Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.148
Peer-review started: April 21, 2015
First decision: May 13, 2015
Revised: May 26, 2015
Accepted: July 21, 2015
Article in press: July 23, 2015
Published online: August 26, 2015
Aerobic glycolysis, i.e., the Warburg effect, may contribute to the aggressive phenotype of hepatocellular carcinoma. However, increasing evidence highlights the limitations of the Warburg effect, such as high mitochondrial respiration and low glycolysis rates in cancer cells. To explain such contradictory phenomena with regard to the Warburg effect, a metabolic interplay between glycolytic and oxidative cells was proposed, i.e., the “reverse Warburg effect”. Aerobic glycolysis may also occur in the stromal compartment that surrounds the tumor; thus, the stromal cells feed the cancer cells with lactate and this interaction prevents the creation of an acidic condition in the tumor microenvironment. This concept provides great heterogeneity in tumors, which makes the disease difficult to cure using a single agent. Understanding metabolic flexibility by lactate shuttles offers new perspectives to develop treatments that target the hypoxic tumor microenvironment and overcome the limitations of glycolytic inhibitors.
Core tip: The Warburg effect plays a vital role in cancer cell proliferation and survival, and contributes to the initiation of tumor metastasis. To adapt to rapidly changing microenvironment for survival such as from normoxia to hypoxia, cancer cells vary in metabolic phenotype; “metabolic flexibility”. Even in a hypoxic condition, oxidative cancer cells and/or stromal cells should theoretically exist to support the metabolic fuel for glycolytic cancer cells and handle lactate via the dynamic shuttle; “the reverse Warburg effect”. Treatments against tumor metabolism may aim to target two distinct metabolic pathways of glycolysis and mitochondrial oxidative phosphorylation.
- Citation: Lee M, Yoon JH. Metabolic interplay between glycolysis and mitochondrial oxidation: The reverse Warburg effect and its therapeutic implication. World J Biol Chem 2015; 6(3): 148-161
- URL: https://www.wjgnet.com/1949-8454/full/v6/i3/148.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i3.148
Hepatocellular carcinoma (HCC) is the seventh most common cancer and the third cause of cancer-related mortality all over the world[1]. Despite the recent development of various types of targeted agents, minimal improvements have been identified in the survival of patients with advanced HCC since the introduction of sorafenib 10 years ago[2]. To overcome the limitations of current anticancer agents, new strategies must be developed.
Most previous studies have strongly suggested the metabolic reprogramming of cancer cells into aerobic glycolysis, i.e., the Warburg effect, in the process of carcinogenesis and adjustment for the hypoxic tumor microenvironment. Based on this concept, the development of anticancer agents that target the enzymes involved in glycolysis appears to be promising. 3-bromopyruvate (3-BP), is a potent anticancer agent that inhibits the glycolytic pathway primarily leads to a depletion of energy reserves. Previous studies have demonstrated that 3-BP could have strong anticancer effects in various cancer types. However, in a case study performed in Egypt, the killing effect of 3-BP was not as potent as expected in the treatment of a 28-year-old man who presented with stage IV metastatic melanoma. The patient died of cancer progression even though he had received 3-BP treatment[3].
Based on the cancer progression despite 3-BP treatment in this case, mechanisms beyond the Warburg effect may contribute to cancer cell survival, i.e., evading the glycolytic pathway or attenuating the 3-BP anticancer effect. One potential explanation of the mechanism is that cancer cells might have a preference to produce energy reserves via mitochondrial oxidative phosphorylation (OXPHOS) rather than high glycolysis according to their surrounding conditions. The cells use lactate from tumor stromal cells, which is the end product of glycolysis and can fuel mitochondrial OXPHOS after conversion to pyruvate. This phenomenon has been referred to as the ‘‘reverse Warburg effect’’, which indicates increased aerobic glycolysis of stromal cells adjacent to tumor cells[4]. Another potential reason for 3-BP resistance might be the capability of tumor cells to balance the redox potentials, which play a key role in drug detoxification and cellular protection from oxidative injury by free radicals and peroxides, i.e., “chemoresistance” to 3-BP[5].
In this review, we describe the clinical implication of the Warburg effect with a high redox potential, and the roles of mitochondrial OXPHOS with a focus on lactate shuttles beyond the Warburg effect. Given the importance as modulators of tumor cell metabolism, these approaches may represent promising therapeutic targets to potentiate the anticancer effect of 3-BP, which has been well-known as the strongest inhibitor of glycolysis in cancer.
Warburg first reported an anomalous characteristic of energy metabolism in cancer cells[6,7], even in the presence of oxygen, cancer cells can accelerate glycolysis rather than mitochondrial OXPHOS; ‘‘aerobic glycolysis”. Aerobic glycolysis is seemingly contradictory phenomena because cancer cells must compensate for the 18-fold lower efficiency of ATP production afforded by glycolysis as compared to mitochondrial OXPHOS. To compensate this lower efficiency, the cells upregulate glucose transporters such as GLUT1[8-10]. This inefficient energy metabolism provides cancer cells with several advantages: (1) balancing the redox potential inner cell; and (2) increased biosynthesis of intermediate macromolecules, anti-apoptosis, and efficient signaling through metabolites as compared with mitochondrial OXPHOS[9].
In hypoxic tumor cells, the overexpression of glucose transporters and glycolytic enzymes such as hexokinase II (HK II), phosphofructokinase (PFK), phosphoglycerate kinase, and lactate dehydrogenase (LDH), has been investigated[11]. It was reported that high serum levels of glucose transporter (GLUT) 1, GLUT 3, aldolase-B, and HK II have been significantly associated with poor prognosis in various types of malignancies[12]. Among them, HK II, involved in the first rate-limiting step of glycolysis, has been related to anti-apoptosis. A predominant fraction of HK II was bound to the voltage-dependent anion channel (VDAC) at the outer membrane of the mitochondria. Among the proposed mechanism of chemoresistance in cancer cells was the decreased availability of free VDAC sites that interact with pro-apoptotic proteins (Bax). HK II binds to the VDAC site and this interaction prevents the activation of Bax[13]. In other words, HK II play a key role for prevention of chemotherapy-induced, mitochondria-mediated, tumor cell apoptosis. In addition, this glycolysis pathway can be further accentuated under the hypoxic conditions in many tumors: the hypoxic condition can upregulate glucose transporters and induce multiple enzymes of the glycolytic pathway[10,14,15]. For example, both Ras oncoprotein and hypoxia can independently increase HIF1a and HIF2a transcription factor levels which upregulate glycolysis[14,16,17]. Our group demonstrated that hypoxia stimulates HCC cellular growth through hexokinase II induction, which thereby may participate in HCC progression, and the blockage of this enzyme may be therapeutically efficacious in human HCCs[18,19]. Although tumor cells can redirect energy metabolism of “aerobic glycolysis”, it has become apparent that oxygenation, which ranges from normoxia to hypoxia, is not always static in tumor microenvironment, but instead dynamically fluctuates as a result of the dynamic changes of the tumor-associated neovasculature[20].
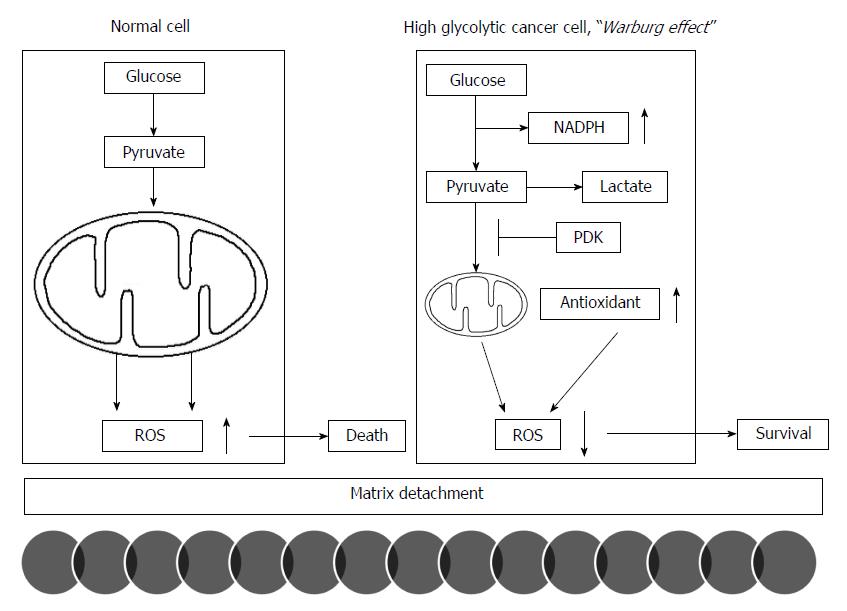
The acceleration of glycolysis in cancer cell energy metabolism could reduce the production of reactive oxygen species (ROS) by less reliance on mitochondrial OXPHOS and simultaneously enhance the redox potential via an increase in NADPH in the pentose phosphate pathway as byproducts for biosynthetic pathways of proliferation. This contribution of the Warburg effect to the balance of redox potential plays a pivotal role in the initiation of metastasis; matrix detachment. While normal cells attenuate mitochondrial OXPHOS in response to matrix detachment for their survival, many cancer cells already limit mitochondrial OXPHOS before detachment because of the Warburg effect. Normal cells activate PDK4 to inhibit PDH following detachment to upregulate glycolytic pathway. However, cancer cells already express high levels of PDK1 under attached conditions[21]. PDK1 and PDK3 expression in various cancers significantly correlates with patients’ prognosis: tumor histological grades and disease-free survival[22,23]. PDK inhibition or PDH activation in cancer cells stimulates mitochondrial OXPHOS and thereby increase ROS production. Excess production of intracellular ROS levels increase their susceptibility to cell death after matrix detachment, which leads to a decreased metastatic potential[21]. Therefore, the Warburg effect allows cancer cells to evade cellular oxidative stress that would be produced by mitochondrial OXPHOS for glucose metabolism[24]. Thus, the reduction of ROS levels, which promotes metastasis, may represent an advantage given by the Warburg effect (Figure 1). Increased glucose consumption diverts more glucose carbon into the oxidative branch of the pentose phosphate pathway, which represents a major source to generate NADPH[25]. NADPH is a critical cofactor for the replenishment of reduced glutathione (GSH) in a cell. Cancer cells can further enhance this antioxidant generation pathway via PKM inhibition when oxidative stress increases[26].
