is a true risk factor for cardiovascular disease or whether its impact on vascular risk is mediated solely through interrelations with glucose intolerance, insulin resistance, hypertension, physical inactivity, and dyslipidemia[28,29].
The adrenergic stimulation of mental stress can clearly augment myocardial oxygen requirements, can cause coronary vasoconstriction, particularly in atherosclerotic coronary arteries, and hence can also influence myocardial oxygen supply. Catecholamines can also promote alterations in thrombosis[30].
Before the menopause, women have lower age-adjusted incidence and mortality rates for coronary heart disease than men. This effect results from the beneficial actions of estrogen on lipid fractions, but is also due to direct vascular mechanisms such as improved endothelial-dependent vasomotion, reduced LDL oxidation, altered adhesion molecule levels, increased fibrinolytic capacity, and enhanced glucose metabolism.
Despite these facts, exogenous estrogen use among young women as a form of oral contraception is associated with increased rates of intravascular thrombosis; these effects are particularly prominent among smokers[28-31].
Several novel markers of atherothrombotic risk have emerged from epidemiological studies and might prove useful clinically.
Hyperhomocystinemia is linked to atherosclerosis. The mechanisms that account for these effects remain uncertain but may include endothelial toxicity, accelerated oxidation of LDL-C, impairment of endothelial-derived relaxing factor, and reduced flow-mediated arterial vasodilation[32].
Plasma fibrinogen critically influences platelet aggregation and blood viscosity, interacts with plasminogen binding, and in combination with thrombin mediates the final step in clot formation. In addition, fibrinogen associates positively with age, obesity, smoking, diabetes, and LDL-C and inversely with HDL-C, alcohol use, physical activity, and exercise level. Several studies consider fibrinogen an independent marker of risk for coronary heart disease[33-35].
The normal function of Lp(a) is unknown; the close homology between Lp(a) and plasminogen has raised the possibility that this unusual lipoprotein may inhibit endogenous fibrinolysis by competing with plasminogen for binding on the endothelial surface. More recent data demonstrate accumulation of Lp(a) and co-localization with fibrin within atherosclerotic lesions, both in stable patients and among those with unstable angina pectoris. Apo(a) may also induce monocyte chemotactic activity in the vascular endothelium, whereas Lp(a) may increase release of PAI. Thus, several mechanisms may contribute to a role for Lp(a) in atherothrombosis. As yet, many studies have not established the importance of Lp(a) as a marker for all future cardiovascular events or whether an increased risk is restricted to those with the highest levels or with an absence of other traditional risk factors[34-36].
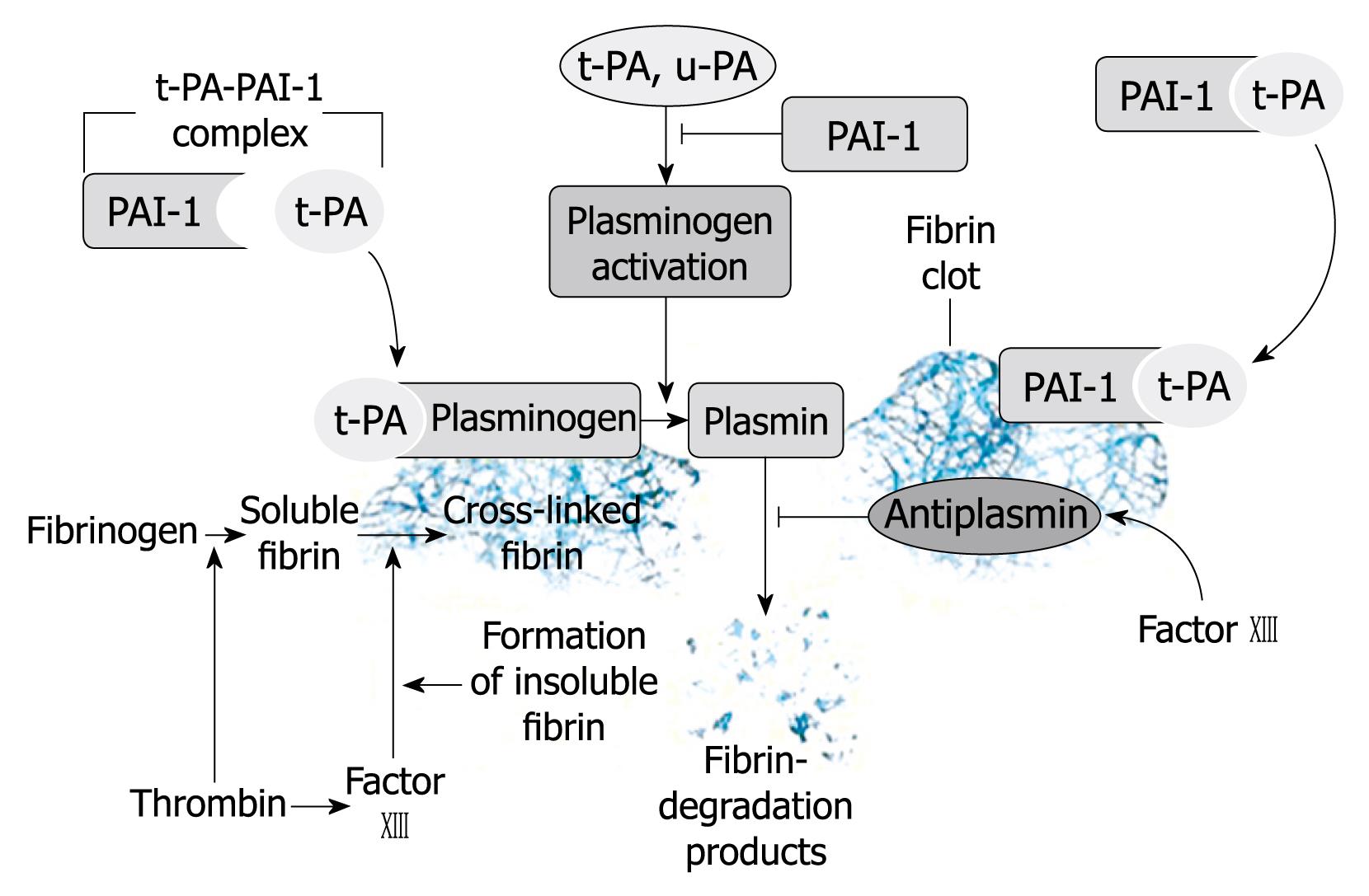
A role for either t-PA or PAI-1 in the development of venous thrombosis remains controversial. In contrast, a highly consistent series of studies have linked abnormalities of fibrinolysis to increased risk of arterial thrombosis. Finally, several studies indicate that levels of D-dimer also predict myocardial infarction, peripheral atherothrombosis, and recurrent coronary events. Despite these data, the clinical use of fibrinolytic markers to determine coronary risk may offer little marginal value (Figure 1)[37,38].
Recently, interest has increased in the possibility that infections, and perhaps chronic infection, may cause atherosclerosis. Inflammation characterizes all phases of atherosclerosis from foam cell formation to plaque progression and rupture.
In fact, there are a lot of data related to this issue; atherosclerotic lesions are heavily infiltrated by cellular components associated with inflammation, especially lymphocytes [T cells of both the helper (CD4+) type and cytotoxic/suppressor (CD8+) immunologically activated type], macrophages, and foam cells. It is evident that plaque complexities, especially intraplaque hemorrhages, are connected with the intensity of inflammation. Several studies have shown a link between baseline elevations of CRP, or other acute phase proteins, and the risk of future cardiac events, and the evaluation of this marker of infection and inflammation may be of importance for an effective prevention of cardiovascular events[21-28].
