Published online Feb 26, 2016. doi: 10.4331/wjbc.v7.i1.14
Peer-review started: August 27, 2015
First decision: October 27, 2015
Revised: November 25, 2015
Accepted: January 16, 2016
Article in press: January 19, 2016
Published online: February 26, 2016
Bioavailability of vitamin E is influenced by several factors, most are highlighted in this review. While gender, age and genetic constitution influence vitamin E bioavailability but cannot be modified, life-style and intake of vitamin E can be. Numerous factors must be taken into account however, i.e., when vitamin E is orally administrated, the food matrix may contain competing nutrients. The complex metabolic processes comprise intestinal absorption, vascular transport, hepatic sorting by intracellular binding proteins, such as the significant α-tocopherol-transfer protein, and hepatic metabolism. The coordinated changes involved in the hepatic metabolism of vitamin E provide an effective physiological pathway to protect tissues against the excessive accumulation of, in particular, non-α-tocopherol forms. Metabolism of vitamin E begins with one cycle of CYP4F2/CYP3A4-dependent ω-hydroxylation followed by five cycles of subsequent β-oxidation, and forms the water-soluble end-product carboxyethylhydroxychroman. All known hepatic metabolites can be conjugated and are excreted, depending on the length of their side-chain, either via urine or feces. The physiological handling of vitamin E underlies kinetics which vary between the different vitamin E forms. Here, saturation of the side-chain and also substitution of the chromanol ring system are important. Most of the metabolic reactions and processes that are involved with vitamin E are also shared by other fat soluble vitamins. Influencing interactions with other nutrients such as vitamin K or pharmaceuticals are also covered by this review. All these processes modulate the formation of vitamin E metabolites and their concentrations in tissues and body fluids. Differences in metabolism might be responsible for the discrepancies that have been observed in studies performed in vivo and in vitro using vitamin E as a supplement or nutrient. To evaluate individual vitamin E status, the analytical procedures used for detecting and quantifying vitamin E and its metabolites are crucial. The latest methods in analytics are presented.
Core tip: Several factors influence vitamin E bioavailability. Gender, age and genetic constitution cannot be modified but life-style and vitamin E intake can be. Physiological handling of vitamin E involves intestinal absorption, vascular transport, hepatic sorting by intracellular binding proteins, and hepatic metabolism. These processes involve kinetics which vary between the different vitamin E forms. The coordinated metabolism of vitamin E is an effective physiological pathway to prevent excessive accumulation of non-α-tocopherol forms. Interactions with other nutrients or pharmaceutics occur. To evaluate vitamin E status, analytical procedures to detect and quantify vitamin E and metabolites are crucial. Current state-of-the-art analytics are presented.
- Citation: Schmölz L, Birringer M, Lorkowski S, Wallert M. Complexity of vitamin E metabolism. World J Biol Chem 2016; 7(1): 14-43
- URL: https://www.wjgnet.com/1949-8454/full/v7/i1/14.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v7.i1.14
Vitamin E is recognized as being essential for human health, yet fundamental questions remain. In the words of Eduardo Cardenas[1]: “This is a very exciting time in vitamin E research, yet it is evident that we are far away from making the final decision on the benefit vs risk for the potential use of vitamin E in human health”. Underlying this lack of understanding is the immense complexity involved in the metabolism of vitamin E. Despite many decades of research on vitamin E, many relevant processes remain puzzling. In this review we will outline what is known today including more uncertain claims about vitamin E, to deepen insight into the physiological mechanisms and its metabolism.
Evans and Bishop were the first in 1922 to describe the relevance of vitamin E in the reproduction of rats and to characterize tocopherols (TOH) and tocotrienols (T3) including their α-, β-, γ- and δ-forms as vitamins[2]. Vitamin E belongs to the group of fat-soluble vitamins and occurs dominantly in oily plants; therefore, nuts, seeds and oils are good sources for vitamin E. Almonds, hazelnuts, germ oil and sunflower oil contain high amounts of α-TOH while walnuts, palm oil and soybeans predominantly contain γ-TOH[1]. T3 are widely found in some cereals, palm oil and rice bran oil[3]. Coconut oil, cocoa butter, soybeans, barley and wheat germ are also naturally occurring sources of T3s[4], whereas vegetables and fruits - with the exception of dried apricots, some legumes, avocado and green olives - contain lower levels of vitamin E forms[5]. The concentration of vitamin E forms contained in food depends on several factors, such as growing, harvesting and any further processing (refining or cooking)[5,6].
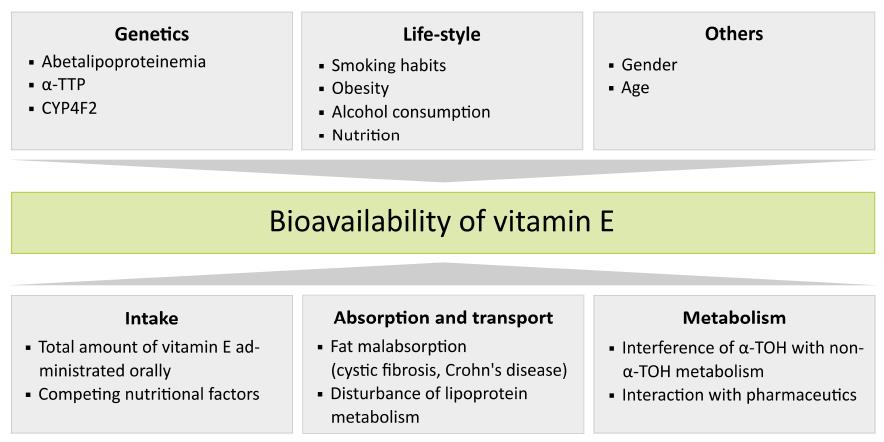
Bioavailability of vitamin E is influenced by numerous factors including: (1) the amount of vitamin E and intake of interfering nutrients; (2) proteins involved in vitamin E absorption and individual differences in the efficiency of vitamin E absorption, influenced by for example diseases; (3) vitamin E metabolism; (4) life-style factors; (5) gender; and (6) genetic polymorphisms. For an overview, see Figure 1.
When considering dietary vitamin E, official intake recommendations are provided by various boards and institutes, and are theoretically defined amounts of a nutrient providing an adequate intake for the major part of a healthy population[7]. Here there exists a subtle difference in definitions describing levels of vitamin intake; whereas vitamin deficiency is caused by diseases or metabolic disorders, vitamin undersupply is characterized as an intake issue which does not achieve reference values[7].
As of today there is no generally accepted recommendation defining the value for an adequate intake of vitamin E. This is due to different references used to validate the recommended dietary allowance (RDA) for vitamin E, or α-TOH. Whereas in the United States the correlation of hydrogen peroxide-induced erythrocyte lysis and plasma α-TOH concentrations is used[8], in Germany, Austria and Switzerland the RDA for vitamin E is based on the effects of vitamin E on the prevention of lipid peroxidation[9]. At present, the German Society of Nutrition (Deutsche Gesellschaft für Ernährung) recommends a daily vitamin E intake of 12 mg/d for women and 13-15 mg/d for men, for both adolescents and adults; intake should be higher during pregnancy (13 mg/d) and breast-feeding (17 mg/d). The required amount of vitamin E increases with age for infants and children and decreases in the elderly independent of gender[10]. Generally, the recommended intake of vitamin E should correlate with the amount of polyunsaturated fatty acids in food: 1 g of diene fatty acid or rather diene equivalent requires an intake of 0.5 mg RRR-α-TOH.
Although several foods contain naturally occurring sources of vitamin E, it is frequently the case that the intake recommendations are not achieved. In Germany infants and children up to age twelve commonly do not reach the recommended levels of vitamin E intake[7], as shown in a number of studies including the VELS investigation (Verzehrsstudie zur Ermittlung der Lebensmittelaufnahme von Säuglingen und Kleinkindern; Food Consumption Survey of Babies and Infants) and the EsKiMo study (Ernährungsstudie als KiGGS-Modul, Nutritional Study as KiGGS modul), a follow-up of the KiGGS study (Kinder- und Jugendgesundheitssurvey; Children and Adolescence Health Survey). In addition some studies show that the elderly are often undersupplied with vitamin E[7,11-13]. Numerous research groups analyzing compliance to the vitamin E intake recommendations in Americans have found that a significant number of individuals consume insufficient amounts[14-16]. Dietary intake surveys by the United Kingdom and the United States National Health and Nutrition Examination Survey have revealed that at least 75% of the population consumes vitamin E far below recommended values[17]. Although the recommended amount of vitamin E is higher for men than for women, Dutch women consume less vitamin E more often compared to Dutch men[17]. Data by Traber[18] published last year suggest that more than 90% of United States Americans consume insufficient amounts of vitamin E.
As vitamin E is primarily stored in adipose tissue (about 90% of the body’s total vitamin E content[19]), vitamin E deficiency is almost unknown under normal physiological conditions[19]. Symptoms of slight vitamin E undersupply become apparent after many years, usually after decades[19], since vitamin E can be mobilized from adipose tissue for years[20]. In contrast, serious vitamin E deficiency manifests in acute symptoms, such as neuropathy and myopathy, as vitamin E is essential for the development and maintenance of the central nervous system[21]. Ulatowski and Manor[16] categorized metabolic vitamin E deficiencies as: (1) a primary deficiency “(…) arising from specific alteration in vitamin E status”; and (2) a secondary deficiency, “(…) in which low levels of vitamin E are secondary to other global perturbations such as disorders in lipid malabsorption or lipoprotein metabolism and transport”. Next to dietary habits, hereditary disorders are known to cause primary and secondary vitamin E deficiencies or inadequate vitamin E bioavailability[19].
Because the official intake recommedations of vitamen E are so seldom met, along with the rare occurance of deficiency symptoms (apart from being caused by disease, addressed later), Traber[18] recently questioned whether the recommended α-TOH intake is set too high and whether a diet low in dietary α-TOH intake has any biological significance. However as of now, circulating α-TOH concentrations below 9 mmol/L for men or below 12 mmol/L for women are considered as deficient and only marginal for healthy adults, respectively[18].
When supplements are used to maintain vitamin E balance, either self-medicated or by medical prescription, questions of toxicity and other safety concerns must be considered. Recent animal studies on reproduction or investigations on the developmental toxic effects of natural or non-natural vitamin E components have been negative but anti-mutagenic activity has been shown[22]. Physiological vitamin E intake of 100 mg/d (150 IU/d) can be increased up to 300 mg/d (450 IU/d) without any complications[23,24]. Even for short-term, high-dose administration of vitamin E no adverse effects have been described[22]. However, persistent high-dose supplementation has been shown to interfere with blood clotting and has been associated with increased risk of hemorrhagic stroke in animal studies[22]. In the past, TOH was considered to be a safe food additive[25]. But, Miller et al[26] reported an increase of total mortality after high-dose vitamin E intake for at least one year. Since adverse effects, such as increased tendency to hemorrhage, have been observed at high vitamin E intake, the tolerable upper intake level for adults was set at 1000 mg/d α-TOH[8].
Concentrations of serum nutrients such as vitamin E are influenced by age and lifestyle factors (e.g., obesity, tobacco smoking, alcohol consumption)[27]. Age-related differences or changes in vitamin E levels in serum and tissue have been discussed in numerous studies. Campbell et al[28] showed that vitamin E decreased after the age of 80 years and argued that this finding is possibly connected with reduced food intake of elder people. In contrast, hepatic levels of vitamin E have been found to not be significantly affected by age[29]. Other studies have reported enhanced serum concentrations of vitamin E in the elderly from 60 years[27,30,31], which may be attributed to the age-dependent increases in serum cholesterol/lipoprotein concentrations[30]; this may be protective against increased lipid peroxidation during aging[29,32]. Another explanation was given by Succari et al[31], who suggested that life-style and age-associated changes independent of serum cholesterol/lipoprotein concentrations are responsible for the increased vitamin E levels in aged French women.
Numerous clinical trials have demonstrated a gender-independent inverse relationship between human obesity and serum α-TOH concentrations[33,34]. However, clear correlations between serum α-TOH concentrations and general obesity [defined by body mass index (BMI) or body fat percentage] have not been shown, but other parameters for obesity, such as waist circumference and waist-to-hip ratio, were positively associated with α-TOH serum concentrations in both men and women[35-37]. Indeed, Wallström et al[35] reported from the Malmö Diet and Cancer Study cohort that serum levels of α-TOH were associated with central adiposity after adjustment for body fat. In contrast, Gunanti et al[34] found inverse associations between BMI and serum α-TOH concentrations adjusted for total cholesterol, i.e., the α-TOH:total cholesterol ratio in Mexican-American children of the United States NHANES study. In addition to the association between BMI and α-TOH, BMI has also been positively associated with serum γ-TOH concentrations[32].
It has been observed in several studies that smoking affects serum levels of antioxidants, such as vitamin E. For example, Al-Azemi et al[33] and Shah et al[38] observed that smokers had lower serum concentrations of α-TOH compared to non-smokers. These findings were supported by Miwa et al[39] and Galan et al[27] analyzing the female participants of a Japanese study and the cohort of the SU.VI.MAX study, respectively. It is suggested that the dietary patterns of smokers differ from that of non-smokers ultimately leading to differences in vitamin E intake. Interestingly, others have not found differences in vitamin E plasma concentrations between smokers and non-smokers[40,41]. Whereas Mezzetti et al[42] observed no differences in plasma vitamin E concentrations between the two groups, vitamin E arterial tissue content was significantly lower in the group of smokers compared to that of non-smokers. This observation may indicate that vitamin E acts as a lipid-soluble antioxidant for the prevention of oxidative damage in the arterial wall. Increased serum concentrations of γ-TOH have also been found both in active and passive smokers by Dietrich et al[41]. This finding suggests that excretion of non-α-TOH forms of vitamin E may be decreased to mobilize additional anti-oxidative agents, such as γ-TOH, to increase the body’s anti-oxidative capacity. Chronic alcohol consumption also leads to decreased serum levels of α-TOH, partly due to malnutrition[43]. Animal studies have suggested that chronic administration of alcohol results in significantly lower hepatic α-TOH concentrations, probably due to decreased amounts of α-TOH in the mitochondria of hepatocytes[43,44].
When vitamin E is consumed, intestinal absorption is an important factor that limits vitamin E bioavailability. It is known that vitamin E, as a fat-soluble vitamin, follows the intestinal absorption, hepatic metabolism and cellular uptake processes of other lipophilic molecules and lipids[45]. Therefore, intestinal absorption of vitamin E requires the presence of other lipid-rich foods.
In the gastro-intestinal system the absorption rate of vitamin E varies interindividually between 20%-80%[43,45], and is lower than for other fat-soluble vitamins, such as vitamin A[46]. Reasons for individual differences in the absorption rate are diverse. Increased administration of α-TOH with parallel intake of additional food ingredients can decrease the absorption of α-TOH and non-α-TOH forms of vitamin E[47]. For example, retinoic acid[48], plant sterols[49], eicosapentaenoic acid[43], chronic alcohol consumption[43], and dietary fiber[50] are natural food components known to compete with the absorption of vitamin E. How vitamin E is presented to the intestinal surface is crucial for its absorption[19]. In addition, the supplied form of α-TOH, either as a free molecule or, for example, as α-TOH acetate, a common food additive, is of particular importance for its bioavailability[51].
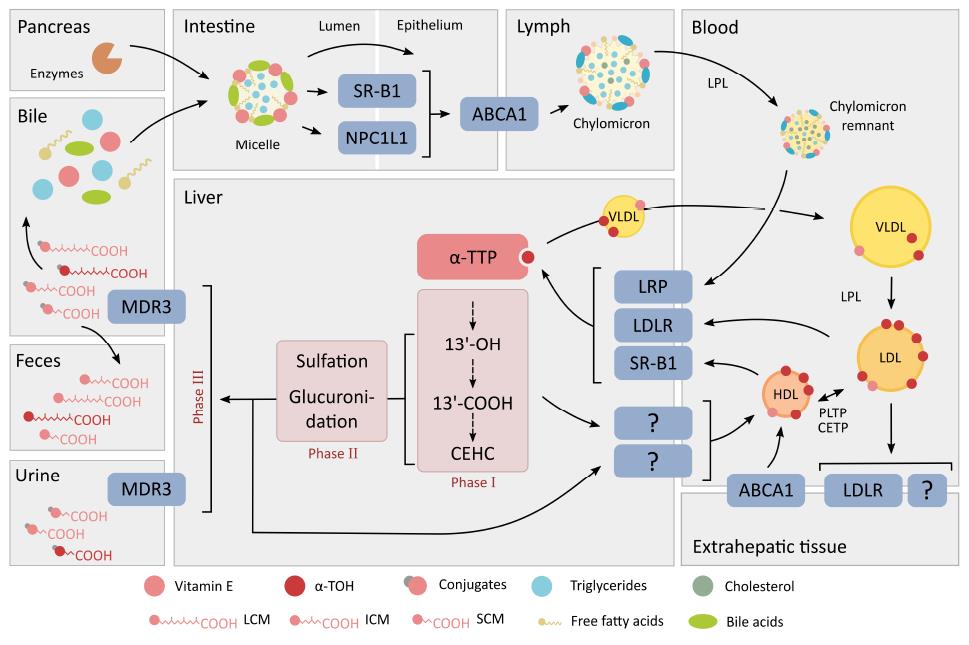
First, triacylglycerols and esterified fat-soluble compounds are partly processed enzymatically in the stomach by gastric lipase[46]. Digestive enzymes including pancreatic lipase, carboxyl esterase and phospholipase A, secreted into the intestinal lumen, continue the digestion of dietary lipids[52]. As vitamin E in the human diet is mostly not esterified, the importance of the lipolytic degradation in the digestion system is likely limited[46], but the need for gastric hydrolysis of the stabilized forms of vitamin E, such as α-TOH acetate, has not been studied in detail so far[46]. The more stable α-TOH acetate, however, requires further hydrolysis by the bile acid-dependent lipase in the pancreas or an intestinal mucosal esterase[43]. Subsequent absorption of vitamin E in the duodenum is characterized by the transfer from emulsion fat globules to water-soluble multi- and unilamellar vesicles and mixed micelles comprised of phospholipids and bile acids. This is the fundamental step in the gastric digestion and uptake of lipids, and also a crucial phase in the absorption of vitamin E (Figure 2). Since the uptake of vitamin E into enterocytes is less efficient compared to other types of lipids, this may explain the relatively low bioavailability of vitamin E[46]. As mentioned by Desmarchelier et al[53], α-TOH acetate is embedded in matrices where its hydrolysis and its uptake by intestinal cells are markedly less efficient than in mixed micelles. The intestinal cellular uptake of vitamin E from mixed micelles follows in principle two different pathways across enterocytes, as has been shown in vitro and in vivo: (1) passive diffusion; and (2) receptor-mediated transport. Receptors facilitating α-TOH transport across the enterocyte membrane, similar to the intestinal uptake of cholesterol[54], are the class B type 1 scavenger receptor class B type 1 (SR-B1)[55] and the Niemann-Pick C1-like protein 1 (NPC1L1), an apical membrane receptor of the small intestine[56]. The ATP-binding cassette (ABC) transporters ABCG5/ABCG8, at the luminal site, and ABCA1, at the apical site, are responsible for steroid efflux into the intestinal lumen and transport into the lymph system, respectively[57,58]. It has been shown in rats that vitamin E up-regulates these transporters, highlighting the contribution of vitamin E to cholesterol absorption and release[59]. Finally, ABCA1 is directly involved in the export of vitamin E from cells[60]. Presently, an increased intestinal absorption of RRR-α-TOH as compared to other stereoisomers[61], or other vitamin E forms such as γ-TOH[62], has not been uncovered. Thereby the efficiency in absorption, and of the integration of α-TOH or γ-TOH into chylomicrons, appears to be comparably equivalent.
No specific plasma transport protein for α-TOH has yet been described[43,63]. The transport of vitamin E in blood circulation follows largely that of cholesterol within lipoprotein metabolism[64]. Lipoproteins serve as carriers for lipophilic molecules, such as cholesterol, triglycerides and vitamin E, to distribute them between the liver and different organs and tissues[43]. In principle, the transport of vitamin E is independent of the type of stereoisomer[65,66]. Under normal physiological conditions, α-TOH is mostly transported via chylomicrons, very low density lipoproteins (VLDL) and high density lipoproteins (HDL), whereas under fasting conditions low density lipoproteins (LDL) take on this task[67]. The distribution ratio of α-TOH between the different types of lipoproteins is difficult to define. In a study on fasting normolipemic volunteers, Kostner et al[68] found a ratio of 1.0:1.9:1.4 of α-TOH distribution between VLDL, LDL and HDL, respectively. In contrast, Behrens et al[69] calculated a ratio of 1.0:9.4:8.4. These variations are most likely due to individual differences in α-TOH distribution in the lipoproteins caused by differing dietary α-TOH intake, metabolic states[68], and other factors influencing bioavailability and the status of vitamin E. In addition, results obtained from human studies regarding the distribution of α-TOH to lipoproteins differ from studies performed on rodents. As shown by Bjørneboe et al[67], under non-fasting conditions in rats 51% of serum α-TOH were associated with chylomicrons and VLDL whereas 47% were found within HDL. These differences across species highlight clearly the complexity of this topic.
The concentrations of vitamin E in chylomicrons are independent of the vitamin E form or stereoisomers. Vitamin E is secreted via chylomicrons by enterocytes into the intestinal lymph system to begin systemic circulation. The triglycerides in chylomicrons are subjected to hydrolysis by lipoprotein lipase (LPL)[56,70]. During lipolysis, vitamin E remains in the lipoprotein particle and its further transport through circulation occurs via chylomicron remnants. Vitamin E is then imported into the liver via LDL receptor (LDLR)-related proteins - and LDLR-mediated uptake of chylomicron remnants[60,71].