In addition to the Warburg effect, cancer cells also potentiate antioxidant systems to cope with increased oxidative stress[25]. For example, while MnSOD is induced following matrix detachment in normal cells[27], MnSOD is constitutively overexpressed in cancer cells. Furthermore, increased MnSOD expression in cancer is significantly associated with poor prognosis[27-29]. An enhanced antioxidant capacity allows cancer cells to better survive detachment-induced oxidative stress and initiate to metastasize. In a lung cancer mouse model, antioxidant treatments have consistently reduced oxidative stress and accelerated lung cancer progression[30].
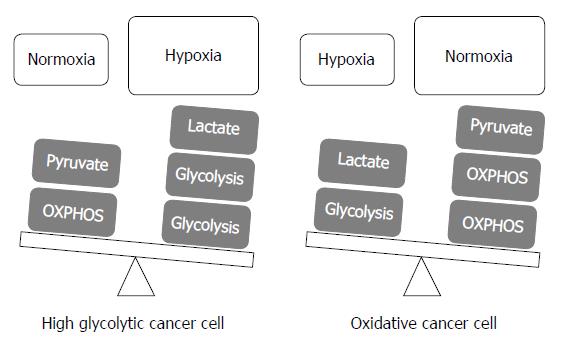
To adapt to rapidly changing microenvironment for their survival such as from normoxia to hypoxia[31,32], each cancer cells may vary in metabolic phenotype even in a single tumor mass; “metabolic flexibility”[33]. Because total ATP production via the glycolytic pathway does not generally exceed 50%-60%[34], mitochondrial OXPHOS still, to a certain extent, contributes to ATP generation in cancer cells, i.e., a mixture of glycolysis and mitochondrial OXPHOS[35]. The ratio of contribution to ATP production in cancer cells can be rapidly changed to maintain pace with the alteration of the tumor microenvironment. Herst et al[36] reported that energy production for tumor cell growth was altered from mitochondrial OXPHOS to accelerated glycolysis according to changes from normoxia to hypoxia: mitochondrial OXPHOS contributes to total ATP production, which was 91% in normoxia and reduced to 36% in hypoxia.
Given this flexibility, all cancer cells did not completely depend on accelerated glycolysis. Previous studies reported that mitochondrial OXPHOS in many cancers can be well-functioned to produce ATPs[9,37-40]. These authors have concluded that the Warburg effect is a result of accelerated glycolysis which suppressed mitochondrial OXPHOS rather than the initial impairments in mitochondrial OXPHOS. If glycolysis is suppressed in cancer cells, the mitochondrial OXPHOS function can be recovered[35,39,41,42]. In a noticeable investigation, Fantin et al[39] demonstrated that when glycolysis was inhibited by suppression of LDH-A in cancer cells, mitochondrial OXPHOS could be restored to compensate for energy production. This study reported that both LDH-A and mitochondrial function were modulated by the metabolite level such as pyruvate and the NADH/NAD+ ratio. This result indicates that cancer cells have the capacity of regulating ATP production by mitochondrial OXPHOS to adapt to the rapidly changing microenvironment.
According to a proposal by Smolková et al[41], metabolic phenotypes in cancer cells can be simply divided into two subgroups with each condition of the tumor microenvironment: (1) enhanced glycolysis and suppressed mitochondrial OXPHOS with a hypoxic condition; and (2) relatively suppressed glycolysis and restoration of mitochondrial OXPHOS with nutrient shortage because of high proliferation rates. This proposal explained that the Warburg phenotype is not a universal finding, and mitochondrial respiration impairment is not a fixed feature of cancer cells[41].
Although the glycolytic inhibitors targeting the Warburg effect have been investigated in various cancer types, the glycolytic inhibitors with the exception of 3-BP (a lactate analog)[18,19,43-70], 3-BrOP (a 3-bromopyruvate derivative)[71-74], and dichloroacetate (DCA)[75-98] have demonstrated low efficacy in arresting tumor growth when used alone[99]; these inhibitors include 2-deoxy-D-glucose (a glucose analog)[70,100-112], lonidamine (a derivative of indazole-3-carboxylic acid)[113-132], methyl jasmonate on HK[133-161], 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one on PFK[162-166], and iodoacetate on glyceraldehydes-3-phosphate dehydrogenase (GAPDH)[167-170].
Among various glycolytic inhibitors, 3-BP could only target two energy pathways of glycolysis and mitochondrial OXPHOS. The mechanism for 3-BP action suggested that mitochondrial HK II is essential for the high glycolytic capacity via the utilization of mitochondrial ATP rather than cytosolic ATP, and the lowering of mitochondrial OXPHOS capacity by limiting Pi and ADP delivery to the mitochondria[171,172]. In contrast, DCA would affect only in cancer cells having impaired mitochondria function by PDK inhibition: otherwise alternative energy sources could compensate for the inhibited glycolysis through the competent mitochondria[173]. No phase III randomized clinical trial of glycolytic inhibitors has exhibited satisfactory clinical outcomes[174].
Of note, the previously described studies that have reported the anticancer effects of these glycolytic inhibitors, with the exception of 3-BP, have exhibited common characteristics: (1) a low efficacy for anticancer effects when used alone; (2) capable of sensitizing cancer cells to conventional chemotherapy drugs, such as 5-fluorouracil, cisplatin, doxorubicin, and sorafenib; (3) the role of a sensitizer to make cancer cells vulnerable to radiotherapy and photodynamic therapy; and (4) an increase in the intracellular ROS levels as an important mechanism to induce their apoptosis.
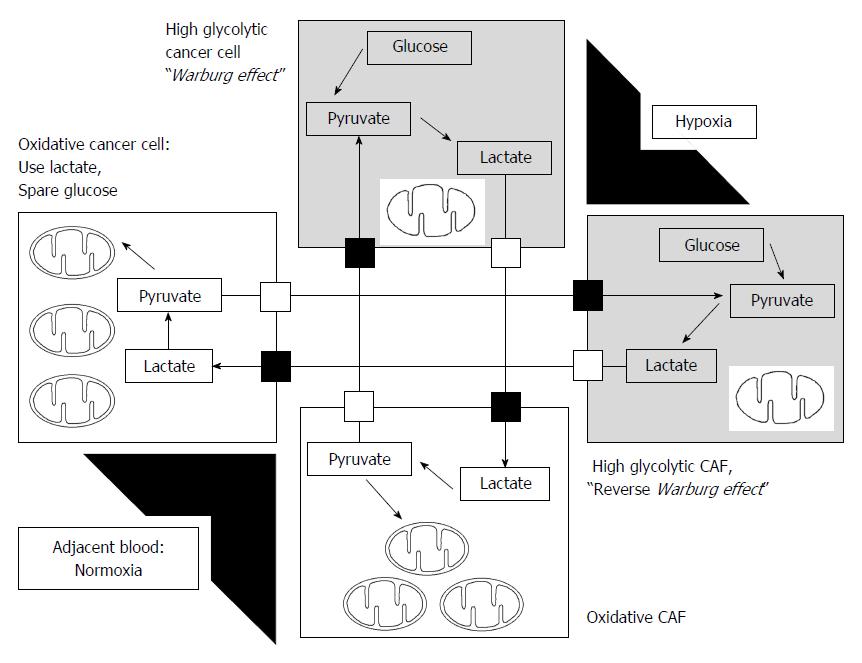
To explain metabolic flexibility, i.e., mitochondrial OXPHOS, the reverse Warburg effect was suggested[175]; cancer cells educate carcinoma-associated fibroblasts (CAFs) to enhance aerobic glycolysis, and CAFs thereby produce lactate, which was converted to pyruvate and utilized for mitochondrial OXPHOS in cancer cells[175,176]. Tumor cells and CAFs influence each other in energy metabolites for co-evolution in cancer progression. A growing body of evidence indicates that lactate as an end product of glycolysis in hypoxic cancer cells and/or CAFs is not a waste product. Lactate can be used as energy fuel for oxygenated tumor cells and/or oxidative CAFs as shown in Figure 2. Lactate is converted to pyruvate by LDH-B, which can enter the mitochondria to produce ATP in the cells with restored mitochondrial OXPHOS[31,40,176-181]. Particularly, oxidative tumor cells in normoxic microenvironment use mitochondrial OXPHOS to spare glucose, which can be utilized by glycolytic tumor cells located in hypoxic microenvironment.