However, the potential mechanisms for infection-induced atherosclerosis remain speculative.
Inflammation may promote the process by acting both directly and indirectly. Infection could indirectly influence this process without infiltrating the artery wall. Host defenses to extravascular infections usually elicit proinflammatory cytokines and stimulate increased expression of cellular adhesion molecules, enhancing leukocyte adhesion. These cytokines could promote a second wave or “echo” from inflammatory cells already at sites of atherogenesis, such as arterial wall cells or macrophages. Circulating microbial products such as endotoxin can also produce an echo. Similarly, cytokines induced by extravascular infection [specifically interleukin (IL)-6] characteristically elicit hepatic synthesis of acute-phase reactants, some of which might promote atheromata complicated by thrombosis. Accordingly, levels of the acute-phase reactant fibrinogen correlate prospectively with risk for coronary events, and plasminogen activator inhibitor can promote clot stability by interfering with fibrinolysis. However, direct infection of the arterial wall could promote evolution of atherosclerotic lesions or precipitate acute cardiovascular events as suggested by histological findings in unstable coronary plaques, evidence of systemic release of thromboxanes and leukotrienes, and the presence of activated circulating leucocyte[15,39].
The earliest lesions of atherogenesis, consisting of altered endothelial permeability (endothelial dysfunction), can be induced by hemodynamic forces, by a variety of vasoactive substances, by mediators from blood cells, and directly from risk factors for atherosclerosis, with resulting arterial intimal accumulations of leukocytes, foam cells (primarily lipid-laden macrophages) and T lymphocytes intermixed with smooth muscle cells. After crossing the surface of the endothelium, the leukocytes accumulate within the intima. As the process continues, monocytes are converted to activated macrophages and take up oxidized LDL particles, thus becoming foam cells. The formation and accumulation of foam cells within the intima create the fatty streaks of atherosclerotic lesions. If the precipitating risk factors or offending agents are not removed, this process continues and leads to complex lesions. These complex lesions contain layers of smooth muscle, connective tissues, macrophages, and T lymphocytes. The presence of activated T lymphocytes in the atherosclerotic plaque suggests a local immune response, and it has been postulated that such a response may be directed against local antigens in the plaque. Activated T lymphocytes secrete growth factors and cytokines that may affect other cell types and the process of atherosclerosis. Interleukins, complement factor fragments, and tumour necrosis factors (TNF) can enhance monocyte adhesiveness and chemotaxis and so form an amplification mechanism for recruitment of further monocytes into the lesion. Following endothelial adhesion and transmigration into the arterial intima, these cells express markers of activation. Furthermore, released mitogens, such as macrophage derived growth factor, may play a key role in smooth muscle cell migration and subsequent proliferation and hence the progression of plaques. Activation of circulating leucocytes may be facilitated at the endothelium covering an atherosclerotic plaque, with upregulation of adhesion molecules and tethering of circulating cells. These inflammatory responses may further promote the infiltration of activated leucocytes into the atherosclerotic lesion, which in turn may directly activate smooth muscle cells, macrophages, and T cells inside the vessel wall. Lesional macrophages produce proteolytic enzymes, including members of the metalloproteinase family, which contribute to weakness of the protective fibrous cap of the plaque and hence promote the propensity of those plaques to rupture and trigger thrombosis. Over time, fibrous caps, consisting of smooth muscle, collagen, and elastic fibers, form to cover the complex lesions and intrude into the arterial wall. Along with impeding blood flow in the lumen, these fibrous plaques may rupture, causing thrombus formation, plaque progression, or death.