In the liver, vitamin E undergoes several sorting steps [see section “α-TOH transfer protein (α-TTP)” in the following chapter “intracellular binding proteins”] or metabolic processes (see section “metabolism of vitamin E”). α-Tocopherol is the form of vitamin E that is almost exclusively secreted into circulation via VLDL. Whereas VLDL have the highest capacity to carry α-TOH, these particles represent the smallest fraction of lipoproteins involved in vitamin E transport in the circulation in the fasting state[67]. Unfortunately, the mechanisms by which VLDL are enriched with α-TOH are poorly understood[72] (for further details, see the section on α-TTP in “intracellular binding proteins”). Alternatively, analogous mechanisms for α-TOH and free cholesterol have been discussed to explain the intracellular incorporation of α-TOH into nascent VLDL[72]. In support of this hypothesis, Bjornson et al[73] found that α-TOH and unesterified cholesterol translocate spontaneously from cellular membranes to lipoproteins. Further, ABCA1 has been shown to enrich HDL with α-TOH, which can be then transfered spontaneously or via the plasma phospholipid transfer protein (PLTP) to VLDL[68,74,75]. A similar mechanism has been suggested for the transfer of α-TOH to HDL via ABCA1[75].
Similar to chylomicrons, triglycerides in VLDL particles are enzymatically hydrolyzed by LPL resulting in the stepwise formation of LDL. Kono et al[76] suggested recently that LDL particles carry the major portion of plasma α-TOH and that LDLR-mediated endocytosis contributes significantly to the uptake of α-TOH into cells[77]. However, studies performed on apolipoprotein B (Apob)-knockout mice and heritable hyperlipidemic Watanabe rabbits lacking the LDLR showed discrepancies in circulating α-TOH levels and tissue distribution, thus questioning the importance of LDL for α-TOH transport[78,79]. Cohn et al[79] therefore concluded that α-TOH in LDL can be taken up by tissues via LDLR but is also independent of this lipoprotein receptor. Uptake transporters and specific intracellular transport proteins involved in α-TOH trafficking are handled in more detail in a following section.
High-density lipoproteins provides the means for α-TOH to be secreted out of the extrahepatic tissues and enter into circulation to be transported back to the liver. High-density lipoprotein particles contain the lowest concentration of vitamin E per particle but HDL is the most potent donor of vitamin E to several target tissues[64,74,80], despite the larger amounts of LDL in plasma. One possible explanation for this observation was given by Clevidence et al[81], who speculated that HDL contain more components for binding α-TOH besides serum ApoAI[69]. The in vivo importance of HDL in providing α-TOH to the central nervous system was highlighted by Goti et al[80]. In agreement with this finding, Kolleck et al[64] found that HDL might be the primary source of vitamin E for type II pneumocytes. Furthermore, it has been suggested that the supply of vitamin E by HDL could be more important under pathophysiological conditions, such as oxidative stress, most likely independent from the HDL-mediated uptake of cholesterol[64]. The HDL-interacting scavenger receptor SR-B1 controls the uptake and accumulation of α-TOH in specific tissues[82]. In vitro experiments using type II pneumocytes identified SR-B1 as a potential α-TOH uptake promoter, whereas the role of SR-B2 in this process is still speculative[64].
Vitamin E or rather α-TOH has been shown to move actively between lipoproteins of different density classes[74]. As previously mentioned, triglycerides in lipoproteins are catabolized via LPL[56,70]. During this lipolysis step, vitamin E remains incorporated either in chylomicron remnants (in the case of chylomicrons) or in LDL (in the case of VLDL). PLTP mediates the transfers of α-TOH between different classes of lipoproteins and also the exchange of α-TOH between HDL and cell membranes[68]. Another member of the group of lipid transfer proteins, the cholesteryl ester transfer protein, has also been suggested as having a role in vitamin E transport and metabolism[60] (Figure 2).
As already outlined, Ulatowski and Manor[16] categorized metabolic deficiencies of vitamin E into primary and secondary deficiencies, where the latter includes disorders of lipid absorption and transport as well as impaired lipoprotein metabolism, such as cholestatic liver disease, short bowl syndrome, Crohn’s disease, abetalipoproteinemia and Niemann-Pick disease type C as well as Tangier disease. Depending on the disease, the severity of vitamin E deficiency varies[83]. The causes for fat malabsorption are diverse and usually result in vitamin E deficiency presenting already in early childhood[19]; these include, inter alia cholestatic liver diseases, cystic fibrosis[84], Crohn’s disease, thrombosis, intestinal pseudoobstruction and chronic pancreatitis. The Marinesco-Sjögren syndrome and chylomicron retention disease, also known as Anderson’s disease, are further causes of severe vitamin E deficiency. The symptoms of Anderson’s disease are much milder compared to that of the Marinesco-Sjögren syndrome[85,86]. However, both diseases are caused by the inability of enterocytes to assemble or deliver chylomicrons leading to disturbed intestinal fat transport. Abetalipoproteinemia is caused by mutations in the microsomal triglyceride transfer protein that result in impaired intestinal absorption of lipids and severe vitamin E deficiency. In addition, the transport of vitamin E via VLDL to extrahepatic tissues is disturbed. Reduced levels of vitamin E are also found in patients with homozygous hypobetalipoproteinemia[83], which is caused by mutations in the APOB gene.
Finding a cure for vitamin E deficiency has remained elusive. Administration of α-TOH in micellar form in moderate doses[43] and supplementation of vitamin E are recommended but should be performed without any interfering components, such as some food ingredients. Intramuscular injection or oral uptake as water-soluble vitamin E ester has also been considered[19].
As outlined above the vascular transport of vitamin E is performed by lipoproteins. However, in body fluids with low lipoprotein concentrations, such as follicular fluids, an alternative carrier protein for vitamin E, namely afamin, has been described[87-89]. Afamin is a liver-derived plasma glycoprotein and a member of the albumin protein family[90-92]; therefore it is also called α-albumin[93]. Afamin is partly (13%) bound to plasma lipoproteins[88], but circulates mostly in free form. Since afamin has 18 predicted binding sites for vitamin E and shows binding affinity for both α-TOH and γ-TOH, it has been suggested to be an alternative vitamin E transporter in body fluids under conditions where the lipoprotein system is not sufficient for vitamin E transport[89]. Originally, vitamin E was discovered as a resorption-gestations factor in female rats[2,94]. Afamin was therefore suggested as playing a role in female fertility[92], and indeed it has been shown to bind vitamin E and to increase in maternal serum during pregnancy[92]. Yet the precise role of afamin in pregnancy or for infertility is still not known[95]. On the one hand, afamin increases during pregnancy and decreases after childbirth to baseline levels, but on the other hand, a pilot study of Hubalek et al[92] showed that women suffering from pregnancy complications had significantly higher median afamin concentrations than women with uncomplicated pregnancy. In addition to being found in follicular fluids, afamin has also been detected in other extravascular fluids, such as cerebrospinal fluids, although the concentration is tenfold lower than in follicular fluids[88]. Since vitamin E deficiency is also known to cause cerebral complications[96], the detection of afamin in cerebrospinal fluids may indicate a role of afamin in neuroprotection[88]. Supporting the suggestion that afamin plays a potential role in fertility and neuroprotection, Jerkovic et al[88] found significant correlations of afamin and vitamin E concentrations in follicular and cerebrospinal fluids.
In contrast to the lipoprotein-mediated transport of vitamin E in the vascular system, cellular vitamin E is specifically bound to intracellular transport proteins, such as α-TTP in the liver where this protein is highly expressed[97]. As α-TTP is also abundantly expressed in the placenta, the importance of α-TOH in preventing fetus resorption is evident[98]. Furthermore, α-TTP is also expressed in several other tissues[76], such as rat brain, spleen, lung and kidney[99], the pregnant mouse uterus[100], retina[101] and central nervous system[21], suggesting an ubiquitous role for α-TTP in intra-organ trafficking[102].
Hosomi et al[103] estimated the relative affinities of α-TTP to the different vitamin E forms and stereoisomers starting from RRR-α-TOH set to 100%: β-TOH (38%), α-T3 (12%), SRR-α-TOH (11%), γ-TOH and trolox (9%) followed by δ-TOH, α-TOH acetate and α-TOH quinone with 2%. Requirements for the binding of vitamin E forms and derivatives to α-TTP are: Three methyl groups at the chromanol ring system (especially at position C5), one free hydroxyl group and the phythyl side-chain[103]. As α-TOH fulfills all of these criteria best within the group of vitamin E forms, it binds efficiently to α-TTP into a deep cavity lined with hydrophobic residues[16], while α-TTP does not readily bind[104] to or transfer γ-TOH[103]. Since the affinity of different vitamin E forms to α-TTP reflects the biological activity from rat resorption-gestations assays[94], the hypothesis is supported that α-TTP is responsible for the discrimination of α-TOH. However, a current study from Grebenstein et al[105] raises the suggestion that the metabolism of vitamin E, but not α-TTP, is responsible for discrimination against mainly non-α-TOH forms, as α-TTP protects the side-chain of the different vitamin E forms from ω-hydroxylase-induced degradation. High levels of expression of α-TTP were found to lead to higher intracellular concentrations of γ-TOH in combination with a reduced production of γ-CEHC, which confirms the concept that binding to α-TTP protects from metabolic degradation[105].
While α-TTP binds both TOHs and T3s, the binding affinity is modulated by the presence of α-TOH, leading to decreased binding of non-α-TOH forms and T3s[106] and, in turn, to improved metabolism of the non-α-TOH forms. Several studies support the observation that α-TOH supplementation depletes plasma and tissue γ-TOH because of the enhanced metabolism of non-α-TOH forms[107,108].
The main function of α-TTP is to maintain normal α-TOH concentrations in the plasma and extrahepatic tissues[72]. This function is ensured by facilitating the transport of α-TOH from the lysosomes to the plasma membranes[109], followed by the continuous export of α-TOH from the liver to the plasma[110]. It is assumed that α-TTP is required for the incorporation of α-TOH into VLDL particles, but the underlying mechanisms are still not understood[72]. Traber et al[72] have systematically reviewed the enrichment of VLDL with α-TOH in the ribosomal endoplasmic reticulum, the Golgi apparatus, and the plasma membrane. It is suggested that α-TTP transfers α-TOH into nascent VLDL from the endosome, multi-vesicular bodies and lysosome[72]. Further, Kono and Arai suggested that α-TTP translocates from the cytosol to late endosomes/lysosomes to acquire α-TOH, which has been taken up by endocytosis or released from lipoproteins[76]. The outer membrane of the endocytic vesicles is enriched with both RRR-α-TOH and SRR-α-TOH by ABCA1, followed by the selective uptake of RRR-α-TOH viaα-TTP[72]. Non-α-TOH forms are not protected against ω-hydroxylation after endocytosis and are further transported to the endoplasmic reticulum and the late endosomal compartment[105]. The α-TTP/α-TOH complex moves to the plasma membrane where it is targeted by phosphatidylinositol bisphosphates (PIP2), which are essential interaction partners of α-TTP[76]. In this case, α-TTP has been shown to transfer α-TOH between membranes through direct protein-membrane interactions[111-113]. In brief, experiments with liposomes have revealed that α-TTP acts as an α-TOH/PIP2 exchanger[76]. A flippase has been suggested to be involved in the transfer of α-TOH to the outer leaflet of the plasma membrane[114]. Next is the spontaneous transfer of α-TOH to nascent VLDL particles in the perisinusoidal space[72]. Interestingly, the effectiveness of the enrichment of nascent VLDL and pre-VLDL with the different stereoisomers RRR-α-TOH and SRR-α-TOH is similar, although α-TTP is known to be more specific for RRR-α-TOH[72]. Hence, the enrichment of VLDL seems to be more complex. However, the Golgi apparatus is probably not involved in this process. As shown by Arita et al[115], suppression of the endoplasmic reticulum/Golgi secretory pathway using brefeldin A did not affect the release of α-TOH. These findings suggest that α-TOH can be secreted by the liver independently from VLDL, although post-secretory nascent VLDL has not yet been eliminated as a physiological α-TOH acceptor[72].
Familial isolated vitamin E deficiency, which is also known as ataxia with isolated vitamin E deficiency (AVED), is categorized as a primary vitamin E deficiency. Symptoms of AVED are ataxia, dysarthria, reduced or absent tendon jerks, and impaired vibration sense. AVED is an autosomal recessive disorder caused by mutations in the TTPA gene[76], which result in the inadequate distribution of vitamin E to peripheral tissues[116,117]. Many of the 20 different mutations known to underlie AVED encode for truncated and therefore defective α-TTP proteins[76]. While absorption and vascular transport of vitamin E is normal in these patients, the release of α-TOH from the liver into circulation is disturbed[118]. Due to the dysfunctional α-TTP, AVED patients show impaired selectivity between α- and γ-TOH[119] and no differentiation between RRR- and SRR-α-TOH[72]. As a consequence of the lack of α-TTP, lower α-TOH plasma concentrations are found in AVED patients, likely due to increased rates of metabolic degradation of α-TOH, measureable as significantly increased urinary excretion of α-CEHC, the end-product of vitamin E catabolism, compared to healthy individuals[116]. It was therefore concluded that the capacity of α-TTP rather than the plasma concentration of α-TOH regulates the rate of vitamin E degradation[120].
Kono et al[76] found in mice with different genetic α-TTP backgrounds, with respect to the Ttpa gene encoding α-TTP, such as Ttpa+/+, Ttpa+/- and Ttpa-/-, that α-TOH plasma levels correlate with the number of functional Ttpa alleles, which supports the aforementioned conclusion. Similarly to human AVED patients, Ttpa knockout mice are “characterized by vitamin E deficiency, oxidative stress, late-onset ataxia, and female infertility, all of which can be prevented by timely supplementation with α-tocopherol”[16]; the Ttpa knockout mouse seems to be therefore a suitable animal model to study the role of α-TTP in vitamin E homeostasis.
In addition to α-TTP, further intracellular α-TOH binding and transport proteins are known, namely the tocopherol-associated protein (TAP) and the tocopherol-binding protein. In humans, three highly homologous TAP proteins, namely TAP1/SEC14-like 2 protein (SEC14L2; synonymously, supernatant protein factor, SPF), TAP2/SEC14L3 and TAP3/SEC14L4, have been described[121,122], but the contribution of TAP2 and TAP3 to the transport and metabolism of vitamin E has not yet been investigated. TAP 1 and α-TTP belong to a family of ligand-binding proteins that have a cis-retinal binding motif sequence, the so-called CRAL-TRIO domain. All members of this protein family bind α-TOH, but to a lesser extent than α-TTP, and the physiological relevance is unknown[19]. It has been suggested that TAP1 is involved in intracellular trafficking of α-TOH[56,123]; TAP1 mediates anti-proliferative effects in LNCaP and DU-145 prostate cancer cell lines by promoting vitamin E uptake but also by effects independent of α-TOH[1,124]. TAP 1 is involved in cholesterol synthesis and forms complexes with RRR-α-TOH quinone, the oxidation product of α-TOH; however, for a better understanding of the function of TAP1, further studies are needed[19,125].
In addition to the binding and transport proteins with affinity and specificity for α-TOH, the cytosolic protein saposin B has a specific binding site for γ-TOH[126]. Saposin B binds γ-TOH more effectively than α-TOH when these vitamin E forms are competing for binding to saposin B in vitro[126]. However, in vivo the concentration of α-TOH is tenfold higher than of γ-TOH, leading to widely differing conditions which characterize the in vitro binding affinity studies in which a ratio of γ-TOH to α-TOH of 1:5 was used.
Many decades ago it was thought that the metabolism of vitamin E was initiated by a radical attack on the chroman structure resulting in a ring opening and the building thereby of TOH quinone[127]. Subsequent side-chain degradation would lead to α-tocopheronic acid and its lactone, α-tocopheronelactone (α-TL), the so-called Simon metabolites described in conjugated and non-conjugated forms in urine of mice and humans[120,128-130]. This pathway has since been questioned because: (1) urinary TOH metabolites with an intact chroman ring system, the carboxyethylhydroxychromanols (CEHC), have been discovered[131-133]; and (2) an almost complete conversion of α-CEHC to α-TL is possible at least in vitro by bubbling oxygen through a solution of 70 mmol/L α-CEHC in 0.1 mol/L HCl for 24 h at room temperature[132,134]. In contrast, a recent study by Sharma et al[135] provided evidence that conjugates of α-TL are indeed real metabolites and not methodological artefacts. In addition, the study characterized α-TL as a biomarker of oxidative stress in children with type 1 diabetes, as the mean concentrations of the glucuronides and sulfate conjugates of α-TL were all significantly increased in these children[135]. Further research is required to answer the question of whether α-TL is a definite marker or an analytical artefact.
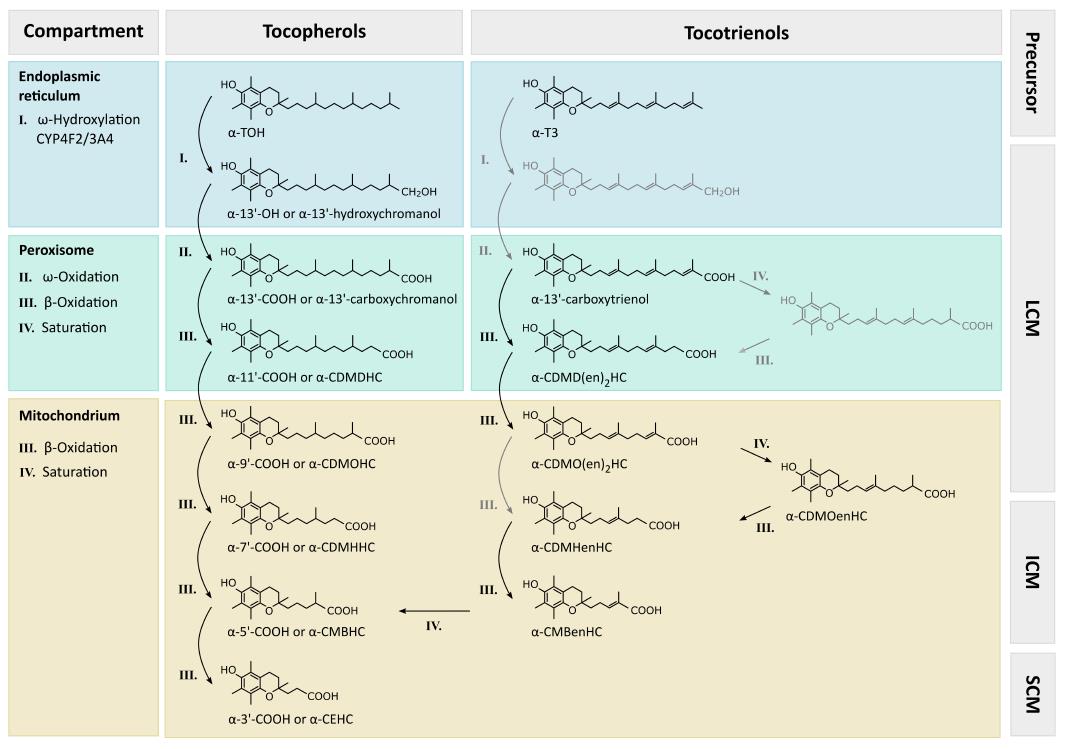
Degradation processes in the hepatic metabolism of vitamin E remain poorly understood. Initial mechanisms are generally accepted, i.e., all vitamers are degraded to vitamer-specific physiological metabolites with an intact chromanol ring (therefore the nomenclature as α-, β-, γ- and δ-metabolites is used), leading to changes in the side-chain. The metabolites have been found in different tissues and body fluids conjugated similarly to xenobiotics and in non-conjugated form. For a detailed overview, see Figure 3.