During communication between glycolytic and oxidative cells, MCT1 and MCT4 are key players in this metabolic cross-talk. Influx of lactate by oxidative cancer cells occurs through MCT1, whereas lactate is released through MCT4[177]. MCT1 inhibition can make a metabolic shift from mitochondrial oxidation to glycolysis, which thereby induce glucose consumption. MCT4 inhibition can directly induce cell death via accumulation of intracellular lactic acid in hypoxic tumor cells[177]. Clinical phase I trials investigating MCT1 inhibitors are ongoing (http://clinicaltrials.gov/show/NCT01791595).
In addition to metabolic fuel by lactate shuttle, this phenomenon can maintain an acid-base balance via the prevention of the development of a fatal acidic environment in cancer cells[181,182]. Koukourakis et al[181,182] reported that increased expression of MCT1, LDH, and PDH in CAFs metabolically utilize lactate produced by tumor cells. In the other way, previous studies reported that some CAFs can undergo aerobic glycolysis and provide nearby oxidative cancer cells with the released lactate[176,182,183] (Figure 2).
Furthermore, accumulation of lactic acid, which represents high glycolysis rates in hypoxic conditions, reflects poor vascularity caused by rapid tumor growth. Previous studies demonstrated that lactate released from glycolytic tumor cells through MCT4 can stimulate angiogenesis and tumor growth via IL-8 dependent pathway[184]. Taken together, these findings establish important roles for lactate shuttles in tumors: (1) it acts as both a metabolic fuel; and (2) maintains an acid-base balance in cancer cells; and (3) signals for angiogenesis in hypoxic microenvironment. Finally, cancer cells can adapt to rapid changes in the tumor microenvironment through the metabolic interplay between oxidative and glycolytic cells, such as glycolytic and oxidative tumor cells and glycolytic and oxidative stromal cells, through lactate shuttle: “tumor heterogeneity and metabolic flexibility” (Figure 2).
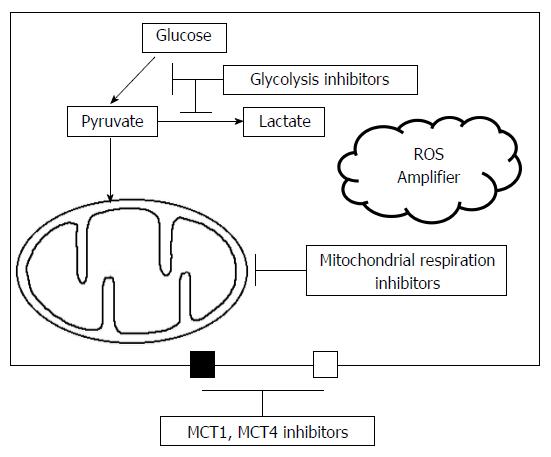
Although increasing evidence has recently indicated that mitochondrial OXPHOS and lactate shuttle may contribute to cancer cell survival and progression, the Warburg effect still plays a pivotal role in cancer cell metabolism and the initiation of metastasis in hypoxic conditions as previously discussed. Therefore, therapeutic strategies should focus on both targets, glycolysis and mitochondrial OXPHOS. Even though some studies reported that mitochondrial OXPHOS is also one of 3-BP targets[185], 3-BP has been mainly demonstrated to be the most potent glycolytic inhibitor among various types of inhibitors. However, including our studies, the results of in vivo studies that have used human HCC cell lines did not exhibit complete remission, but showed only partial remission after 3-BP treatment[18,19,43-70]. One reason why 3-BP did not completely suppress tumor growth might be the low efficiency to suppress mitochondrial OXPHOS, the lactate shuttle, and high redox potential in cancer cells. To overcome the weakness of glycolytic inhibitors, the inhibitors to target mitochondrial OXPHOS and other involved mechanisms such as the suppression of ROS production, might be effective when used simultaneously with glycolytic inhibitors as a combination treatment.
The metabolic interplay (lactate shuttle) between glycolytic and oxidative cells in tumors is modulated by two simple compartments for ATP production; glycolytic pathway and mitochondrial OXPHOS. Therefore, a combined treatment targeting both glycolysis and mitochondrial OXPHOS, could potentially be effective in suppression of tumor growth. Although various agents have been introduced to suppress mitochondrial metabolism, two agents, including metformin and glutamate dehydrogenase 1, were noticeable regarding their safety and potency. The metformin is widely used for Type II diabetes in practical fields and has anti-cancer effects in animal models. Previous studies demonstrated that metformin inhibits complex I via the inhibition of ubiquinone reduction and independently stimulates ROS production by the complex I flavin[186-188]. It might be more clinically meaningful in terms of tolerable safety for a long period as demonstrated in diabetic patients.
Another promising agent that targets mitochondrial metabolism is the mitochondrial enzyme glutamate dehydrogenase 1 (GDH1). Previous reports demonstrated that glutamine may be utilized as the energy fuel for mitochondrial OXPHOS in cancer cells[189-192]. GDH1 is upregulated in human cancers and important for redox homeostasis by controlling the intracellular levels of its product alpha-ketoglutarate and subsequent metabolite fumarate, which subsequently activates glutathione peroxidase 1, i.e., the antioxidant system. Targeting GDH1 by a small molecule inhibitor, the purpurin analog R162, resulted in an imbalanced redox homeostasis, which led to suppress tumor growth[193].
As described above, the common characteristics of glycolytic inhibitors were to increase ROS levels in cancer cells. Thus, a further increase in ROS stress using exogenous ROS enhancers combined with glycolytic inhibitors might effectively increase ROS levels above the threshold stimulating cell death pathways. The vulnerability for ROS stress in normal and cancer cells is quite different. Normal cells have tolerability for a certain level of exogenous ROS stress because of their high antioxidant capacity for lowering the ROS level and thereby prevent to reach the cell-death threshold[194,195]. In cancer cells, the increased ROS production from metabolic disarrangement and rapid proliferation may induce an upregulation of antioxidant capacity, i.e., vulnerable redox equilibrium with high ROS production and elimination to maintain the ROS levels below the threshold for cell death[196,197]. Thus, cancer cells would be more vulnerable to increased oxidative stress induced by exogenous ROS enhancers that directly or indirectly suppress the antioxidant system[198-201]. These characteristics may provide a biochemical basis for a combination treatment of glycolytic inhibitors and ROS enhancers.
There have been a lot of advances to understand the importance of the Warburg effect and the metabolic interplay between glycolytic and oxidative cells in terms of lactate shuttle in previous decades. As previously discussed, the Warburg effect plays a vital role in cancer cell proliferation and survival in hypoxia, and also contributes to the initiation of tumor metastasis as matrix detachment. This effect is also highly linked to lowering the ROS levels to remain away from the cell death threshold. However, to survive under hypoxic conditions, a small portion of cancer cells and/or stromal cells should potentiate their mitochondrial OXPHOS rather than glycolysis to prevent a hostile acidic environment of lactate accumulation, which is the end product of the glycolytic pathway in hypoxic cancer cells. Thus, even in a hypoxic condition, oxidative cancer cells and/or stromal cells should theoretically exist to support the metabolic fuel for glycolytic cancer cells and handle their waste of lactate via the dynamic shuttle of lactate. Tumor heterogeneity exists in tumor mass under hypoxia, and metabolic flexibility is one adaptation mechanism to oxygen gradients (Figure 3).
Treatments against tumor metabolism may aim to target two distinct metabolic pathways of glycolysis and mitochondrial OXPHOS as a combination treatment (Figure 4). Although targeting one specific element of the tumor metabolism could often be ineffective because of the dynamic changes between glycolysis and mitochondrial OXPHOS, targeted treatments against glycolytic-oxidative cell interactions through the inhibition of glycolysis and mitochondrial metabolism might become a promising treatment for advanced stage HCC in clinical practice.
Given the unsatisfactory results of tyrosine kinase inhibitors in HCC treatment, a better understanding of the dynamic interactions between intracellular interactions, such as glycolysis and sensitivity of ROS amplification, and the intercellular interplay, such as glycolytic and mitochondrial OXPHOS cells in cancers, is critical to elucidate the heterogeneous biological features of HCC and identify effective strategies.