Raised concentrations of LDL and possibly Lp(a) may attract monocytes to adhere to endothelium and induce their transformation into macrophages. The proinflammatory effects of oxidised LDL involve peroxides and other reactive oxygen intermediates generated by the oxidation of LDL. These molecules activate nuclear transcription factor κB (NF-κB), which plays a key role in the orchestration of inflammatory and immune responses by controlling the transcription of the genes encoding several of the adhesion molecules, ILs, TNFα, class II antigen, and antibodies. NF-κB recognises various activators, among which are the proinflammatory cytokines and CRP[38-41].
Most important is the role of cytokines such as TNFα or IL-1 isoforms, which can stimulate the expression of IL-6, IL-8, and leucocyte-platelet adhesion molecules such as ICAM-1. These cytokines are produced by neutrophils and macrophages which are located in atheromatous plaques. They may be derived from non-vascular sources and reflect generalised inflammatory states, such as chronic infection, which have been linked to atherogenesis and its clinical manifestations. The contribution of vascular and extravascular sources of inflammatory cytokines may vary between individuals. Primary cytokines (TNFα, IL-1) stimulate the production, by endothelial and other cells, of adhesion molecules, procoagulants, and other mediators that may be released in soluble form into circulating blood. Primary cytokines also stimulate the production of messenger cytokine, IL-6, which induces expression of hepatic genes encoding acute phase reactants found in the blood, including CRP and serum amyloid A. Moreover, CRP may activate complement and thus participate in sustaining inflammation. Serum amyloid A can bind to HDL particles, perhaps rendering them less protective against vascular inflammation[1-12].
Inflammatory cytokines modulate the homeostatic properties of the endothelium. The local effects of inflammatory cells on digestion of the fibrous cap lead to plaque disruption and thrombus formation. Tissue factor is normally expressed in exposed intima and activates factor VII which in turn activates factors IX and X. Collagen in exposed intima binds von Willebrand factor, which mediates platelet adherence by binding to the glycoprotein Ib/V/IX platelet surface receptor complex under high shear stress conditions. Von Willebrand factor itself is the carrier protein for factor VIII, an essential component of the amplifying mechanism of the factor X-Xa conversion. Furthermore, platelets activated by adhesion then adhere to other platelets through the glycoprotein IIb/IIIa receptor and its ligand, von Willebrand factor and fibrinogen. Such activated platelets release PAI-1, which locally inhibits the fibrinolytic mechanism[39,40].
Inflammation may promote thrombosis by acting both locally and systemically. Local mechanisms include the cytokine-stimulated expression of tissue factor by endothelial cells and macrophages. Indirectly, inflammation may act locally to induce thrombosis by weakening the fibrous cap of the atheromatous plaque, leading to plaque rupture. However, this role of inflammation, and specifically the role of macrophages, remains controversial. Inflammation can affect systemic hemostatic activity via IL-6-mediated stimulation of hepatocytes to produce acute phase reactants. These include certain coagulation factors, such as increased levels of fibrinogen and PAI-1, which induce a prothrombotic state. An enhanced CD40L-CD40 interaction also promotes thrombotic activity by enhancing tissue factor expression in macrophages and through the direct regulation of endothelium procoagulant activity. Intravascular fibrinolysis induced by tissue type plasminogen activator may contribute to atherosclerosis by inducing P-selectin and platelet activating factor, as well as contributing to plaque rupture by activating metalloproteinases. Oxidised LDL also induces tissue factor expression in macrophages and decreases the anticoagulant activity of the endothelium by interfering with thrombomodulin expression and inactivating tissue factor pathway inhibitor. Its expression is upregulated in circulating and endothelium adherent monocytes, and tissue factor has been found to be increased in coronary tissue of the culprit lesion from patients with unstable angina.
It is also now accepted that platelets may promote inflammatory responses. Studies have shown that activated platelets may mediate the homing of leucocytes by interaction with the subendothelial matrix under shear stresses that do not allow neutrophil adhesion. They may also contribute to the oxidative modification of LDL, provide a source of lipids for foam cell generation, and contribute to smooth muscle cell proliferation[40].