Hepatic metabolism of TOH is initiated by CYP4F2/CYP3A4 (for details, see the sections on CYP3A4 and CYP4F2 in chapter “metabolism of vitamin E”) dependent ω-hydroxylation of the aliphatic side-chain, forming 13’-hydroxychromanol (13’-OH), which can be analyzed using GC-MS (γ-, δ-13’-OH[139,140]). Next, ω-oxidation leads to 13’-carboxychromanol (13’-COOH), which can be detected via LC-ESI-MS (γ-, δ-13’-COOH[139,136]), HPLC-FD or HPLC-ECD (γ-, δ-13’-COOH[136,141]) or GC-MS (γ-13’-COOH[140]). An overview of all metabolites of TOH, analytical methods and identified matrices is provided in Table 1. Subsequent β-oxidation steps shorten the side-chain, thus forming carboxydimethyldecylhydroxychromanol (CDMDHC, 11’-COOH) followed by carboxymethyloctylhydroxychromanol (CDMOHC, 9’-COOH), both of which can be analyzed by LC-ESI-MS (γ-, δ-11’-COOH and γ-, δ-9’-COOH[139,136,141]), HPLC-FD or HPLC-ECD (γ-, δ-11’-COOH and γ-, δ-9’-COOH[136,141]) or GC-MS (γ-11’-COOH and γ-9’-COOH[140]). These metabolites with a side-chain length of between 13 to 9 carbon units can be summarized as long-chain metabolites (LCM) of vitamin E. According to their hydrophobicity, the LCMs are not excreted into urine and have been found in human and rat liver microsomes (α-, γ-13’-OH, and α-, γ-13’-COOH[139,140]), murine serum (γ-, δ-11’-COOH[136]), as well as human serum (α-13’-COOH[142]), in human and mice feces (α-13’-COOH; γ-, δ-11’-COOH; γ-, δ-9’-COOH[136]), and in vitro in cell culture supernatants of human lung epithelial A549 cells (γ-, δ-13’-OH and γ-, δ-13’-COOH[139]), as well as in HepG2 cells (γ-, δ-13’-OH; γ-, δ-13’-COOH; γ-11’-COOH; γ-9’-COOH[140,143]).
| Metabolite | Methods | Matrix |
| 13’-Hydroxychromanol | GC-MS[139,140] | Human and rat liver microsomes[139,140] |
| (13’-OH) | Cell culture medium of A549 cells[139] | |
| HepG2 cells[140,143] | ||
| 13‘-Carboxychromanol | LC-ESI-MS[136,139] | Human liver microsomes, serum, feces[136,140,142] |
| (13’-COOH) | HPLC-FD or HPLC-ECD[136,141] | Rat liver microsomes[139,140] |
| GC-MS[140] | Mouse feces[136] | |
| Cell culture medium of A549 cells[139] | ||
| HepG2 cells[140,143] | ||
| Carboxydimethyldecyl-hydroxychroman | LC-ESI-MS[136,139,141] | Human feces[136] |
| (CDMDHC) | HPLC-FD or HPLC-ECD[136,141] | Mouse serum and feces[136] |
| (11’-COOH) | GC-MS[140] | HepG2 cells[140,143] |
| Carboxymethyloctyl-hydroxychroman | LC-ESI-MS[136,139,141] | Human and mouse feces[136] |
| (CDMOHC) | HPLC-FD or HPLC-ECD[136,141] | HepG2 cells[140,143] |
| (9’-COOH) | GC-MS[140] | |
| Carboxymethylhexyl-hydroxychroman | GC-MS[134,137,140,144] | Human and mouse feces[136] |
| (CDMHHC) | HPLC-ECD[136,137] | HepG2 cells[120,140,144] |
| (7’-COOH) | LC-ESI-MS[136] | |
| Carboxymethylbutyl-hydroxychroman | GC-MS[134,137,140,144] | Human serum or plasma, urine and feces[116,134,136,144] |
| (CMBHC) | HPLC-ECD[136,137] | Mouse liver, serum, urine and feces[136] |
| (5’-COOH) | LC-ESI-MS[136] | HepG2 cells[120,140,144] |
| Carboxyethyl-hydroxychroman | LC-ESI-MS[136,139,141] | Human plasma or serum, urine, feces[134-136,140,144,150-152,155,156] |
| (CEHC) | HPLC-ECD[136,137,149-152] | Rat liver, plasma, bile and urine[141,149,154] |
| (3’-COOH) | GC-MS[134,137,144,153] | Mouse serum or plasma, urine, feces and liver[134-136,144,151,155,156] |
| A549 and HepG2 cells[137,140,141,144] |
Intermediate-chain metabolites (ICM) are the products of two further β-oxidation steps. Carboxymethylhexylhydroxychromanol (CDMHHC, 7’-COOH) and carboxymethylbutylhydroxychromanol (CMBHC, 5’-COOH) have been detected via GC-MS (γ-7’-COOH and α-, γ-5’-COOH[134,140,144,137]), HPLC-ECD (γ-, δ-7’-COOH and α-, γ-, δ-5’-COOH[136,137]), and LC-ESI-MS (γ-, δ-7’-COOH and α-, γ-, δ-5’-COOH[136]) in murine liver (γ-, δ-5’-COOH[136]), in murine serum (γ-, δ-5’-COOH[136]), as well as in human serum or plasma (α-, γ-5’-COOH[136,116]), in murine and human feces (γ-, δ-7’-COOH; α-, γ-, δ-5’-COOH[136]), and in murine urine (γ-, δ-5’-COOH[136]), as well as in human urine (α-, γ-, δ-5’-COOH[134,136,144]). The ICMs have also been found in cultured HepG2 cells (α-, γ-7’-COOH and α-, γ-5’-COOH[120,140,144]).
The catabolic end-products of vitamin E metabolism are the CEHC (CEHC, sometimes also referred to 3’-COOH or short-chain metabolites, SCM), which were an early focus of research on vitamin E metabolism[145,146]. In the 1980s and 1990s different CEHCs were identified as the first known metabolites of vitamin E degradation: α-CEHC[132,146], γ-CEHC[147] and δ-CEHC[131]. Shortly after the discovery of the SCMs, it was shown that not only γ-TOH metabolism but also the degradation of γ-T3 results in γ-CEHC[148]. Meanwhile, this has been confirmed for all T3s and their corresponding CEHCs[136]. Many different analytical procedures and detection methods for the SCMs have been described: LC-ESI-MS (α-, γ-, δ-CEHC[139,136,141]), HPLC-ECD (α-, γ-CEHC[136,137,149-152]), and GC-MS (α-, γ-, δ-CEHC[134,144,137,153]). Usually, trolox or 1-naphthol are used as internal standards for the analysis of SCMs[150,151,154]. The CEHCs have been analyzed in rat and murine liver (α-, γ-, δ-CEHC[136,154]), in rat plasma and in mice serum (γ-, δ-CEHC[136,141]), as well as in human plasma and serum (α-, γ-, δ-CEHC[140,136,150,152,155]), in human and mouse urine (α-, γ-, δ-CEHC[134-136,144,151,155,156]) and in human and mouse feces (α-, γ-, δ-CEHC[136]) as well as in rat bile (α-, γ-CEHC[149]) and in vitro in A549 and HepG2 cells (γ-CEHC[140,141,144,137]).
Recently, two new metabolites, namely 12’-hydroxychromanol (12’-OH: γ- and δ-12’-OH) and 11’-hydroxychromanol (11’-OH: γ- and δ-11’-OH) have been identified using GC-MS in fecal pellets of mice fed a diet rich in γ-TOH[157]. These products provide evidence for ω-1 and ω-2 hydroxylation activity and that 12’-OH is not able to undergo oxidation followed by side-chain truncation. These metabolites are therefore excreted via bile and are found in the feces of mice and humans[157].
The metabolism of T3s was first analyzed by Birringer et al[137] in HepG2 cells; it is in principal comparable to the metabolism of TOHs starting with an ω-hydroxylation followed by five cycles of β-oxidation and resulting in the end-product CEHC. Meanwhile almost all expected metabolites have been found in mouse and human samples after supplementation with their respective metabolic precursors, TOHs and T3s[136]. The corresponding metabolites were found: Carboxytrienol (13’), carboxydimethyldecadienylhydroxychromanol [CDMD(en)2HC; 11’], carbodimethyloctenylhydroxychromanol (CDMOenHC; 9’), as well as carboxydimethyloctadienylhydroxychromanols [CDMO(en)2HC; 9’], carboxymethylhexenylhydroxychromanol (CMHenHC; 7’), and carboxymethylbutadienylhydroxychromanol (CMBenHC; 5’). This leads to the conclusion that the side-chain of T3s is saturated before shortening. We speculate that auxiliary enzymes also needed for the degradation of unsaturated fatty acids, e.g., 2,4-dienoyl-CoA reductase and 3,2-enoyl-CoA isomerase, are therefore required for the saturation of the double bonds in the side chain of T3 metabolites, as was originally suggested by Birringer et al[137].
Mustacich et al[138] were the first to identify the intracellular compartmentation of vitamin E metabolism. Based on their work, the ω-hydroxylation catalyzed by alcohol dehydrogenase (resulting in 13’-OH) and the following reaction catalyzed by aldehyde dehydrogenase (forming 13’-COOH), take place at the endoplasmic reticulum. The carboxylated side-chain of TOHs are similar in structure to 2-methyl branched-chain fatty acids, which are subsequently β-oxidized in peroxisomes by their activation to an acyl-CoA ester[138]. This pattern seems to also characterize degradation of LCMs as two cycles of peroxisomal β-oxidation (resulting in 11’-COOH and 9’-COOH) with separation of propionyl-CoA or acetyl-CoA were suggested for TOHs[138]. Cho et al[156] noted that the sterol carrier protein-x (SCP-x) is involved in peroxisomal oxidation of branched-chain lipids, serves as a peroxisomal 3-ketoacyl-CoA thiolase, and shows reduced expression in response to pregnane X receptor (PXR) activation (for further details on PXR and vitamin E interactions, see the section on CYP3A4 in chapter “metabolism of vitamin E”). Based on their data the authors suggested that SCP-x is involved in the formation of vitamin E metabolites. It is not known yet whether LCMs are transported into peroxisomes as “free”molecules or as CoA esters, as it is known for long-chain, very long-chain or branched-chain fatty acids[158]. Based on knowledge of the transport of very long-chain acyl-CoA into the peroxisomes via ABCD[159], an involvement of related proteins for the import of vitamin E metabolites could be possible. As the last degradation steps are located in the mitochondria[138], the transport mechanisms out of peroxisomes and the subsequent import into mitochondria also needs to be unraveled. The import into mitochondria might occur through carnithin-acyl-transferases, as reported for metabolites of vitamin K[160]. Grebenstein et al[105] suggested that the LCMs are possible ligands for α-TTP and/or other hepatic TOH binding proteins. However, the importance of these proteins for the transfer of LCMs to mitochondria needs to be investigated further[105]. The mitochondrial production of ICMs and SCMs (three β-oxidation steps) is indicated by the analysis of CEHCs solely in mitochondria but not in peroxisomes[138]. It was emphasized by Mustacich et al[138] that these data do not exclude a model of exclusive mitochondrial β-oxidation.
As mentioned above, catabolism of TOHs and T3s begins with an ω-hydroxylation of the side-chain which is catalyzed by cytochrome P450 (CYP) enzymes, namely CYP4F2 or CYP3A4. This oxidation is the rate-limiting step in vitamin E metabolism[161]. CYP enzymes are heme-thiolate proteins that differ in substrate selectivity but catalyse monooxygenation reactions via activation of molecular oxygen[162]. In the following sections, we provide an overview of the two CYP enzymes known to be involved in vitamin E metabolism.
CYP3A4: The enzyme CYP3A4 is the most important CYP enzyme in humans, as the majority of administrated drugs are metabolized via CYP3A4 due to its wide range of substrates[162]. The first evidence for the involvement of CYP3A4 in vitamin E metabolism was provided by Parker et al[163]. Ketoconazole, an inhibitor of CYP3A4, blocked the metabolism of TOHs [α- and γ-TOH in primary rat hepatocytes or γ- and δ-TOH in HepG2/C3A (25 μmol/L each)] to their corresponding SCMs by approximately 90% after incubation with 1 mmol/L or 0.25 mmol/L for 48 h[163]. Similar effects were seen for γ-TOH when 1 mmol/L sesamin was used, a major sesame seed lignin and natural inhibitor of CYP3A4[163]. When 50 mg/kg body weight of ketoconazole were applied by oral gavage to rats simultaneously with a mixture of 10 mg/kg body weight of α-TOH, 10 mg/kg body weight of γ-TOH or 29.5 mg/kg body weight of T3 the catabolism of the vitamin E forms to their respective SCMs and excretion via urine was clearly reduced compared to the controls[164]. Using 50 μmol/L rifampicin, an inducer of CYP3A4 activity, Birringer et al[120] demonstrated an up to fivefold increase in all-rac-α-TOH degradation in HepG2 cells. It should be noted that preconditioning of the cells with 100 μmol/L α-TOH for 10 d was necessary, as the standard cell culture medium is deficient for α-TOH[120]. This might be the reason why Parker et al[163] were not able to detect α-TOH metabolism in HepG2 cells, as they did not perform a preconditioning and instead incubated the cells only for 24 h to 48 h with 0.25 μmol/L or 0.5 μmol/L α-TOH. Further evidence is given by the α-TOH-dependent regulation of CYP3A4[165]. Feeding mice with 200 mg/d of α-TOH for nine months resulted in 1.7- fold higher Cyp3a11 (i.e., the murine orthologue of human CYP3A4) mRNA expression levels compared to after three months, while γ-T3 did not increase Cyp3a11 mRNA levels[166]. Similar effects on Cyp3a protein levels were observed when 10 mg/100 g body weight of α-TOH was injected subcutaneously into rats[167]. Traber et al[165] showed that C57BL/6 mice receiving a diet sufficiently enriched with α-TOH (31 mg α-TOH per kg diet) for five weeks have increased hepatic Cyp3a levels compared to mice fed an α-TOH-deficient diet with less than < 2 mg α-TOH per kg diet.
Several CYP enzymes, including CYP3A4, are regulated by structurally diverse xenobiotics via PXR, a nuclear receptor that regulates the expression of metabolic enzymes and transporters involved in the metabolism of xenobiotics and endobiotics[156,168]. Landes et al[169] showed that vitamin E acts as an agonist of PXR. Thereby, the inductive effect of vitamin E on chloramphenicol acetyl transferase activity was dependent on the vitamin E form in the following order: γ-T3 approximately equal α-T3 > rifampicin > δ-TOH > RRR-α-TOH ≥γ-TOH. The treatment of HepG2 cells with γ-T3 led to an up-regulation of CYP3A4 and CYP3A5 mRNA levels[169] and a dose-dependent activation of chloramphenicol acetyl transferase at 1 μmol/L to 10 μmol/L, concentrations which can be reached also in human plasma[23]. These striking contrary effects of γ-T3, up-regulation of CYP3A4 expression in vitro[168] and no effect[166] or even a reduction of Cyp3a expression[165] in vivo might be explained by different availabilities of the individual vitamin E forms at the site of action. Whereas the substance in vitro is applied onto the cells directly, all physiological processes of vitamin E handling, especially α-TTP dependent sorting of non-α-TOH forms in combination with the high metabolic degradation and elimination rates for γ-T3[166], may interfere in vivo, thus resulting in contradictory effects.
However, the induction of Cyp3a11, the mouse homologue of human CYP3A4, by α-TOH and the involvement of PXR was confirmed in wild-type mice by Johnson et al[170]. In this study, mice kept vitamin E-deficient were fed daily for two weeks with 500 mg/kg DL-α-TOH acetate, which is equivalent to a typical supplementation with 400 - 600 mg/d α-TOH for a 70 kg human. Expression of Cyp3a11 was induced in wild-type mice, but remained unchanged in Pxr-null mice and in humanized PXR mice as well, while dosing with known murine and human PXR-specific agonists up-regulated expression of Cyp3a11 in both the wild-type and the humanized PXR mice, but not the Pxr-deficient mice. This led to the conclusion that α-TOH is a partial agonist of mouse Pxr and that Pxr is required for the induction of Cyp3a11 by α-TOH in mice[170].
Apart from these, there are further contradictory findings. Parker et al[171] emphasized that the hypo-thesis of involvement of CYP3A4 in the metabolism of vitamin E is only based on the assumption of ketoconazole specificity, which proved incorrect[172]. Testing recombinant human CYP3A4 in insect cell derived microsomes revealed no activity towards α- or γ-TOH[140], whereas a systematic screening of other CYP enzymes showed tocopherol-ω-hydroxylase activity only for CYP4F2[171] (for further details, see the section on CYP4F2 in chapter “metabolism of vitamin E”). In addition, Birringer et al[120] showed that production of γ-CEHC from γ-TOH was not affected by rifampicin in HepG2 cells, leading to the conclusion that either CYP3A4 may not degrade all vitamin E forms to the same extent or other CYP enzymes may be involved in γ-TOH metabolism. Furthermore, Schuetz et al[173] reported in 1993 that HepG2 cells do not express CYP3A4 but CYP3A7. With respect to PXR, more discrepancies have been reported. Cho et al[156] used Pxr-deficient vs wild-type mice both treated with and without pregnenolone 16α-carbonitrile (an activator of rodent Pxr) to analyze the impact of Pxr on vitamin E degradation. The study revealed that urinary excretion of α-CEHC glucuronide was significantly decreased down to 16% and γ-CEHC glucoside was attenuated down to 40% in pregnenolone 16α-carbonitrile-treated wild-type mice compared with control wild-type mice, while urinary excretion of both metabolites were unaffected in the Pxr-null mice. Johnson et al[170] suggested that these findings are the result of a down-regulation of β-oxidation by pregnenolone 16α-carbonitrile. To sum up, the role of CYP3A4 in the metabolism of vitamin E remains unclear.
CYP4F2: The CYP4 subfamily of CYP enzymes catalyzes the ω-hydroxylation of saturated, branched-chain fatty acids as well as unsaturated fatty acids, whereas members of the CYP4F subfamily metabolize long-chain and very long-chain fatty acids[158]. As mentioned above, Sontag and Parker[140] reported the involvement of CYP4F2 in vitamin E metabolism using reporter-gene assays combined with a systematical screening of CYP enzymes in this context. Among the CYP enzymes tested, only CYP4F2 exhibited tocopherol-ω-hydroxylase activity, which was higher for γ-TOH than for α-TOH. In a subsequent study, Sontag and Parker[108] characterized the substrate specificity of CYP4F2. According to this study, the unsubstituted carbon at position C5 of the chromanol ring system induces activity of CYP4F2, so that γ- and δ-TOH are metabolized more efficiently than α-TOH, which in turn stimulates the metabolism of other vitamin E forms. The authors found higher Vmax values for T3s than for their corresponding TOHs, suggesting that CYP4F2 contributes to the preferential physiological retention of α-TOH compared to other vitamin E forms. This finding supports the central role of this pathway in modulating the vitamin E biopotencies of TOHs and T3s[140,108]. Bardowell et al[161] identified Cyp4f14 as the murine orthologue of human CYP4F2 and analyzed vitamin E homeostasis in Cyp4f14 knock-out mice. Cyp4f14-deficient mice had higher tissue concentrations of non-α-TOH forms, such as γ-TOH in plasma and tissues and δ-TOH in fat tissue, whereas tissue and plasma levels of α-TOH remained unchanged (except for lower concentrations in heart tissue). In line with these findings, reduced elimination of γ-, δ-, and α-TOH metabolites via urine and feces was found, but increased fecal excretion of γ- and δ-TOH[161]. Due to the reduction of vitamin E metabolism instead of a complete abolishment, Bardowell et al[161] suggested the involvement of other enzymes in murine vitamin E catabolism.
While expression of CYP3A4 is regulated by α-TOH, CYP4F2 levels are not influenced by α-TOH as reported by Mustacich et al[167] in rats that were injected subcutaneously daily with 10 mg/100 g body weight α-TOH for up to 18 d. In these rats, protein levels of Cyp3a, Cyp2b, and Cyp2c were increased. Johnson et al[170] found that only the mouse orthologue of CYP4F2, Cyp4f13, but not Cyp4f14 was upregulated by α-TOH in wild-type mice, while in Pxr-null or humanized PXR mice no influence on the expression of Cyp4f13 and Cyp4f14 was observed. In Ttpa-/- and wild-type mice, Traber et al[165] revealed no influence of γ-TOH on the expression of the Cyp4f13 protein. However, a synthetic inhibitor of CYP4F2/Cyp4f13, namely (R)-2-[9-(1H-imidazol-1-yl)nonyl]-2,5,7,8-tetramethylchroman-6-ol, decreased formation of γ-CEHC from γ-TOH in HepG2 cells in culture[174]. The stable expression of CYP4F2 in vivo regardless of elevated vitamin E intake or availability, as indicated by higher concentrations of the metabolites, calls into question the suggestion that CYP4F2 alone is responsible for the initial step of vitamin E degradation. Taken together, the evidence for the involvement of CYP4F2 in vitamin E metabolism is convincing, but the participation of other CYP enzymes such as CYP3A4 cannot be excluded yet.
Conjugation of metabolites: Non-α-TOH forms of vitamin E are preferentially handled in the human body as xenobiotics involving phase I enzymes, and further degradation steps seem to also follow the track of xenobiotic metabolism, as vitamin E degradation products are found as sulfate and glucuronide conjugates. See Table 2 for an overview on the conjugates of vitamin E metabolites identified thus far.
Sulfates are thought to be the main conjugation products of the LCMs (γ- and δ-LCMs: 13’-COOH, 11’-COOH and 9’-COOH in rats and in vitro)[139,141], as an unknown peak occurring only after supplementation with γ-TOH in rats had the theoretical weight of sulfated γ-CEHC. In agreement with this finding, the peak decreased after treatment with sulfatase[139] or a combination of β-glucuronidase and sulfatase[141]. As the LCMs were found in both conjugated and non-conjugated form in cell culture medium of human A549 cells, it was suggested that conjugation and subsequent β-oxidation are parallel processes. This was confirmed in studies by Hashiguchi et al[175]. α-5’-COOH ether glucuronides[176] in urine of both humans and mice as well as α-5’-COOH sulfate in human urine[177] are known conjugates of ICMs. More is known about the conjugates of the SCMs. In humans, the majority of CEHCs are excreted via urine as glucuronides[136,147,152,155], whereas sulfates were found only in trace amounts[177] or in significant amounts[135,136,176]. In addition, other conjugates are known for α-CEHC, such as taurine, glycine or glycine glucuronide conjugates[176]. In mice, glucuronides and sulfates of α-, γ-, δ-CEHC have been found[136,156,176]. While glucoside conjugates of γ-CEHC[156] and α-CEHC glutamine[176] have been only found in mice, taurine, glycine or glycine glucuronides conjugates were found in murine urine as well as in urine of humans[176]. The glucoside conjugate of γ-CEHC was found by Cho et al[156] and appeared to be the main conjugated form of γ-CEHC in mice. A comparative experiment revealed that β-glucuronidase treatment hydrolyzed not only glucuronide conjugates but also glucose-conjugated metabolites; it has been therefore suggested that glucosides originally contained in the samples were not detectable when β-glucuronidase treatment was applied[156]. As this procedure is common in vitamin E metabolite analysis, it is possible that glucosides remained mostly undetected. Tanabe et al[178] found sulfated γ-CEHC to be the main excretion product in rats, when γ-CEHC was applied; a conjugation product with the expected weight of sulfated γ-CEHC was detected and was also sensitive to β-glucuronidase/sulfatase treatment. This finding was confirmed by others, when sulfated γ-CEHC in rat urine or plasma was found[139,141,179].