P- Reviewer: Bai G, Echtay KS, Migliaccio E S- Editor: Tian YL L- Editor: A E- Editor: Wang CH
| 1. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [PubMed] [Cited in This Article: ] |
| 2. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9016] [Cited by in F6Publishing: 9499] [Article Influence: 593.7] [Reference Citation Analysis (1)] |
| 3. | El Sayed SM, Mohamed WG, Seddik MA, Ahmed AS, Mahmoud AG, Amer WH, Helmy Nabo MM, Hamed AR, Ahmed NS, Abd-Allah AA. Safety and outcome of treatment of metastatic melanoma using 3-bromopyruvate: a concise literature review and case study. Chin J Cancer. 2014;33:356-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Lisanti MP, Martinez-Outschoorn UE, Chiavarina B, Pavlides S, Whitaker-Menezes D, Tsirigos A, Witkiewicz A, Lin Z, Balliet R, Howell A. Understanding the “lethal” drivers of tumor-stroma co-evolution: emerging role(s) for hypoxia, oxidative stress and autophagy/mitophagy in the tumor micro-environment. Cancer Biol Ther. 2010;10:537-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | Wu JH, Batist G. Glutathione and glutathione analogues; therapeutic potentials. Biochim Biophys Acta. 2013;1830:3350-3353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Warburg O. On the origin of cancer cells. Science. 1956;123:309-314. [PubMed] [Cited in This Article: ] |
| 7. | Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269-270. [PubMed] [Cited in This Article: ] |
| 8. | DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2779] [Cited by in F6Publishing: 2821] [Article Influence: 176.3] [Reference Citation Analysis (0)] |
| 9. | Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1605] [Cited by in F6Publishing: 1708] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 10. | Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23:537-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 720] [Cited by in F6Publishing: 751] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 11. | Moreno-Sánchez R, Rodríguez-Enríquez S, Saavedra E, Marín-Hernández A, Gallardo-Pérez JC. The bioenergetics of cancer: is glycolysis the main ATP supplier in all tumor cells? Biofactors. 2014;35:209-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 812] [Cited by in F6Publishing: 840] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 13. | Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166-1173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 741] [Cited by in F6Publishing: 724] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 14. | Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 883] [Cited by in F6Publishing: 961] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 15. | Hayakawa N, Nakamoto Y, Nakatani K, Hatano E, Seo S, Higashi T, Saga T, Uemoto S, Togashi K. Clinical utility and limitations of FDG PET in detecting recurrent hepatocellular carcinoma in postoperative patients. Int J Clin Oncol. 2014;19:1020-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1318] [Cited by in F6Publishing: 1296] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 17. | Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1553] [Cited by in F6Publishing: 1593] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 18. | Gwak GY, Yoon JH, Kim KM, Lee HS, Chung JW, Gores GJ. Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J Hepatol. 2005;42:358-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Kim W, Yoon JH, Jeong JM, Cheon GJ, Lee TS, Yang JI, Park SC, Lee HS. Apoptosis-inducing antitumor efficacy of hexokinase II inhibitor in hepatocellular carcinoma. Mol Cancer Ther. 2007;6:2554-2562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Hardee ME, Dewhirst MW, Agarwal N, Sorg BS. Novel imaging provides new insights into mechanisms of oxygen transport in tumors. Curr Mol Med. 2009;9:435-441. [PubMed] [Cited in This Article: ] |
| 21. | Kamarajugadda S, Stemboroski L, Cai Q, Simpson NE, Nayak S, Tan M, Lu J. Glucose oxidation modulates anoikis and tumor metastasis. Mol Cell Biol. 2012;32:1893-1907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 22. | Lu CW, Lin SC, Chien CW, Lin SC, Lee CT, Lin BW, Lee JC, Tsai SJ. Overexpression of pyruvate dehydrogenase kinase 3 increases drug resistance and early recurrence in colon cancer. Am J Pathol. 2011;179:1405-1414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Wigfield SM, Winter SC, Giatromanolaki A, Taylor J, Koukourakis ML, Harris AL. PDK-1 regulates lactate production in hypoxia and is associated with poor prognosis in head and neck squamous cancer. Br J Cancer. 2008;98:1975-1984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J. 1997;11:388-395. [PubMed] [Cited in This Article: ] |
| 25. | Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3445] [Cited by in F6Publishing: 3492] [Article Influence: 268.6] [Reference Citation Analysis (0)] |
| 26. | Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278-1283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 808] [Cited by in F6Publishing: 851] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 27. | Kamarajugadda S, Cai Q, Chen H, Nayak S, Zhu J, He M, Jin Y, Zhang Y, Ai L, Martin SS. Manganese superoxide dismutase promotes anoikis resistance and tumor metastasis. Cell Death Dis. 2013;4:e504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Landriscina M, Maddalena F, Laudiero G, Esposito F. Adaptation to oxidative stress, chemoresistance, and cell survival. Antioxid Redox Signal. 2009;11:2701-2716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 29. | Pani G, Colavitti R, Bedogni B, Fusco S, Ferraro D, Borrello S, Galeotti T. Mitochondrial superoxide dismutase: a promising target for new anticancer therapies. Curr Med Chem. 2004;11:1299-1308. [PubMed] [Cited in This Article: ] |
| 30. | Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6:221ra15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 525] [Cited by in F6Publishing: 561] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 31. | Chen JL, Lucas JE, Schroeder T, Mori S, Wu J, Nevins J, Dewhirst M, West M, Chi JT. The genomic analysis of lactic acidosis and acidosis response in human cancers. PLoS Genet. 2008;4:e1000293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta. 2010;1805:105-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 796] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 33. | Miccheli A, Tomassini A, Puccetti C, Valerio M, Peluso G, Tuccillo F, Calvani M, Manetti C, Conti F. Metabolic profiling by 13C-NMR spectroscopy: [1,2-13C2]glucose reveals a heterogeneous metabolism in human leukemia T cells. Biochimie. 2006;88:437-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Zu XL, Guppy M. Cancer metabolism: facts, fantasy, and fiction. Biochem Biophys Res Commun. 2004;313:459-465. [PubMed] [Cited in This Article: ] |
| 35. | Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 705] [Cited by in F6Publishing: 769] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 36. | Herst PM, Berridge MV. Cell surface oxygen consumption: a major contributor to cellular oxygen consumption in glycolytic cancer cell lines. Biochim Biophys Acta. 2007;1767:170-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Scott DA, Richardson AD, Filipp FV, Knutzen CA, Chiang GG, Ronai ZA, Osterman AL, Smith JW. Comparative metabolic flux profiling of melanoma cell lines: beyond the Warburg effect. J Biol Chem. 2011;286:42626-42634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 38. | Lim HY, Ho QS, Low J, Choolani M, Wong KP. Respiratory competent mitochondria in human ovarian and peritoneal cancer. Mitochondrion. 2011;11:437-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1141] [Cited by in F6Publishing: 1158] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 40. | Griguer CE, Oliva CR, Gillespie GY. Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines. J Neurooncol. 2005;74:123-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Smolková K, Plecitá-Hlavatá L, Bellance N, Benard G, Rossignol R, Ježek P. Waves of gene regulation suppress and then restore oxidative phosphorylation in cancer cells. Int J Biochem Cell Biol. 2011;43:950-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 42. | Jose C, Bellance N, Rossignol R. Choosing between glycolysis and oxidative phosphorylation: a tumor’s dilemma? Biochim Biophys Acta. 2011;1807:552-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 340] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 43. | Ko YH, Pedersen PL, Geschwind JF. Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Cancer Lett. 2001;173:83-91. [PubMed] [Cited in This Article: ] |
| 44. | Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL. Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res. 2002;62:3909-3913. [PubMed] [Cited in This Article: ] |