It is now known that acute cardiovascular events are linked with a non-stenotic, but a vulnerable plaque. The difference between the mature and the unstable plaque is related to both core and fibrous cap. The former consist of two main components, a soft lipid-rich atheromatous “gruel” and hard collagen-rich sclerotic tissue. The latter contain a core of soft atheromatous gruel that is separated from the vascular lumen by a thin cap of fibrous tissue. The fibrous cap is infiltrated by foam cells indicating ongoing disease activity. Such a thin and macrophage-infiltrated cap is probably very weak and vulnerable, and it can indeed be disrupted nearby, explaining why erythrocytes can be seen in the gruel just beneath the macrophage-infiltrated cap. Sclerosis is relatively innocuous because fibrous tissue appears to stabilize plaques, protecting them against disruption. In contrast, the usually less voluminous atheromatous component is the more dangerous component, because the soft atheromatous gruel destabilizes plaques, making them vulnerable to rupture, whereby the highly thrombogenic gruel is exposed to the flowing blood, leading to thrombosis - a potentially life-threatening event.
The risk of plaque disruption is related to intrinsic properties of individual plaques (their vulnerability) and extrinsic forces acting on plaques (rupture triggers). Plaque disruption occurs most frequently where the fibrous cap is thinnest, most heavily infiltrated by foam cells, and therefore weakest. The vulnerability to rupture depends on size and consistency of the atheromatous core, thickness and collagen content of the fibrous cap covering the core, inflammation within the cap and the cap “fatigue” caused by cyclic stretching, compression, bending, flexion, shear, and pressure fluctuations. Regarding the cap during inflammation, macrophages play an important role as they are capable of degrading extracellular matrix by phagocytosis or by secreting proteolytic enzymes such as plasminogen activators and a family of MMPs such as collagenases, gelatinases, and stromelysins that may weaken the fibrous cap, predisposing it to rupture, and also promoting thrombin generation and luminal thrombosis through the tissue factor pathway. Neutrophils are also capable of destroying tissue by secreting proteolytic enzymes, but neutrophils are rare in intact plaques. They may occasionally be found in disrupted plaques beneath coronary thrombi, probably entering these plaques shortly after disruption, and neutrophils may also migrate into the arterial wall shortly after reperfusion of occluded arteries in response to ischemia/reperfusion. The rupture of a cap is linked, presumably, with digestion by macrophages but also with senescense or apoptosis of smooth muscle cells caused by inflammatory cytokines.
Coronary plaques are constantly stressed by a variety of biomechanical and hemodynamic forces that may precipitate or “trigger” disruption of vulnerable plaques. The circumferential wall tension (tensile stress) caused by the blood pressure establishes a stress which is redistributed to adjacent structures and may be concentrated at critical points. The consistency of the gruel may be important for this stress redistribution, as indeed are the characteristics of the cap; the thinner the fibrous cap, the higher the stress that develops within it. Furthermore, mechanical shear stresses may develop in plaques at the interface between tissues of different stiffness, resulting, for example, in shear failure, calcified plates and adjacent noncalcified tissue[29,30].
Plaque disruption may occur when there are increases in the intraplaque pressure, caused by vasospasm, bleeding from vasa vasorum, plaque edema, and/or collapse of compliant stenoses. Vasospasm reduces the circumferential tension in fibrous caps by narrowing the lumen (Laplace’s law). Nevertheless, spasm could theoretically rupture plaques by compressing the atheromatous core, “blowing” the fibrous cap out into the lumen. Bleeding and/or transudation (edema) into plaques from the thin-walled new vessels originating from vasa vasorum and frequently found at the plaque base could theoretically increase the intraplaque pressure, with resultant cap rupture from the inside. High-grade stenosis may be subjected to strong compressive forces due to the accelerated velocities in the throat. Collapse of severe but compliant stenoses due to negative transmural pressures may produce highly concentrated compressive stresses from buckling of the wall with bending deformation, preferentially involving plaque edges, and theoretically, this could contribute to plaque disruption.
Another factor important for the rupture is the propagating pulse wave during the cardiac cycle that causes changes in lumen size and shape with deformation and bending of plaques, and particularly of eccentric plaques[12-15].
Onset of acute coronary syndromes does not occur at random; in fact, a large fraction appear to be triggered by external factors or conditions such as emotional stress, vigorous exercise or cold. The pathophysiological mechanisms responsible for the nonrandom and (apparently often) triggered onset of infarction are unknown but probably related to (1) plaque disruption, most likely caused by surges in sympathetic activity with a sudden increase in blood pressure, pulse rate, heart contraction, and coronary blood flow; (2) thrombosis, occurring on previously disrupted or intact plaques when the systemic thrombotic tendency is high because of platelet hyperaggregability, hypercoagulability, and/or impaired fibrinolysis; and (3) vasoconstriction, occurring locally around a coronary plaque or generalized[23-25].