To address the ratio of conjugated to non-conjugated metabolites in different body fluids, parallel analyses with or without enzymatic hydrolysis were used. Most CEHCs in urine are conjugated, as several groups have reported. Zhao et al[136] found a six- to tenfold increase of free CEHC in mouse urine after treatment with β-glucuronidase and sulfatase. Freiser and Jiang[141] suggested that between 88% to 98% of γ-CEHC is conjugated in the plasma of rats, and Leonard et al[154] found that between 30% to 40% of the tested α- and γ-CEHCs are conjugated in the liver. Lodge et al[151] suggested analysis of the portion of non-conjugated SCMs, as γ-CEHC was shown to be a powerful natriuretic factor in human urine[133].
The conjugates of vitamin E metabolites identified so far indicate an involvement of phase II enzymes, mainly UDP glucuronosyltransferases (UGT) and sulfotransferases (SULT). UDP glucuronosyltransferase enzymes are involved in the conjugation of CEHCs with glucuronic acid or glucose[156], whereas SULT catalyze the transformation to sulfate conjugates. Interestingly, the different vitamin E forms have no effect on the expression of phase II enzymes, as in vitro none of the eight vitamin E forms showed an altered expression of UGT1A1 mRNA in human primary hepatocytes[180]. Furthermore, the majority of UGT isoforms were not regulated in mice fed α-TOH deficient or enriched diets[170], and hepatic UGT activity was not influenced by feeding rats daily with 200 mg/kg body weight α-TOH for two weeks. It is of note that expression of UGT1A1 mRNA was increased 1.7-fold after treatment of wild-type mice with the PXR agonist pregnenolone 16α-carbonitrile compared to untreated control mice[156]. As UGTs are regulated via PXR[181,182], the effect of pregnenolone 16α-carbonitrile is in line with the latter finding, while the lack of UGT activation by α-TOH was unexpected. In a comparative screening of 14 members of the SULT enzyme family, Hashiguchi et al[175] found evidence for the involvement of SULT1 enzymes in vitamin E metabolism. Sulfotransferase 1 showed a stronger preference for γ-TOH than for α-TOH and for γ-CEHC over all other CEHCs. Contradictory results were obtained in mice where expression of Sult2a mRNA was increased 10.8-fold by α-TOH compared to the control mice[183]. Mustacich et al[138] noted that the presence of sulfated metabolites is difficult to clarify with respect to the exclusive peroxisomal degradation of vitamin E and the cytosolic localization of phase II enzymes. However, when metabolites are generated in peroxisomes and mitochondria, they must be transported through the cytoplasm and can be subjected as substrates to the cytosolic SULTs[138]. Jiang et al[139] noted that sulfated metabolites of vitamin E may not only contribute to detoxification but could also perform regulatory functions. As mentioned before, in humans and mice, the concentration of glucuronides is higher than that of sulfates and a ratio of glucuronide:sulfate of approximately 8 was reported by Sharma et al[135]. The authors argued that this is in line with the fact that humans have higher capacity for glucuronidation than sulfation because of the high activity of hepatic UGT.
Another enzyme involved in the xenobiotic metabolism is glutathione S-transferase (GST). Van Haaften et al[184,185] found that TOHs and T3s inhibit isolated human GST P1-1 with IC50 values of 0.7 mmol/L for α-TOH, 0.8 mmol/L for δ-TOH, 1.8 mmol/L for α-T3 and 0.7 mmol/L for γ-T3. Contradictory findings were reported by Podszun and Frank[186] in rats fed high doses of α-TOH (2500 mg/kg diet for ten days), in which GST activity was increased 2- to 3-fold. When mice were fed with 1000 mg/kg diet of all rac-α-TOH acetate for four months expression of hepatic Gstm3 mRNA, responsible for the detoxification of electrophilic compounds, increased about twofold compared to the controls (35 mg all rac-α-TOH acetate/kg diet)[183].
Excretion: Due to their polarity, ICMs and SCMs, namely 5’-COOH and CEHCs, are excreted via urine, mostly as glucoside conjugates[136]. Feces contain the whole set of vitamin E metabolites, including precursors (TOHs and T3s) and water-soluble SCMs in humans[136] and mice[157,161,187]; LCMs (especially 13’-COOH) are the main fecal metabolites with > 60% of total metabolites[187]. Zhao et al[136] reported that the LCMs are unconjugated in feces. Because of the lack of β-glucuronidase treatment, Jiang et al[187] could not distinguish between conjugated and non-conjugated metabolites in their fecal samples, which in turn could support the results from Zhao et al[136]. Wu and Croft[188] concluded that part of the total vitamin E undergoes enterohepatic circulation (estimated to be about 60% in rats) and that the remaining is likely lost via the fecal route[189]. However, the fecal portion of metabolite excretion was estimated to be about 80%[161]. As mentioned before, intestinal absorption was reported to be between 20%-80% for α-TOH, leaving a considerable portion that remains in the intestinal tract[188]. Zhao et al[136] tested whether intestinal flora is able to degrade TOHs by incubating fecal extracts with TOHs, but this experiment failed to produce vitamin E metabolites. To summarize, SCMs are transported via blood and are excreted through urine, while TOH, T3 and all other metabolites circulate through the vascular system (shown for α-13’-COOH in humans by our group[142]) and are secreted into bile and eliminated via feces.
Thus far, no specific transport or binding proteins for CEHCs or CEHC conjugates have been reported. However, some phase III transporters are involved in the elimination of vitamin E, or their expression is regulated by vitamin E[63]. Early findings by Bjørneboe et al[190] in rats injected with radioactively labeled α-TOH revealed that 14% of the radioactivity was recovered during 24 h of bile draining, thus indicating an involvement of biliary excretion pathways. Since then, the underlying molecular mechanisms have been of particular interest. Apob seems to be involved in the secretion of α-TOH from liver cells into blood, as in Apob-knockout mice the biliary secretion of α-TOH is significantly decreased after a single i.v. injection of 25 mg/kg α-TOH compared to the controls[191]. Mardones et al[82] provided evidence that SR-B1 encoded by the Scarb1 gene contributes to biliary excretion of α-TOH, as the hepatic concentration of α-TOH is normal compared to the controls but biliary excreted α-TOH was 74%-81% lower in Scarb1-knockout mice. Expression of SR-B1 is also reported to be regulated by α-TOH. Mice kept vitamin E-deficient showed elevated hepatic SR-B1 protein levels, which were reversible by feeding them a vitamin E-enriched diet, whereas HepG2 cells cultured in the presence of vitamin E-loaded HDL showed decreased SR-B1 levels[192]. According to Takada and Suzuki[193], SR-B1 might be responsible for the import of vitamin E into hepatocytes when located at basolateral membranes and also for the export into bile when expressed at the canalicular site. Therefore, many studies have aimed to clarify the differing expression patterns of SR-B1 between sinusoidal and canalicular membranes[194]. Comparison of human and mouse liver tissues as well as HepG2 cells have revealed pronounced differences. While ex vivo SR-B1 was highly enriched in sinusoidal membranes and was also found in canalicular membranes, HepG2 cells clearly showed enrichment of SR-B1 in bile canalicular-like structures[194].
Multidrug resistance proteins (MDR) are also involved in the elimination of α-TOH, as inhibition or deletion of canalicular Mdr2 (also named Ppg or p-glycoprotein) leads to a decrease in the basal release of α-TOH into bile in rats and mice[189]. Results from in vitro studies on MDR1 did not reveal clear results. Primary human hepatocytes showed no response in the expression of MDR1 to T3 treatment, whereas intestinal LS180 cells reacted with a clear increase of MDR1 expression (depending on the type of T3)[180]. In line with the latter finding, rats daily injected subcutaneously with 10 mg/100 g body weight α-TOH had increased levels of hepatic Mdr1 protein beginning at day 9 and reaching a peak at day 15 of injections[167]. Further, mice fed with 1000 IU/d all-rac-α-TOH acetate for four months showed elevated levels of Mdr1a protein, the mouse orthologue of human MDR1, compared to the controls, while expressions of Mdr1b, Mdr2, Abcc2, Abcc6 and breast cancer resistance protein 1 (Bcrp1/Abcg2) remained unchanged[183]. When rats were daily injected subcutaneously with 100 mg/kg body weight α-TOH, the hepatic efflux transporters Abcb1b and Abcg2 were upregulated, while the organic anion transporting polypeptide 2 (Oatp), a liver influx transporter, was downregulated[195]. Traber et al[195] noted that Abcg2 transports sulfates and glucuronides and suggested that this protein is involved in the excretion of conjugated vitamin E metabolites, such as CEHCs, while Oatp might be involved in the uptake of CEHCs into liver cells. Summarizing the current knowledge on excretion of vitamin E metabolites, it has to be emphasized that many aspects regarding the involvement of transporters in vitamin E and vitamin E metabolites still lack clarity. It remains to be resolved whether and which specialized proteins for the regulated excretion of vitamin E metabolites exist.
Kinetics of vitamin E degradation: All vitamin E forms are in principle degraded via the same pathways independent from their substitution pattern with methyl groups at the chromanol ring system. However, the rate of degradation depends on the methylation pattern of the chromanol ring, the saturation of the side-chain, and on the source of vitamin E (i.e., natural vs synthetic).
Natural forms of vitamin E, i.e., enantiopure vitamin E forms with the RRR configuration, are partially protected from degradation compared to synthetic forms, i.e., vitamin E forms with the all-rac configuration, as was shown by Traber et al[196], who supplemented humans with 150 mg d3-RRR-α- or d6-all rac-α-TOH acetates. While d3-RRR-α-TOH accumulated in plasma, d6-α-CEHC derived from d6-all rac-α-TOH was found almost only in urine. Vitamin E forms with unsaturated side-chains are degraded faster than the saturated forms. T3 were reported to appear in human plasma with half-lives of 4.3, 4.4, and 2.3 h for α-, γ- and δ-T3, respectively[23], whereas for RRR-α-TOH a half-life of 45 h[110] to 60 h[197] was found. When humans were supplemented with a single dose of 125 mg or 500 mg γ-T3, urinary excretion levels of γ-CEHC rose about 4- to 6-fold with a maximum at 9 h after ingestion and a decline to baseline by the following day[198]. An increase of urinary α-CEHC was only observed after ingestion of a very high dose of α-tocotrienyl acetate (500 mg compared to 125 mg)[198]. This study found 1%-2% of α-tocotrienyl acetates and 4%-6% of γ-tocotrienyl acetates as urinary metabolites, suggesting alternative elimination routes for T3s[198].
As outlined above, the elimination of vitamin E forms depends on the methylation pattern of the chromanol ring system. Zhao et al[136] analyzed human serum after one-time supplementation with a mixture of different vitamin E forms (2400 mg γ-TOH, 1596 mg α-TOH, 936 mg δ-TOH and 24 mg T3s) and found concentrations of α-, γ- and δ-TOHs of 21.1, 6.19, and 0.5 μmol/L, respectively, as well as ICM and SCM concentrations after enzymatic hydrolysis of 0.03 μmol/L (α-5’-COOH), 0.21 μmol/L (γ-5’-COOH), 0.08 μmol/L (δ-5’-COOH), 0.02 μmol/L (α-CEHC), 0.35 μmol/L (γ-CEHC), 0.09 μmol/L (δ-CEHC) 12 h post dose. In support of these findings the estimated half-life of γ-TOH is, at 12 ± 4 h[197], shorter than that of α-TOH at 45 h[110] to 60 h[197]. Leonard et al[197] administrated humans orally with about 50 mg of an equimolar mixture of d6-α-TOH and d2-γ-TOH acetates and found no increase of α-CEHC (detection limit in this study: 1 nmol/L), while γ-CEHC plasma concentrations doubled (129 ± 20 to 258 ± 40 nmol/L in women) after 12 h. Schuelke et al[116] concluded that α-CEHC excretion follows α-TOH plasma levels only when a threshold of 30-40 μmol/L α-TOH in plasma is exceeded. Rats treated orally with an oil containing 10 mg of γ-TOH or a combination of α- and γ-TOH (10 mg each) had urinary γ-CEHC levels which reached their highest levels 24 h to 30 h post application in both groups, but the concentration of γ-CEHC was 20%-50% higher in the α- and γ-TOH treated group compared to the group receiving only γ-TOH[149]. In addition, a shift from biliary to urinary excretion of γ-CEHC was observed, with γ-CEHC concentrations being higher for the combined treatment with α- and γ-TOH than for the γ-TOH-treated group[149]. Analyzing the absolute contents of γ-CEHC in bile and urine, they found 130 μg or 190 μg for γ-TOH or α- and γ-TOH in bile, respectively, and 250 μg γ-CEHC in the γ-TOH treated group and 280 μg in the α- and γ-TOH treated group. The authors therefore suggested that the major excretion pathway for γ-CEHC is via urine[149]. Mustacich et al[167] injected rats daily with 10 mg/100 g body weight α-TOH and analyzed the hepatic concentrations of LCM α-13’-OH and ICM α-5’-COOH. The study revealed an increase in both metabolites for the first measurement after three days post first application, with higher concentrations for α-13’-OH (up to 6.4 ± 0.7 nmol/g; 20-fold higher than prior to injection) than for α-5’-COOH (1.0 ± 0.3 nmol/g at day 3; undetectable prior to injection). None of the other known metabolites were found in the analyzed livers. The levels of α-13’-OH decreased to 1.2 ± 0.2 nmol/g at day 18 and levels of α-5’-COOH decreased to 0.4 ± 0.1 nmol/g at day 12; both values remained unaffected by the subsequent injections of α-TOH. Bardowell et al[157] speculated that mechanisms other than ω-hydroxylation might contribute to the elimination of non-α-TOH forms of vitamin E. They proposed formation and fecal excretion of ω-1 and ω-2 metabolites γ-TOH, namely γ-12’-OH and γ-11’-OH, as well as fecal elimination of non-metabolized TOH, as these metabolites and their precursor TOHs were found in human feces. Zhao et al[136] found several vitamin E metabolites in mouse feces (see Table 1) after supplementation with a diet enriched with 0.3% of a mixture of different vitamin E forms (20.2% α-T3, 4.0% β-T3, 16.1% γ-T3, 9.9% δ-T3, 14.8% α-TOH, and 3.1% γ-TOH) for four weeks and reported increases in the concentrations of almost all of these metabolites in human fecal samples over time after a single dose supplementation of 2400 mg γ-TOH, 1596 mg α-TOH, 936 mg δ-TOH und 24 mg T3s. To sum up, elimination of non-α-TOH forms is not only greater than of α-TOH, but α-TOH also increases elimination rates of non-α-TOH forms of vitamin E. This can be explained by the physiological action of α-TTP (see the corresponding section on α-TTP in chapter “Intracellular binding proteins”) and vitamin E ω-hydroxylase CYP4F2 (see the section on “Enzymatic degradation of vitamin E”).
Parker et al[171] published a hypothesis to explain the underlying physiological importance of the different rates of metabolism and elimination of the different forms of vitamin E. When murine macrophages (RAW264.7 cells) were incubated with α-TOH, γ-TOH, δ-TOH or δ-T3, different cell viabilities were found: Cell viability for α-TOH was not impaired but was intermediately reduced for γ-TOH and cell viabilities for δ-TOH or δ-T3 were substantially lower. The catabolism rates of these vitamin E forms in HepG2 cells, however, were inverse, as almost no metabolites for α-TOH but increasing amounts of metabolites for the other vitamin E forms investigated were found, with highest amounts for δ-T3. Therefore, the authors suggested an inverse correlation between the different cytotoxicities of the different vitamin E forms and their elimination rates.
Gender-specific bioavailability of vitamin E: Several years ago, differences in the bioavailability of vitamin E between genders were described. Feingold et al[199] observed in rats that α-TOH levels were higher in adrenal glands and livers as well as in all subcellular fractions derived from female rats compared to male rats. However, no sex differences in the subcellular distribution of α-TOH were observed. Notable differences in the kinetics of vitamin E metabolism were observed also in humans orally administered with a single dose of 50 mg deuterium-labeled d6-α-TOH acetate and 50 mg d2-γ-TOH acetate. Women showed higher maximum plasma concentrations of d2-γ-CEHC and excreted four times more d2-γ-CEHC in urine as men[197]. However, in both sexes urinary d2-γ-CEHCs concentrations were decreased within 24 h after consumption of sesame oil, containing sesamine, a known inhibitor of vitamin E metabolism[200]. In line with this, urinary excretion of non-deuterated γ-CEHC was also higher in women compared to men. But, in contrast to d6-α-TOH, urinary excretion of non-deuterated α-CEHC was also higher in women than in men. This suggests that women have faster γ-TOH disappearance rates in plasma than men[200], possibly because of their higher HDL TOH levels[197]. In contrast to γ-TOH, no differences in plasma disappearance rate have been found for α-TOH. But, serum concentrations of α-TOH are higher in females compared to men[33,39].
As postulated by Miwa et al[39], the observed gender-specific differences in α-TOH/lipids ratio in serum may be due to effects of the female hormone estrogen. Unfortunately, studies measuring serum vitamin E concentrations in humans have revealed contradictory results. In the SU.VI.MAX cohort of middle-aged French participants, lower serum vitamin E concentrations in women than in men were found[27]. But, when serum vitamin E levels were compared to energy intake or serum cholesterol concentrations, which are both lower in women than in men, vitamin E density per kcal intake was higher in women[27]. Reasons for the gender-specific differences in vitamin E serum levels in humans are most likely: (1) the hormonal differences; and (2) gender-dependent differences in the activation of the CYP enzymes involved in vitamin E metabolism[201]. In many species, expression of liver-expressed genes depends on hormones and growth factor profiles, which show sexual dimorphism[202-206]. For example, the transcription factor Stat5b is a key factor in maintaining the sexual dimorphic response of CYP gene expression[202,207]. As a consequence, protein levels of CYP enzymes, such as Cyp4f1, Cyp4f4, Cyp4f5 and Cyp4f6, are usually higher in liver, kidneys, lungs and brain of female rats compared to male rats[208]. In the case of the CYP4F family, Kalsotra et al[208] suggested the importance of estrogen in regulating the sex-specific expressions of Cyp4f1, Cyp4f4 and Cyp4f6 in the kidneys and liver of rats. It is questionable whether the sex-specific differences in hepatic CYP expression profiles can be directly transferred to the case of humans. Whereas sex differences in drug metabolism have been shown in humans, too, larger interindividual differences in CYP activity have been also discussed[201,202]. Indeed, in general studies have shown that CYP3A activity is higher in women than in men[201]. In line with the observation that estradiol and testosterone regulate the expression of CYP3A isoforms in the liver[209], Parkinson et al[210] and Wang et al[204] demonstrated that women metabolize some CYP3A4 and CYP3A9 substrates faster than men do.
It is known that vitamin E levels change under metabolic challenges, such as age-associated diseases or oxidative stress (see the section “Life-style and age influence the bioavailability of vitamin E” in the chapter on the bioavailability of vitamin E). Furthermore, sex-related differences occur under these conditions, as observed by Aryamanesh et al[211]. This group described increased serum levels of α-TOH in women compared to men under ischemic preconditioning. Cavalca and colleges[212] found interactions between coronary artery disease (CAD) status, gender and α-TOH concentrations; in this study α-TOH concentrations were lower in female CAD patients than in male patients. Further analysis revealed that women with CAD, but not men, had lower serum levels of α-TOH compared to women with no CAD. Interestingly, no gender-specific differences were observed for γ-TOH[212].
Extrahepatic vitamin E metabolism: In addition to the established importance of hepatic metabolism of vitamin E, there is evidence for extrahepatic, namely intestinal vitamin E catabolism. First hints were obtained from studies by Abe et al[164], in which ketoconazole decreased γ-CEHC concentrations in jejunum after oral gavage of γ-TOH. A preferential degradation of γ-TOH and γ-T3 over α-TOH was also found. Further evidence was provided by Bardowell et al[157], as mice lacking hepatic microsomal CYP enzyme activity (including Cyp4f14) had only 70% reduced vitamin E metabolism after supplementation with γ- and δ-TOH instead of the expected total absence of metabolites. Supporting this observation, Cyp4f14 is expressed in the small intestine[213] and ω-hydroxylase activity for several forms of vitamin E was found by Bardowell et al[157]; this activity comprised about 10% of the ω-hydroxylase activity found in the liver. Mice deficient for Cyp4f14 had no ω-hydroxylase activity, leading to the conclusion that the small intestine, in contrast to the liver, may have only one enzyme capable of hydroxylating vitamin E[157].