| 45. | Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, Torbenson MS, Hullihen J, Pedersen PL. Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun. 2004;324:269-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 286] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 46. | Vali M, Liapi E, Kowalski J, Hong K, Khwaja A, Torbenson MS, Georgiades C, Geschwind JF. Intraarterial therapy with a new potent inhibitor of tumor metabolism (3-bromopyruvate): identification of therapeutic dose and method of injection in an animal model of liver cancer. J Vasc Interv Radiol. 2007;18:95-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Schaefer NG, Geschwind JF, Engles J, Buchanan JW, Wahl RL. Systemic administration of 3-bromopyruvate in treating disseminated aggressive lymphoma. Transl Res. 2012;159:51-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Ko YH, Verhoeven HA, Lee MJ, Corbin DJ, Vogl TJ, Pedersen PL. A translational study “case report” on the small molecule “energy blocker” 3-bromopyruvate (3BP) as a potent anticancer agent: from bench side to bedside. J Bioenerg Biomembr. 2012;44:163-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 49. | Icard P, Zhang XD, Lemoisson E, Louis MH, Allouche S, Lincet H, Poulain L. Experimental results using 3-bromopyruvate in mesothelioma: in vitro and in vivo studies. J Bioenerg Biomembr. 2012;44:81-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Zhang Q, Pan J, North PE, Yang S, Lubet RA, Wang Y, You M. Aerosolized 3-bromopyruvate inhibits lung tumorigenesis without causing liver toxicity. Cancer Prev Res (Phila). 2012;5:717-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Yu SJ, Yoon JH, Yang JI, Cho EJ, Kwak MS, Jang ES, Lee JH, Kim YJ, Lee HS, Kim CY. Enhancement of hexokinase II inhibitor-induced apoptosis in hepatocellular carcinoma cells via augmenting ER stress and anti-angiogenesis by protein disulfide isomerase inhibition. J Bioenerg Biomembr. 2012;44:101-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Ganapathy-Kanniappan S, Kunjithapatham R, Torbenson MS, Rao PP, Carson KA, Buijs M, Vali M, Geschwind JF. Human hepatocellular carcinoma in a mouse model: assessment of tumor response to percutaneous ablation by using glyceraldehyde-3-phosphate dehydrogenase antagonists. Radiology. 2012;262:834-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Ota S, Geschwind JF, Buijs M, Wijlemans JW, Kwak BK, Ganapathy-Kanniappan S. Ultrasound-guided direct delivery of 3-bromopyruvate blocks tumor progression in an orthotopic mouse model of human pancreatic cancer. Target Oncol. 2013;8:145-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Lea MA, Qureshi MS, Buxhoeveden M, Gengel N, Kleinschmit J, Desbordes C. Regulation of the proliferation of colon cancer cells by compounds that affect glycolysis, including 3-bromopyruvate, 2-deoxyglucose and biguanides. Anticancer Res. 2013;33:401-407. [PubMed] [Cited in This Article: ] |
| 55. | Calviño E, Estañ MC, Sánchez-Martín C, Brea R, de Blas E, Boyano-Adánez Mdel C, Rial E, Aller P. Regulation of death induction and chemosensitizing action of 3-bromopyruvate in myeloid leukemia cells: energy depletion, oxidative stress, and protein kinase activity modulation. J Pharmacol Exp Ther. 2014;348:324-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Rieber M, Strasberg-Rieber M. p53 inactivation decreases dependence on estrogen/ERK signalling for proliferation but promotes EMT and susceptility to 3-bromopyruvate in ERα+ breast cancer MCF-7 cells. Biochem Pharmacol. 2014;88:169-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Majkowska-Skrobek G, Augustyniak D, Lis P, Bartkowiak A, Gonchar M, Ko YH, Pedersen PL, Goffeau A, Ułaszewski S. Killing multiple myeloma cells with the small molecule 3-bromopyruvate: implications for therapy. Anticancer Drugs. 2014;25:673-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Liu Z, Zhang YY, Zhang QW, Zhao SR, Wu CZ, Cheng X, Jiang CC, Jiang ZW, Liu H. 3-Bromopyruvate induces apoptosis in breast cancer cells by downregulating Mcl-1 through the PI3K/Akt signaling pathway. Anticancer Drugs. 2014;25:447-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 59. | Xian SL, Cao W, Zhang XD, Lu YF. Inhibitory effects of 3-bromopyruvate on human gastric cancer implant tumors in nude mice. Asian Pac J Cancer Prev. 2014;15:3175-3178. [PubMed] [Cited in This Article: ] |
| 60. | Gong L, Wei Y, Yu X, Peng J, Leng X. 3-Bromopyruvic acid, a hexokinase II inhibitor, is an effective antitumor agent on the hepatoma cells: in vitro and in vivo findings. Anticancer Agents Med Chem. 2014;14:771-776. [PubMed] [Cited in This Article: ] |
| 61. | Bean JF, Qiu YY, Yu S, Clark S, Chu F, Madonna MB. Glycolysis inhibition and its effect in doxorubicin resistance in neuroblastoma. J Pediatr Surg. 2014;49:981-984; discussion 984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Zhang Q, Zhang Y, Zhang P, Chao Z, Xia F, Jiang C, Zhang X, Jiang Z, Liu H. Hexokinase II inhibitor, 3-BrPA induced autophagy by stimulating ROS formation in human breast cancer cells. Genes Cancer. 2014;5:100-112. [PubMed] [Cited in This Article: ] |
| 63. | Wicks RT, Azadi J, Mangraviti A, Zhang I, Hwang L, Joshi A, Bow H, Hutt-Cabezas M, Martin KL, Rudek MA. Local delivery of cancer-cell glycolytic inhibitors in high-grade glioma. Neuro Oncol. 2015;17:70-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Isayev O, Rausch V, Bauer N, Liu L, Fan P, Zhang Y, Gladkich J, Nwaeburu CC, Mattern J, Mollenhauer M. Inhibition of glucose turnover by 3-bromopyruvate counteracts pancreatic cancer stem cell features and sensitizes cells to gemcitabine. Oncotarget. 2014;5:5177-5189. [PubMed] [Cited in This Article: ] |
| 65. | Warmoes MO, Locasale JW. Heterogeneity of glycolysis in cancers and therapeutic opportunities. Biochem Pharmacol. 2014;92:12-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 66. | Glick M, Biddle P, Jantzi J, Weaver S, Schirch D. The antitumor agent 3-bromopyruvate has a short half-life at physiological conditions. Biochem Biophys Res Commun. 2014;452:170-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 67. | Chapiro J, Sur S, Savic LJ, Ganapathy-Kanniappan S, Reyes J, Duran R, Thiruganasambandam SC, Moats CR, Lin M, Luo W. Systemic delivery of microencapsulated 3-bromopyruvate for the therapy of pancreatic cancer. Clin Cancer Res. 2014;20:6406-6417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 68. | Wu L, Xu J, Yuan W, Wu B, Wang H, Liu G, Wang X, Du J, Cai S. The reversal effects of 3-bromopyruvate on multidrug resistance in vitro and in vivo derived from human breast MCF-7/ADR cells. PLoS One. 2014;9:e112132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 69. | Nilsson H, Lindgren D, Mandahl Forsberg A, Mulder H, Axelson H, Johansson ME. Primary clear cell renal carcinoma cells display minimal mitochondrial respiratory capacity resulting in pronounced sensitivity to glycolytic inhibition by 3-Bromopyruvate. Cell Death Dis. 2015;6:e1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 70. | Feng X, Zhang Y, Wang P, Liu Q, Wang X. Energy metabolism targeted drugs synergize with photodynamic therapy to potentiate breast cancer cell death. Photochem Photobiol Sci. 2014;13:1793-1803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 71. | Levy AG, Zage PE, Akers LJ, Ghisoli ML, Chen Z, Fang W, Kannan S, Graham T, Zeng L, Franklin AR. The combination of the novel glycolysis inhibitor 3-BrOP and rapamycin is effective against neuroblastoma. Invest New Drugs. 2012;30:191-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | Yuan S, Wang F, Chen G, Zhang H, Feng L, Wang L, Colman H, Keating MJ, Li X, Xu RH. Effective elimination of cancer stem cells by a novel drug combination strategy. Stem Cells. 2013;31:23-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 73. | Tang Z, Yuan S, Hu Y, Zhang H, Wu W, Zeng Z, Yang J, Yun J, Xu R, Huang P. Over-expression of GAPDH in human colorectal carcinoma as a preferred target of 3-bromopyruvate propyl ester. J Bioenerg Biomembr. 2012;44:117-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 74. | Akers LJ, Fang W, Levy AG, Franklin AR, Huang P, Zweidler-McKay PA. Targeting glycolysis in leukemia: a novel inhibitor 3-BrOP in combination with rapamycin. Leuk Res. 2011;35:814-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Vella S, Conti M, Tasso R, Cancedda R, Pagano A. Dichloroacetate inhibits neuroblastoma growth by specifically acting against malignant undifferentiated cells. Int J Cancer. 2012;130:1484-1493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 76. | Tong J, Xie G, He J, Li J, Pan F, Liang H. Synergistic antitumor effect of dichloroacetate in combination with 5-fluorouracil in colorectal cancer. J Biomed Biotechnol. 2011;2011:740564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 77. | Niewisch MR, Kuçi Z, Wolburg H, Sautter M, Krampen L, Deubzer B, Handgretinger R, Bruchelt G. Influence of dichloroacetate (DCA) on lactate production and oxygen consumption in neuroblastoma cells: is DCA a suitable drug for neuroblastoma therapy? Cell Physiol Biochem. 2012;29:373-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Kumar A, Kant S, Singh SM. Novel molecular mechanisms of antitumor action of dichloroacetate against T cell lymphoma: Implication of altered glucose metabolism, pH homeostasis and cell survival regulation. Chem Biol Interact. 2012;199:29-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | Ayyanathan K, Kesaraju S, Dawson-Scully K, Weissbach H. Combination of sulindac and dichloroacetate kills cancer cells via oxidative damage. PLoS One. 2012;7:e39949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Zheng MF, Shen SY, Huang WD. DCA increases the antitumor effects of capecitabine in a mouse B16 melanoma allograft and a human non-small cell lung cancer A549 xenograft. Cancer Chemother Pharmacol. 2013;72:1031-1041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Xuan Y, Hur H, Ham IH, Yun J, Lee JY, Shim W, Kim YB, Lee G, Han SU, Cho YK. Dichloroacetate attenuates hypoxia-induced resistance to 5-fluorouracil in gastric cancer through the regulation of glucose metabolism. Exp Cell Res. 2014;321:219-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 82. | Strum SB, Adalsteinsson O, Black RR, Segal D, Peress NL, Waldenfels J. Case report: Sodium dichloroacetate (DCA) inhibition of the “Warburg Effect” in a human cancer patient: complete response in non-Hodgkin’s lymphoma after disease progression with rituximab-CHOP. J Bioenerg Biomembr. 2013;45:307-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 83. | Shen YC, Ou DL, Hsu C, Lin KL, Chang CY, Lin CY, Liu SH, Cheng AL. Activating oxidative phosphorylation by a pyruvate dehydrogenase kinase inhibitor overcomes sorafenib resistance of hepatocellular carcinoma. Br J Cancer. 2013;108:72-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 84. | Sanchez WY, McGee SL, Connor T, Mottram B, Wilkinson A, Whitehead JP, Vuckovic S, Catley L. Dichloroacetate inhibits aerobic glycolysis in multiple myeloma cells and increases sensitivity to bortezomib. Br J Cancer. 