The possibility that various microbial agents may trigger a cascade of reactions leading to inflammation, atherogenesis, and thrombotic events in the vascular system has been raised in the last two decades. Chronic infection with various agents, both bacteria and viruses, such as Chlamydia pneumoniae (C. pneumoniae), Cytomegalovirus (CMV), HSV, Helicobacter pylori (H. pylori), Mycoplasma pneumoniae, anaerobic periodontal organisms, etc., has been implicated in the pathogenesis of CAD. Specific agents have been proposed as direct initiators or accelerators of atherosclerosis, through nonspecific stimulation of the inflammatory cascade. However, the role of these infection agents must be proven; there are often confounding factors which should be carefully considered, although it is quite plausible that infections may potentiate the action of traditional risk factors.
C. pneumoniae is an important respiratory pathogen associated with 5% to 10% of community-acquired cases of pneumonia, pharyngitis, bronchitis, and sinusitis. It is an obligatory intracellular bacterium that has the tendency to cause persistent infection, and may drive a chronic inflammatory reaction in coronary vasculature or other tissues. C. pneumoniae has been proposed as an etiologic factor for atherosclerosis, contributing either directly or indirectly, by modifying traditional risk factors[34-40].
In particular, cytokines produced by C. pneumoniae-infected macrophages, located in coronary atherosclerotic plaques, may trigger an ongoing inflammatory response, and thus an increased prothrombotic state and smooth muscle cell proliferation, all of which favor atherothrombotic complications and restenosis after stenting. Lines of evidence associating C. pneumoniae with atherosclerosis include seroepidemiologic studies, direct detection of bacterial components in atherosclerotic lesions by polymerase chain reaction or electron microscopic studies, occasional isolation of viable organisms from coronary and carotid atheromatous tissue, and in vitro and animal experiments. The strongest evidence associating C. pneumoniae with atherosclerotic cardiovascular disease has been detection of bacterial components in atherosclerotic lesions. C. pneumoniae appears to have a tropism for atheromata. In addition, it is rarely found in normal arteries[34-40].
C. pneumoniae may infect circulatory components, which may attach to the endothelium and smooth muscle cells and kill them by apoptosis. The probable molecular mechanism of atherosclerosis pathogenesis can be explained by up-regulation of expression of heat shock protein 60 (HSP-60) by C. pneumoniae infection, which induces production of cytokines such as TNF-α, IL-1β and IL-6, and MMPs by macrophages. Furthermore, C. pneumoniae could lead to elevation of CRP and contribute to instability or progression of atherosclerotic plaques. The bacterium replicates in endothelial and smooth muscle cells and macrophages, and it can activate CD4+ and CD8+ T lymphocytes. C. pneumoniae initiates inflammatory activation via the NF-κB pathway, resulting in increased expression of vascular cell adhesion molecule-1, enhanced recruitment of inflammatory leukocytes to the vessel wall, impaired activity of endothelial nitric oxide, increased platelet adhesion to endothelial cells and procoagulant activity in endothelial cells, as well as causing oxidation of LDL-C. Therefore, chronic infection may contribute to the risk of CHD by initiating a high level of immunologic activity, by raising triglyceride levels and decreasing HDL levels, and by increasing the concentrations of acute-phase reactants such as fibrinogen, CRP, and sialic acid.
Specific microbial products such as lipopolysaccharides, heat-shock proteins, or other virulence factors might act locally at the level of the artery wall to potentiate atherosclerosis in infected lesions. Extravascular infection might also influence the development of atheromatous lesions and provoke their complication. For example, circulating endotoxin or cytokines produced in response to a remote infection can act locally at the level of the artery wall to promote the activation of vascular cells and of leukocytes in pre-existing lesions, producing an “echo” at the level of the artery wall of a remote infection. Also, the acute phase response to an infection in a nonvascular site might affect the incidence of thrombotic complications of atherosclerosis by increasing fibrinogen or PAI-1 levels or otherwise altering the balance between coagulation and fibrinolysis. Such disturbance in the prevailing prothrombotic/fibrinolytic balance may critically influence whether a given plaque disruption will produce a clinically inapparent, transient or nonocclusive thrombus, or sustained and occlusive thrombi that could cause an acute coronary event. Acute infections might also produce hemodynamic alterations that could trigger coronary events such as tachycardia; increased metabolic demands of fever could augment the oxygen requirements of the heart, precipitating ischemia in an otherwise compensated individual. Infectious processes, either local in the atheroma or extravascular, might aggravate atherogenesis, particularly in preexisting lesions or in concert with traditional risk factors.
The presence of C. pneumoniae in atherosclerotic lesions raises the possibility that antibiotic treatment might have a favourable effect on the course of CAD. However, a few large randomized antibiotic trials have not shown any beneficial effect of long term antibiotic therapy (azithromycin and rifampin) suggesting that large randomized antibiotic trials may not show clinical benefit in patients with established acute or chronic CAD. After failure of antibiotic trials, it was postulated that C. pneumoniae has a pathogenetic role in the early development of atherosclerosis. It is possible, therefore, that infection with C. pneumoniae plays a part in the initiation of atherosclerosis early on in life; however, once plaque and inflammation are established, antichlamydial antibiotics cannot alter the progression of coronary disease[34-42].