Interindividual differences in vitamin E metabolism are known and have been reported for, for example, γ-CEHC excretion after γ-TOH supplementation[198], as well as for α-TOH metabolism[176]. Both studies suggest that differences in the absorption and metabolism of vitamin E are responsible for these effects. In agreement with this suggestion, genetic polymorphisms are known for genes involved in vitamin E homeostasis, which could cause such interindividual differences. An overview on known polymorphisms in genes encoding for proteins involved in vitamin E homeostasis is provided in Table 3.
| Gene | Polymorphism | RefSNP ID | Functional consequence | Ref. |
| ABCA1 | NM_005502.3:c.2828+426A > G | rs4149314 | Intron variant | [215] |
| NM_005502.3:c.814-1304T > C | rs4149297 | Intron variant | [215] | |
| NM_005502.3:c.161-170G > A | rs11789603 | Intron variant | [215] | |
| NM_005502.3:c.936C > A | rs2274873 | Synonymous codon | [215] | |
| NM_005502.3:c.936C > T | ||||
| ABCG1 | NC_000021.9:g.42167452C > T | rs468320 | [215] | |
| APOA5 | NM_052968.4:c.-644C > T | rs662799 | Upstream variant 2KB | [219] |
| APOB | NC_000002.12:g.20937665C > T | rs4643493 | [215] | |
| NM_000384.2:c.12541G > A | rs1042031 | Intron variant | [215] | |
| Missense: E4181K | ||||
| NC_000002.12:g.21048451A > G | rs1713222 | [215] | ||
| BET1 | NC_000007.14:g.94352727G > A | rs10464587 | Intron variant | [215] |
| CD36 | NM_001289911.1:c.-108-13288T > C | rs1527479 | Intron variant | [220] |
| CYP4F2 | NM_001082.4:c.1297G > A | rs2108622 | Missense: V433M | [217,225] |
| NM_001082.4:c.34T > G | rs3093105 | Missense: W12G | [225] | |
| IRS1 | NC_000002.12:g.226683594A > G | rs1316328 | [215] | |
| LIPC | NC_000015.9:g.58688186A > C | rs4238329 | [215] | |
| NM_000236.2:c.89-47937A > G | rs8041525 | Intron variant | [215] | |
| NC_000015.10:g.58394555C > T | rs7164909 | Intron variant | [215] | |
| NC_000015.10:g.58353522T > C | rs8035357 | Intron variant | [215] | |
| NM_000236.2:c.88+49790T > C | rs12591216 | Intron variant | [215] | |
| NM_000236.2:c.89-50891C > A | rs12593880 | Intron variant | [215] | |
| NAT2 | NC_000008.11:g.18439195T > C | rs4921920 | [215] | |
| NPC1L1 | NM_013389.2:c.1184C > T | rs62001882 | UTR variant 5 prime | [216] |
| Missense: A395V | ||||
| NM_013389.2:c.1204G > A | rs141973731 | UTR variant 5 prime | [216] | |
| Missense: G402S | ||||
| NM_001300967.1:c.1249C > T | rs139659653 | UTR variant 5 prime | [216] | |
| Missense: R417W | ||||
| NM_013389.2:c.1300G > A | rs114375162 | UTR variant 5 prime | [216] | |
| Missense: G434R | ||||
| PNLIP | NM_000936.2:c.930+205T > C | rs2915775 | Intron variant | [215] |
| NC_000010.11:g.116569152G > T | rs3010494 | [215] | ||
| SCARB1 | NM_001082959.1:c.127-4800C > T | rs11057830 | Intron variant | [217] |
| SEC14L2 | NM_033382.2:c.32G > A | rs757660 | UTR variant 5 prime | [224] |
| Missense: R11K | ||||
| NM_016498.4:c.-2240T > C | rs1061660 | Upstream variant 2KB, UTR variant 3 prime | [224] | |
| NM_016498.4:c.-2110G > A | rs1061664 | Upstream variant 2KB, UTR variant 3 prime | [224] | |
| SLC10A2 | NC_000013.11:g.103037119C > T | rs1571513 | [215] | |
| NC_000013.11:g.103898490C > T | rs9558203 | [215] | ||
| NC_000013.11:g.103040492G > A | rs16961116 | [215] | ||
| NC_000013.11:g.103444248G > A | rs12874168 | [215] | ||
| NC_000013.11:g.103452026G > A | rs2065550 | [215] | ||
| SREBF2 | NM_004599.3:c.2208+3245C > T | rs2839715 | Intron variant | [215] |
| NM_004599.3:c.1761+160G > A | rs4822062 | Intron variant | [215] | |
| TTPA | NM_000370.3:c.421G > A | rs397515524 | Intron variant | [222] |
| Missense: E141K | ||||
| NM_000370.3:c.175C > T | rs397515522 | Missense: R59W | [222] | |
| NM_000370.3:c.-1753G > A | rs12056582 | Upstream variant 2KB | [223] | |
| NM_000370.3:c.-1410A > T | rs6472071 | Upstream variant 2KB | [223] | |
| NM_000370.3:c.-1410A > C | ||||
| -345C/T1 | rs75371508 | [223] | ||
| NM_000370.3:c.-981T > A | rs6994076 | Upstream variant 2KB | [223,224] | |
| NM_000370.3:c.-945G > A | rs34358293 | Upstream variant 2KB | [223] | |
| NM_000370.3:c.-675G > A | rs80169698 | Upstream variant 2KB | [223] | |
| NM_000370.3:c.-440C > T | rs73684515 | Upstream variant 2KB | [223] | |
| -344C/T1 | rs74684018 | [223] | ||
| ZNF664 | NM_001204299.1:c.-234+53341T > C | rs7296124 | Intron variant | [215] |
| NM_152437.2:c.*2065G > A | rs1048497 | Intron variant, | [215] | |
| UTR variant 3 prime | ||||
| ZPR1 | NM_003904.3:c.*724C > G | rs964184 | Downstream variant 500B | [217] |
Döring et al[214] were the first to systematically evaluate single nucleotide polymorphisms (SNPs) in genes encoding for proteins involved in vitamin E homeostasis. They analyzed TTPA, TAP, LPL, ABCC3/MRP2, PXR and CYP genes such as CYP3A4, CYP3A5 and CYP4F2 for the frequency of SNPs in coding regions and found LPL, ABCC3/MRP2, PXR, CYP3A4 and CYP4F2 to be highly polymorphic in their coding region. Recently, a new screening for candidates was published by Borel et al[215]. The postprandial chylomicron α-TOH response was positively correlated to fasting plasma α-TOH levels and 28 SNPs in eleven genes were identified that may contribute to 82% of the postprandial chylomicron α-TOH response, namely ABCA1, ABCG1, APOB, BET1, IRS1, LIPC, NAT2, PNLIP, SLC10A2, SREBF2 and ZNF664. Among these genes are seven which are involved in chylomicron metabolism and are therefore indirectly responsible for vitamin E transport in serum. Borel et al[215] noted that TTPA, CD36, SCARB1 and SEC14L were not found to be associated with the response of serum α-TOH levels in this analysis.
The importance of variants of a transporter involved in the intestinal uptake of lipids and lipophilic nutrients, namely NPC1L1, was analyzed in vitro and four variants, located on the predicted extracellular loop of NPC1L1 protein (i.e., A395V, G402S, R417W, and G434R) showed lower transport efficiencies for both cholesterol and α-TOH[216]. Borel et al[217] analyzed polymorphisms in genes involved in lipid metabolism, namely APOA4, APOB, APOE, LPL and SRBI, and found α- or γ-TOH levels to be different in subjects with SNPs in APO4, APOE and SRBI or APO4 and SRBI, respectively. Major et al[218] found three SNPs to be associated with circulating α-TOH serum levels, rs964184 near APOA1, APOA4, APOA5 and APOC3, rs2108622 in CYP4F2 as well as rs11057830 in SCARB1. As the importance of proteins involved in lipoprotein metabolism for vitamin E transport and serum levels has been highlighted by these findings, Sundl et al[219] emphasized that vitamin E serum levels should be adjusted for plasma or serum lipids. The authors reported that the association of the APOA5 -1131T>C variant (rs662799) with vitamin E serum concentrations was no longer statistically significant, when the vitamin E values were adjusted for serum triglyceride or total serum cholesterol concentrations. The scavenger receptor cluster of differentiation 36 (CD36) might also be involved in cellular vitamin E uptake[220], and indeed a SNP (rs1527479) was identified to be associated with lower α-TOH serum concentrations[221].
As already mentioned, hepatic α-TTP plays a central role in vitamin E homoestasis. As some genetic variants in the TTPA gene encoding α-TTP cause reduced circulating α-TOH levels in serum, Bromley et al[222] analyzed two SNPs in the TTPA gene, uncovering the variants E141K and R59W, which are known to be associated with AVED. These variants are located in or close to the proposed ligand-binding domain of α-TTP, and the authors showed that the variants are responsible for reduced binding of α-TOH to α-TTP in vitro. Ulatowski et al[223] analyzed SNPs in the promotor region of the TTPA gene and found that the substitutions -1752C/T (rs12056582), -1408G/T (rs6472071), and -345C/T (rs75371508) increased promotor activity about 3-fold, whereas the SNPs -1408A/T (rs6472071), -980A/T (rs6994076), -943A/G (rs34358293), -674C/T (rs80169698), -439A/G (rs73684515), and -344C/T (rs74684018) repressed promoter activity. To support the physiological relevance of their findings the authors noted that Wright et al[224] found reduced vitamin E plasma levels to be associated with the SNP -980A/T in the promotor region of the TTPA gene. In addition, three SNPs in the coding region of the SEC14L2 gene encoding TAP1 are associated with increased serum vitamin E concentrations[224]. Major et al[218] identified a new SNP in the CYP4F2 gene (rs2108622) which encodes the vitamin E ω-hydroxylase. The physiological relevance of this SNP and the SNP rs3093105 has been characterized by Bardowell et al[225]. The CYP4F2 W12G variant leads to a 2.3 to 2.8-fold increased specific enzyme activity for both TOHs and T3s, whereas the CYP4F2 V433M (rs2108622) variant causes reduced enzyme activity for TOHs, without affecting the activity for T3s. Athinarayanan et al[226] analyzed the impact of these two genetic variants on vitamin E plasma levels in children and adults with non-alcoholic fatty liver disease. The study revealed moderate effects on plasma vitamin E levels during vitamin E treatment only for the V433M variant but showed no effects on histological parameters, such as fibrosis, ballooning or steatosis.
In summary, genes involved in vitamin E homeostasis are polymorphic and some genetic variants, in particular in coding and promotor regions, are associated with vitamin E plasma/serum levels.
As the pathways responsible for vitamin E metabolism are not restricted to vitamin E, interactions with other metabolic pathways can occur that make use of: (1) the same enzymes; (2) the vitamin E-dependent regulation of gene expression; or (3) the enzyme activity of xenobiotic pathways. Two possible categories of interactions are discussed here; firstly, interaction with vitamin K metabolism and secondly, with xenobiotics and drugs.
The interference of vitamin E with vitamin K metabolism and, as a result, with blood coagulation has been known for decades[227]. As shown by several supplementation studies with vitamin E in rats[228] or humans[229] an increased risk of bleeding was noted. The underlying molecular mechanisms are still unclear, but recent research has shed new light on this aspect of vitamin E metabolism.
Vitamin E and vitamin K share the same metabolic pathways, as the degradation of both vitamins is initiated with an ω-hydroxylation followed by subsequent β-oxidation of the aliphatic side-chain, thus resulting in urinary and biliary excretion of the respective carboxylic acids or conjugates with shortened side-chains[230]. When vitamin E increases the expression or activity of enzymes involved in its own degradation, it is possible that vitamin K metabolism is also enhanced under elevated vitamin E status; this may lead to higher rates of vitamin K excretion and in turn to vitamin K deficiency with enhanced bleeding risk[160]. Evidence for this hypothesis was provided by Hanzawa et al[231], as rats treated with a diet consisting of 20% sesame seeds, containing sesamin as an inhibitor of vitamin E metabolism, for 40 d had increased vitamin K tissue concentrations. It should be noted that the vitamin E form used in this diet was solely γ-TOH. Farley et al[232] investigated the influence of α-TOH on phylloquinone or menadione metabolism and excretion. They fed rats with either a phylloquinone or a menadione containing diet (2 μmol/kg body weight) for 2.5 wk and injected after ten days a daily dose of 100 mg/kg body weight α-TOH for a further seven days. Tissue levels of menaquinone-4, a tissue-specific metabolite of phylloquinone and menadione, were decreased in brain, lung, kidney and heart and levels of phylloquinone were decreased in lung independent of the type of diet. The authors observed a downregulation of the expression of CYP enzymes CYP3A, CYP4F4 and CYP4F1, which was explained by an alternative mode of interference of vitamin E with vitamin K metabolism apart from the induced degradation of vitamin K. The induction of the xenobiotic exporters ABCB1/MDR1 and ABCG2/BCRP1 provided the first hints for an increased excretion of vitamin K metabolites into bile. This concept is supported by the 100-fold increased urinary excretion of α-CEHC in response to the application of α-TOH, whereas urinary excretion of vitamin K metabolites remained unchanged. In another study, Farley et al[233] focused on CYP4F2, as this protein is involved in the degradation of both vitamin E[140] and vitamin K[234]. The catalytic efficiency of CYP4F2 for the hydroxylation of phylloquinone is higher than for α-TOH, while the co-incubation of phylloquinone and α-TOH had no influence on the metabolism of phylloquinone. Thus, this study indicates that the activity of CYP4F2 is not enhanced by high concentrations of α-TOH. In their most recent study, Farley et al[235] concluded that α-TOH, or even α-CEHC, interferes with an intermediate step in the formation of tissue-specific menaquinone-4 from phylloquinone, such as alterations in the transport via chylomicrons or other lipoproteins or reductions of the cellular uptake of phylloquinone or menadione, an intermediate product of phylloquinone in the menaquinone formation pathway. This hypothesis is supported by findings of Hanzawa et al[236] who found that rats fed a diet with 0.75 mg/kg phylloquinone for six weeks have decreased phylloquinone tissue concentrations with supplementation of 100 mg α-TOH while in rats fed with 0.75 mg/kg menaquinone diet the tissue concentrations of menaquinone remained unchanged with an increased application of α-TOH.
Finally, the form of vitamin E also contributes to the interference with vitamin K metabolism, as γ-TOH is not as potent as α-TOH in decreasing extrahepatic phylloquinone concentrations[236]. Taken together, vitamin K metabolism is disturbed by vitamin E at the level of extrahepatic synthesis of menaquinone from phylloquinone or menadione and the different vitamin E forms also lead to differing effects on the metabolic interactions.
As CYP3A4 is responsible for the metabolism of more than 50% of xenobiotics[237], the upregulation of CYP3A4 expression by α-TOH may be of concern regarding the interactions of α-TOH with drug metabolism. Clarke et al[238] investigated the effect of α-TOH on midazolam plasma concentrations and found no influence. When the interaction with cyclosporine A was analyzed, Bárány et al[239] found reduced plasma levels after six weeks of supplementation with 800 IU/d α-TOH. These results indicate possible interactions of α-TOH with drug metabolism that need further investigation.
Vitamin E was a promising candidate as a supplement to prevent atherosclerosis and its complications. Data obtained from in vitro studies have been very promising, yet large intervention studies have revealed inconsistent results (for an overview, see Wallert et al[240]). There may be many reasons to explain this finding, but the details still need to be unraveled. With respect to patients who take medications, the situation may be even more complicated. For example, in patients treated with statins to reduce LDL cholesterol levels, interactions with simultaneously ingested vitamin E supplements may occur. A combined treatment with atorvastatin and α-TOH in stable, non-diabetic dialysis patients revealed a reduced in vitro oxidizability of LDL[241]. Podszun et al[242] found no interactions in guinea pigs fed with high-dose α-TOH (250 mg/kg diet) for six weeks when atorvastatin (300 mg/kg diet) was simultaneously applied. In 2001, however, two studies were published which questioned the benefit of the combined therapies. Patients treated with simvastatin, niacin and a combination of different antioxidants (400 IU bis in die (BID; twice a day) vitamin E, 500 mg BID vitamin C, 12.5 mg BID β-carotene and 50 mg BID selenium) profited less than the simvastatin and niacin groups as the protective increase in HDL2 with simvastatin plus niacin was attenuated by concurrent therapy with antioxidants[243,244]. Tousoulis et al[245] treated patients suffering from ischemic heart failure with low dose atorvastatin (10 mg/d) alone or in combination with 400 IU/d vitamin E and found that vitamin E impairs the positive effects of atorvastatin, on, for example, endothelial function or inflammatory response.
Many studies focusing on the effects of statins on human lipid levels also analyzed other plasma parameters, such as vitamin E status. The results depend, among others, on the evaluation of the data. When vitamin E serum levels were measured without any corrections most researchers found a decrease[241,246-250]; however, when vitamin E serum levels were normalized to plasma lipids, such as LDL cholesterol, total serum cholesterol or serum triglycerides, increased or unchanged ratios of vitamin E and serum lipids were observed[247,249-252]. The underlying processes are still unclear but on the one hand, the decrease of circulating lipoproteins could directly cause reduced levels of vitamin E (in terms of absolute concentrations), whereas the ratio of vitamin E to serum lipids could at the same time be increased, as statins mostly decrease specific lipid fractions, namely LDL cholesterol and total cholesterol. On the other hand, Werba et al[253] suggested an inhibitory effect of some statins, such as simvastatin, on CYP3A4.
When assessing vitamin E status, analytical parameters which have proven the most reliable must be primarily considered. As noted by Traber[18] in 2014, circulating α-TOH concentrations are not necessarily a reliable marker for an adequate vitamin E status in humans. Supplementation of α-TOH, for example, increases serum levels but leads simultaneously to increased amounts of α-CEHC in urine. Hence, α-CEHC in urine can be used as a marker for α-TOH status in healthy humans[132] or at a minimum as a marker for an adequate level of α-TOH[116]. In addition to serum α-TOH levels and urinary α-CEHC, the quest for another more reliable marker for adequate supply of vitamin E would be helpful for determining vitamin E status. Our group has been the first to reveal preliminary evidence for the systemic bioavailability of the first hepatic metabolites, α-13’-OH and α-13’-COOH, and their extrahepatic presence[142]. We suggest that the serum concentrations of these α-LCMs may be promising candidates in the search for new reliable markers of α-TOH levels. Both LCMs increase after supplementation with 1000 IU RRR-α-TOH for a few days. It should be also noted that the assessment of absolute food and energy intake is needed when vitamin E concentrations are measured and correlated to gender, age and life-style[118]. Determining the right analytical parameters for evaluating vitamin E status is critically important; however it is also crucial that new analytical parameters and procedures be validated, optimized and standardized for ensuring optimal diagnosis and comparability.
The analytics here involve numerous elements which should be considered before beginning measurements. Yu et al[254] demonstrated nicely that the reproducibility of the measurement of vitamin E metabolites is better in plasma than in serum samples, while the concentrations measured in serum are higher. The sample matrix also matters in the determination of conjugated metabolites. Freiser and Jiang[141] showed that conjugated metabolites are more difficult to hydrolyze in plasma compared to other biological fluids, such as urine. In principle, there are two methods for analyzing conjugated metabolites, the simultaneous analysis of conjugated and non-conjugated metabolites or the pre-treatment of samples with the respective enzymes glucuronidase and sulfatase[139]. With respect to the simultaneous analysis, Jiang et al[139] stated that “neither electrochemical detection nor GC-MS can be used to detect sulfated carboxychromanols because of their lack of redox activity or the inertness of the conjugated phenolic group to chemical derivatization, respectively”. Pope et al[177] developed the first tandem MS-MS approach for the reliable and simultaneous detection of conjugated and non-conjugated metabolites. Since then, the analytical procedures for measuring the metabolites in both conditions were adopted for LC-MS[135] and UPLC-TOF-MS[176].
Treatment of samples with deconjugation enzymes must be performed carefully. Only a cleanup-procedure with methanol/hexane extraction for the removal of proteins and lipids in the sample and an enzymatic hydrolysis overnight ensures complete deconjugation; otherwise the conjugated CEHCs are significantly underestimated[141]. It must be considered that the length of the side-chain may also influence the efficiency of deconjugation, because sulfatase treatment of relatively hydrophilic compounds (e.g., sulfated 9’-COOH) is more efficient than the treatment of more hydrophobic compounds (e.g., sulfated 13’-COOH)[141]. Freiser et al[141] optimized deconjugation of vitamin E metabolites and found that only an overnight treatment with β-glucosidase/sulfates ensures optimal recovery. Although enzymatic hydrolysis is a standard procedure, the type of enzymes used differs[255]. In some studies recombinant enzymes with distinct activities are used, such as β-glucuronidase from E. coli[144,147,157], whereas others[149,151,154-156,177] used enzymes with the combined activity of β-glucosidase and sulfatase. In these cases, glucuronidase activity is usually higher than sulfatase activity. Here it is important to consider the study by Freiser et al[141] who reported that type Β-1 β-glucuronidase from bovine liver has the lowest sulfatase activity of all enzymes tested, which is consistent with the common use of type H-1 enzymes from H. pomatia. Pope et al[177] noted that deconjugation of CEHCs may produce artefacts, such as a conversion of CEHC to tocopheronolactone. In addition, extraction conditions are important in terms of yield and recovery. According to Lodge et al[151], acidification and ether extraction ensure the highest recovery rates for α- or γ-CEHC from urine. To sum up, no standardized analytical method is available today which is suitable for use on all metabolites and conjugates. However, the establishment and careful evaluation of standardized analytics enables the reliable validation of biomarkers. This in turn offers the opportunity to obtain an accurate impression about the distribution and importance of vitamin E metabolites.
The coordinated mechanisms involved in the metabolism of vitamin E provide an effective but complex physiological pathway to protect the body against critical imbalances, i.e., an excessive accumulation of non-α-TOH forms to unphysiological levels. However, vitamin E metabolism is complex and large gaps remain in the understanding of crucial processes which are involved. Thus far, the contribution of enzymes other than CYP enzymes and the mechanisms regulating the intracellular and interorgan trafficking and metabolism are still not understood in sufficient detail.