2013;108:1624-1633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 85. | Park JM, Recht LD, Josan S, Merchant M, Jang T, Yen YF, Hurd RE, Spielman DM, Mayer D. Metabolic response of glioma to dichloroacetate measured in vivo by hyperpolarized (13)C magnetic resonance spectroscopic imaging. Neuro Oncol. 2013;15:433-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | Ohashi T, Akazawa T, Aoki M, Kuze B, Mizuta K, Ito Y, Inoue N. Dichloroacetate improves immune dysfunction caused by tumor-secreted lactic acid and increases antitumor immunoreactivity. Int J Cancer. 2013;133:1107-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 87. | Kumar A, Kant S, Singh SM. Antitumor and chemosensitizing action of dichloroacetate implicates modulation of tumor microenvironment: a role of reorganized glucose metabolism, cell survival regulation and macrophage differentiation. Toxicol Appl Pharmacol. 2013;273:196-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 88. | Hur H, Xuan Y, Kim YB, Lee G, Shim W, Yun J, Ham IH, Han SU. Expression of pyruvate dehydrogenase kinase-1 in gastric cancer as a potential therapeutic target. Int J Oncol. 2013;42:44-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 89. | Dunbar EM, Coats BS, Shroads AL, Langaee T, Lew A, Forder JR, Shuster JJ, Wagner DA, Stacpoole PW. Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Invest New Drugs. 2014;32:452-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 90. | Ruggieri V, Agriesti F, Scrima R, Laurenzana I, Perrone D, Tataranni T, Mazzoccoli C, Lo Muzio L, Capitanio N, Piccoli C. Dichloroacetate, a selective mitochondria-targeting drug for oral squamous cell carcinoma: a metabolic perspective of treatment. Oncotarget. 2015;6:1217-1230. [PubMed] [Cited in This Article: ] |
| 91. | Lin G, Hill DK, Andrejeva G, Boult JK, Troy H, Fong AC, Orton MR, Panek R, Parkes HG, Jafar M. Dichloroacetate induces autophagy in colorectal cancer cells and tumours. Br J Cancer. 2014;111:375-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 92. | Ho N, Coomber BL. Pyruvate dehydrogenase kinase expression and metabolic changes following dichloroacetate exposure in anoxic human colorectal cancer cells. Exp Cell Res. 2015;331:73-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Haugrud AB, Zhuang Y, Coppock JD, Miskimins WK. Dichloroacetate enhances apoptotic cell death via oxidative damage and attenuates lactate production in metformin-treated breast cancer cells. Breast Cancer Res Treat. 2014;147:539-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 94. | Garon EB, Christofk HR, Hosmer W, Britten CD, Bahng A, Crabtree MJ, Hong CS, Kamranpour N, Pitts S, Kabbinavar F. Dichloroacetate should be considered with platinum-based chemotherapy in hypoxic tumors rather than as a single agent in advanced non-small cell lung cancer. J Cancer Res Clin Oncol. 2014;140:443-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 95. | Gang BP, Dilda PJ, Hogg PJ, Blackburn AC. Targeting of two aspects of metabolism in breast cancer treatment. Cancer Biol Ther. 2014;15:1533-1541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Delaney LM, Ho N, Morrison J, Farias NR, Mosser DD, Coomber BL. Dichloroacetate affects proliferation but not survival of human colorectal cancer cells. Apoptosis. 2015;20:63-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 97. | Dai Y, Xiong X, Huang G, Liu J, Sheng S, Wang H, Qin W. Dichloroacetate enhances adriamycin-induced hepatoma cell toxicity in vitro and in vivo by increasing reactive oxygen species levels. PLoS One. 2014;9:e92962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 98. | Abildgaard C, Dahl C, Basse AL, Ma T, Guldberg P. Bioenergetic modulation with dichloroacetate reduces the growth of melanoma cells and potentiates their response to BRAFV600E inhibition. J Transl Med. 2014;12:247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | Rodríguez-Enríquez S, Marín-Hernández A, Gallardo-Pérez JC, Carreño-Fuentes L, Moreno-Sánchez R. Targeting of cancer energy metabolism. Mol Nutr Food Res. 2009;53:29-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 100. | Zhang F, Aft RL. Chemosensitizing and cytotoxic effects of 2-deoxy-D-glucose on breast cancer cells. J Cancer Res Ther. 2009;5 Suppl 1:S41-S43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 101. | Piña Y, Houston SK, Murray TG, Boutrid H, Celdran M, Feuer W, Shi W, Hernandez E, Lampidis TJ. Focal, periocular delivery of 2-deoxy-D-glucose as adjuvant to chemotherapy for treatment of advanced retinoblastoma. Invest Ophthalmol Vis Sci. 2010;51:6149-6156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 102. | Cheng G, Zielonka J, Dranka BP, McAllister D, Mackinnon AC, Joseph J, Kalyanaraman B. Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 2012;72:2634-2644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 103. | Aghaee F, Pirayesh Islamian J, Baradaran B. Enhanced radiosensitivity and chemosensitivity of breast cancer cells by 2-deoxy-d-glucose in combination therapy. J Breast Cancer. 2012;15:141-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 104. | Sullivan EJ, Kurtoglu M, Brenneman R, Liu H, Lampidis TJ. Targeting cisplatin-resistant human tumor cells with metabolic inhibitors. Cancer Chemother Pharmacol. 2014;73:417-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 105. | Liu H, Kurtoglu M, Cao Y, Xi H, Kumar R, Axten JM, Lampidis TJ. Conversion of 2-deoxyglucose-induced growth inhibition to cell death in normoxic tumor cells. Cancer Chemother Pharmacol. 2013;72:251-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 106. | Issaq SH, Teicher BA, Monks A. Bioenergetic properties of human sarcoma cells help define sensitivity to metabolic inhibitors. Cell Cycle. 2014;13:1152-1161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 107. | Farooque A, Singh N, Adhikari JS, Afrin F, Dwarakanath BS. Enhanced antitumor immunity contributes to the radio-sensitization of ehrlich ascites tumor by the glycolytic inhibitor 2-deoxy-D-glucose in mice. PLoS One. 2014;9:e108131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 108. | Fan LX, Liu CM, Gao AH, Zhou YB, Li J. Berberine combined with 2-deoxy-d-glucose synergistically enhances cancer cell proliferation inhibition via energy depletion and unfolded protein response disruption. Biochim Biophys Acta. 2013;1830:5175-5183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 109. | Estañ MC, Calviño E, de Blas E, Boyano-Adánez Mdel C, Mena ML, Gómez-Gómez M, Rial E, Aller P. 2-Deoxy-D-glucose cooperates with arsenic trioxide to induce apoptosis in leukemia cells: involvement of IGF-1R-regulated Akt/mTOR, MEK/ERK and LKB-1/AMPK signaling pathways. Biochem Pharmacol. 2012;84:1604-1616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 110. | Dilip A, Cheng G, Joseph J, Kunnimalaiyaan S, Kalyanaraman B, Kunnimalaiyaan M, Gamblin TC. Mitochondria-targeted antioxidant and glycolysis inhibition: synergistic therapy in hepatocellular carcinoma. Anticancer Drugs. 2013;24:881-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 111. | Cheng Y, Diao D, Zhang H, Guo Q, Wu X, Song Y, Dang C. High glucose-induced resistance to 5-fluorouracil in pancreatic cancer cells alleviated by 2-deoxy-D-glucose. Biomed Rep. 2014;2:188-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 112. | Cheng G, Zielonka J, McAllister D, Tsai S, Dwinell MB, Kalyanaraman B. Profiling and targeting of cellular bioenergetics: inhibition of pancreatic cancer cell proliferation. Br J Cancer. 2014;111:85-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 113. | Fanciulli M, Valentini A, Bruno T, Citro G, Zupi G, Floridi A. Effect of the antitumor drug lonidamine on glucose metabolism of adriamycin-sensitive and -resistant human breast cancer cells. Oncol Res. 1996;8:111-120. [PubMed] [Cited in This Article: ] |
| 114. | Villa R, Orlandi L, Berruti A, Dogliotti L, Zaffaroni N. Modulation of cytotoxic drug activity by mitotane and lonidamine in human adrenocortical carcinoma cells. Int J Oncol. 1999;14:133-138. [PubMed] [Cited in This Article: ] |
| 115. | Ricotti L, Tesei A, De Paola F, Milandri C, Amadori D, Frassineti GL, Ulivi P, Zoli W. Potentiation of antiproliferative drug activity by lonidamine in hepatocellular carcinoma cells. J Chemother. 2003;15:480-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 116. | Pacini P, Rinaldini M, Algeri R, Guarneri A, Tucci E, Barsanti G, Neri B, Bastiani P, Marzano S, Fallai C. FEC (5-fluorouracil, epidoxorubicin and cyclophosphamide) versus EM (epidoxorubicin and mitomycin-C) with or without lonidamine as first-line treatment for advanced breast cancer. A multicentric randomised study. Final results. Eur J Cancer. 2000;36:966-975. [PubMed] [Cited in This Article: ] |
| 117. | Oudard S, Carpentier A, Banu E, Fauchon F, Celerier D, Poupon MF, Dutrillaux B, Andrieu JM, Delattre JY. Phase II study of lonidamine and diazepam in the treatment of recurrent glioblastoma multiforme. J Neurooncol. 2003;63:81-86. [PubMed] [Cited in This Article: ] |
| 118. | Li YC, Fung KP, Kwok TT, Lee CY, Suen YK, Kong SK. Mitochondrial targeting drug lonidamine triggered apoptosis in doxorubicin-resistant HepG2 cells. Life Sci. 2002;71:2729-2740. [PubMed] [Cited in This Article: ] |
| 119. | Kaplan O. Correspondence re: M. Fanciulli et al., Energy metabolism of human LoVo colon carcinoma cells: correlation to drug resistance and influence fo lonidamine. Clin. Cancer Res., 6: 1590-1597, 2000. Clin Cancer Res. 2000;6:4166-4167. [PubMed] [Cited in This Article: ] |
| 120. | Gebbia V, Borsellino N, Testa A, Latteri MA, Milia V, Valdesi M, Giotta F, Gebbia N, Colucci G. Cisplatin and epirubicin plus oral lonidamine as first-line treatment for metastatic breast cancer: a phase II study of the Southern Italy Oncology Group (GOIM). Anticancer Drugs. 1997;8:943-948. [PubMed] [Cited in This Article: ] |