Specific infectious agents other than C. pneumoniae have the potential to play a role in atherosclerosis as demonstrated by experimental models, the presence of organisms within plaques and inflammatory cells, and by seroepidemiologic associations.
Several investigators suggested a role for other bacteria and viruses in cardiovascular disease such as oral infections caused by Porphyromonas gingivalis, Bacteroides forsythus, Campylobacter rectus, Fusobacterium nucleatum, Treponema spp., and Prevotella species. Like C. pneumoniae, oral bacteria might affect atherosclerosis through direct invasion of vascular endothelial cells or indirectly through products that stimulate proinflammatory and prothrombotic functions of vascular cells.
The literature linking H. pylori, HIV, HSV-1, and HSV-2 to atherogenesis is less extensive than for C. pneumoniae or CMV[12]. CMV, a herpes-family DNA virus, is a common human pathogen and a candidate atherogenic organism. CMV can also accelerate atherosclerosis in animal models. Infection may induce a systemic inflammatory response that promotes atherosclerosis. CMV may contribute to CAD by several mechanisms, including impaired fibrinolysis, increased Lp(a), enhanced procoagulant activity, and upregulation of the macrophage oxidized LDL scavenger receptor. CMV can inactivate p53, an apoptosis-related protein, facilitating excessive proliferation of vascular smooth muscle cells[38].
The presence of any chronic bacterial infection (e.g. respiratory, urinary, dental, or other) increases considerably the risk of developing atherosclerosis[17]. H. pylori, the etiologic organism of peptic ulcer disease, has also received attention as a potential pathogen in atherosclerosis, although the early positive serologic associations have not been confirmed. H. pylori organisms have not been demonstrated in atherosclerotic plaques, nor have animal models demonstrated a pathologic role. However, H. pylori infection can increase CRP and fibrinogen levels and promote platelet aggregation. Thus, a role for H. pylori in atherogenesis also remains to be established[39,40].
The best way to prevent cardiovascular disease is the prevention of atherosclerosis and its development through removal of risk factors. Statins are the best therapeutic option to modulate inflammation, in fact several studies have demonstrated their anti-inflammatory, antioxidant and plaque-stabilizing effects in addition to their cholesterol-lowering effects. Statin treatment for global cardiovascular risk prevention is indicated and must be proposed in high risk patients. In addition, treatments for hypertension, diabetes, weight loss, exercise and smoking cessation are vitally important measures for cardiovascular risk reduction.
Peer reviewer: Wei-Chuan Tsai, MD, Department of Internal Medicine, National Cheng Kung University Hospital, 138 Sheng-Li Road, Tainan 704, Taiwan, China
S- Editor Cheng JX L- Editor Logan S E- Editor Zheng XM
| 1. | Zipes DP, Libby P, Bonow RO, Braunwald E, editors . Braunwald's heart disease: a textbook of cardiovascular medicine. 7th ed. Philadelphia: Elsevier Saunders 2005; . [Cited in This Article: ] |
| 2. | Fazio G, Sutera L, Zito R, Cascio C, Briguglia D, Taormina S, Giammanco A, Assennato P, Novo S. [Rupture of the atherosclerotic plaque: is Chlamydia pneumoniae a possible agent?]. G Ital Cardiol (Rome). 2006;7:809-814. [Cited in This Article: ] |
| 3. | Lastas A, Graziene V, Barkauskas E, Salkus G, Rimkevicius A. Carotid artery atherosclerotic plaque: clinical and morphological-immunohistochemical correlation. Med Sci Monit. 2004;10:CR606-CR614. [Cited in This Article: ] |
| 4. | Shishehbor MH, Patel T, Bhatt DL. Using statins to treat inflammation in acute coronary syndromes: Are we there yet? Cleve Clin J Med. 2006;73:760-766. [Cited in This Article: ] |
| 5. | Vijayvergiya R. Association of infection with coronary artery disease. Indian J Med Res. 2007;125:112-114. [Cited in This Article: ] |
| 6. | Yamamoto H, Watanabe T, Miyazaki A, Katagiri T, Idei T, Iguchi T, Mimura M, Kamijima K. High prevalence of Chlamydia pneumoniae antibodies and increased high-sensitive C-reactive protein in patients with vascular dementia. J Am Geriatr Soc. 2005;53:583-589. [Cited in This Article: ] |