In addition to mechanistic insights into vitamin E metabolism, the physiological functions of the different vitamin E metabolites remains incompletely understood. While many researchers have focused in the past on the SCMs, there is a significant lack of knowledge on the physiological importance of the ICMs and, in particular, the LCMs. As of yet only a few studies have investigated the principle modes of action of the LCMs in vitro, while studies in vivo have not yet been performed as LCMs have not been available as pure compounds until recently. As absolute concentrations of LCMs in the blood circulation of mammals are far too low for isolation, a natural compound, called garcinoic acid, isolated from the African bitternut Garcinia kola has proven to be a usefull resource to synthesize the α- and δ-LCMs of α- and δ-TOH[143]. Recent studies on α- and δ-LCMs have provided first preliminary insights[142,143,256-258] into the molecular modes of action of the LCMs, which seem to be specific and distinct from that of their metabolic precursors. We therefore hypothesize that at least some of the discrepancies in the results obtained from in vitro and in vivo studies on vitamin E might be explained by individual differences in the physiological metabolism of vitamin E, and in particular under pathological conditions. We suggest that the LCMs comprise a new class of regulatory metabolites, which must be investigated in more detail to unravel their proposed physiological relevance. In the meantime, a promising new approach may be to analyze serum concentrations of the LCMs in human intervention studies on vitamin E supplementation, to determine whether correlations exist to vitamin E intake and to the incidence of defined diseases.
P- Reviewer: Cairo G S- Editor: Qi Y L- Editor: A E- Editor: Jiao XK
| 1. | Cardenas E, Ghosh R. Vitamin E: a dark horse at the crossroad of cancer management. Biochem Pharmacol. 2013;86:845-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Evans HM, Bishop KS. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science. 1922;56:650-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 672] [Cited by in F6Publishing: 532] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 3. | Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: the other half of the natural vitamin E family. Mol Aspects Med. 2007;28:692-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 4. | Wong RS, Radhakrishnan AK. Tocotrienol research: past into present. Nutr Rev. 2012;70:483-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Dutta A, Dutta SK. Vitamin E and its role in the prevention of atherosclerosis and carcinogenesis: a review. J Am Coll Nutr. 2003;22:258-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Chun J, Lee J, Ye L, Exler J, Eitenmiller RR. Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States diet. J Food Composit Analy. 2006;19:196-204. [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 214] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 7. | Bechthold A, Albrecht V, Leschik-Bonnet E, Heseker H. Beurteilung der Vitaminversorgung in Deutschland. Teil 1: Daten zur Vitaminzufuhr. Ernährungs Umschau. 2012;324-336. [Cited in This Article: ] |
| 8. | Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. A report of the panel on dietary antioxidants and related compounds, subcommittees on upper reference levels of nutrients and interpretation and uses of dietary reference intakes, and the standing committee on the scientific evaluation of dietary reference intakes. Washington D.C. : National Academy Press 2000; . [Cited in This Article: ] |
| 9. | Deutsche Gesellschaft für Ernährung, Österreichische Gesellschaft für Ernährung, Schweizerische Gesellschaft für Ernährungsforschung, Schweizerische Vereinigung für Ernährung. Referenzwerte für die Nährstoffzufuhr. Neuer Umschau Buchverlag. 2008;87-93. [Cited in This Article: ] |
| 10. | Vitamin E (Tocopherole). Available from: https://www.dge.de/wissenschaft/referenzwerte/vitamin-e/. [Cited in This Article: ] |
| 11. | Bechthold A, Albrecht V, Leschik-Bonnet E, Heseker H. Beurteilung der Vitaminversorgung in Deutschland. Teil 2: Kritische Vitamine und Vitaminzufuhr in besonderen Lebenssituationen. Ernährungs Umschau. 2012;396-401. [Cited in This Article: ] |
| 12. | Schwarzpaul S, Strassburg A, Luhrmann PM, Neuhauser-Berthold M. Intake of vitamin and mineral supplements in an elderly german population. Ann Nutr Metab. 2006;50:155-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Wolters M, Hermann S, Golf S, Katz N, Hahn A. Selenium and antioxidant vitamin status of elderly German women. Eur J Clin Nutr. 2006;60:85-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Fulgoni VL, Keast DR, Auestad N, Quann EE. Nutrients from dairy foods are difficult to replace in diets of Americans: food pattern modeling and an analyses of the National Health and Nutrition Examination Survey 2003-2006. Nutr Res. 2011;31:759-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Fulgoni VL, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J Nutr. 2011;141:1847-1854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 289] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 16. | Ulatowski L, Manor D. Vitamin E trafficking in neurologic health and disease. Annu Rev Nutr. 2013;33:87-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Troesch B, Hoeft B, McBurney M, Eggersdorfer M, Weber P. Dietary surveys indicate vitamin intakes below recommendations are common in representative Western countries. Br J Nutr. 2012;108:692-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Traber MG. Vitamin E inadequacy in humans: causes and consequences. Adv Nutr. 2014;5:503-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 19. | Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ. Modern Nutrition in Health and Disease. 11th ed. Philadelphia, USA: Lippincott Williams and Wilkins 2012; . [Cited in This Article: ] |
| 20. | Handelman GJ, Epstein WL, Peerson J, Spiegelman D, Machlin LJ, Dratz EA. Human adipose alpha-tocopherol and gamma-tocopherol kinetics during and after 1 y of alpha-tocopherol supplementation. Am J Clin Nutr. 1994;59:1025-1032. [PubMed] [Cited in This Article: ] |
| 21. | Ulatowski LM, Manor D. Vitamin E and neurodegeneration. Neurobiol Dis. 2015;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Zondlo Fiume M. Final report on the safety assessment of Tocopherol, Tocopheryl Acetate, Tocopheryl Linoleate, Tocopheryl Linoleate/Oleate, Tocopheryl Nicotinate, Tocopheryl Succinate, Dioleyl Tocopheryl Methylsilanol, Potassium Ascorbyl Tocopheryl Phosphate, and Tocophersolan. Int J Toxicol. 2002;21 Suppl 3:51-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Yap SP, Yuen KH, Wong JW. Pharmacokinetics and bioavailability of alpha-, gamma- and delta-tocotrienols under different food status. J Pharm Pharmacol. 2001;53:67-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Yu SG, Thomas AM, Gapor A, Tan B, Qureshi N, Qureshi AA. Dose-response impact of various tocotrienols on serum lipid parameters in 5-week-old female chickens. Lipids. 2006;41:453-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Tomassi G, Silano V. An assessment of the safety of tocopherols as food additives. Food Chem Toxicol. 1986;24:1051-1061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1820] [Cited by in F6Publishing: 1562] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 27. | Galan P, Viteri FE, Bertrais S, Czernichow S, Faure H, Arnaud J, Ruffieux D, Chenal S, Arnault N, Favier A. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur J Clin Nutr. 2005;59:1181-1190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 28. | Campbell D, Bunker VW, Thomas AJ, Clayton BE. Selenium and vitamin E status of healthy and institutionalized elderly subjects: analysis of plasma, erythrocytes and platelets. Br J Nutr. 1989;62:221-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Miyagi SJ, Brown IW, Chock JM, Collier AC. Developmental changes in hepatic antioxidant capacity are age-and sex-dependent. J Pharmacol Sci. 2009;111:440-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Krasinski SD, Russell RM, Otradovec CL, Sadowski JA, Hartz SC, Jacob RA, McGandy RB. Relationship of vitamin A and vitamin E intake to fasting plasma retinol, retinol-binding protein, retinyl esters, carotene, alpha-tocopherol, and cholesterol among elderly people and young adults: increased plasma retinyl esters among vitamin A-supplement users. Am J Clin Nutr. 1989;49:112-120. [PubMed] [Cited in This Article: ] |
| 31. | Succari M, Garric B, Ponteziere C, Miocque M, Cals MJ. Influence of sex and age on vitamin A and E status. Age Ageing. 1991;20:413-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Chai W, Novotny R, Maskarinec G, Le Marchand L, Franke AA, Cooney RV. Serum coenzyme Q10, α-tocopherol, γ-tocopherol, and C-reactive protein levels and body mass index in adolescent and premenopausal females. J Am Coll Nutr. 2014;33:192-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Al-Azemi MK, Omu AE, Fatinikun T, Mannazhath N, Abraham S. Factors contributing to gender differences in serum retinol and alpha-tocopherol in infertile couples. Reprod Biomed Online. 2009;19:583-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Gunanti IR, Marks GC, Al-Mamun A, Long KZ. Low serum concentrations of carotenoids and vitamin E are associated with high adiposity in Mexican-American children. J Nutr. 2014;144:489-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Wallström P, Wirfält E, Lahmann PH, Gullberg B, Janzon L, Berglund G. Serum concentrations of beta-carotene and alpha-tocopherol are associated with diet, smoking, and general and central adiposity. Am J Clin Nutr. 2001;73:777-785. [PubMed] [Cited in This Article: ] |
| 36. | Ohrvall M, Tengblad S, Vessby B. Lower tocopherol serum levels in subjects with abdominal adiposity. J Intern Med. 1993;234:53-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 92] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Virtanen SM, van’t Veer P, Kok F, Kardinaal AF, Aro A. Predictors of adipose tissue tocopherol and toenail selenium levels in nine countries: the EURAMIC study. European Multicentre Case-Control Study on Antioxidants, Myocardial Infarction, and Cancer of the Breast. Eur J Clin Nutr. 1996;50:599-606. [PubMed] [Cited in This Article: ] |
| 38. | Shah AA, Khand F, Khand TU. Effect of smoking on serum xanthine oxidase, malondialdehyde, ascorbic acid and α-tocopherol levels in healthy male subjects. Pak J Med Sci. 2015;31:146-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Miwa K, Fujita M. Gender difference in oxidative stress and its genesis by analysis of serum alpha-tocopherol concentrations in a Japanese population. Int J Cardiol. 2008;129:453-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Duthie GG, Arthur JR, James WP. Effects of smoking and vitamin E on blood antioxidant status. Am J Clin Nutr. 1991;53:1061S-1063S. [PubMed] [Cited in This Article: ] |
| 41. | Dietrich M, Block G, Norkus EP, Hudes M, Traber MG, Cross CE, Packer L. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increase gamma-tocopherol in vivo after adjustment for dietary antioxidant intakes. Am J Clin Nutr. 2003;77:160-166. [PubMed] [Cited in This Article: ] |
| 42. | Mezzetti A, Lapenna D, Pierdomenico SD, Calafiore AM, Costantini F, Riario-Sforza G, Imbastaro T, Neri M, Cuccurullo F. Vitamins E, C and lipid peroxidation in plasma and arterial tissue of smokers and non-smokers. Atherosclerosis. 1995;112:91-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 124] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Bjørneboe A, Bjørneboe GE, Drevon CA. Absorption, transport and distribution of vitamin E. J Nutr. 1990;120:233-242. [PubMed] [Cited in This Article: ] |
| 44. | Bjørneboe GE, Bjørneboe A, Hagen BF, Mørland J, Drevon CA. Reduced hepatic alpha-tocopherol content after long-term administration of ethanol to rats. Biochim Biophys Acta. 1987;918:236-241. [PubMed] [Cited in This Article: ] |
| 45. | Rigotti A. Absorption, transport, and tissue delivery of vitamin E. Mol Aspects Med. 2007;28:423-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 46. | Borel P, Pasquier B, Armand M, Tyssandier V, Grolier P, Alexandre-Gouabau MC, Andre M, Senft M, Peyrot J, Jaussan V. Processing of vitamin A and E in the human gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2001;280:G95-G103. [PubMed] [Cited in This Article: ] |
| 47. | Traber MG, Kayden HJ, Green JB, Green MH. Absorption of water-miscible forms of vitamin E in a patient with cholestasis and in thoracic duct-cannulated rats. Am J Clin Nutr. 1986;44:914-923. [PubMed] [Cited in This Article: ] |
| 48. | Bieri JG, Wu AL, Tolliver TJ. Reduced intestinal absorption of vitamin E by low dietary levels of retinoic acid in rats. J Nutr. 1981;111:458-467. [PubMed] [Cited in This Article: ] |
| 49. | Richelle M, Enslen M, Hager C, Groux M, Tavazzi I, Godin JP, Berger A, Métairon S, Quaile S, Piguet-Welsch C. Both free and esterified plant sterols reduce cholesterol absorption and the bioavailability of beta-carotene and alpha-tocopherol in normocholesterolemic humans. Am J Clin Nutr. 2004;80:171-177. [PubMed] [Cited in This Article: ] |
| 50. | Doi K, Matsuura M, Kawara A, Tanaka T, Baba S. Influence of dietary fiber (konjac mannan) on absorption of vitamin B12 and vitamin E. Tohoku J Exp Med. 1983;141 Suppl:677-681. [PubMed] [Cited in This Article: ] |
| 51. | Burton GW, Ingold KU, Foster DO, Cheng SC, Webb A, Hughes L, Lusztyk E. Comparison of free alpha-tocopherol and alpha-tocopheryl acetate as sources of vitamin E in rats and humans. Lipids. 1988;23:834-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Weng W, Li L, van Bennekum AM, Potter SH, Harrison EH, Blaner WS, Breslow JL, Fisher EA. Intestinal absorption of dietary cholesteryl ester is decreased but retinyl ester absorption is normal in carboxyl ester lipase knockout mice. Biochemistry. 1999;38:4143-4149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | Desmarchelier C, Tourniaire F, Prévéraud DP, Samson-Kremser C, Crenon I, Rosilio V, Borel P. The distribution and relative hydrolysis of tocopheryl acetate in the different matrices coexisting in the lumen of the small intestine during digestion could explain its low bioavailability. Mol Nutr Food Res. 2013;57:1237-1245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Narushima K, Takada T, Yamanashi Y, Suzuki H. Niemann-pick C1-like 1 mediates alpha-tocopherol transport. Mol Pharmacol. 2008;74:42-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 55. | Reboul E, Klein A, Bietrix F, Gleize B, Malezet-Desmoulins C, Schneider M, Margotat A, Lagrost L, Collet X, Borel P. Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. J Biol Chem. 2006;281:4739-4745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 56. | Hacquebard M, Carpentier YA. Vitamin E: absorption, plasma transport and cell uptake. Curr Opin Clin Nutr Metab Care. 2005;8:133-138. [PubMed] [Cited in This Article: ] |
| 57. | Hui DY, Labonté ED, Howles PN. Development and physiological regulation of intestinal lipid absorption. III. Intestinal transporters and cholesterol absorption. Am J Physiol Gastrointest Liver Physiol. 2008;294:G839-843. [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Li G, Gu H, Zhang D. ATP-binding cassette transporters and cholesterol translocation. IUBMB Life. 2013;65:505-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Rogi T, Tomimori N, Ono Y, Kiso Y. The mechanism underlying the synergetic hypocholesterolemic effect of sesamin and α-tocopherol in rats fed a high-cholesterol diet. J Pharmacol Sci. 2011;115:408-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Lemaire-Ewing S, Desrumaux C, Néel D, Lagrost L. Vitamin E transport, membrane incorporation and cell metabolism: Is alpha-tocopherol in lipid rafts an oar in the lifeboat? Mol Nutr Food Res. 2010;54:631-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Kiyose C, Muramatsu R, Fujiyama-Fujiwara Y, Ueda T, Igarashi O. Biodiscrimination of alpha-tocopherol stereoisomers during intestinal absorption. Lipids. 1995;30:1015-1018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Traber MG, Kayden HJ. Preferential incorporation of alpha-tocopherol vs gamma-tocopherol in human lipoproteins. Am J Clin Nutr. 1989;49:517-526. [PubMed] [Cited in This Article: ] |
| 63. | Traber MG. Mechanisms for the prevention of vitamin E excess. J Lipid Res. 2013;54:2295-2306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 64. | Kolleck I, Schlame M, Fechner H, Looman AC, Wissel H, Rüstow B. HDL is the major source of vitamin E for type II pneumocytes. Free Radic Biol Med. 1999;27:882-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 65. | Traber MG, Burton GW, Ingold KU, Kayden HJ. RRR- and SRR-alpha-tocopherols are secreted without discrimination in human chylomicrons, but RRR-alpha-tocopherol is preferentially secreted in very low density lipoproteins. J Lipid Res. 1990;31:675-685. [PubMed] [Cited in This Article: ] |
| 66. | Traber MG, Burton GW, Hughes L, Ingold KU, Hidaka H, Malloy M, Kane J, Hyams J, Kayden HJ. Discrimination between forms of vitamin E by humans with and without genetic abnormalities of lipoprotein metabolism. J Lipid Res. 1992;33:1171-1182. [PubMed] [Cited in This Article: ] |
| 67. | Bjørneboe A, Bjørneboe GE, Bodd E, Hagen BF, Kveseth N, Drevon CA. Transport and distribution of alpha-tocopherol in lymph, serum and liver cells in rats. Biochim Biophys Acta. 1986;889:310-315. [PubMed] [Cited in This Article: ] |
| 68. | Kostner GM, Oettl K, Jauhiainen M, Ehnholm C, Esterbauer H, Dieplinger H. Human plasma phospholipid transfer protein accelerates exchange/transfer of alpha-tocopherol between lipoproteins and cells. Biochem J. 1995;305:659-667. [PubMed] [Cited in This Article: ] |
| 69. | Behrens WA, Thompson JN, Madère R. Distribution of alpha-tocopherol in human plasma lipoproteins. Am J Clin Nutr. 1982;35:691-696. [PubMed] [Cited in This Article: ] |
| 70. | Kiyose C, Muramatsu R, Kameyama Y, Ueda T, Igarashi O. Biodiscrimination of alpha-tocopherol stereoisomers in humans after oral administration. Am J Clin Nutr. 1997;65:785-789. [PubMed] [Cited in This Article: ] |
| 71. | Herz J, Qiu SQ, Oesterle A, DeSilva HV, Shafi S, Havel RJ. Initial hepatic removal of chylomicron remnants is unaffected but endocytosis is delayed in mice lacking the low density lipoprotein receptor. Proc Natl Acad Sci USA. 1995;92:4611-4615. [PubMed] [Cited in This Article: ] |
| 72. | Traber MG, Burton GW, Hamilton RL. Vitamin E trafficking. Ann N Y Acad Sci. 2004;1031:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 73. | Bjornson LK, Gniewkowski C, Kayden HJ. Comparison of exchange of alpha-tocopherol and free cholesterol between rat plasma lipoproteins and erythrocytes. J Lipid Res. 1975;16:39-53. [PubMed] [Cited in This Article: ] |
| 74. | Traber MG, Lane JC, Lagmay NR, Kayden HJ. Studies on the transfer of tocopherol between lipoproteins. Lipids. 1992;27:657-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 84] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Oram JF, Vaughan AM, Stocker R. ATP-binding cassette transporter A1 mediates cellular secretion of alpha-tocopherol. J Biol Chem. 2001;276:39898-39902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 76. | Kono N, Arai H. Intracellular transport of fat-soluble vitamins A and E. Traffic. 2015;16:19-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 77. | Traber MG, Kayden HJ. Vitamin E is delivered to cells via the high affinity receptor for low-density lipoprotein. Am J Clin Nutr. 1984;40:747-751. [PubMed] [Cited in This Article: ] |
| 78. | Homanics GE, Smith TJ, Zhang SH, Lee D, Young SG, Maeda N. Targeted modification of the apolipoprotein B gene results in hypobetalipoproteinemia and developmental abnormalities in mice. Proc Natl Acad Sci USA. 1993;90:2389-2393. [PubMed] [Cited in This Article: ] |
| 79. | Cohn W, Goss-Sampson MA, Grun H, Muller DP. Plasma clearance and net uptake of alpha-tocopherol and low-density lipoprotein by tissues in WHHL and control rabbits. Biochem J. 1992;287:247-254. [PubMed] [Cited in This Article: ] |
| 80. | Goti D, Hammer A, Galla HJ, Malle E, Sattler W. Uptake of lipoprotein-associated alpha-tocopherol by primary porcine brain capillary endothelial cells. J Neurochem. 2000;74:1374-1383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Clevidence BA, Lehmann J. Alpha- and gamma-tocopherol levels in lipoproteins fractionated by affinity chromatography. Lipids. 1989;24:137-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Mardones P, Strobel P, Miranda S, Leighton F, Quiñones V, Amigo L, Rozowski J, Krieger M, Rigotti A. Alpha-tocopherol metabolism is abnormal in scavenger receptor class B type I (SR-BI)-deficient mice. J Nutr. 2002;132:443-449. [PubMed] [Cited in This Article: ] |