| 121. | De Lena M, Lorusso V, Bottalico C, Brandi M, De Mitrio A, Catino A, Guida M, Latorre A, Leone B, Vallejo C. Revertant and potentiating activity of lonidamine in patients with ovarian cancer previously treated with platinum. J Clin Oncol. 1997;15:3208-3213. [PubMed] [Cited in This Article: ] |
| 122. | De Cesare M, Pratesi G, Giusti A, Polizzi D, Zunino F. Stimulation of the apoptotic response as a basis for the therapeutic synergism of lonidamine and cisplatin in combination in human tumour xenografts. Br J Cancer. 1998;77:434-439. [PubMed] [Cited in This Article: ] |
| 123. | Angioli R, Janicek M, Sevin B, Estape R, Averette H, Koechli O, Untch M, Penalver M. Use of lonidamine to potentiate the effect of cisplatin and carboplatin on platinum resistant human ovarian cancer cells. Int J Oncol. 1997;11:777-780. [PubMed] [Cited in This Article: ] |
| 124. | Prabhakara S, Kalia VK. Optimizing radiotherapy of brain tumours by a combination of temozolomide & amp; lonidamine. Indian J Med Res. 2008;128:140-148. [PubMed] [Cited in This Article: ] |
| 125. | Nath K, Nelson DS, Ho AM, Lee SC, Darpolor MM, Pickup S, Zhou R, Heitjan DF, Leeper DB, Glickson JD. (31) P and (1) H MRS of DB-1 melanoma xenografts: lonidamine selectively decreases tumor intracellular pH and energy status and sensitizes tumors to melphalan. NMR Biomed. 2013;26:98-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 126. | Nath K, Nelson DS, Heitjan DF, Leeper DB, Zhou R, Glickson JD. Lonidamine induces intracellular tumor acidification and ATP depletion in breast, prostate and ovarian cancer xenografts and potentiates response to doxorubicin. NMR Biomed. 2015;28:281-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 127. | Miyato Y, Ando K. Apoptosis of human melanoma cells by a combination of lonidamine and radiation. J Radiat Res. 2004;45:189-194. [PubMed] [Cited in This Article: ] |
| 128. | Milane L, Duan Z, Amiji M. Therapeutic efficacy and safety of paclitaxel/lonidamine loaded EGFR-targeted nanoparticles for the treatment of multi-drug resistant cancer. PLoS One. 2011;6:e24075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 129. | Macchioni L, Davidescu M, Roberti R, Corazzi L. The energy blockers 3-bromopyruvate and lonidamine: effects on bioenergetics of brain mitochondria. J Bioenerg Biomembr. 2014;46:389-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 130. | Li N, Zhang CX, Wang XX, Zhang L, Ma X, Zhou J, Ju RJ, Li XY, Zhao WY, Lu WL. Development of targeting lonidamine liposomes that circumvent drug-resistant cancer by acting on mitochondrial signaling pathways. Biomaterials. 2013;34:3366-3380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 131. | Fuchs AG. [Treatment and post-treatment with lonidamine in human colon carcinoma HT-29 cell line]. Medicina (B Aires). 2008;68:13-22. [PubMed] [Cited in This Article: ] |
| 132. | Calviño E, Estañ MC, Simón GP, Sancho P, Boyano-Adánez Mdel C, de Blas E, Bréard J, Aller P. Increased apoptotic efficacy of lonidamine plus arsenic trioxide combination in human leukemia cells. Reactive oxygen species generation and defensive protein kinase (MEK/ERK, Akt/mTOR) modulation. Biochem Pharmacol. 2011;82:1619-1629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 133. | Yeruva L, Pierre KJ, Carper SW, Elegbede JA, Toy BJ, Wang RC. Jasmonates induce apoptosis and cell cycle arrest in non-small cell lung cancer lines. Exp Lung Res. 2006;32:499-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 134. | Yeruva L, Elegbede JA, Carper SW. Methyl jasmonate decreases membrane fluidity and induces apoptosis through tumor necrosis factor receptor 1 in breast cancer cells. Anticancer Drugs. 2008;19:766-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 135. | Tong QS, Jiang GS, Zheng LD, Tang ST, Cai JB, Liu Y, Zeng FQ, Dong JH. Methyl jasmonate downregulates expression of proliferating cell nuclear antigen and induces apoptosis in human neuroblastoma cell lines. Anticancer Drugs. 2008;19:573-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 136. | Rotem R, Heyfets A, Fingrut O, Blickstein D, Shaklai M, Flescher E. Jasmonates: novel anticancer agents acting directly and selectively on human cancer cell mitochondria. Cancer Res. 2005;65:1984-1993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 137. | Rotem R, Fingrut O, Moskovitz J, Flescher E. The anticancer agent methyl jasmonate induces activation of stress-regulated c-Jun N-terminal kinase and p38 protein kinase in human lymphoid cells. Leukemia. 2003;17:2230-2234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 138. | Kim JH, Lee SY, Oh SY, Han SI, Park HG, Yoo MA, Kang HS. Methyl jasmonate induces apoptosis through induction of Bax/Bcl-XS and activation of caspase-3 via ROS production in A549 cells. Oncol Rep. 2004;12:1233-1238. [PubMed] [Cited in This Article: ] |
| 139. | Ishii Y, Kiyota H, Sakai S, Honma Y. Induction of differentiation of human myeloid leukemia cells by jasmonates, plant hormones. Leukemia. 2004;18:1413-1419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 140. | Ezekwudo DE, Wang RC, Elegbede JA. Methyl jasmonate induced apoptosis in human prostate carcinoma cells via 5-lipoxygenase dependent pathway. J Exp Ther Oncol. 2007;6:267-277. [PubMed] [Cited in This Article: ] |
| 141. | Ezekwudo D, Shashidharamurthy R, Devineni D, Bozeman E, Palaniappan R, Selvaraj P. Inhibition of expression of anti-apoptotic protein Bcl-2 and induction of cell death in radioresistant human prostate adenocarcinoma cell line (PC-3) by methyl jasmonate. Cancer Lett. 2008;270:277-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 142. | Yeruva L, Pierre KJ, Bathina M, Elegbede A, Carper SW. Delayed cytotoxic effects of methyl jasmonate and cis-jasmone induced apoptosis in prostate cancer cells. Cancer Invest. 2008;26:890-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 143. | Yeruva L, Hall C, Elegbede JA, Carper SW. Perillyl alcohol and methyl jasmonate sensitize cancer cells to cisplatin. Anticancer Drugs. 2010;21:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 144. | Xiao XY, Jiang GS, Wang L, Lv L, Zeng FQ. Predominant enhancement of apoptosis induced by methyl jasmonate in bladder cancer cells: therapeutic effect of the Antp-conjugated Smac peptide. Anticancer Drugs. 2011;22:853-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 145. | Tsumura H, Akimoto M, Kiyota H, Ishii Y, Ishikura H, Honma Y. Gene expression profiles in differentiating leukemia cells induced by methyl jasmonate are similar to those of cytokinins and methyl jasmonate analogs induce the differentiation of human leukemia cells in primary culture. Leukemia. 2009;23:753-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 146. | Raviv Z, Zilberberg A, Cohen S, Reischer-Pelech D, Horrix C, Berger MR, Rosin-Arbesfeld R, Flescher E. Methyl jasmonate down-regulates survivin expression and sensitizes colon carcinoma cells towards TRAIL-induced cytotoxicity. Br J Pharmacol. 2011;164:1433-1444 [. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 147. | Park C, Jin CY, Kim GY, Cheong J, Jung JH, Yoo YH, Choi YH. A methyl jasmonate derivative, J-7, induces apoptosis in human hepatocarcinoma Hep3B cells in vitro. Toxicol In Vitro. 2010;24:1920-1926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 148. | Park C, Jin CY, Hwang HJ, Kim GY, Jung JH, Kim WJ, Yoo YH, Choi YH. J7, a methyl jasmonate derivative, enhances TRAIL-mediated apoptosis through up-regulation of reactive oxygen species generation in human hepatoma HepG2 cells. Toxicol In Vitro. 2012;26:86-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 149. | Palmieri B, Iannitti T, Capone S, Flescher E. A preliminary study of the local treatment of preneoplastic and malignant skin lesions using methyl jasmonate. Eur Rev Med Pharmacol Sci. 2011;15:333-336. [PubMed] [Cited in This Article: ] |
| 150. | Jiang G, Zhao J, Xiao X, Tao D, Gu C, Tong Q, Luo B, Wang L, Zeng F. AN N-terminal Smac peptide sensitizes human prostate carcinoma cells to methyl jasmonate-induced apoptosis. Cancer Lett. 2011;302:37-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 151. | Elia U, Flescher E. PI3K/Akt pathway activation attenuates the cytotoxic effect of methyl jasmonate toward sarcoma cells. Neoplasia. 2008;10:1303-1313. [PubMed] [Cited in This Article: ] |
| 152. | Zheng L, Li D, Xiang X, Tong L, Qi M, Pu J, Huang K, Tong Q. Methyl jasmonate abolishes the migration, invasion and angiogenesis of gastric cancer cells through down-regulation of matrix metalloproteinase 14. BMC Cancer. 2013;13:74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 153. | Wang Y, Xiang W, Wang M, Huang T, Xiao X, Wang L, Tao D, Dong L, Zeng F, Jiang G. Methyl jasmonate sensitizes human bladder cancer cells to gambogic acid-induced apoptosis through down-regulation of EZH2 expression by miR-101. Br J Pharmacol. 2014;171:618-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 154. | Wang CF, Wang YQ, Huang FZ, Nie WP, Liu XY, Jiang XZ. Association between reversal of multidrug resistance by methyl jasmonate and P-glycoprotein ATPase activity in hepatocellular carcinoma. J Int Med Res. 2013;41:964-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 155. | Milrot E, Jackman A, Kniazhanski T, Gonen P, Flescher E, Sherman L. Methyl jasmonate reduces the survival of cervical cancer cells and downregulates HPV E6 and E7, and survivin. Cancer Lett. 2012;319:31-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 156. | Milrot E, Jackman A, Flescher E, Gonen P, Kelson I, Keisari Y, Sherman L. Enhanced killing of cervical cancer cells by combinations of methyl jasmonate with cisplatin, X or alpha radiation. Invest New Drugs. 2013;31:333-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 157. | Liu AG, Juvik JA, Jeffery EH, Berman-Booty LD, Clinton SK, Erdman JW. Enhancement of broccoli indole glucosinolates by methyl jasmonate treatment and effects on prostate carcinogenesis. J Med Food. 2014;17:1177-1182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 158. | Klippel S, Jakubikova J, Delmore J, Ooi M, McMillin D, Kastritis E, Laubach J, Richardson PG, Anderson KC, Mitsiades CS. Methyljasmonate displays in vitro and in vivo activity against multiple myeloma cells. Br J Haematol. 