| 7. | Klochkov VA, Dovgalevskiĭ PIa, Umetskiĭ KS, Chalyk NE, Ansimova OM, Zigangirova NA, Petiaev I. [Effect of therapy with antibiotics on lipid metabolism and antioxidant reserve of patients with ischemic heart disease during Chlamydia pneumoniae infection]. Kardiologiia. 2005;45:58-61. [Cited in This Article: ] |
| 8. | O'Connor S, Taylor C, Campbell LA, Epstein S, Libby P. Potential infectious etiologies of atherosclerosis: a multifactorial perspective. Emerg Infect Dis. 2001;7:780-788. [Cited in This Article: ] |
| 9. | Kinjo K, Sato H, Sato H, Ohnishi Y, Hishida E, Nakatani D, Mizuno H, Ohgitani N, Kubo M, Shimazu T. Joint effects of Chlamydia pneumoniae infection and classic coronary risk factors on risk of acute myocardial infarction. Am Heart J. 2003;146:324-330. [Cited in This Article: ] |
| 10. | Sessa R, Di Pietro M, Santino I, del Piano M, Varveri A, Dagianti A, Penco M. Chlamydia pneumoniae infection and atherosclerotic coronary disease. Am Heart J. 1999;137:1116-1119. [Cited in This Article: ] |
| 11. | Zairis MN, Papadaki OA, Psarogianni PK, Thoma MA, Andrikopoulos GK, Batika PC, Poulopoulou CG, Trifinopoulou KG, Olympios CD, Foussas SG. Serologic markers of persistent Chlamydia pneumonia infection and long-term prognosis after successful coronary stenting. Am Heart J. 2003;146:1082-1089. [Cited in This Article: ] |
| 12. | Wong BY, Gnarpe J, Teo KK, Ohman EM, Prosser C, Gibler WB, Langer A, Chang WC, Armstrong PW. Does chronic Chlamydia pneumoniae infection increase the risk of myocardial injury? Insights from patients with non-ST-elevation acute coronary syndromes. Am Heart J. 2002;144:987-994. [Cited in This Article: ] |
| 13. | Zebrack JS, Anderson JL. The role of infection in the pathogenesis of cardiovascular disease. Prog Cardiovasc Nurs. 2003;18:42-49. [Cited in This Article: ] |
| 14. | Aguilar D, Fisher MR, O'Connor CM, Dunne MW, Muhlestein JB, Yao L, Gupta S, Benner RJ, Cook TD, Edwards D. Metabolic syndrome, C-reactive protein, and prognosis in patients with established coronary artery disease. Am Heart J. 2006;152:298-304. [Cited in This Article: ] |
| 15. | Schmermund A, Möhlenkamp S, Stang A, Grönemeyer D, Seibel R, Hirche H, Mann K, Siffert W, Lauterbach K, Siegrist J. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL Study. Risk Factors, Evaluation of Coronary Calcium and Lifestyle. Am Heart J. 2002;144:212-218. [Cited in This Article: ] |
| 16. | Tousoulis D, Davies G, Stefanadis C, Toutouzas P, Ambrose JA. Inflammatory and thrombotic mechanisms in coronary atherosclerosis. Heart. 2003;89:993-997. [Cited in This Article: ] |
| 17. | Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657-671. [Cited in This Article: ] |
| 18. | Casscells W, Naghavi M, Willerson JT. Vulnerable atherosclerotic plaque: a multifocal disease. Circulation. 2003;107:2072-2075. [Cited in This Article: ] |
| 19. | Ieven MM, Hoymans VY. Involvement of Chlamydia pneumoniae in atherosclerosis: more evidence for lack of evidence. J Clin Microbiol. 2005;43:19-24. [Cited in This Article: ] |
| 20. | Rizzo M, Corrado E, Coppola G, Muratori I, Novo G, Novo S. Prediction of cardio- and cerebro-vascular events in patients with subclinical carotid atherosclerosis and low HDL-cholesterol. Atherosclerosis. 2008;200:389-395. [Cited in This Article: ] |
| 21. | Corrado E, Rizzo M, Tantillo R, Muratori I, Bonura F, Vitale G, Novo S. Markers of inflammation and infection influence the outcome of patients with baseline asymptomatic carotid lesions: a 5-year follow-up study. Stroke. 2006;37:482-486. [Cited in This Article: ] |
| 22. | Higuchi Mde L, Ramires JA. Infectious agents in coronary atheromas: a possible role in the pathogenesis of plaque rupture and acute myocardial infarction. Rev Inst Med Trop Sao Paulo. 2002;44:217-224. [Cited in This Article: ] |
| 23. | Kaźmierski R, Podsiadły E, Tylewska-Wierzbanowska S, Kozubski W. [Association between carotid atherosclerosis, inflammatory markers and Chlamydia pneumoniae infection]. Neurol Neurochir Pol. 2005;39:277-286. [Cited in This Article: ] |
| 24. | Andrié R, Braun P, Welsch U, Straube E, Höpp HW, Erdmann E, Lüderitz B, Bauriedel G. [Chlamydial and human heat shock protein 60 homologues in acute coronary syndromes. (Auto-)immune reactions as a link between infection and atherosclerosis]. Z Kardiol. 2003;92:455-465. [Cited in This Article: ] |
| 25. | Fernández-Miranda C, Paz M, Aranda JL, Fuertes A, Gómez De La Cámara A. [Chronic Chlamydia pneumoniae infection in patients with coronary disease. Relation with increased fibrinogen values]. Med Clin (Barc). 2002;119:561-564. [Cited in This Article: ] |