| 83. | Hentati F, El-Euch G, Bouhlal Y, Amouri R. Ataxia with vitamin E deficiency and abetalipoproteinemia. Handb Clin Neurol. 2012;103:295-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 84. | Rana M, Wong-See D, Katz T, Gaskin K, Whitehead B, Jaffe A, Coakley J, Lochhead A. Fat-soluble vitamin deficiency in children and adolescents with cystic fibrosis. J Clin Pathol. 2014;67:605-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Aguglia U, Annesi G, Pasquinelli G, Spadafora P, Gambardella A, Annesi F, Pasqua AA, Cavalcanti F, Crescibene L, Bagalà A. Vitamin E deficiency due to chylomicron retention disease in Marinesco-Sjögren syndrome. Ann Neurol. 2000;47:260-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 86. | Gordon N. Hereditary vitamin-E deficiency. Dev Med Child Neurol. 2001;43:133-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 87. | Fujimoto VY, Kane JP, Ishida BY, Bloom MS, Browne RW. High-density lipoprotein metabolism and the human embryo. Hum Reprod Update. 2010;16:20-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | Jerkovic L, Voegele AF, Chwatal S, Kronenberg F, Radcliffe CM, Wormald MR, Lobentanz EM, Ezeh B, Eller P, Dejori N. Afamin is a novel human vitamin E-binding glycoprotein characterization and in vitro expression. J Proteome Res. 2005;4:889-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 89. | Voegele AF, Jerković L, Wellenzohn B, Eller P, Kronenberg F, Liedl KR, Dieplinger H. Characterization of the vitamin E-binding properties of human plasma afamin. Biochemistry. 2002;41:14532-14538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 90. | Angelucci S, Ciavardelli D, Di Giuseppe F, Eleuterio E, Sulpizio M, Tiboni GM, Giampietro F, Palumbo P, Di Ilio C. Proteome analysis of human follicular fluid. Biochim Biophys Acta. 2006;1764:1775-1785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 91. | Lichenstein HS, Lyons DE, Wurfel MM, Johnson DA, McGinley MD, Leidli JC, Trollinger DB, Mayer JP, Wright SD, Zukowski MM. Afamin is a new member of the albumin, alpha-fetoprotein, and vitamin D-binding protein gene family. J Biol Chem. 1994;269:18149-18154. [PubMed] [Cited in This Article: ] |
| 92. | Hubalek M, Buchner H, Mörtl MG, Schlembach D, Huppertz B, Firulovic B, Köhler W, Hafner E, Dieplinger B, Wildt L. The vitamin E-binding protein afamin increases in maternal serum during pregnancy. Clin Chim Acta. 2014;434:41-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 93. | Bélanger L, Roy S, Allard D. New albumin gene 3’ adjacent to the alpha 1-fetoprotein locus. J Biol Chem. 1994;269:5481-5484. [PubMed] [Cited in This Article: ] |
| 94. | Leth T, Sondergaard H. Biological activity of vitamin E compounds and natural materials by the resorption-gestation test, and chemical determination of the vitamin E activity in foods and feeds. J Nutr. 1977;107:2236-2243. [PubMed] [Cited in This Article: ] |
| 95. | Seeber BE, Czech T, Buchner H, Barnhart KT, Seger C, Daxenbichler G, Wildt L, Dieplinger H. The vitamin E-binding protein afamin is altered significantly in the peritoneal fluid of women with endometriosis. Fertil Steril. 2010;94:2923-2926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 96. | Niki E, Traber MG. A history of vitamin E. Ann Nutr Metab. 2012;61:207-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 97. | Sato Y, Arai H, Miyata A, Tokita S, Yamamoto K, Tanabe T, Inoue K. Primary structure of alpha-tocopherol transfer protein from rat liver. Homology with cellular retinaldehyde-binding protein. J Biol Chem. 1993;268:17705-17710. [PubMed] [Cited in This Article: ] |
| 98. | Traber MG, Sies H. Vitamin E in humans: demand and delivery. Annu Rev Nutr. 1996;16:321-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 363] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 99. | Hosomi A, Goto K, Kondo H, Iwatsubo T, Yokota T, Ogawa M, Arita M, Aoki J, Arai H, Inoue K. Localization of alpha-tocopherol transfer protein in rat brain. Neurosci Lett. 1998;256:159-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 89] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 100. | Kaempf-Rotzoll DE, Igarashi K, Aoki J, Jishage K, Suzuki H, Tamai H, Linderkamp O, Arai H. Alpha-tocopherol transfer protein is specifically localized at the implantation site of pregnant mouse uterus. Biol Reprod. 2002;67:599-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 101. | Yokota T, Shiojiri T, Gotoda T, Arita M, Arai H, Ohga T, Kanda T, Suzuki J, Imai T, Matsumoto H. Friedreich-like ataxia with retinitis pigmentosa caused by the His101Gln mutation of the alpha-tocopherol transfer protein gene. Ann Neurol. 1997;41:826-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 85] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 102. | Brigelius-Flohé R. Vitamin E: the shrew waiting to be tamed. Free Radic Biol Med. 2009;46:543-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 103. | Hosomi A, Arita M, Sato Y, Kiyose C, Ueda T, Igarashi O, Arai H, Inoue K. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997;409:105-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 506] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 104. | Panagabko C, Morley S, Hernandez M, Cassolato P, Gordon H, Parsons R, Manor D, Atkinson J. Ligand specificity in the CRAL-TRIO protein family. Biochemistry. 2003;42:6467-6474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 105. | Grebenstein N, Schumacher M, Graeve L, Frank J. α-Tocopherol transfer protein is not required for the discrimination against γ-tocopherol in vivo but protects it from side-chain degradation in vitro. Mol Nutr Food Res. 2014;58:1052-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 106. | Khanna S, Patel V, Rink C, Roy S, Sen CK. Delivery of orally supplemented alpha-tocotrienol to vital organs of rats and tocopherol-transport protein deficient mice. Free Radic Biol Med. 2005;39:1310-1319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 107. | Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714-722. [PubMed] [Cited in This Article: ] |
| 108. | Sontag TJ, Parker RS. Influence of major structural features of tocopherols and tocotrienols on their omega-oxidation by tocopherol-omega-hydroxylase. J Lipid Res. 2007;48:1090-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 109. | Qian J, Morley S, Wilson K, Nava P, Atkinson J, Manor D. Intracellular trafficking of vitamin E in hepatocytes: the role of tocopherol transfer protein. J Lipid Res. 2005;46:2072-2082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 110. | Traber MG, Ramakrishnan R, Kayden HJ. Human plasma vitamin E kinetics demonstrate rapid recycling of plasma RRR-alpha-tocopherol. Proc Natl Acad Sci USA. 1994;91:10005-10008. [PubMed] [Cited in This Article: ] |
| 111. | Zhang WX, Frahm G, Morley S, Manor D, Atkinson J. Effect of bilayer phospholipid composition and curvature on ligand transfer by the alpha-tocopherol transfer protein. Lipids. 2009;44:631-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 112. | Morley S, Cecchini M, Zhang W, Virgulti A, Noy N, Atkinson J, Manor D. Mechanisms of ligand transfer by the hepatic tocopherol transfer protein. J Biol Chem. 2008;283:17797-17804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 113. | Zhang WX, Thakur V, Lomize A, Pogozheva I, Panagabko C, Cecchini M, Baptist M, Morley S, Manor D, Atkinson J. The contribution of surface residues to membrane binding and ligand transfer by the α-tocopherol transfer protein (α-TTP). J Mol Biol. 2011;405:972-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 114. | Horiguchi M, Arita M, Kaempf-Rotzoll DE, Tsujimoto M, Inoue K, Arai H. pH-dependent translocation of alpha-tocopherol transfer protein (alpha-TTP) between hepatic cytosol and late endosomes. Genes Cells. 2003;8:789-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 115. | Arita M, Nomura K, Arai H, Inoue K. alpha-tocopherol transfer protein stimulates the secretion of alpha-tocopherol from a cultured liver cell line through a brefeldin A-insensitive pathway. Proc Natl Acad Sci USA. 1997;94:12437-12441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 102] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 116. | Schuelke M, Elsner A, Finckh B, Kohlschütter A, Hübner C, Brigelius-Flohé R. Urinary alpha-tocopherol metabolites in alpha-tocopherol transfer protein-deficient patients. J Lipid Res. 2000;41:1543-1551. [PubMed] [Cited in This Article: ] |
| 117. | Ouahchi K, Arita M, Kayden H, Hentati F, Ben Hamida M, Sokol R, Arai H, Inoue K, Mandel JL, Koenig M. Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha-tocopherol transfer protein. Nat Genet. 1995;9:141-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 371] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 118. | Traber MG, Sokol RJ, Burton GW, Ingold KU, Papas AM, Huffaker JE, Kayden HJ. Impaired ability of patients with familial isolated vitamin E deficiency to incorporate alpha-tocopherol into lipoproteins secreted by the liver. J Clin Invest. 1990;85:397-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 196] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 119. | Kayden HJ, Traber MG. Absorption, lipoprotein transport, and regulation of plasma concentrations of vitamin E in humans. J Lipid Res. 1993;34:343-358. [PubMed] [Cited in This Article: ] |
| 120. | Birringer M, Drogan D, Brigelius-Flohe R. Tocopherols are metabolized in HepG2 cells by side chain omega-oxidation and consecutive beta-oxidation. Free Radic Biol Med. 2001;31:226-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 121. | Stocker A, Zimmer S, Spycher SE, Azzi A. Identification of a novel cytosolic tocopherol-binding protein: structure, specificity, and tissue distribution. IUBMB Life. 1999;48:49-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 122. | Kempna P, Ricciarelli R, Azzi A, Zingg JM. Alternative splicing and gene polymorphism of the human TAP3/SEC14L4 gene. Mol Biol Rep. 2010;37:3503-3508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 123. | Dutta-Roy AK, Leishman DJ, Gordon MJ, Campbell FM, Duthie GG. Identification of a low molecular mass (14.2 kDa) alpha-tocopherol-binding protein in the cytosol of rat liver and heart. Biochem Biophys Res Commun. 1993;196:1108-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 124. | Ni J, Wen X, Yao J, Chang HC, Yin Y, Zhang M, Xie S, Chen M, Simons B, Chang P. Tocopherol-associated protein suppresses prostate cancer cell growth by inhibition of the phosphoinositide 3-kinase pathway. Cancer Res. 2005;65:9807-9816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 125. | Stocker A, Baumann U. Supernatant protein factor in complex with RRR-alpha-tocopherylquinone: a link between oxidized Vitamin E and cholesterol biosynthesis. J Mol Biol. 2003;332:759-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 126. | Jin G, Horinouchi R, Sagawa T, Orimo N, Kubo H, Yoshimura S, Fujisawa A, Kashiba M, Yamamoto Y. Coenzyme Q10-Binding/Transfer Protein Saposin B also Binds gamma-Tocopherol. J Clin Biochem Nutr. 2008;43:95-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 127. | Dutton PJ, Hughes LA, Foster DO, Burton GW, Ingold KU. Simon metabolites of alpha-tocopherol are not formed via a rate-controlling scission of the 3’C-H bond. Free Radic Biol Med. 1990;9:435-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 128. | Pope SA, Burtin GE, Clayton PT, Madge DJ, Muller DP. New synthesis of (+/-)-alpha-CMBHC and its confirmation as a metabolite of alpha-tocopherol (vitamin E). Bioorg Med Chem. 2001;9:1337-1343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 129. | Gross CS, Milhorat AT, Simon EJ. The metabolism of vitamin E. I. The absorption and excretion of d-alpha-tocopheryl-5-methyl-C14-succinate. J Biol Chem. 1956;221:797-805. [PubMed] [Cited in This Article: ] |
| 130. | Eisengart A, Milhorat At, Simon Ej, Sundheim L. The metabolism of vitamin E. II. Purification and characterization of urinary metabolites of alpha-tocopherol. J Biol Chem. 1956;221:807-817. [PubMed] [Cited in This Article: ] |
| 131. | Chiku S, Hamamura K, Nakamura T. Novel urinary metabolite of d-delta-tocopherol in rats. J Lipid Res. 1984;25:40-48. [PubMed] [Cited in This Article: ] |
| 132. | Schultz M, Leist M, Petrzika M, Gassmann B, Brigelius-Flohé R. Novel urinary metabolite of alpha-tocopherol, 2,5,7,8-tetramethyl-2(2’-carboxyethyl)-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am J Clin Nutr. 1995;62:1527S-1534S. [PubMed] [Cited in This Article: ] |
| 133. | Wechter WJ, Kantoci D, Murray ED, D’Amico DC, Jung ME, Wang WH. A new endogenous natriuretic factor: LLU-alpha. Proc Natl Acad Sci USA. 1996;93:6002-6007. [PubMed] [Cited in This Article: ] |
| 134. | Pope SA, Clayton PT, Muller DP. A new method for the analysis of urinary vitamin E metabolites and the tentative identification of a novel group of compounds. Arch Biochem Biophys. 2000;381:8-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 135. | Sharma G, Muller DP, O’Riordan SM, Bryan S, Dattani MT, Hindmarsh PC, Mills K. Urinary conjugated α-tocopheronolactone--a biomarker of oxidative stress in children with type 1 diabetes. Free Radic Biol Med. 2013;55:54-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 136. | Zhao Y, Lee MJ, Cheung C, Ju JH, Chen YK, Liu B, Hu LQ, Yang CS. Analysis of multiple metabolites of tocopherols and tocotrienols in mice and humans. J Agric Food Chem. 2010;58:4844-4852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 137. | Birringer M, Pfluger P, Kluth D, Landes N, Brigelius-Flohé R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J Nutr. 2002;132:3113-3118. [PubMed] [Cited in This Article: ] |
| 138. | Mustacich DJ, Leonard SW, Patel NK, Traber MG. Alpha-tocopherol beta-oxidation localized to rat liver mitochondria. Free Radic Biol Med. 2010;48:73-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 139. | Jiang Q, Freiser H, Wood KV, Yin X. Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats. J Lipid Res. 2007;48:1221-1230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 140. | Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J Biol Chem. 2002;277:25290-25296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 340] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 141. | Freiser H, Jiang Q. Optimization of the enzymatic hydrolysis and analysis of plasma conjugated gamma-CEHC and sulfated long-chain carboxychromanols, metabolites of vitamin E. Anal Biochem. 2009;388:260-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 142. | Wallert M, Mosig S, Rennert K, Funke H, Ristow M, Pellegrino RM, Cruciani G, Galli F, Lorkowski S, Birringer M. Long-chain metabolites of α-tocopherol occur in human serum and inhibit macrophage foam cell formation in vitro. Free Radic Biol Med. 2014;68:43-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 143. | Birringer M, Lington D, Vertuani S, Manfredini S, Scharlau D, Glei M, Ristow M. Proapoptotic effects of long-chain vitamin E metabolites in HepG2 cells are mediated by oxidative stress. Free Radic Biol Med. 2010;49:1315-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 144. | Parker RS, Swanson JE. A novel 5’-carboxychroman metabolite of gamma-tocopherol secreted by HepG2 cells and excreted in human urine. Biochem Biophys Res Commun. 2000;269:580-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 145. | Schultz M, Leist M, Elsner A, Brigelius-Flohé R. alpha-Carboxyethyl-6-hydroxychroman as urinary metabolite of vitamin E. Methods Enzymol. 1997;282:297-310. [PubMed] [Cited in This Article: ] |
| 146. | Schönfeld A, Schultz M, Petrizka M, Gassmann B. A novel metabolite of RRR-alpha-tocopherol in human urine. Nahrung. 1993;37:498-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 147. | Swanson JE, Ben RN, Burton GW, Parker RS. Urinary excretion of 2,7, 8-trimethyl-2-(beta-carboxyethyl)-6-hydroxychroman is a major route of elimination of gamma-tocopherol in humans. J Lipid Res. 1999;40:665-671. [PubMed] [Cited in This Article: ] |
| 148. | Hattori A, Fukushima T, Yoshimura H, Abe K, Imai K. Production of LLU-alpha following an oral administration of gamma-tocotrienol or gamma-tocopherol to rats. Biol Pharm Bull. 2000;23:1395-1397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 149. | Kiyose C, Saito H, Kaneko K, Hamamura K, Tomioka M, Ueda T, Igarashi O. Alpha-tocopherol affects the urinary and biliary excretion of 2,7,8-trimethyl-2 (2’-carboxyethyl)-6-hydroxychroman, gamma-tocopherol metabolite, in rats. Lipids. 2001;36:467-472. [PubMed] [Cited in This Article: ] |
| 150. | Galli F, Floridi AG, Floridi A, Buoncristiani U. Accumulation of vitamin E metabolites in the blood of renal failure patients. Clin Nutr. 2004;23:205-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 151. | Lodge JK, Traber MG, Elsner A, Brigelius-Flohé R. A rapid method for the extraction and determination of vitamin E metabolites in human urine. J Lipid Res. 2000;41:148-154. [PubMed] [Cited in This Article: ] |
| 152. | Stahl W, Graf P, Brigelius-Flohé R, Wechter W, Sies H. Quantification of the alpha- and gamma-tocopherol metabolites 2,5,7, 8-tetramethyl-2-(2’-carboxyethyl)-6-hydroxychroman and 2,7, 8-trimethyl-2-(2’-carboxyethyl)-6-hydroxychroman in human serum. Anal Biochem. 1999;275:254-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 153. | Galli F, Lee R, Dunster C, Kelly FJ. Gas chromatography mass spectrometry analysis of carboxyethyl-hydroxychroman metabolites of alpha- and gamma-tocopherol in human plasma. Free Radic Biol Med. 2002;32:333-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 154. | Leonard SW, Gumpricht E, Devereaux MW, Sokol RJ, Traber MG. Quantitation of rat liver vitamin E metabolites by LC-MS during high-dose vitamin E administration. J Lipid Res. 2005;46:1068-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 155. | Morinobu T, Yoshikawa S, Hamamura K, Tamai H. Measurement of vitamin E metabolites by high-performance liquid chromatography during high-dose administration of alpha-tocopherol. Eur J Clin Nutr. 2003;57:410-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 156. | Cho JY, Kang DW, Ma X, Ahn SH, Krausz KW, Luecke H, Idle JR, Gonzalez FJ. Metabolomics reveals a novel vitamin E metabolite and attenuated vitamin E metabolism upon PXR activation. J Lipid Res. 2009;50:924-937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 157. | Bardowell SA, Ding X, Parker RS. Disruption of P450-mediated vitamin E hydroxylase activities alters vitamin E status in tocopherol supplemented mice and reveals extra-hepatic vitamin E metabolism. J Lipid Res. 2012;53:2667-2676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 158. | Hardwick JP. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem Pharmacol. 2008;75:2263-2275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 159. | Morita M, Imanaka T. Peroxisomal ABC transporters: structure, function and role in disease. Biochim Biophys Acta. 2012;1822:1387-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 160. | Card DJ, Gorska R, Cutler J, Harrington DJ. Vitamin K metabolism: current knowledge and future research. Mol Nutr Food Res. 2014;58:1590-1600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 161. | Bardowell SA, Duan F, Manor D, Swanson JE, Parker RS. Disruption of mouse cytochrome p450 4f14 (Cyp4f14 gene) causes severe perturbations in vitamin E metabolism. J Biol Chem. 2012;287:26077-26086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 162. | Sevrioukova IF, Poulos TL. Understanding the mechanism of cytochrome P450 3A4: recent advances and remaining problems. Dalton Trans. 2013;42:3116-3126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 163. | Parker RS, Sontag TJ, Swanson JE. Cytochrome P4503A-dependent metabolism of tocopherols and inhibition by sesamin. Biochem Biophys Res Commun. 2000;277:531-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 174] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 164. | Abe C, Uchida T, Ohta M, Ichikawa T, Yamashita K, Ikeda S. Cytochrome P450-dependent metabolism of vitamin E isoforms is a critical determinant of their tissue concentrations in rats. Lipids. 2007;42:637-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 165. | Traber MG, Siddens LK, Leonard SW, Schock B, Gohil K, Krueger SK, Cross CE, Williams DE. Alpha-tocopherol modulates Cyp3a expression, increases gamma-CEHC production, and limits tissue gamma-tocopherol accumulation in mice fed high gamma-tocopherol diets. Free Radic Biol Med. 2005;38:773-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 166. | Kluth D, Landes N, Pfluger P, Müller-Schmehl K, Weiss K, Bumke-Vogt C, Ristow M, Brigelius-Flohé R. Modulation of Cyp3a11 mRNA expression by alpha-tocopherol but not gamma-tocotrienol in mice. Free Radic Biol Med. 2005;38:507-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 167. | Mustacich DJ, Leonard SW, Devereaux MW, Sokol RJ, Traber MG. Alpha-tocopherol regulation of hepatic cytochrome P450s and ABC transporters in rats. Free Radic Biol Med. 2006;41:1069-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 168. | Landes N, Birringer M, Brigelius-Flohé R. Homologous metabolic and gene activating routes for vitamins E and K. Mol Aspects Med. 2003;24:337-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 169. | Landes N, Pfluger P, Kluth D, Birringer M, Rühl R, Böl GF, Glatt H, Brigelius-Flohé R. Vitamin E activates gene expression via the pregnane X receptor. Biochem Pharmacol. 2003;65:269-273. [PubMed] [Cited in This Article: ] |