2012;159:340-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 159. | Farooqi AA, Butt G, Razzaq Z. Algae extracts and methyl jasmonate anti-cancer activities in prostate cancer: choreographers of ‘the dance macabre’. Cancer Cell Int. 2012;12:50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 160. | Cesari IM, Carvalho E, Figueiredo Rodrigues M, Mendonça Bdos S, Amôedo ND, Rumjanek FD. Methyl jasmonate: putative mechanisms of action on cancer cells cycle, metabolism, and apoptosis. Int J Cell Biol. 2014;2014:572097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 161. | Bruchim I, Sarfstein R, Reiss A, Flescher E, Werner H. IGF1R tyrosine kinase inhibitor enhances the cytotoxic effect of methyl jasmonate in endometrial cancer. Cancer Lett. 2014;352:214-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 162. | Klarer AC, O’Neal J, Imbert-Fernandez Y, Clem A, Ellis SR, Clark J, Clem B, Chesney J, Telang S. Inhibition of 6-phosphofructo-2-kinase (PFKFB3) induces autophagy as a survival mechanism. Cancer Metab. 2014;2:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 163. | Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquière B, Cauwenberghs S, Kuchnio A, Wong BW, Quaegebeur A, Goveia J. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 2014;19:37-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 373] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 164. | Vuyyuri SB, Rinkinen J, Worden E, Shim H, Lee S, Davis KR. Ascorbic acid and a cytostatic inhibitor of glycolysis synergistically induce apoptosis in non-small cell lung cancer cells. PLoS One. 2013;8:e67081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 165. | Clem BF, O’Neal J, Tapolsky G, Clem AL, Imbert-Fernandez Y, Kerr DA, Klarer AC, Redman R, Miller DM, Trent JO. Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther. 2013;12:1461-1470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 166. | Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A, Rasku MA, Arumugam S, Dean WL, Eaton J. Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther. 2008;7:110-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 299] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 167. | Sánchez-Aragó M, Cuezva JM. The bioenergetic signature of isogenic colon cancer cells predicts the cell death response to treatment with 3-bromopyruvate, iodoacetate or 5-fluorouracil. J Transl Med. 2011;9:19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 168. | Bhardwaj V, Rizvi N, Lai MB, Lai JC, Bhushan A. Glycolytic enzyme inhibitors affect pancreatic cancer survival by modulating its signaling and energetics. Anticancer Res. 2010;30:743-749. [PubMed] [Cited in This Article: ] |
| 169. | Fahim FA, Esmat AY, Mady EA, Ibrahim EK. Antitumor activities of iodoacetate and dimethylsulphoxide against solid Ehrlich carcinoma growth in mice. Biol Res. 2003;36:253-262. [PubMed] [Cited in This Article: ] |
| 170. | Andrés MI, Repetto G, Sanz P, Repetto M. Comparative effects of the metabolic inhibitors 2,4-dinitrophenol and iodoacetate on mouse neuroblastoma cells in vitro. Toxicology. 1996;110:123-132. [PubMed] [Cited in This Article: ] |
| 171. | Pedersen PL, Mathupala S, Rempel A, Geschwind JF, Ko YH. Mitochondrial bound type II hexokinase: a key player in the growth and survival of many cancers and an ideal prospect for therapeutic intervention. Biochim Biophys Acta. 2002;1555:14-20. [PubMed] [Cited in This Article: ] |
| 172. | Pedersen PL. Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr. 2007;39:211-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 324] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 173. | Suh DH, Kim MK, No JH, Chung HH, Song YS. Metabolic approaches to overcoming chemoresistance in ovarian cancer. Ann N Y Acad Sci. 2011;1229:53-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 174. | Pathania D, Millard M, Neamati N. Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Adv Drug Deliv Rev. 2009;61:1250-1275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 175. | Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984-4001. [PubMed] [Cited in This Article: ] |
| 176. | Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, Frank PG, Flomenberg N, Howell A, Martinez-Outschoorn UE. Ketones and lactate “fuel” tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9:3506-3514. [PubMed] [Cited in This Article: ] |
| 177. | Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930-3942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 792] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 178. | Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front Pharmacol. 2011;2:49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 316] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 179. | Sandulache VC, Ow TJ, Pickering CR, Frederick MJ, Zhou G, Fokt I, Davis-Malesevich M, Priebe W, Myers JN. Glucose, not glutamine, is the dominant energy source required for proliferation and survival of head and neck squamous carcinoma cells. Cancer. 2011;117:2926-2938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 180. | Feron O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol. 2009;92:329-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 377] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 181. | Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res. 2006;66:632-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 357] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 182. | Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL. Pyruvate dehydrogenase and pyruvate dehydrogenase kinase expression in non small cell lung cancer and tumor-associated stroma. Neoplasia. 2005;7:1-6. [PubMed] [Cited in This Article: ] |
| 183. | Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, Ertel A, Flomenberg N, Witkiewicz AK, Birbe RC, Howell A, Pavlides S, Gandara R. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10:1772-1783. [PubMed] [Cited in This Article: ] |
| 184. | Végran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550-2560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 482] [Cited by in F6Publishing: 542] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 185. | Pereira da Silva AP, El-Bacha T, Kyaw N, dos Santos RS, da-Silva WS, Almeida FC, Da Poian AT, Galina A. Inhibition of energy-producing pathways of HepG2 cells by 3-bromopyruvate. Biochem J. 2009;417:717-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 186. | Andrzejewski S, Gravel SP, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014;2:12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 297] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 187. | Ramsay EE, Hogg PJ, Dilda PJ. Mitochondrial metabolism inhibitors for cancer therapy. Pharm Res. 2011;28:2731-2744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 188. | Bridges HR, Jones AJ, Pollak MN, Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J. 2014;462:475-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 413] [Cited by in F6Publishing: 453] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 189. | Guppy M, Leedman P, Zu X, Russell V. Contribution by different fuels and metabolic pathways to the total ATP turnover of proliferating MCF-7 breast cancer cells. Biochem J. 2002;364:309-315. [PubMed] [Cited in This Article: ] |
| 190. | Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009;69:7986-7993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 316] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 191. | Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 469] [Cited by in F6Publishing: 520] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 192. | Ko YH, Lin Z, Flomenberg N, Pestell RG, Howell A, Sotgia F, Lisanti MP, Martinez-Outschoorn UE. Glutamine fuels a vicious cycle of autophagy in the tumor stroma and oxidative mitochondrial metabolism in epithelial cancer cells: implications for preventing chemotherapy resistance. Cancer Biol Ther. 2011;12:1085-1097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 193. | Jin L, Li D, Alesi GN, Fan J, Kang HB, Lu Z, Boggon TJ, Jin P, Yi H, Wright ER. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell. 2015;27:257-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 194. | Toyokuni S. Novel aspects of oxidative stress-associated carcinogenesis. Antioxid Redox Signal. 2006;8:1373-1377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 195. | Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 882] [Cited by in F6Publishing: 908] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 196. | Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 2006;387:365-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 316] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 197. | Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794-798. [PubMed] [Cited in This Article: ] |
| 198. | Bey EA, Bentle MS, Reinicke KE, Dong Y, Yang CR, Girard L, Minna JD, Bornmann WG, Gao J, Boothman DA. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by beta-lapachone. Proc Natl Acad Sci USA. 2007;104:11832-11837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 277] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 199. | Alexandre J, Batteux F, Nicco C, Chéreau C, Laurent A, Guillevin L, Weill B, Goldwasser F. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int J Cancer. 2006;119:41-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 200. | Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 870] [Cited by in F6Publishing: 836] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 201. | Fruehauf JP, Meyskens FL. Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 685] [Cited by in F6Publishing: 679] [Article Influence: 39.9] [Reference Citation Analysis (0)] |