| 26. | Paz M, de Otero J, Codinach P, Ferrer-Ruscalleda F, Gayà M, Ibernón M. [Infection and coronary atherosclerosis: the role Chlamydia pneumonia]. Rev Esp Cardiol. 1998;51:857-863. [Cited in This Article: ] |
| 27. | Wyplosz B, Capron L. [Infectious features of atherosclerosis]. Med Sci (Paris). 2004;20:169-174. [Cited in This Article: ] |
| 28. | Ramires JA, Higuchi Mde L. [Mycoplasma pneumoniae and Chlamydia pneumoniae are associated to inflammation and rupture of the atherosclerotic coronary plaques]. Rev Esp Cardiol. 2002;55 Suppl 1:2-9. [Cited in This Article: ] |
| 29. | González-Castañeda C, Pérez-Castrillón JL, Romero-Gómez M, Herreros-Fernández V. Antibodies against Chlamydia pneumoniae and their relation to lymphocyte population levels. Int J Cardiol. 2002;82:293-295. [Cited in This Article: ] |
| 30. | Grayston JT. Background and current knowledge of Chlamydia pneumoniae and atherosclerosis. J Infect Dis. 2000;181 Suppl 3:S402-S410. [Cited in This Article: ] |
| 31. | Kim DK, Kim HJ, Han SH, Lee JE, Moon SJ, Kim BS, Kang SW, Choi KH, Lee HY, Han DS. Chlamydia pneumoniae accompanied by inflammation is associated with the progression of atherosclerosis in CAPD patients: a prospective study for 3 years. Nephrol Dial Transplant. 2008;23:1011-8. [Cited in This Article: ] |
| 32. | Gattone M, Iacoviello L, Colombo M, Castelnuovo AD, Soffiantino F, Gramoni A, Picco D, Benedetta M, Giannuzzi P. Chlamydia pneumoniae and cytomegalovirus seropositivity, inflammatory markers, and the risk of myocardial infarction at a young age. Am Heart J. 2001;142:633-640. [Cited in This Article: ] |
| 33. | Mazzoli S, Tofani N, Fantini A, Semplici F, Bandini F, Salvi A, Vergassola R. Chlamydia pneumoniae antibody response in patients with acute myocardial infarction and their follow-up. Am Heart J. 1998;135:15-20. [Cited in This Article: ] |
| 34. | Liu R, Yamamoto M, Moroi M, Kubota T, Ono T, Funatsu A, Komatsu H, Tsuji T, Hara H, Hara H. Chlamydia pneumoniae immunoreactivity in coronary artery plaques of patients with acute coronary syndromes and its relation with serology. Am Heart J. 2005;150:681-688. [Cited in This Article: ] |
| 35. | Zamorano J, García-Tejada J, Suárez A, Culebras E, Castañón J, Moreno R, Reguillo F, Gil M, Picazo J, Sánchez-Harguindey L. Chlamydia pneumoniae in the atherosclerotic plaques of patients with unstable angina undergoing coronary artery bypass grafting: does it have prognostic implications? Int J Cardiol. 2003;90:297-302. [Cited in This Article: ] |
| 36. | Higuchi Mde L, Reis MM, Sambiase NV, Palomino SA, Castelli JB, Gutierrez PS, Aiello VD, Ramires JA. Coinfection with Mycoplasma pneumoniae and Chlamydia pneumoniae in ruptured plaques associated with acute myocardial infarction. Arq Bras Cardiol. 2003;81:12-22, 1-11. [Cited in This Article: ] |
| 37. | Hong MK, Mintz GS, Lee CW, Kim YH, Lee SW, Song JM, Han KH, Kang DH, Song JK, Kim JJ. Comparison of coronary plaque rupture between stable angina and acute myocardial infarction: a three-vessel intravascular ultrasound study in 235 patients. Circulation. 2004;110:928-933. [Cited in This Article: ] |
| 38. | Fukuda D, Shimada K, Tanaka A, Kusuyama T, Yamashita H, Ehara S, Nakamura Y, Kawarabayashi T, Iida H, Yoshiyama M. Comparison of levels of serum matrix metalloproteinase-9 in patients with acute myocardial infarction versus unstable angina pectoris versus stable angina pectoris. Am J Cardiol. 2006;97:175-180. [Cited in This Article: ] |
| 39. | Davidson M, Kuo CC, Middaugh JP, Campbell LA, Wang SP, Newman WP 3rd, Finley JC, Grayston JT. Confirmed previous infection with Chlamydia pneumoniae (TWAR) and its presence in early coronary atherosclerosis. Circulation. 1998;98:628-633. [Cited in This Article: ] |
| 40. | Liu R, Moroi M, Yamamoto M, Kubota T, Ono T, Funatsu A, Komatsu H, Tsuji T, Hara H, Hara H. Presence and severity of Chlamydia pneumoniae and Cytomegalovirus infection in coronary plaques are associated with acute coronary syndromes. Int Heart J. 2006;47:511-519. [Cited in This Article: ] |
| 41. | Zamorano J, Suarez A, Garcia Tejada J, Culebras E, Castañón J, Picazo J, Moreno R, Sanchez-Harguindey L. Prevalence of Chlamydia pneumoniae in the atherosclerotic plaque of patients with unstable angina and its relation with serology. Int J Cardiol. 2003;89:273-279. [Cited in This Article: ] |