| 170. | Johnson CH, Bonzo JA, Cheng J, Krausz KW, Kang DW, Luecke H, Idle JR, Gonzalez FJ. Cytochrome P450 regulation by α-tocopherol in Pxr-null and PXR-humanized mice. Drug Metab Dispos. 2013;41:406-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 171. | Parker RS, Sontag TJ, Swanson JE, McCormick CC. Discovery, characterization, and significance of the cytochrome P450 omega-hydroxylase pathway of vitamin E catabolism. Ann N Y Acad Sci. 2004;1031:13-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 172. | Stresser DM, Broudy MI, Ho T, Cargill CE, Blanchard AP, Sharma R, Dandeneau AA, Goodwin JJ, Turner SD, Erve JC. Highly selective inhibition of human CYP3Aa in vitro by azamulin and evidence that inhibition is irreversible. Drug Metab Dispos. 2004;32:105-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 173. | Schuetz EG, Schuetz JD, Strom SC, Thompson MT, Fisher RA, Molowa DT, Li D, Guzelian PS. Regulation of human liver cytochromes P-450 in family 3A in primary and continuous culture of human hepatocytes. Hepatology. 1993;18:1254-1262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 113] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 174. | Ohnmacht S, Nava P, West R, Parker R, Atkinson J. Inhibition of oxidative metabolism of tocopherols with omega-N-heterocyclic derivatives of vitamin E. Bioorg Med Chem. 2008;16:7631-7638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 175. | Hashiguchi T, Kurogi K, Sakakibara Y, Yamasaki M, Nishiyama K, Yasuda S, Liu MC, Suiko M. Enzymatic sulfation of tocopherols and tocopherol metabolites by human cytosolic sulfotransferases. Biosci Biotechnol Biochem. 2011;75:1951-1956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 176. | Johnson CH, Slanař O, Krausz KW, Kang DW, Patterson AD, Kim JH, Luecke H, Gonzalez FJ, Idle JR. Novel metabolites and roles for α-tocopherol in humans and mice discovered by mass spectrometry-based metabolomics. Am J Clin Nutr. 2012;96:818-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 177. | Pope SA, Burtin GE, Clayton PT, Madge DJ, Muller DP. Synthesis and analysis of conjugates of the major vitamin E metabolite, alpha-CEHC. Free Radic Biol Med. 2002;33:807-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 178. | Tanabe M, Fukushima T, Usui N, Aoyama N, Tsunoda M, Imai K. Intravenous administration of 2,7,8-trimethyl-2-(beta-carboxyethyl)-6-hydroxy chroman (gamma-CEHC) to rats and determination of its plasma concentration and urinary sodium excretion. Biomed Chromatogr. 2004;18:727-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 179. | Freiser H, Jiang Q. Gamma-tocotrienol and gamma-tocopherol are primarily metabolized to conjugated 2-(beta-carboxyethyl)-6-hydroxy-2,7,8-trimethylchroman and sulfated long-chain carboxychromanols in rats. J Nutr. 2009;139:884-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 180. | Zhou C, Tabb MM, Sadatrafiei A, Grün F, Blumberg B. Tocotrienols activate the steroid and xenobiotic receptor, SXR, and selectively regulate expression of its target genes. Drug Metab Dispos. 2004;32:1075-1082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 181. | Buckley DB, Klaassen CD. Induction of mouse UDP-glucuronosyltransferase mRNA expression in liver and intestine by activators of aryl-hydrocarbon receptor, constitutive androstane receptor, pregnane X receptor, peroxisome proliferator-activated receptor alpha, and nuclear factor erythroid 2-related factor 2. Drug Metab Dispos. 2009;37:847-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 182. | Mackenzie PI, Hu DG, Gardner-Stephen DA. The regulation of UDP-glucuronosyltransferase genes by tissue-specific and ligand-activated transcription factors. Drug Metab Rev. 2010;42:99-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 183. | Mustacich DJ, Gohil K, Bruno RS, Yan M, Leonard SW, Ho E, Cross CE, Traber MG. Alpha-tocopherol modulates genes involved in hepatic xenobiotic pathways in mice. J Nutr Biochem. 2009;20:469-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 184. | Van Haaften RI, Evelo CT, Penders J, Eijnwachter MP, Haenen GR, Bast A. Inhibition of human glutathione S-transferase P1-1 by tocopherols and alpha-tocopherol derivatives. Biochim Biophys Acta. 2001;1548:23-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 185. | van Haaften RI, Haenen GR, Evelo CT, Bast A. Tocotrienols inhibit human glutathione S-transferase P1-1. IUBMB Life. 2002;54:81-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 186. | Podszun M, Frank J. Vitamin E-drug interactions: molecular basis and clinical relevance. Nutr Res Rev. 2014;27:215-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 187. | Jiang Q, Jiang Z, Hall YJ, Jang Y, Snyder PW, Bain C, Huang J, Jannasch A, Cooper B, Wang Y. Gamma-tocopherol attenuates moderate but not severe colitis and suppresses moderate colitis-promoted colon tumorigenesis in mice. Free Radic Biol Med. 2013;65:1069-1077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 188. | Wu JH, Croft KD. Vitamin E metabolism. Mol Aspects Med. 2007;28:437–452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 189. | Mustacich DJ, Shields J, Horton RA, Brown MK, Reed DJ. Biliary secretion of alpha-tocopherol and the role of the mdr2 P-glycoprotein in rats and mice. Arch Biochem Biophys. 1998;350:183-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 190. | Bjørneboe A, Bjørneboe GE, Drevon CA. Serum half-life, distribution, hepatic uptake and biliary excretion of alpha-tocopherol in rats. Biochim Biophys Acta. 1987;921:175-181. [PubMed] [Cited in This Article: ] |
| 191. | Yokogawa K, Shima Y, Hashimoto T, Hiyajyo M, Kadoyama K, Ishizki J, Nomura M, Miyamoto K. Disposition kinetics of alpha-tocopherol in apolipoprotein B knockout mice. Pharm Res. 2003;20:368-372. [PubMed] [Cited in This Article: ] |
| 192. | Witt W, Kolleck I, Fechner H, Sinha P, Rüstow B. Regulation by vitamin E of the scavenger receptor BI in rat liver and HepG2 cells. J Lipid Res. 2000;41:2009-2016. [PubMed] [Cited in This Article: ] |
| 193. | Takada T, Suzuki H. Molecular mechanisms of membrane transport of vitamin E. Mol Nutr Food Res. 2010;54:616-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 194. | Fruhwürth S, Kovacs WJ, Bittman R, Messner S, Röhrl C, Stangl H. Differential basolateral-apical distribution of scavenger receptor, class B, type I in cultured cells and the liver. Histochem Cell Biol. 2014;142:645-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 195. | Traber MG, Labut EM, Leonard SW, Lebold KM. α-Tocopherol injections in rats up-regulate hepatic ABC transporters, but not cytochrome P450 enzymes. Free Radic Biol Med. 2011;51:2031-2040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 196. | Traber MG, Elsner A, Brigelius-Flohé R. Synthetic as compared with natural vitamin E is preferentially excreted as alpha-CEHC in human urine: studies using deuterated alpha-tocopheryl acetates. FEBS Lett. 1998;437:145-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 107] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 197. | Leonard SW, Paterson E, Atkinson JK, Ramakrishnan R, Cross CE, Traber MG. Studies in humans using deuterium-labeled alpha- and gamma-tocopherols demonstrate faster plasma gamma-tocopherol disappearance and greater gamma-metabolite production. Free Radic Biol Med. 2005;38:857-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 198. | Lodge JK, Ridlington J, Leonard S, Vaule H, Traber MG. Alpha- and gamma-tocotrienols are metabolized to carboxyethyl-hydroxychroman derivatives and excreted in human urine. Lipids. 2001;36:43-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 199. | Feingold IB, Colby HD. Sex differences in adrenal and hepatic alpha-tocopherol concentrations in rats. Pharmacology. 1992;44:113-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 200. | Frank J, Lee S, Leonard SW, Atkinson JK, Kamal-Eldin A, Traber MG. Sex differences in the inhibition of gamma-tocopherol metabolism by a single dose of dietary sesame oil in healthy subjects. Am J Clin Nutr. 2008;87:1723-1729. [PubMed] [Cited in This Article: ] |
| 201. | Hunt CM, Westerkam WR, Stave GM. Effect of age and gender on the activity of human hepatic CYP3A. Biochem Pharmacol. 1992;44:275-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 262] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 202. | Davey HW, Wilkins RJ, Waxman DJ. STAT5 signaling in sexually dimorphic gene expression and growth patterns. Am J Hum Genet. 1999;65:959-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 203. | Waxman DJ, Ram PA, Pampori NA, Shapiro BH. Growth hormone regulation of male-specific rat liver P450s 2A2 and 3A2: induction by intermittent growth hormone pulses in male but not female rats rendered growth hormone deficient by neonatal monosodium glutamate. Mol Pharmacol. 1995;48:790-797. [PubMed] [Cited in This Article: ] |
| 204. | Wang H, Strobel HW. Regulation of CYP3A9 gene expression by estrogen and catalytic studies using cytochrome P450 3A9 expressed in Escherichia coli. Arch Biochem Biophys. 1997;344:365-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 205. | Pampori NA, Shapiro BH. Gender differences in the responsiveness of the sex-dependent isoforms of hepatic P450 to the feminine plasma growth hormone profile. Endocrinology. 1999;140:1245-1254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 206. | Scandlyn MJ, Stuart EC, Rosengren RJ. Sex-specific differences in CYP450 isoforms in humans. Expert Opin Drug Metab Toxicol. 2008;4:413-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 207. | Park SH, Liu X, Hennighausen L, Davey HW, Waxman DJ. Distinctive roles of STAT5a and STAT5b in sexual dimorphism of hepatic P450 gene expression. Impact of STAT5a gene disruption. J Biol Chem. 1999;274:7421-7430. [PubMed] [Cited in This Article: ] |
| 208. | Kalsotra A, Anakk S, Boehme CL, Strobel HW. Sexual dimorphism and tissue specificity in the expression of CYP4F forms in Sprague Dawley rats. Drug Metab Dispos. 2002;30:1022-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 209. | Sakuma T, Takai M, Endo Y, Kuroiwa M, Ohara A, Jarukamjorn K, Honma R, Nemoto N. A novel female-specific member of the CYP3A gene subfamily in the mouse liver. Arch Biochem Biophys. 2000;377:153-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 210. | Parkinson A, Mudra DR, Johnson C, Dwyer A, Carroll KM. The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicol Appl Pharmacol. 2004;199:193-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 283] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 211. | Aryamanesh S, Ebrahimi SM, Abotaleb N, Nobakht M, Rahimi-Moghaddam P. Role of endogenous vitamin E in renal ischemic preconditioning process: differences between male and female rats. Iran Biomed J. 2012;16:44-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 212. | Cavalca V, Veglia F, Squellerio I, Marenzi G, Minardi F, De Metrio M, Cighetti G, Boccotti L, Ravagnani P, Tremoli E. Glutathione, vitamin E and oxidative stress in coronary artery disease: relevance of age and gender. Eur J Clin Invest. 2009;39:267-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 213. | Shin HC, Kim HR, Cho HJ, Yi H, Cho SM, Lee DG, Abd El-Aty AM, Kim JS, Sun D, Amidon GL. Comparative gene expression of intestinal metabolizing enzymes. Biopharm Drug Dispos. 2009;30:411-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 214. | Döring F, Rimbach G, Lodge JK. In silico search for single nucleotide polymorphisms in genes important in vitamin E homeostasis. IUBMB Life. 2004;56:615-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 215. | Borel P, Desmarchelier C, Nowicki M, Bott R, Tourniaire F. Can genetic variability in α-tocopherol bioavailability explain the heterogeneous response to α-tocopherol supplements? Antioxid Redox Signal. 2015;22:669-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 216. | Yamanashi Y, Takada T, Suzuki H. In-vitro characterization of the six clustered variants of NPC1L1 observed in cholesterol low absorbers. Pharmacogenet Genomics. 2009;19:884-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 217. | Borel P, Moussa M, Reboul E, Lyan B, Defoort C, Vincent-Baudry S, Maillot M, Gastaldi M, Darmon M, Portugal H. Human plasma levels of vitamin E and carotenoids are associated with genetic polymorphisms in genes involved in lipid metabolism. J Nutr. 2007;137:2653-2659. [PubMed] [Cited in This Article: ] |
| 218. | Major JM, Yu K, Wheeler W, Zhang H, Cornelis MC, Wright ME, Yeager M, Snyder K, Weinstein SJ, Mondul A. Genome-wide association study identifies common variants associated with circulating vitamin E levels. Hum Mol Genet. 2011;20:3876-3883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 219. | Sundl I, Guardiola M, Khoschsorur G, Solà R, Vallvé JC, Godàs G, Masana L, Maritschnegg M, Meinitzer A, Cardinault N. Increased concentrations of circulating vitamin E in carriers of the apolipoprotein A5 gene - 1131T& gt; C variant and associations with plasma lipids and lipid peroxidation. J Lipid Res. 2007;48:2506-2513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 220. | Goncalves A, Roi S, Nowicki M, Niot I, Reboul E. Cluster-determinant 36 (CD36) impacts on vitamin E postprandial response. Mol Nutr Food Res. 2014;58:2297-2306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 221. | Lecompte S, Szabo de Edelenyi F, Goumidi L, Maiani G, Moschonis G, Widhalm K, Molnár D, Kafatos A, Spinneker A, Breidenassel C. Polymorphisms in the CD36/FAT gene are associated with plasma vitamin E concentrations in humans. Am J Clin Nutr. 2011;93:644-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 222. | Bromley D, Anderson PC, Daggett V. Structural consequences of mutations to the α-tocopherol transfer protein associated with the neurodegenerative disease ataxia with vitamin E deficiency. Biochemistry. 2013;52:4264-4273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 223. | Ulatowski L, Dreussi C, Noy N, Barnholtz-Sloan J, Klein E, Manor D. Expression of the α-tocopherol transfer protein gene is regulated by oxidative stress and common single-nucleotide polymorphisms. Free Radic Biol Med. 2012;53:2318-2326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 224. | Wright ME, Peters U, Gunter MJ, Moore SC, Lawson KA, Yeager M, Weinstein SJ, Snyder K, Virtamo J, Albanes D. Association of variants in two vitamin e transport genes with circulating vitamin e concentrations and prostate cancer risk. Cancer Res. 2009;69:1429-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 225. | Bardowell SA, Stec DE, Parker RS. Common variants of cytochrome P450 4F2 exhibit altered vitamin E-{omega}-hydroxylase specific activity. J Nutr. 2010;140:1901-1906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 226. | Athinarayanan S, Wei R, Zhang M, Bai S, Traber MG, Yates K, Cummings OW, Molleston J, Liu W, Chalasani N. Genetic polymorphism of cytochrome P450 4F2, vitamin E level and histological response in adults and children with nonalcoholic fatty liver disease who participated in PIVENS and TONIC clinical trials. PLoS One. 2014;9:e95366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 227. | Traber MG. Vitamin E and K interactions--a 50-year-old problem. Nutr Rev. 2008;66:624-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 228. | Wheldon GH, Bhatt A, Keller P, Hummler H. d,1-alpha-Tocopheryl acetate (vitamin E): a long term toxicity and carcinogenicity study in rats. Int J Vitam Nutr Res. 1983;53:287-296. [PubMed] [Cited in This Article: ] |
| 229. | Glynn RJ, Ridker PM, Goldhaber SZ, Zee RY, Buring JE. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: report from the Women’s Health Study. Circulation. 2007;116:1497-1503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 230. | Shearer MJ, Newman P. Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis. J Lipid Res. 2014;55:345-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 231. | Hanzawa F, Nomura S, Sakuma E, Uchida T, Ikeda S. Dietary sesame seed and its lignan, sesamin, increase tocopherol and phylloquinone concentrations in male rats. J Nutr. 2013;143:1067-1073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 232. | Farley SM, Leonard SW, Labut EM, Raines HF, Card DJ, Harrington DJ, Mustacich DJ, Traber MG. Vitamin E decreases extra-hepatic menaquinone-4 concentrations in rats fed menadione or phylloquinone. Mol Nutr Food Res. 2012;56:912-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 233. | Farley SM, Leonard SW, Taylor AW, Birringer M, Edson KZ, Rettie AE, Traber MG. ω-Hydroxylation of phylloquinone by CYP4F2 is not increased by α-tocopherol. Mol Nutr Food Res. 2013;57:1785-1793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 234. | McDonald MG, Rieder MJ, Nakano M, Hsia CK, Rettie AE. CYP4F2 is a vitamin K1 oxidase: An explanation for altered warfarin dose in carriers of the V433M variant. Mol Pharmacol. 2009;75:1337-1346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 236] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 235. | Farley SM, Leonard SW, Stevens JF, Traber MG. Deuterium-labeled phylloquinone fed to α-tocopherol-injected rats demonstrates sensitivity of low phylloquinone-containing tissues to menaquinone-4 depletion. Mol Nutr Food Res. 2014;58:1610-1619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 236. | Hanzawa F, Sakuma E, Nomura S, Uchida T, Oda H, Ikeda S. Excess α-tocopherol decreases extrahepatic phylloquinone in phylloquinone-fed rats but not menaquinone-4 in menaquinone-4-fed rats. Mol Nutr Food Res. 2014;58:1601-1609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 237. | Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352:2211-2221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 813] [Cited by in F6Publishing: 720] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 238. | Clarke MW, Burnett JR, Wu JH, Hodgson JM, Ledowski T, Puddey IB, Croft KD. Vitamin E supplementation and hepatic drug metabolism in humans. J Cardiovasc Pharmacol. 2009;54:491-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 239. | Bárány P, Stenvinkel P, Ottosson-Seeberger A, Alvestrand A, Morrow J, Roberts JJ, Salahudeen AK. Effect of 6 weeks of vitamin E administration on renal haemodynamic alterations following a single dose of neoral in healthy volunteers. Nephrol Dial Transplant. 2001;16:580-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 240. | Wallert M, Schmölz L, Galli F, Birringer M, Lorkowski S. Regulatory metabolites of vitamin E and their putative relevance for atherogenesis. Redox Biol. 2014;2:495-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 241. | Diepeveen SH, Verhoeven GW, Van Der Palen J, Dikkeschei LD, Van Tits LJ, Kolsters G, Offerman JJ, Bilo HJ, Stalenhoef AF. Effects of atorvastatin and vitamin E on lipoproteins and oxidative stress in dialysis patients: a randomised-controlled trial. J Intern Med. 2005;257:438-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 242. | Podszun MC, Grebenstein N, Spruss A, Schlueter T, Kremoser C, Bergheim I, Frank J. Dietary α-tocopherol and atorvastatin reduce high-fat-induced lipid accumulation and down-regulate CD36 protein in the liver of guinea pigs. J Nutr Biochem. 2014;25:573-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 243. | Cheung MC, Zhao XQ, Chait A, Albers JJ, Brown BG. Antioxidant supplements block the response of HDL to simvastatin-niacin therapy in patients with coronary artery disease and low HDL. Arterioscler Thromb Vasc Biol. 2001;21:1320-1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 244. | Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583-1592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1505] [Cited by in F6Publishing: 1575] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 245. | Tousoulis D, Antoniades C, Vassiliadou C, Toutouza M, Pitsavos C, Tentolouris C, Trikas A, Stefanadis C. Effects of combined administration of low dose atorvastatin and vitamin E on inflammatory markers and endothelial function in patients with heart failure. Eur J Heart Fail. 2005;7:1126-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 246. | Shin MJ, Chung N, Lee JH, Jang Y, Park E, Jeon KI, Chung JH, Seo BY. Effects of simvastatin on plasma antioxidant status and vitamins in hypercholesterolemic patients. Int J Cardiol. 2007;118:173-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 247. | Human JA, Ubbink JB, Jerling JJ, Delport R, Vermaak WJ, Vorster HH, Lagendijk J, Potgieter HC. The effect of Simvastatin on the plasma antioxidant concentrations in patients with hypercholesterolaemia. Clin Chim Acta. 1997;263:67-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 248. | Jula A, Marniemi J, Huupponen R, Virtanen A, Rastas M, Rönnemaa T. Effects of diet and simvastatin on serum lipids, insulin, and antioxidants in hypercholesterolemic men: a randomized controlled trial. JAMA. 2002;287:598-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 249. | Oranje WA, Sels JP, Rondas-Colbers GJ, Lemmens PJ, Wolffenbuttel BH. Effect of atorvastatin on LDL oxidation and antioxidants in normocholesterolemic type 2 diabetic patients. Clin Chim Acta. 2001;311:91-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 250. | Vasankari T, Ahotupa M, Viikari J, Nuotio I, Strandberg T, Vanhanen H, Gylling H, Miettinen T, Tikkanen MJ. Effect of 12-month statin therapy on antioxidant potential of LDL and serum antioxidant vitamin concentrations. Ann Med. 2004;36:618-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 251. | Salonen R, Nyssönen K, Porkkala-Sarataho E, Salonen JT. The Kuopio Atherosclerosis Prevention Study (KAPS): effect of pravastatin treatment on lipids, oxidation resistance of lipoproteins, and atherosclerotic progression. Am J Cardiol. 1995;76:34C-39C. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 252. | Cangemi R, Loffredo L, Carnevale R, Pignatelli P, Violi F. Statins enhance circulating vitamin E. Int J Cardiol. 2008;123:172-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 253. | Werba JP, Cavalca V, Veglia F, Massironi P, De Franceschi M, Zingaro L, Tremoli E. A new compound-specific pleiotropic effect of statins: modification of plasma gamma-tocopherol levels. Atherosclerosis. 2007;193:229-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 254. | Yu Z, Kastenmüller G, He Y, Belcredi P, Möller G, Prehn C, Mendes J, Wahl S, Roemisch-Margl W, Ceglarek U. Differences between human plasma and serum metabolite profiles. PLoS One. 2011;6:e21230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 312] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 255. | Birringer M. Analysis of vitamin E metabolites in biological specimen. Mol Nutr Food Res. 2010;54:588-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 256. | Mazzini F, Betti M, Netscher T, Galli F, Salvadori P. Configuration of the vitamin E analogue garcinoic acid extracted from Garcinia Kola seeds. Chirality. 2009;21:519-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 257. | Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc Natl Acad Sci USA. 2008;105:20464-20469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 258. | Wallert M, Schmölz L, Koeberle A, Krauth V, Glei M, Galli F, Werz O, Birringer M, Lorkowski S. α-Tocopherol long-chain metabolite α-13’-COOH affects the inflammatory response of lipopolysaccharide-activated murine RAW264.7 macrophages. Mol Nutr Food Res. 2015;59:1524-1534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |